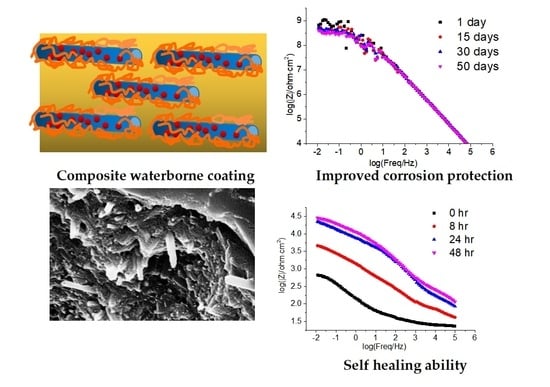
Improving the Protection Performance of Waterborne Coatings with a Corrosion Inhibitor Encapsulated in Polyaniline-Modified Halloysite Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyaniline-Modified HNT (PANI@HNT)
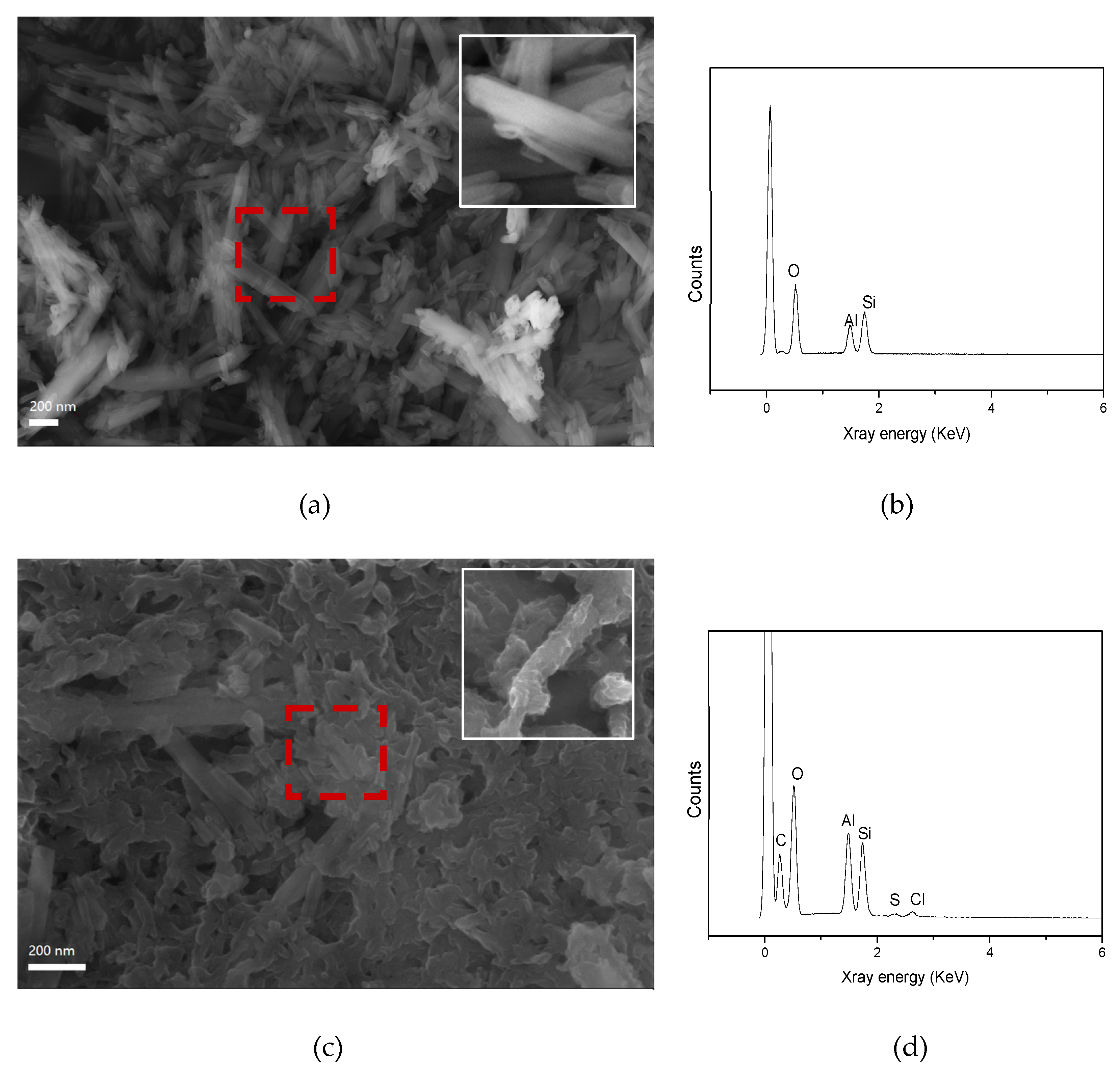
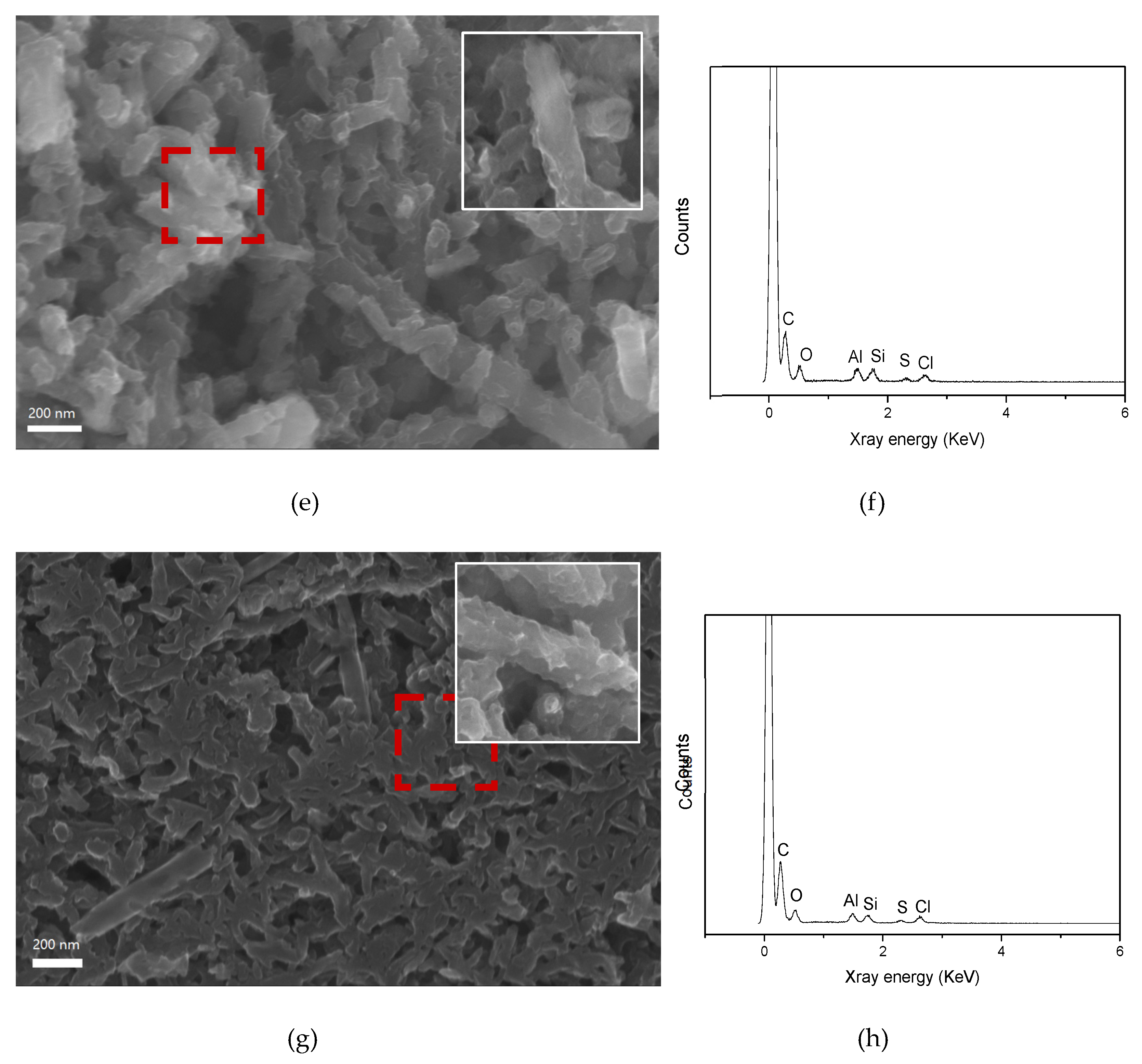
2.3. Scanning Electron Microscopy (SEM)
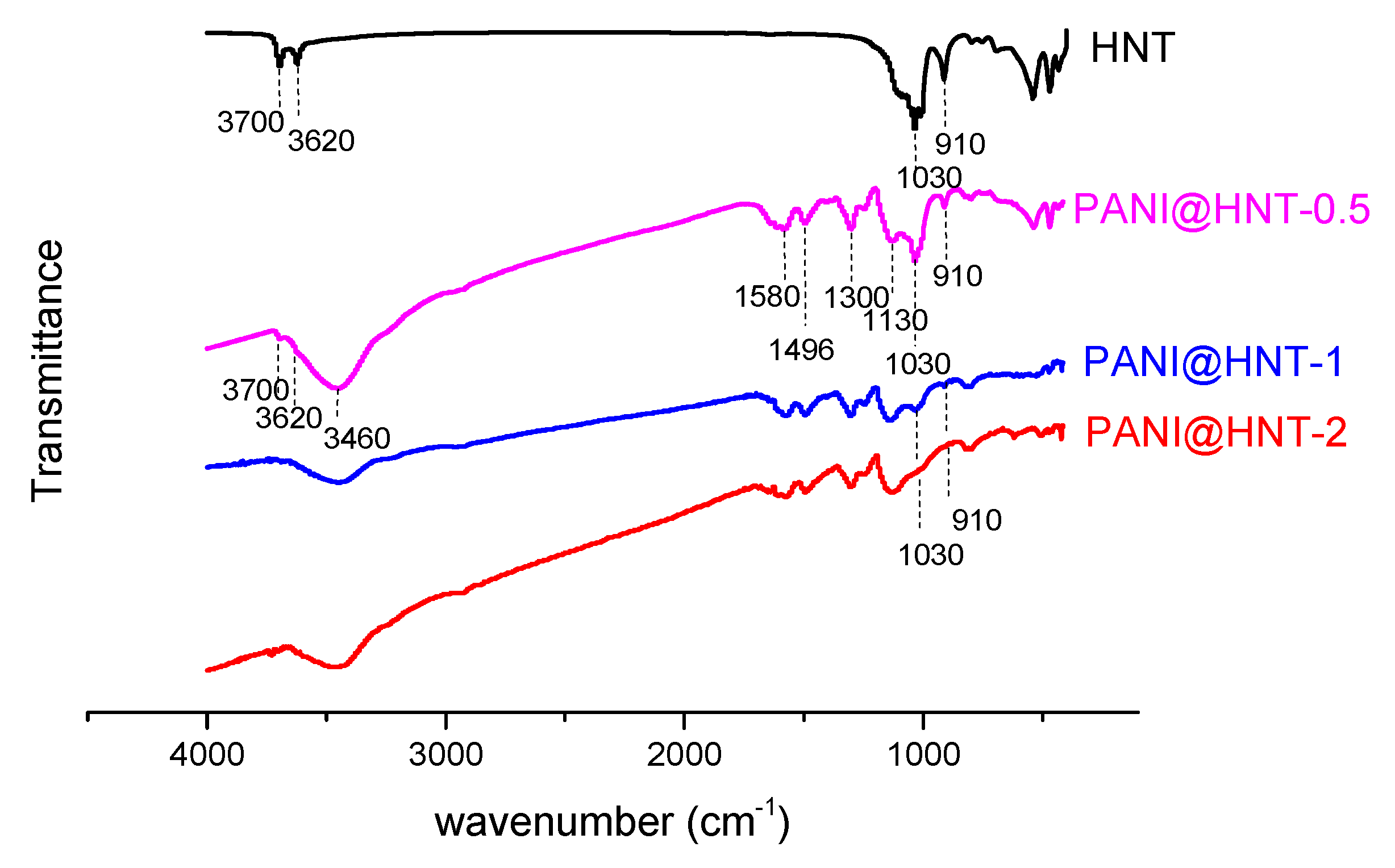
2.4. Fourier Transform Infrared Spectroscopy (FT IR)
2.5. BTA Loading into PANI@HNTs
2.6. Determination of the Loading Ability of PANI@HNT
2.7. Controlled Release of Corrosion Inhibitor
2.8. Coating Preparation and Characterization
2.9. Electrochemical Impedance Spectroscopy (EIS)
2.10. Salt Spray Test
3. Results and Discussion
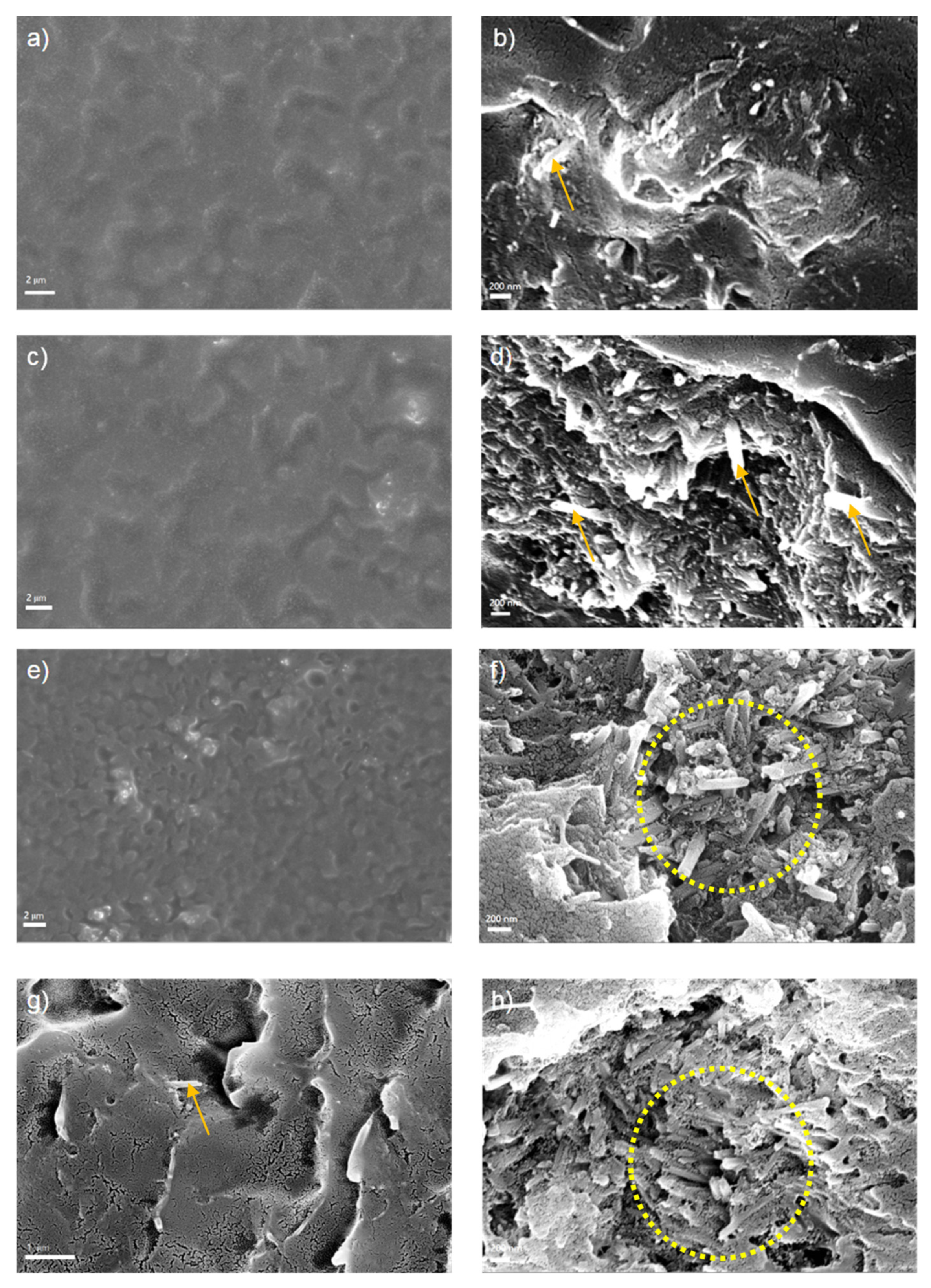
3.1. Synthesis and Characterization of PANI@HNT
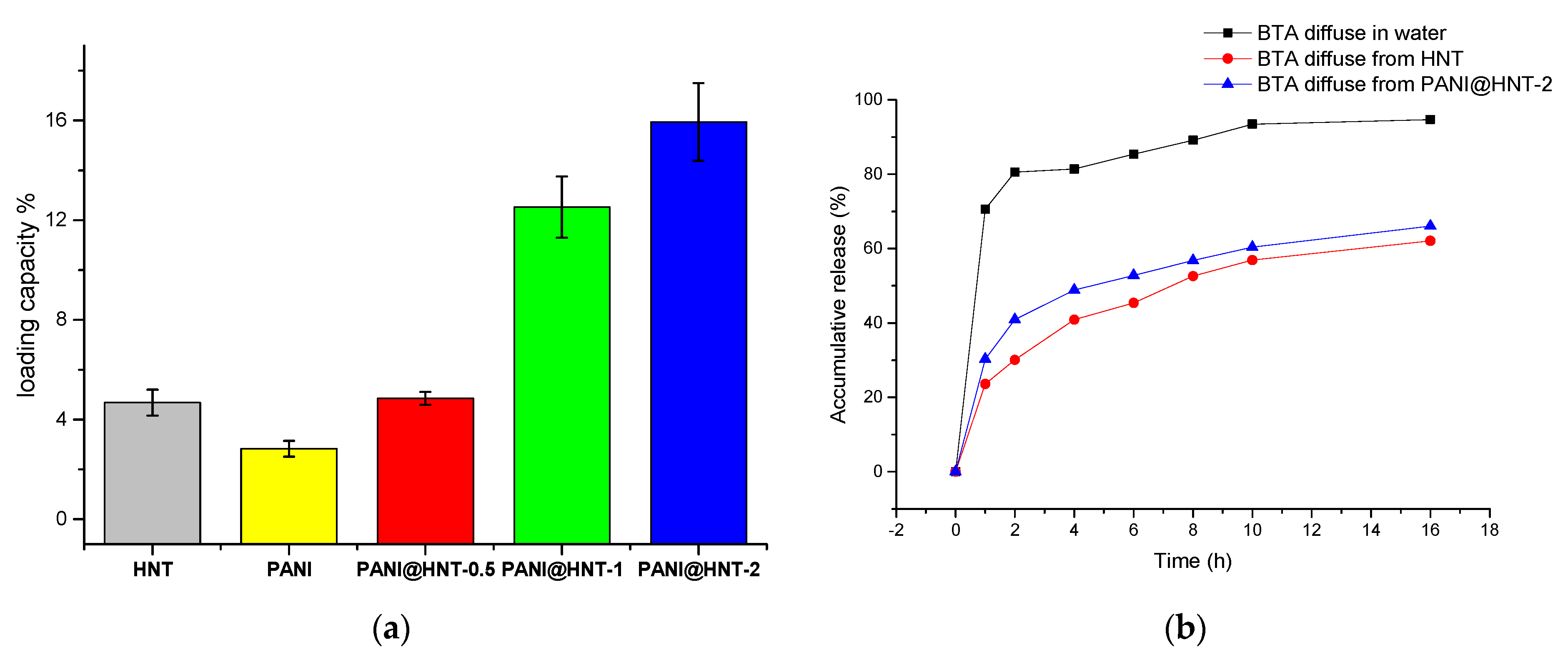
3.2. Corrosion Inhibitor Loading and Release
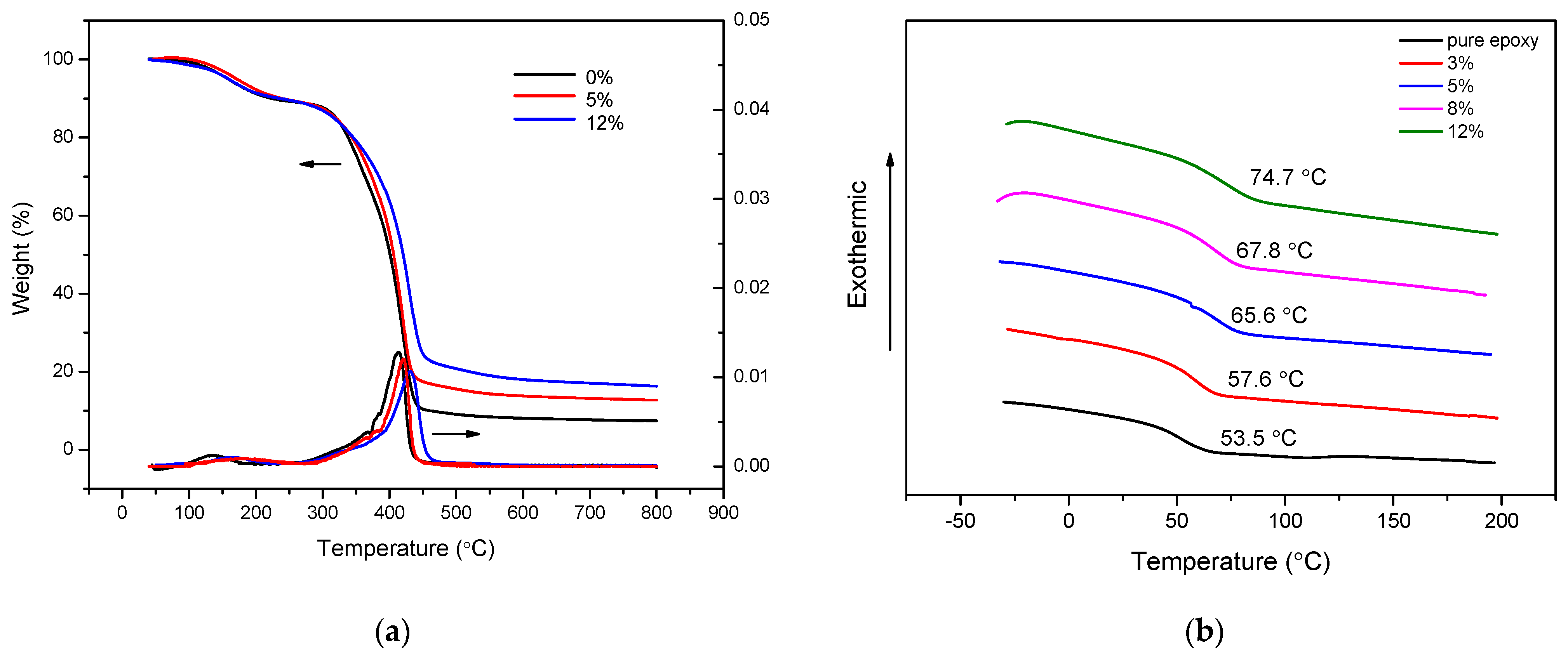
3.3. Coating Preparation and Physical Properties
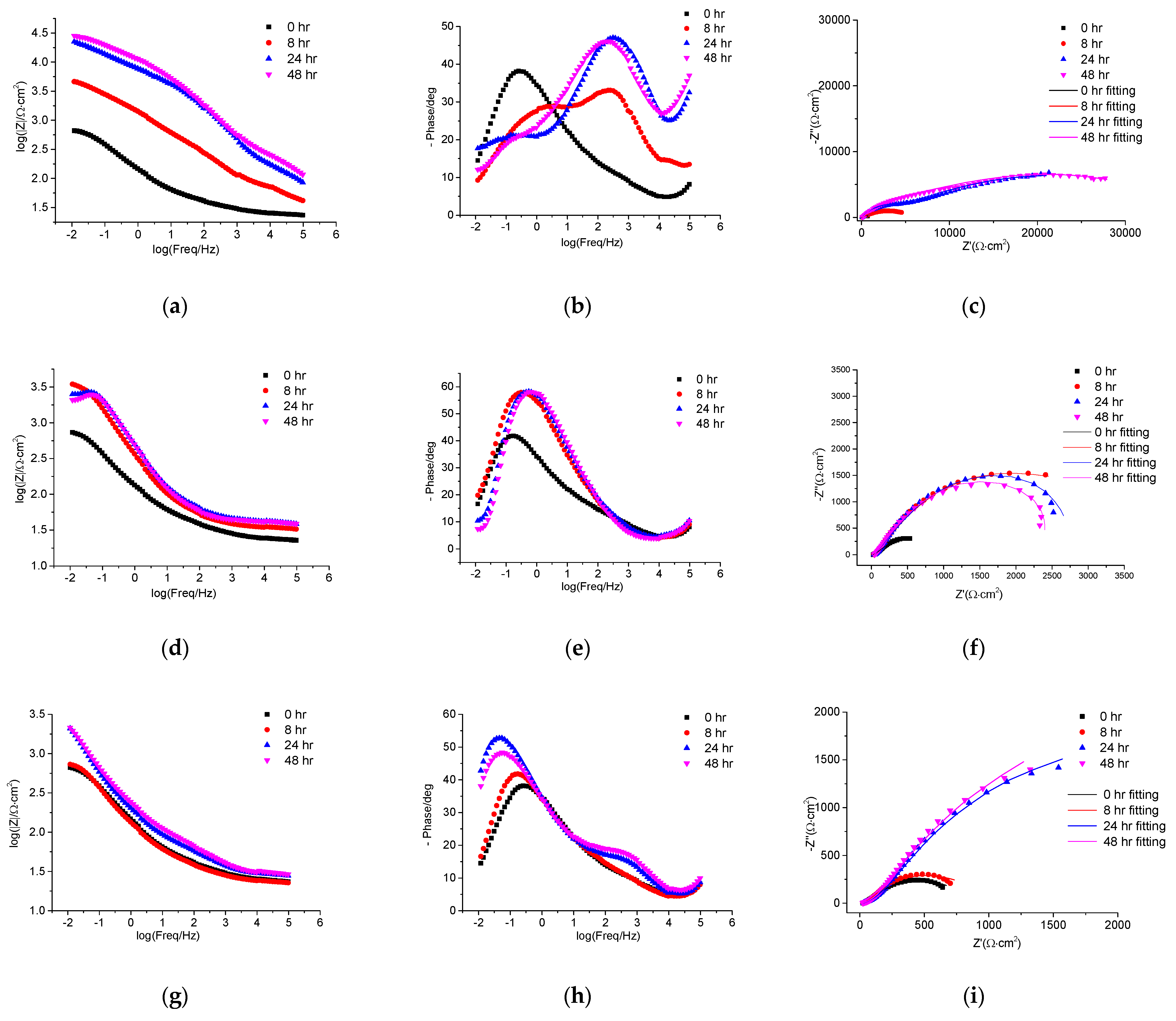
3.4. EIS Corrosion Protection Evaluation of Intact Coatings
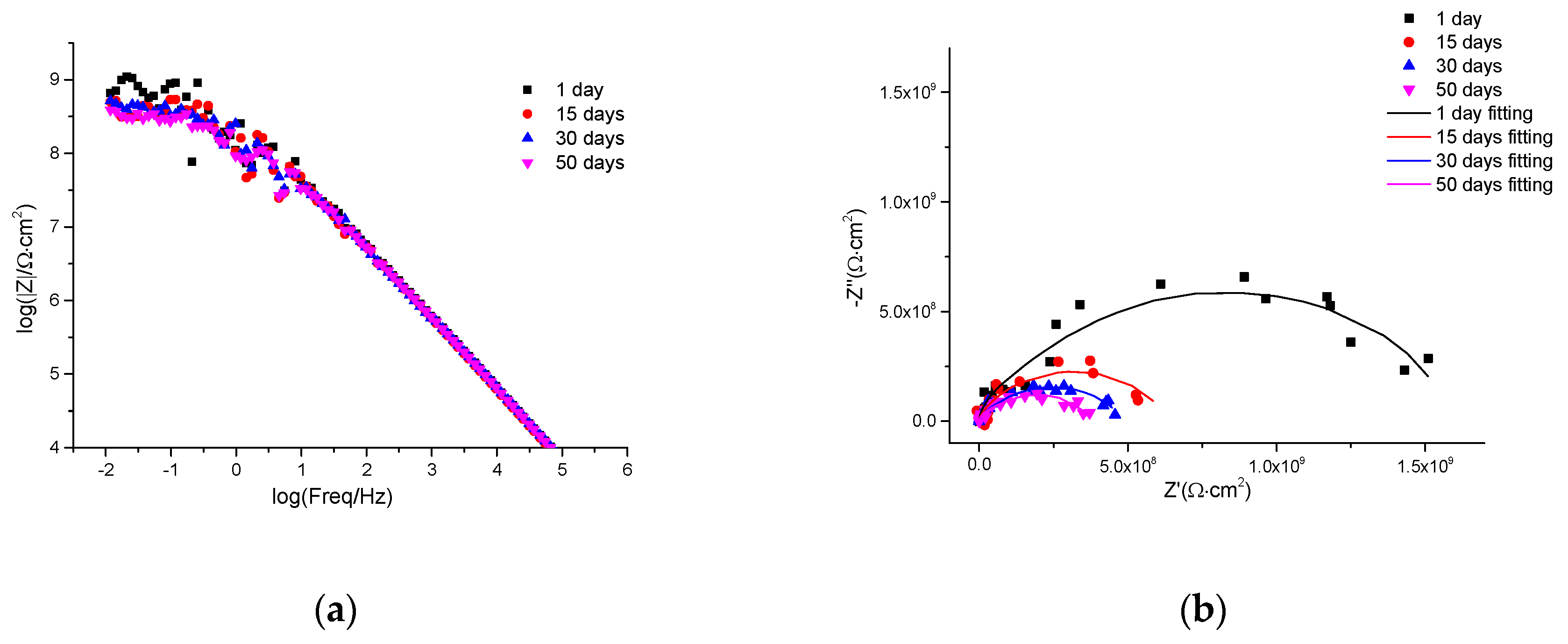
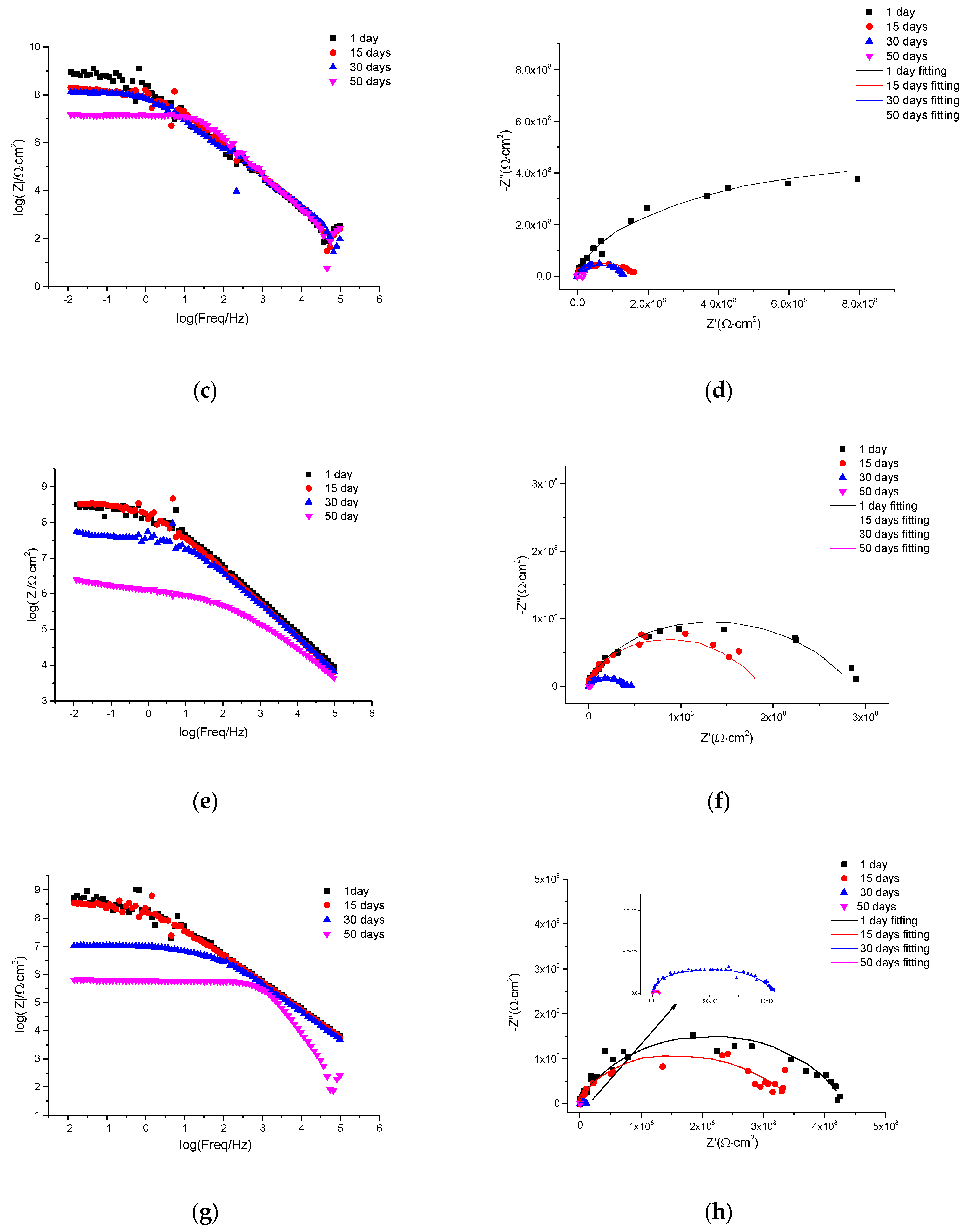
3.5. EIS Self-Healing Evaluation of Damaged Coatings
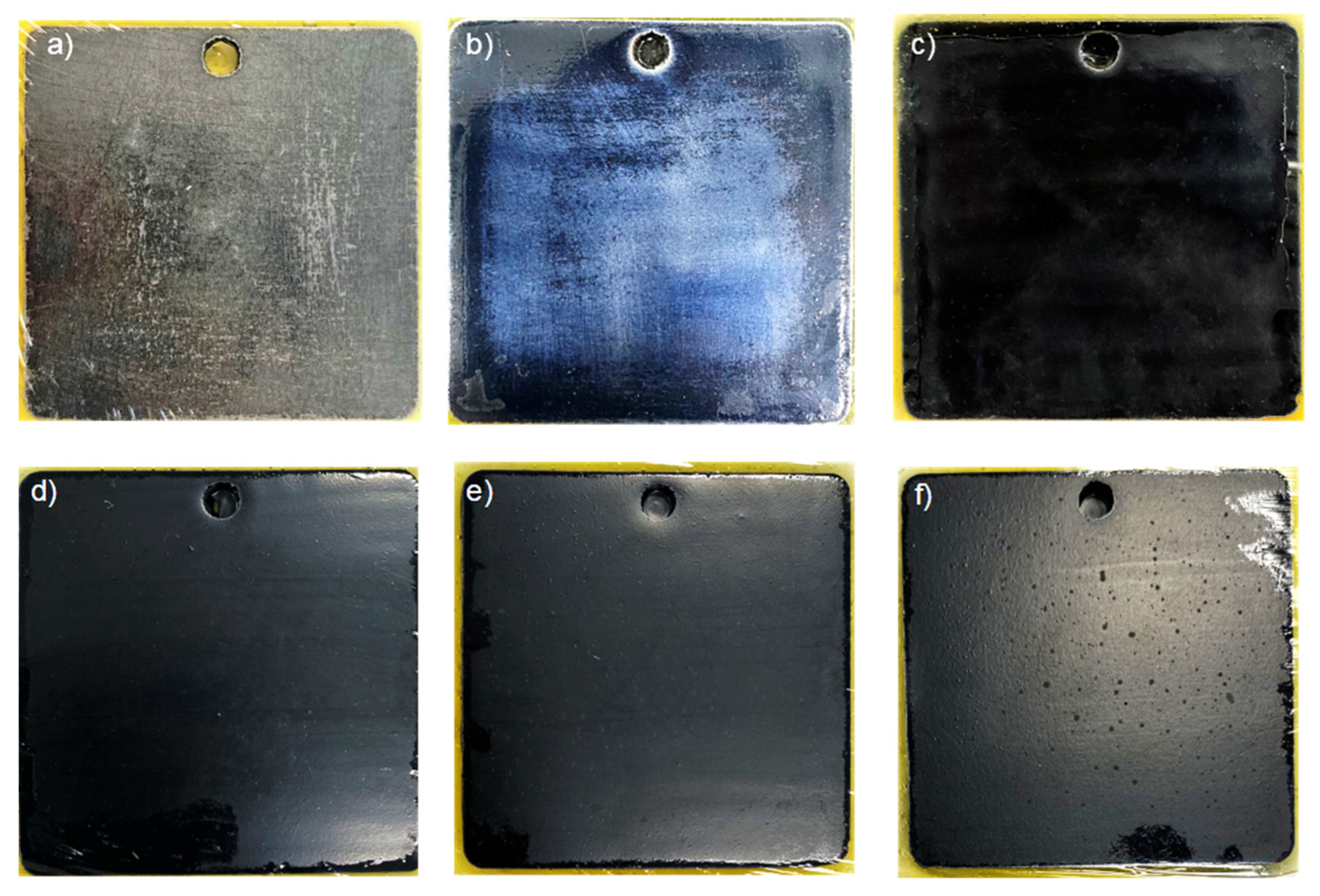
3.6. Salt Spray Test on the Scratched Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Kohl, M.; Alafid, F.; Boštíková, K.; Bouška, M.; Krejčová, A.; Svoboda, J.; Slang, S.; Michalíčková, L.; Kalendová, A.; Hrdina, R.; et al. New azo dyes-based Mg complex pigments for optimizing the anti-corrosion efficiency of zinc-pigmented epoxy ester organic coatings. Coatings 2023, 13, 1276. [Google Scholar] [CrossRef]
- Zafar, F.; Ghosal, A.; Sharmin, E.; Chaturvedi, R.; Nishat, N. A review on cleaner production of polymeric and nanocomposite coatings based on waterborne polyurethane dispersions from seed oils. Prog. Org. Coat. 2019, 131, 259–275. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Clarkson, C.M.; Shrestha, S.; El Awad Azrak, S.M.; Mavlan, M.; Youngblood, J.P. High-performance waterborne polyurethane coating based on a blocked isocyanate with cellulose nanocrystals (CNC) as the polyol. ACS Appl. Polym. Mater. 2020, 2, 385–393. [Google Scholar] [CrossRef]
- Martín-Fabiani, I.; Lesage de la Haye, J.; Schulz, M.; Liu, Y.; Lee, M.; Duffy, B.; D’Agosto, F.; Lansalot, M.; Keddie, J.L. Enhanced water barrier properties of surfactant-free polymer films obtained by macroRAFT-mediated emulsion polymerization. ACS Appl. Mater. Interf. 2018, 10, 11221–11232. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, K.; Wu, H.; Wang, C.; Wang, Q.; Wang, F. Effect of nano-sized mesoporous silica MCM-41 and MMT on corrosion properties of epoxy coating. Prog. Org. Coat. 2012, 75, 386–391. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Chen, J.; Zhang, J.; Fang, Q. Dopamine modified metal-organic frameworks on anti-corrosion properties of waterborne epoxy coatings. Prog. Org. Coat. 2017, 109, 126–134. [Google Scholar] [CrossRef]
- Khodabakhshi, J.; Mahdavi, H.; Najafi, F. Investigation of viscoelastic and active corrosion protection properties of inhibitor modified silica nanoparticles/epoxy nanocomposite coatings on carbon steel. Corros. Sci. 2019, 147, 128–140. [Google Scholar] [CrossRef]
- Ashrafi-Shahri, S.M.; Ravari, F.; Seifzadeh, D. Smart organic/inorganic sol-gel nanocomposite containing functionalized mesoporous silica for corrosion protection. Prog. Org. Coat. 2019, 133, 44–54. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Cui, M.; Li, W.; Zhao, H.; Wang, L. Corrosion protection performance of waterborne epoxy coatings containing self-doped polyaniline nanofiber. Appl. Surf. Sci. 2017, 407, 213–222. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, S.; Hong, R. Graphene oxide/polyaniline nanocomposites used in anticorrosive coatings for environmental protection. Coatings 2020, 10, 1215. [Google Scholar] [CrossRef]
- Pan, W.; Dong, J.; Gui, T.; Liu, R.; Liu, X.; Luo, J. Fabrication of dual anti-corrosive polyaniline microcapsules via Pickering emulsion for active corrosion protection of steel. Soft Matter 2022, 18, 2829–2841. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; He, Y.; Li, H.J.; Zhao, Y.; Li, Z.J.; Sun, D.; Yin, X.Y. Benzotriazole corrosion inhibitor loaded nanocontainer based on g-C3N4 and hollow polyaniline spheres towards enhancing anticorrosion performance of waterborne epoxy coatings. Prog. Org. Coat. 2023, 174, 107276–107289. [Google Scholar] [CrossRef]
- Liu, T.; Ma, L.; Wang, X.; Wang, J.; Qian, H.; Zhang, D.; Li, X. Self-healing corrosion protective coatings based on micro/nanocarriers: A review. Corros. Commun. 2021, 1, 18–25. [Google Scholar] [CrossRef]
- Fix, D.; Andreeva, D.V.; Lvov, Y.M.; Shchukin, D.G.; Möhwald, H. Application of inhibitor-loaded halloysite nanotubes in active anti-corrosive coatings. Adv. Funct. Mater. 2009, 19, 1720–1727. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhang, C.; Zhang, K.; Liu, G. A Hollow porous Mn2O3 microcontainer for encapsulation and release of corrosion inhibitors. ECS Electrochem. Lett. 2013, 2, C39–C42. [Google Scholar] [CrossRef]
- Habib, S.; Hassanein, A.; Kahraman, R.; Mahdi Ahmed, E.; Shakoor, R.A. Self-healing behavior of epoxy-based double-layer nanocomposite coatings modified with Zirconia nanoparticles. Mater. Des. 2021, 207, 109839. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Britz, D.A.; Briggs, G.A.D. Molecules in carbon nanotubes. Acc. Chem. Res. 2005, 38, 901–909. [Google Scholar] [CrossRef]
- Kausar, A. Review on polymer/halloysite nanotube nanocomposite. Polym. Plast. Technol. Eng. 2018, 57, 548–564. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S.; Möhwald, H. Active anticorrosion coatings with halloysite nanocontainers. J. Phys. Chem. C 2008, 112, 958–964. [Google Scholar] [CrossRef]
- Yap, J.Y.; Mat Yaakob, S.; Rabat, N.E.; Shamsuddin, M.R.; Man, Z. Release kinetics study and anti-corrosion behaviour of a pH-responsive ionic liquid-loaded halloysite nanotube-doped epoxy coating. RSC Adv. 2020, 10, 13174–13184. [Google Scholar] [CrossRef]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 15597. [Google Scholar] [CrossRef] [PubMed]
- Njoku, D.I.; Li, B.; Khan, M.S.; Chinonso, U.P.; Njoku, C.N.; Onyeachu, I.B.; Li, Y. Quadruple-action coatings provided by doping epoxy with inhibitor laden clay nanotubes functionalized with layer-by-layer of cross-bridged chitosan and anionic polyelectrolytes. Prog. Org. Coat. 2021, 157, 106312. [Google Scholar] [CrossRef]
- Duan, X.; Duan, Z.; Zhang, Y.; Liu, B.; Li, X.; Zhao, Q.; Yuan, Z.; Jiang, Y.; Tai, H. Enhanced NH3 sensing performance of polyaniline via a facile morphology modification strategy. Sens. Actuators B Chem. 2022, 369, 132302. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, Y.; Han, W.; Xie, H.; Li, C.; Yuan, M. Enhanced solvent-free selective oxidation of cyclohexene to 1,2-cyclohexanediol by polyaniline@halloysite nanotubes. J. Mater. Chem. A 2017, 5, 18230–18241. [Google Scholar] [CrossRef]
- ASTM D3359-09; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: Conshohocken, PA, USA, 2009.
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: Conshohocken, PA, USA, 2019.
- Li, C.; Zhou, T.; Zhu, T.; Li, X. Enhanced visible light photocatalytic activity of polyaniline–crystalline TiO2–halloysite composite nanotubes by tuning the acid dopant in the preparation. RSC Adv. 2015, 5, 98482–98491. [Google Scholar] [CrossRef]
- Tang, J.; Jing, X.; Wang, B.; Wang, F. Infrared spectra of soluble polyaniline. Synth. Met. 1988, 24, 231–238. [Google Scholar] [CrossRef]
- Rodrigues, P.C.; Cantão, M.P.; Janissek, P.; Scarpa, P.C.N.; Mathias, A.L.; Ramos, L.P.; Gomes, M.A.B. Polyaniline/lignin blends: FTIR, MEV and electrochemical characterization. Eur. Polym. J. 2002, 38, 2213–2217. [Google Scholar] [CrossRef]
- Li, C.; Xia, Z.; Yan, H.; Shi, Q.; Weng, J. Benzotriazole functionalized polydimethylsiloxane for reinforcement water-repellency and corrosion resistance of bio-based water-borne epoxy coatings in salt environment. Corros. Sci. 2022, 109, 110150. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, S.; Hu, Z.; Chen, W.; Qu, Z.; Xu, C.; Zhang, Q.; Wu, K.; Shi, J.; Lu, M. pH-responsive self-healing anticorrosion coating based on a lignin microsphere encapsulating inhibitor. Ind. Eng. Chem. Res. 2020, 59, 2657–2666. [Google Scholar] [CrossRef]
- Ge, C.Y.; Yang, X.G.; Hou, B.R. Synthesis of polyaniline nanofiber and anticorrosion property of polyaniline–epoxy composite coating for Q235 steel. J. Coat. Technol. Res. 2012, 9, 59–69. [Google Scholar] [CrossRef]
- Gray, L.G.S.; Appleman, B.R. EIS: Electrochemical impedance spectroscopy: A tool to predict remaining coating life? J. Prot. Coat. Lin. 2003, 20, 66–74. [Google Scholar]
- Trenin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galushek, D. Electrochemical characterization of polymeric coatings for corrosion protection. A review of advances and perspectives. Polymers 2022, 14, 2306. [Google Scholar] [CrossRef]
- Ma, C.; Wang, W.; Wang, Q.; Sun, N.; Hu, S.; Wei, S.; Feng, H.; Hao, X.; Li, W.; Kong, D.; et al. Facile synthesis of BTA@NiCo2O4 hollow structure for excellent microwave absorption and anticorrosion performance. J. Coll. Interf. Sci. 2021, 594, 604–620. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical interpretations of Niquist plots for EDLC electrodes and devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Cao, K.; Yu, Z.; Yin, D.; Chen, L.; Jiang, Y.; Zhu, L. Fabrication of BTA-MOF-TEOS-GO nanocomposite to endow coating systems with active inhibition and durable anticorrosion performances. Prog. Org. Coat. 2020, 143, 105629. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Liu, Z.; Liu, W.; Zhao, H.; Wang, L.; Li, X. Anti-corrosion properties of oligoaniline modified silica hybrid coatings for low-carbon steel. Synth. Met. 2018, 235, 61–70. [Google Scholar] [CrossRef]
- Bao, Y.; Yan, Y.; Chen, Y.; Ma, J.; Zhang, W.; Liu, C. Facile fabrication of BTA@ZnO microcapsules and their corrosion protective application in waterborne polyacrylate coatings. Prog. Org. Coat. 2019, 136, 105233. [Google Scholar] [CrossRef]
- Wei, J.; Li, B.; Jing, L.; Tian, N.; Zhao, X.; Zhang, J. Efficient protection of Mg alloy enabled by combination of a conventional anti-corrosion coating and a superamphiphobic coating. Chem. Eng. J. 2020, 390, 124562. [Google Scholar] [CrossRef]
- Wan, S.; Chen, H.; Ma, X.; Chen, L.; Lei, K.; Liao, B.; Dong, Z.; Guo, X. Anticorrosive reinforcement of waterborne epoxy coating on Q235 steel using NZ/BNNS nanocomposites. Prog. Org. Coat. 2021, 159, 106410. [Google Scholar] [CrossRef]
- Rout, T.K.; Jha, G.; Singh, A.K.; Bandyopadhyay, N.; Mohanty, O.N. Development of conducting polyaniline coating: A novel approach to superior corrosion resistance. Surf. Coat. Technol. 2003, 167, 16–24. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Hu, C.; Zhao, Z.-B.; Ma, Y.; Lin, D.-D.; Du, X.-Y.; Liu, J.-D.; Li, W.-L. Fabrication of BTA-loaded mesoporous silica-encapsulated ZSM-5 molecular sieve for enhancing anti-corrosion performance of waterborne epoxy coatings. Prog. Org. Coat. 2022, 172, 107160. [Google Scholar] [CrossRef]
- Yang, J.; Zou, B.; Fang, Q.; Wang, J.; Wang, L. BTA loaded in various CeO2 nanocontainers in epoxy coating for long-term anticorrosion of low carbon steel. Prog. Org. Coat. 2022, 172, 107156. [Google Scholar] [CrossRef]
- Hassanein, A.; Khan, A.; Fayyad, E.; Abdullah, A.M.; Kahraman, R.; Mansoor, B.; Shakoor, R.A. Multilevel self-healing characteristics of smart polymeric composite coatings. ACS Appl. Mater. Interf. 2021, 13, 51459–51473. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, H.; Wang, L.; Saleem, M.; Ren, F.; Ren, P.; Chen, Y.; Sun, R.; Sun, Y.; Huang, L. Recent progress in the preparation of polyaniline nanostructures and their applications in anticorrosive coatings. RSC Adv. 2014, 4, 28195–28208. [Google Scholar] [CrossRef]
- Yao, J.L.; Ren, B.; Huang, Z.F.; Cao, P.G.; Gu, R.A.; Tian, Z.Q. Extending surface Raman spectroscopy to transition metals for practical applications IV. A study on corrosion inhibition of benzotriazole on bare Fe electrodes. Electrochim. Acta 2003, 48, 1263–1271. [Google Scholar] [CrossRef]
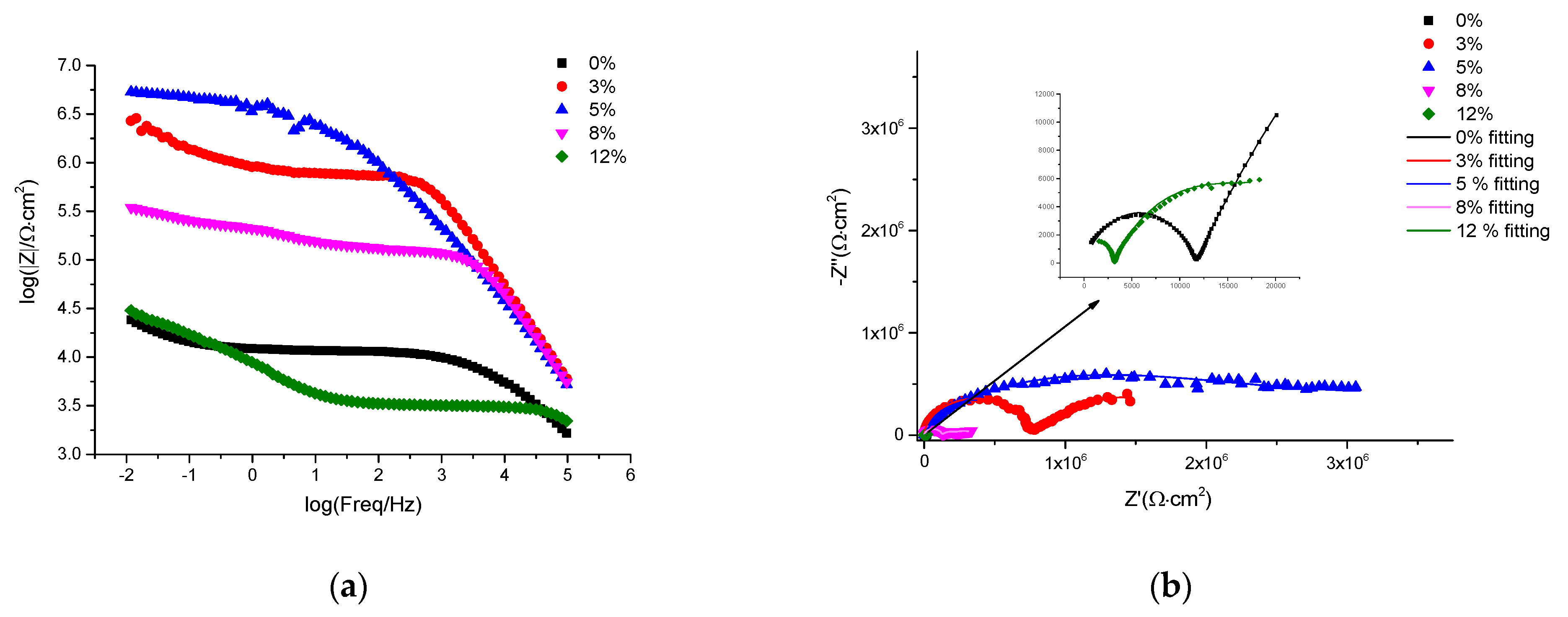
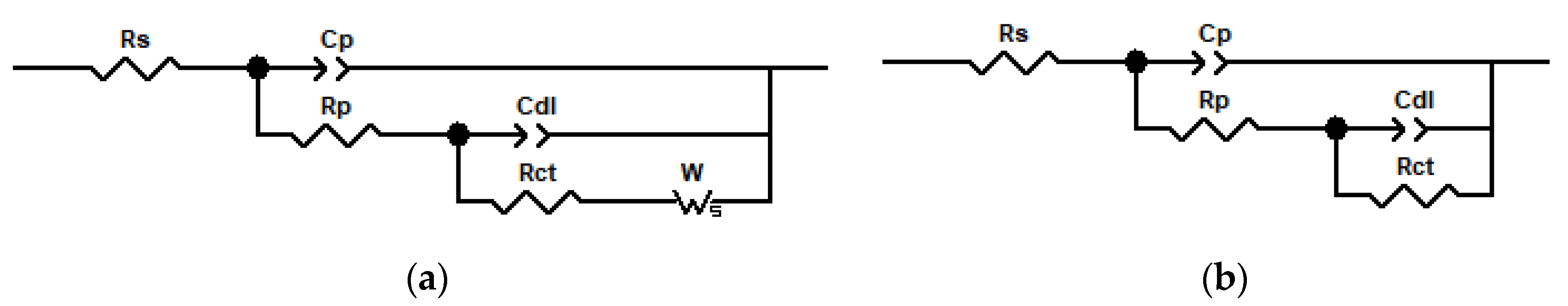

| The Amount of PANI@HNT-2-BTA Doped in the Coatings (%) | Cp | Rp (Ω∙cm2) | Cdl | Rct (Ω∙cm2) | W | Chi Square (×10−3) | ||
|---|---|---|---|---|---|---|---|---|
| Y0 (F∙cm−2) | n | Y0 (F∙cm−2) | n | (Ω∙cm2) | ||||
| 0 | 5.19 × 10−5 | 0.70 | 1.16 × 104 | 3.12 × 10−4 | 0.62 | 5.32 × 104 | 2.57 × 104 | 1.29 |
| 3 | 3.72 × 10−9 | 0.98 | 7.46 × 105 | 1.26 × 10−6 | 0.61 | 2.23 × 106 | 1.04 × 104 | 4.35 |
| 5 | 5.00 × 10−9 | 0.79 | 8.46 × 105 | 5.01 × 10−7 | 0.65 | 2.83 × 106 | 1.96 × 105 | 4.86 |
| 8 | 3.62 × 10−6 | 0.80 | 2.19 × 103 | 2.06 × 10−7 | 0.53 | 1.81 × 105 | 7.50 × 105 | 0.51 |
| 12 | 6.76 × 10−5 | 0.98 | 3.10 × 103 | 4.44 × 10−5 | 0.61 | 2.33 × 104 | 2.12 × 106 | 0.58 |
| Sample | Immersion Time (Days) | Cp | Rp (Ω∙cm2) | Cdl | Rct (Ω∙cm2) | Chi Square (×10−3) | ||
|---|---|---|---|---|---|---|---|---|
| Y0 (F∙cm−2) | n | Y0 (F∙cm−2) | n | |||||
| PANI@HNT-2-BTA-doped coating | 1 | 4.64 × 10−10 | 0.97 | 3.74 × 108 | 8.37 × 10−10 | 0.78 | 1.23 × 109 | 8.94 |
| 15 | 3.73 × 10−10 | 0.99 | 1.14 × 108 | 8.17 × 10−10 | 0.69 | 5.26 × 108 | 3.22 | |
| 30 | 3.13 × 10−10 | 0.95 | 1.04 × 108 | 1.83 × 10−9 | 0.73 | 2.70 × 108 | 2.67 | |
| 50 | 2.98 × 10−10 | 0.98 | 7.06 × 107 | 1.45 × 10−9 | 0.68 | 3.96 × 108 | 1.92 | |
| PANI@HNT-2-doped coating | 1 | 4.99 × 10−10 | 0.98 | 9.41 × 108 | 1.15 × 10−9 | 0.39 | 2.45 × 109 | 6.27 |
| 15 | 3.91 × 10−10 | 0.97 | 3.10 × 108 | 1.98 × 10−9 | 0.64 | 1.33 × 108 | 3.38 | |
| 30 | 3.93 × 10−10 | 0.98 | 2.90 × 108 | 1.11 × 10−8 | 0.65 | 9.33 × 107 | 3.61 | |
| 50 | 3.43 × 10−10 | 0.98 | 7.74 × 107 | 1.98 × 10−8 | 0.78 | 6.96 × 107 | 0.91 | |
| PANI-doped coating | 1 | 1.9 × 10−10 | 0.99 | 4.96 × 108 | 7.95 × 10−10 | 0.63 | 2.86 × 108 | 2.37 |
| 15 | 2.54 × 10−10 | 0.99 | 6.76 × 107 | 6.9 × 10−10 | 0.66 | 1.86 × 108 | 1.91 | |
| 30 | 2.81 × 10−10 | 0.99 | 4.14 × 106 | 1.91 × 10−9 | 0.61 | 4.05 × 107 | 0.21 | |
| 50 | 3.49 × 10−10 | 0.97 | 3.65 × 105 | 1.47 × 10−7 | 0.67 | 1.15 × 106 | 0.32 | |
| Waterborne epoxy varnish | 1 | 2.73 × 10−10 | 1 | 5.34 × 108 | 9.18 × 10−10 | 0.69 | 3.80 × 108 | 8.22 |
| 15 | 3.63 × 10−10 | 0.97 | 3.92 × 107 | 1.26 × 10−9 | 0.38 | 4.59 × 108 | 4.39 | |
| 30 | 4.30 × 10−10 | 0.97 | 3.80 × 106 | 1.15 × 10−8 | 0.64 | 6.98 × 106 | 1.21 | |
| 50 | 5.18 × 10−10 | 0.96 | 2.85 × 105 | 5.65 × 10−7 | 0.73 | 2.92 × 105 | 0.78 | |
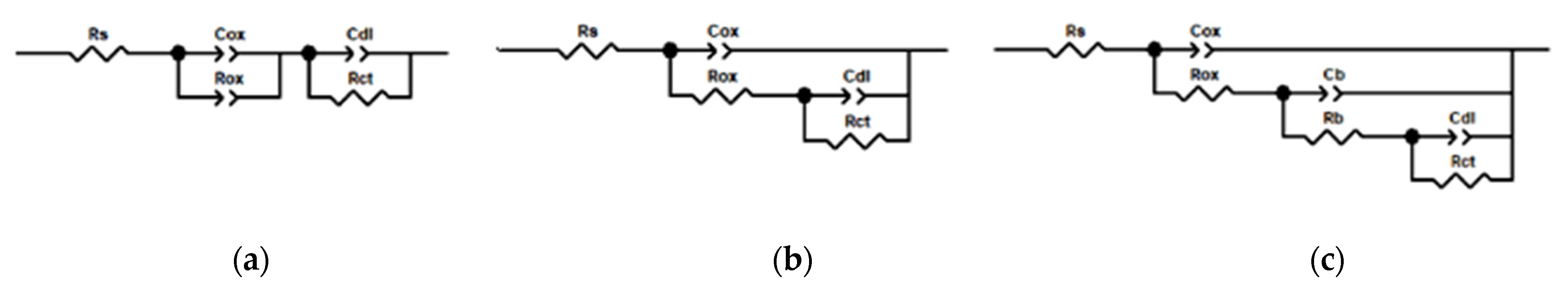
| Immersion Duration | Rox (Ω∙cm2) | Rct (Ω∙cm2) | Rb (Ω∙cm2) | Circuit Model | |
|---|---|---|---|---|---|
| Waterborne epoxy varnish coating | 0 h | 517 | 599 | - | a |
| 8 h | 529 | 858 | - | ||
| 24 h | 388 | 3316 | - | ||
| 48 h | 254 | 4378 | - | ||
| Coating doped with PANI@HNT-2 | 0 h | 529 | 858 | - | a |
| 8 h | 3260 | 16,190 | - | ||
| 24 h | 810 | 5760 | - | b | |
| 48 h | 650 | 5354 | - | ||
| Coating doped with PANI@HNT-2-BTA | 0 h | 582 | 729 | - | a |
| 8 h | 42 | 5181 | 3210 | c | |
| 24 h | 118 | 35,178 | 4245 | ||
| 48 h | 141 | 33,290 | 6181 |
| Sample | Salt Spray Test Duration | |||
|---|---|---|---|---|
| 0 h | 50 h | 100 h | 150 h | |
| Waterborne epoxy varnish |  | |||
| Coating doped with PANI@HNT-2 |  | |||
| Coating doped with PANI@HNT-2-BTA |  | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Gao, Z.; Wang, D.; Yu, F.; Du, B.; Gitsov, I. Improving the Protection Performance of Waterborne Coatings with a Corrosion Inhibitor Encapsulated in Polyaniline-Modified Halloysite Nanotubes. Coatings 2023, 13, 1677. https://doi.org/10.3390/coatings13101677
Liu X, Gao Z, Wang D, Yu F, Du B, Gitsov I. Improving the Protection Performance of Waterborne Coatings with a Corrosion Inhibitor Encapsulated in Polyaniline-Modified Halloysite Nanotubes. Coatings. 2023; 13(10):1677. https://doi.org/10.3390/coatings13101677
Chicago/Turabian StyleLiu, Xin, Zhiyue Gao, Die Wang, Fengjie Yu, Baoshuai Du, and Ivan Gitsov. 2023. "Improving the Protection Performance of Waterborne Coatings with a Corrosion Inhibitor Encapsulated in Polyaniline-Modified Halloysite Nanotubes" Coatings 13, no. 10: 1677. https://doi.org/10.3390/coatings13101677
APA StyleLiu, X., Gao, Z., Wang, D., Yu, F., Du, B., & Gitsov, I. (2023). Improving the Protection Performance of Waterborne Coatings with a Corrosion Inhibitor Encapsulated in Polyaniline-Modified Halloysite Nanotubes. Coatings, 13(10), 1677. https://doi.org/10.3390/coatings13101677