Abstract
Due to the enhancement of people’s environmental awareness, flame-retardant epoxy resin (EP) tends to be non-toxic, efficient, and multi-functional, and its development is systematic. At present, many new flame retardants or intrinsic modification methods reported in studies can effectively improve the flame retardability and thermal stability of EP. However, many aspects still need to be further improved. In this review, the flame-retardant mechanism and method of flame-retardant epoxy resins are briefly analyzed. The research progress of the flame-retardant modification of epoxy resin by physical addition and chemical reaction is summarized and discussed. Furthermore, the research trend of flame-retardant epoxy resin in the field of fire-protective coatings is discussed, and future problems in this field are put forward. This work may provide some new insights for the design of multi-functional integrated epoxy resin fireproof coatings.
1. Introduction
Since the discovery of polymer materials, they have appeared in various fields of modern human life [1,2]. However, high flammability and poor thermal stability greatly limit their applications in some situations [3,4], as they will burn and release a lot of smoke and heat so long as they are exposed to enough energy [5]. According to reports, about 5000 people in Europe and 4000 people in the United States die in fires every year. The direct economic losses caused by these fire accidents are estimated at 0.3% of GDP [6]. Recently, fire safety authorities have stated that it is very necessary to apply suitable materials in different environments, which has led to stricter requirements on the flame retardancy of materials under certain conditions [7].
As an important thermosetting polymer material, epoxy resin (EP) has many advantages, such as high mechanical strength, excellent adhesion, chemical resistance, and electrical insulation, which makes it widely used in various industrial fields of construction, automotive, coatings, electronics, and aerospace [8,9,10,11]. Nevertheless, EP has a limiting oxygen index (LOI) of only about 19.8%. During the combustion process, large amounts of heat and harmful smoke will be released, which may cause huge casualties and property losses. At the same time, generated molten droplets can easily lead to the rapid spread of a fire, which is an important factor limiting the practical application of EP. For example, in the electrical and electronic industries, strict flame-retardant requirements are usually required, such as needing to reach the V-0 level in the vertical burning test. Therefore, there is an urgent need to develop high-performance flame-retardant materials, including high-efficiency flame retardants (FRs) and thermosetting flame-retardant materials.
Flame-retardant additives, especially halogen-based flame retardants, are considered to be the most effective fillers to improve the flame-retardant properties of EP. However, with the enhancement of human awareness of environmental protection, the release of toxic smoke and bromide pollution during the use of halogen flame retardants has become a more and more prominent concern [12,13]. Therefore, for the consideration of environmental protection, some halogen flame retardants are gradually being eliminated, and some halogen-free flame retardants and more environmentally friendly flame-retardant modification methods have emerged as the times require. In this review, two flame-retardant mechanisms are briefly introduced, and two flame-retardant modification methods (including physical addition and chemical reaction) for epoxy resin systems are summarized. After that, the future development direction of flame-retardant epoxy resin in the field of flame-retardant coating and the urgent problems are laid out.
2. Flame-Retardant Mechanism
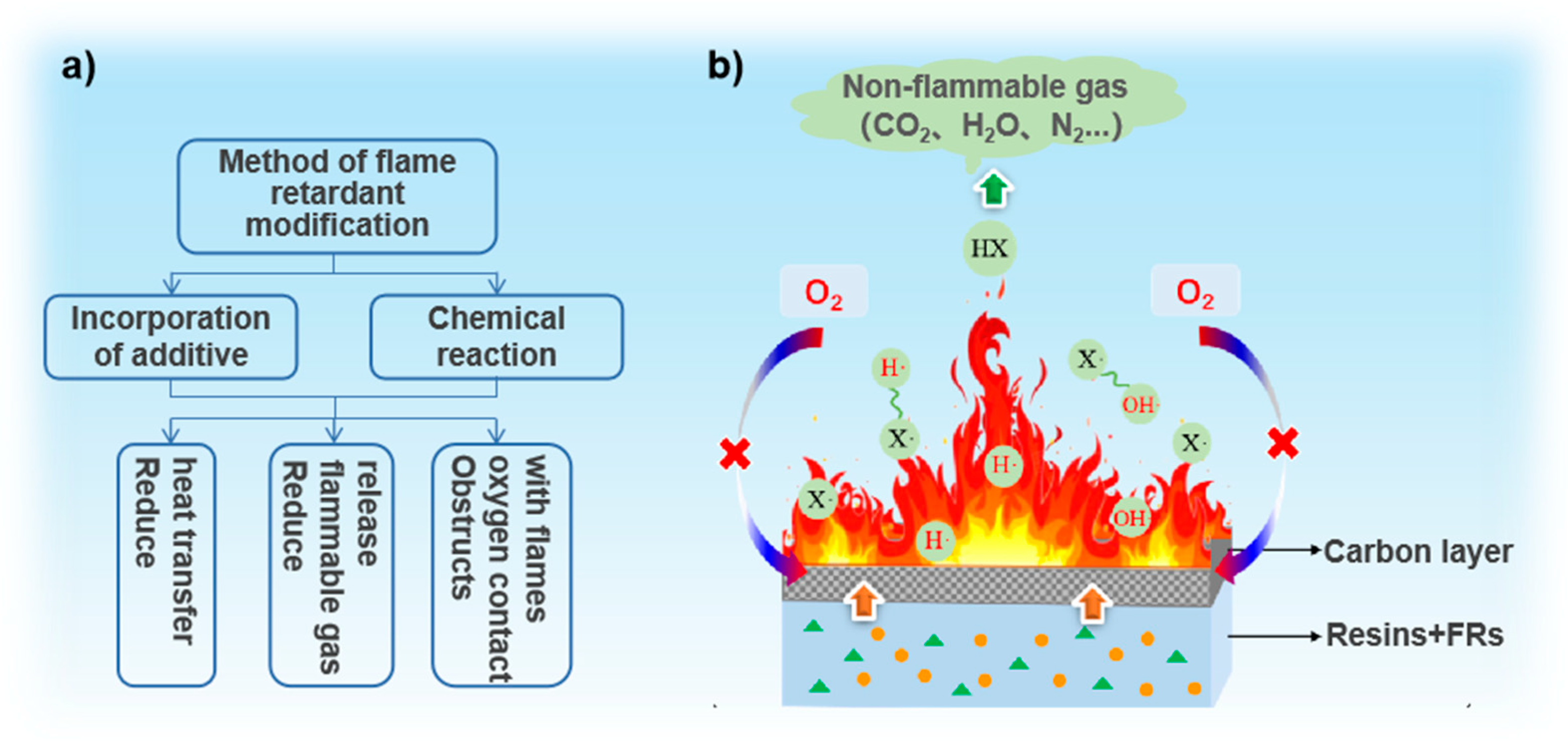
The combustion of a polymer is a multistage process, including complex chemical and physical interactions [14]. The supply of heat, fuel, and oxygen are the three necessary factors required to maintain the combustion cycle [15]. Generally, the combustion process of a polymer is divided into four stages: heat, pyrolysis, ignition, and combustion [16]. Firstly, the polymer is warmed to a certain temperature by a heat source, and the backbone of the polymer begins to break and decompose, after which the following types of products may be formed [7]: (1) combustible gases, such as CO, methane, and ethane; (2) molten fluid produced by thermal decomposition of polymers; (3) incombustible gases such as CO2; (4) fine solid particles composed of polymer fragments or soot; and (5) carbonaceous residues. Afterwards, when the concentration and temperature of released combustible gas reach certain values, they ignite spontaneously in the presence of air. Finally, great amounts of active free radicals such as HO• and O• generated by combustion participate in the chain reaction, accompanied by continuous heat release. Under the influence of heat transfer processes, new decomposition reactions are induced in the polymer, and more combustibles are produced. The combustion cycle is thus maintained [17] until all combustible materials are exhausted without external interruption.
The generation of extensive volumes of flammable gas is one of the direct reasons for increasing the combustibility of polymers; the other is the thermal fluid produced by thermal decomposition. Although not as flammable as gas, the flowing part can spread heat to adjacent parts. Therefore, reducing the release of flammable gases or preventing the spread of flames are the most essential ways to achieve flame retardancy. For the most part, on the basis of understanding polymer combustion, the combustion can be suppressed by breaking the combustion cycle with different methods (see Figure 1b)): (a) reducing heat transfer (the endothermic decomposition of some flame retardants causes a temperature decrease by heat consumption); (b) reducing the release of combustible gas (diluting combustible gases by adding flame retardants to produce inert, non-flammable gases); and (c) obstructing oxygen contact with flames (by forming a carbonaceous layer through the cross-linking reactions of flame retardants or the polymer matrix) [12].
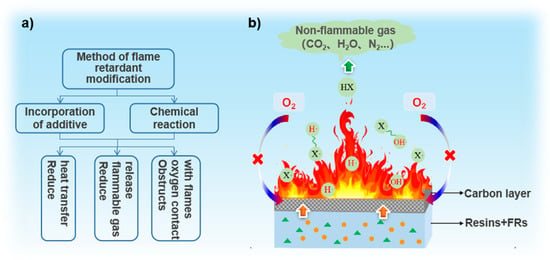
Figure 1.
(a) Fire-retardant methods and (b) schematic diagram of the flame-retardant mechanism.
Available studies have shown that there are two main flame-retardant mechanisms for the inhibition of polymer combustion:
- Condensed phase inhibition, which involves the change of polymer structure after combustion. As the combustion reaction progresses, the polymer gradually forms a low-thickness, high-porosity residual carbonaceous structure. The carbon residue layer has important functions, including protecting the integrity of the matrix structure, isolating the flame contact, and preventing the flame from spreading further. However, the generation of char residues is occasionally accompanied by a violent combustion process and produces a large amount of smoke;
Gas phase suppression, which involves the release of incombustible gases such as H2O and CO2 to dilute the concentration of flammable gases or to interfere with the chemical reactions of flame combustion. Inorganic hydroxides and halogen flame retardants are the most representative examples of using this mechanism. Such as the dilution effect of H2O produced by the thermal decomposition of magnesium hydroxide [18]. And free radical inhibitors formed by volatilization of halogen flame retardants can convert highly active H• and HO• into low-activity X• to inhibit free radical reaction [19]. As shown in Reactions (1)–(4), this conversion first begins with the release of a halogen atom (1), which then reacts with the fuel to produce hydrogen halide (2). The hydrogen halide is believed to be the true combustion inhibitor by disrupting the chain reaction (3,4) [20]. Reactions (1)–(4), free radical inhibition reaction.
MX → M• + X•
X• + RH → R• + HX
H• + HX → H2 + X•
HO• + HX → H2O + X•
3. Different Flame-Retardant Modification Methods
Generally, there are two main methods to improve the flame retardancy of polymer materials: the physical addition of flame retardants and chemical reaction modification [21]. Among them, the addition of flame retardants will greatly improve the flame-retardant performance of polymer materials and extend the survival time in the flame by three times [3].
3.1. Physical Additive Flame-Retardant Method
For epoxy resin, the necessary flame-retardant modification can broaden its application potential in various fields [22]. The flame-retardant performance of epoxy resin is related to chemical structure and flame-retardant formulation. Halogen flame retardants, which are widely used, can improve the flame retardancy of polymers without reducing their physical properties. However, no matter what conditions are applied, the toxic substances produced in the firing process of composite materials containing halogen may cause serious environmental pollution [23]. Therefore, due to the awareness of environmental protection and health, halogen flame retardants have gradually been replaced by other more environmentally friendly and safer flame retardants [24]. As shown in Table 1, the common flame retardants used in epoxy resin systems include silicon-containing compounds, phosphorus/nitrogen compounds, inorganic hydroxides, and carbon-based materials. How to choose the appropriate flame retardants depends on the different resin matrices and the intended use.

Table 1.
Different types of flame-retardant additives applied to the EP system.
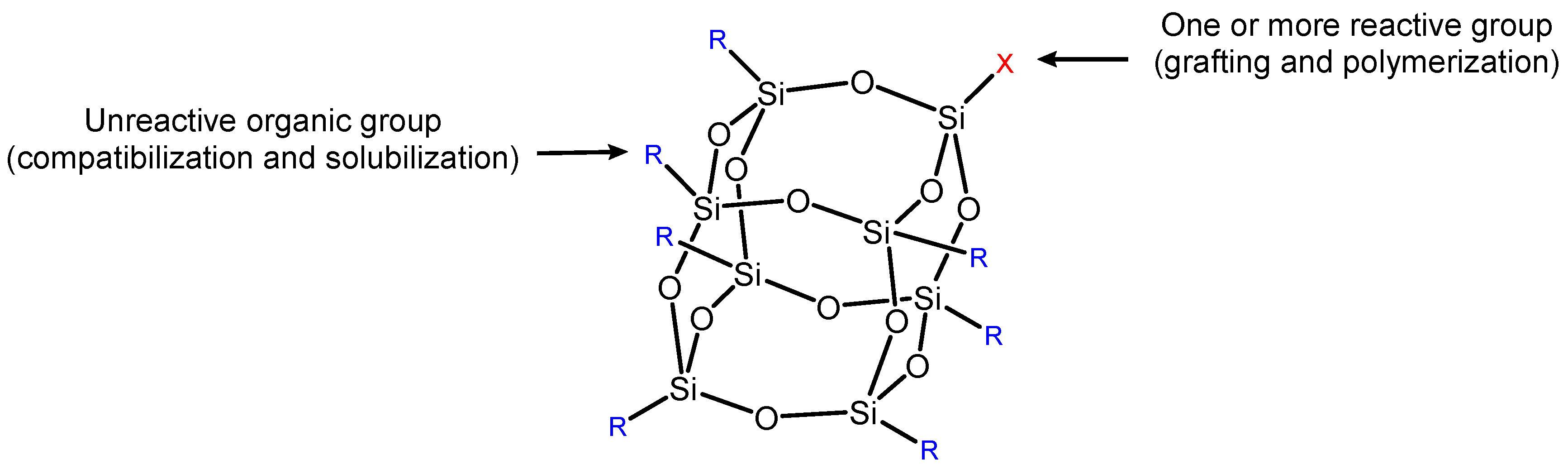
3.1.1. POSS
Silicon-based flame retardants, including siloxane [28], layered silicate [29], and SiO2 [30,31,32], have been at the forefront of flame-retardant additives due to their high versatility, compatibility, low toxicity, and environmentally protective qualities [25]. During combustion, silicon flame-retardant pyrolysis generates non-combustible substances such as SiO2, which promotes the formation of a highly thermally stable char layer, inhibits further decomposition of the underlying polymer, and reduces the rate of heat release. Furthermore, silicon-based flame retardants are considered one of the most promising flame retardants because they do not produce toxic gases and fumes [26]. Among the various silicon-based flame retardants applied to EP, polyhedral oligomeric silsesquioxane (POSS) is one of the most studied structures in recent years. POSS is a well-defined compound consisting of a Si-O framework with the formula (RsiO3/2)n (n = 6, 8, 10, …) (Figure 2) [27]. This special composition structure can significantly improve its thermal performance and flame retardancy [52,53]. The combustion is retarded through both char formation in the condensed phase and trapping of dynamic radicals in the vapor phase if silicone flame retardants are functionalized by phosphorus or nitrogen. However, most reports pay more attention to the effect of POSS on the mechanical and thermal properties of epoxy resins, while less attention is paid to the fire performance of materials [54].
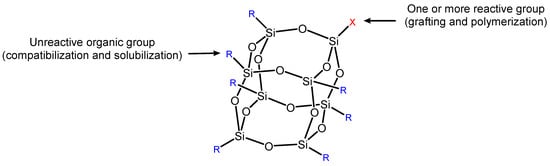
Figure 2.
Polyhedral oligomeric sililoxane (POSS) [27].
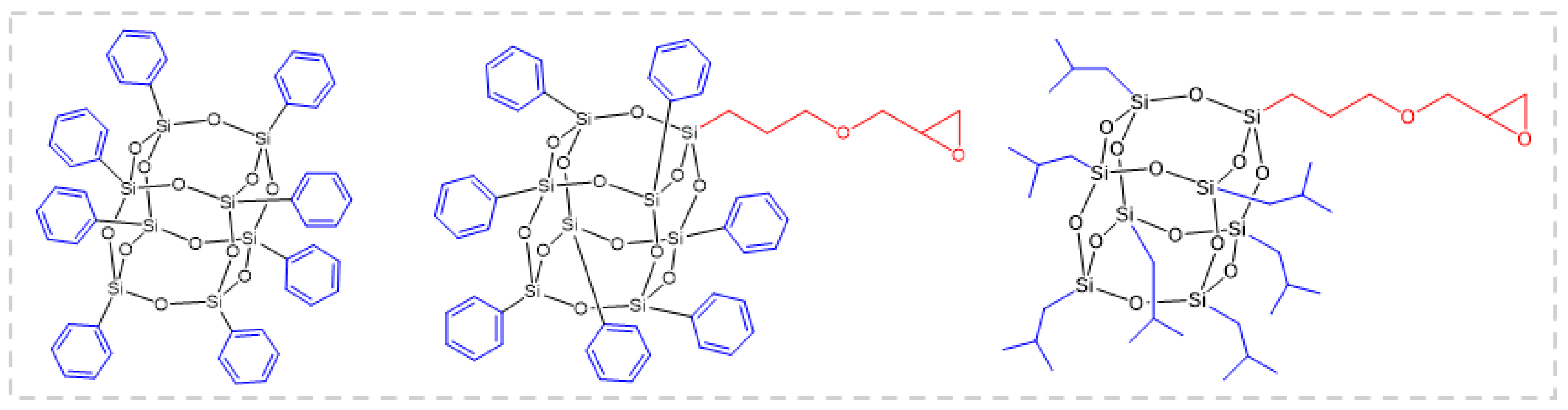
An article reported [55] the effects of POSS with three different structures, octaphenyl POSS, glycidyl oxypropyl heptyl benzene POSS, and glycidyl oxypropyl heptaisobutyl POSS, on the flame-retardant properties of epoxy resin (Figure 3). The PHRR of the composite samples doped with different POSS decreased by 34%, 40%, and 25%, respectively. Among them, the cross-section of the carbon residue after burning the glycidyloxypropylheptylbenzene POSS/EP sample was a sponge-like structure with an outstanding heat insulation effect. This study demonstrates the potential of POSS as an efficient flame retardant for epoxy resins.
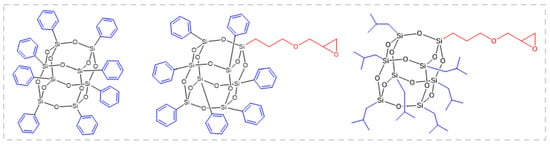
Figure 3.
Octaphenyl-POSS (left), glycidyloxypropyl-heptaphenyl-POSS (middle), and glycidyloxypropyl-heptaisobutyl-POSS (right). (With permission from Ref. [55]; License number: 5617501151054, 2023, Elsevier).
However, the improvement of the flame-retardant properties of the epoxy resin system with a single use of POSS is limited; a more effective method, which has been widely reported, is to modify POSS by introducing other flame-retardant elements or compounding with other flame retardants. Due to the unique structure of polyhedral oligomeric silsesquioxane (POSS) and the synergism of Si and P in the structure, the method of introducing phosphorus element into POSS to form a novel flame retardant has attracted much attention [56,57]. Researchers also claimed that phosphorous derivatives of POSS [58,59] are a new trend in the development of flame-retardant candidates for polymer matrices.
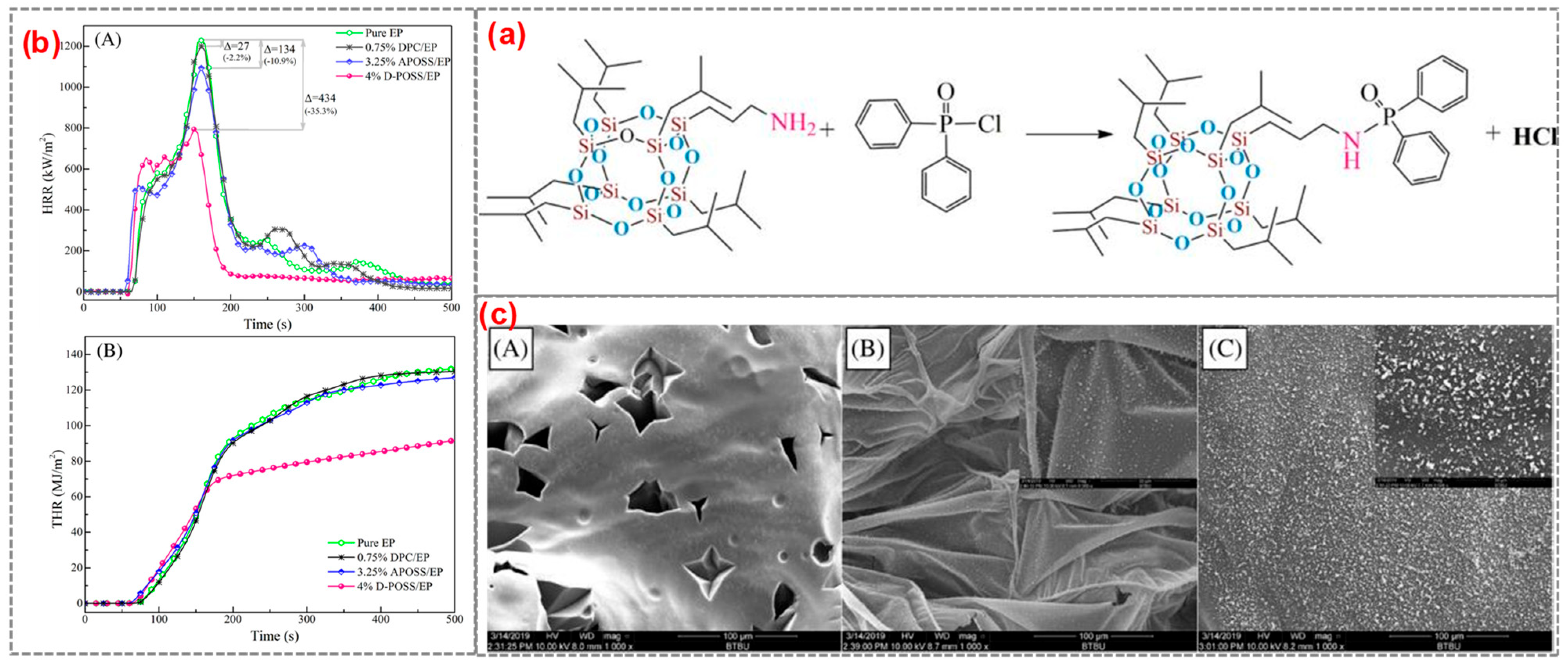
For example, it was reported that the flame-retardant D-POSS for EP was prepared from aminopropylisobutyl POSS and diphenylphosphonyl chloride [60] (Figure 4a). The epoxy resin containing 4 wt% D-POSS reached an LOI value of 29.0% and passed UL94 V-1 grade, and PHRR, THR, and total smoke production were decreased by 35.3%, 30.3%, and 38.3%, respectively. Both the diphenylphosphine group and the POSS group in D-POSS had a strong synergistic effect in controlling PHRR, THR, and smoke production while promoting the carbonization of EP to form an expanded char layer. It is worth noting that some white silica was enriched on the surface of the residue (Figure 4c). White silica and expanded internal coke formed a double carbon layer that blocks heat transfer and improves the flame retardancy of EP composites. Similarly, Liu et al. [61] successfully synthesized a Si/P synergistic flame-retardant POSS-bisDOPO using DOPO, POM, and POSS as raw materials (Figure 5). The surfactant-like structure of POSS-bisDOPO has excellent self-assembly ability, which significantly improves the dispersion uniformity in EP. The EP, with the addition of 20 wt% POSS-bisDOPO, achieved an LOI value of 34.5%. In addition, the mechanical properties of EP were significantly improved due to the self-assembly of POSS-bisDOPO.
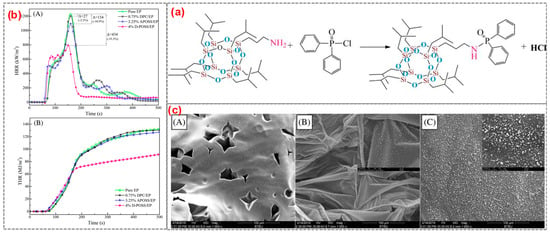
Figure 4.
(a) The synthesis of D-POSS; (b) HRR curve (A) and THR curve (B); and (c) the SEM images of residues: (A) Pure EP; (B) 4%D-POSS/EP external; and (C) 4% DPOSS/EP internal. (With permission from Ref. [60]; License number: 5617510624430, 2023, John Wiley and Sons).
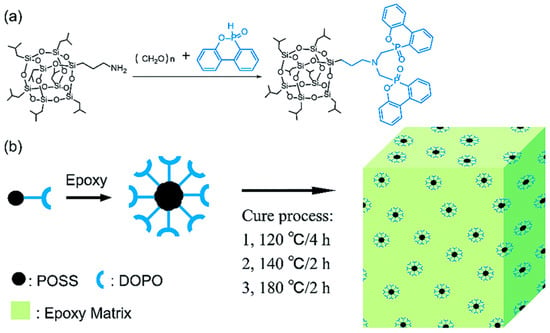
Figure 5.
(a) The synthesis of POSS-bisDOPO and (b) preparation of POSS-bisDOPO-modified epoxy resin. (With permission from Ref. [61]; License number: 1390727-1, 2023, Royal Society of Chemistry).
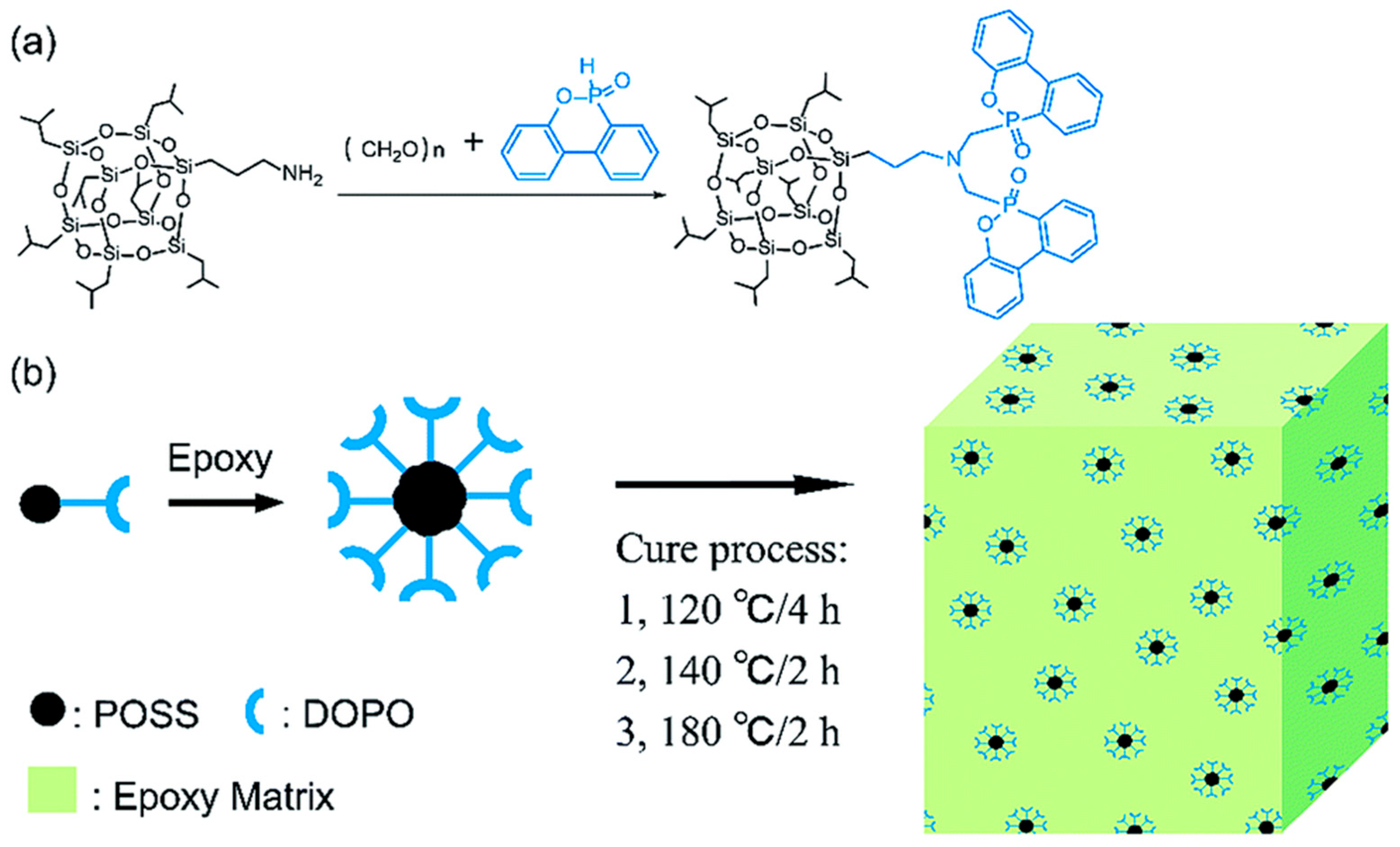
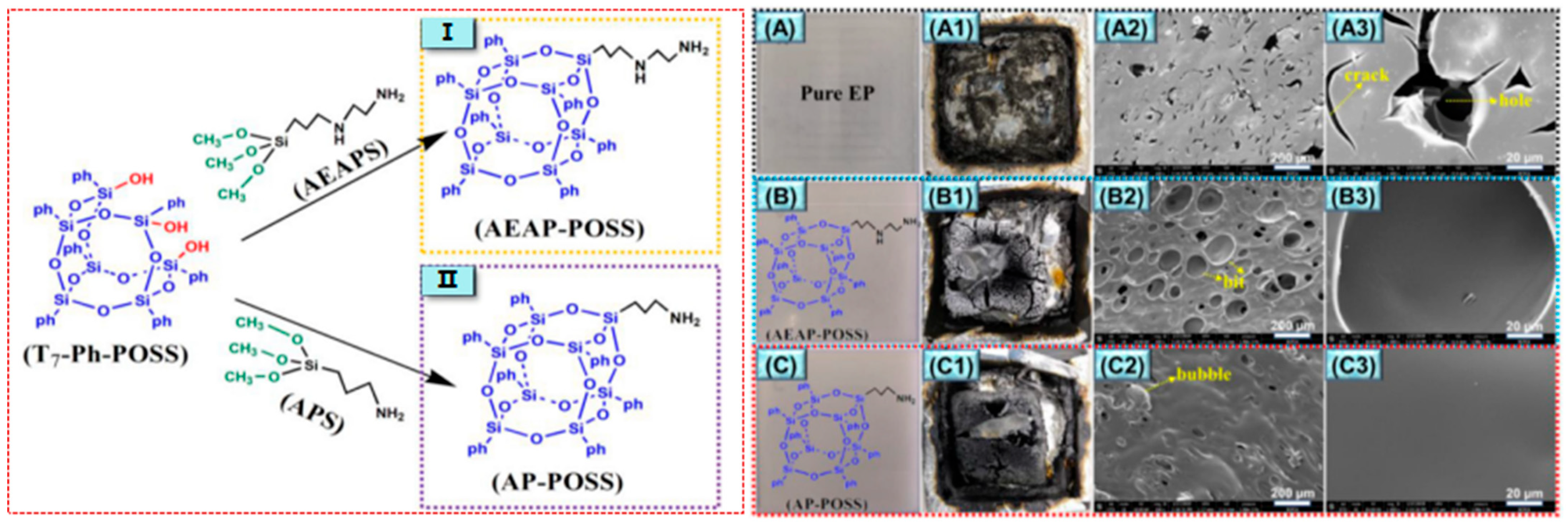
In addition to P and Si, Si and N also have an excellent synergistic effect. As shown in Figure 6 [62], two kinds of nitrogenous polyhedral oligomeric siloxanes (N-POSSs) were prepared by using side chain branches with amino groups in POSS, and the dispersibility, thermal stability, and flame-retardant properties of the two kinds of POSS/EP composites were studied. The results showed that N-POSSs containing Si and N formed a composite-stable, dense carbon layer rich in graphite, Si-C, and Si-N during the combustion process, which effectively suppressed the release of combustible organic volatiles and the generation of smoke (As shown in Table 2, p-SPR, P-COP, and p-CO2P). At the same time, the PHRR of the composite materials was decreased by 46.1% and 60.6%, respectively, compared with pure EP (Table 2), and the synergistic flame-retardant effect was remarkable.
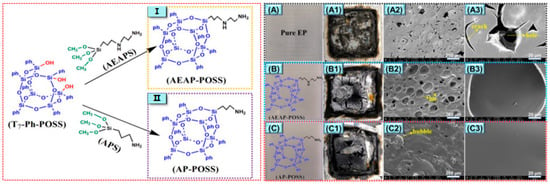
Figure 6.
(Left) The synthetic process of AEAP-POSS and AP-POSS; (Right) digital photos of the cone calorimetry test samples (A–C) and char residues after the cone calorimeter tests (A1,B1,C1); SEM images of the char residue after cone calorimeter tests: EP (A2,A3), EP/AEAP-POSS (B2,B3), and EP/AP-POSS (C2,C3). (With permission from Ref. [62]; License number: 5617531324215, 2023, Elsevier).

Table 2.
Cone calorimeter data of EP, EP/AEAP-POSS, and EP/AP-POSS composites (part).
3.1.2. DOPO
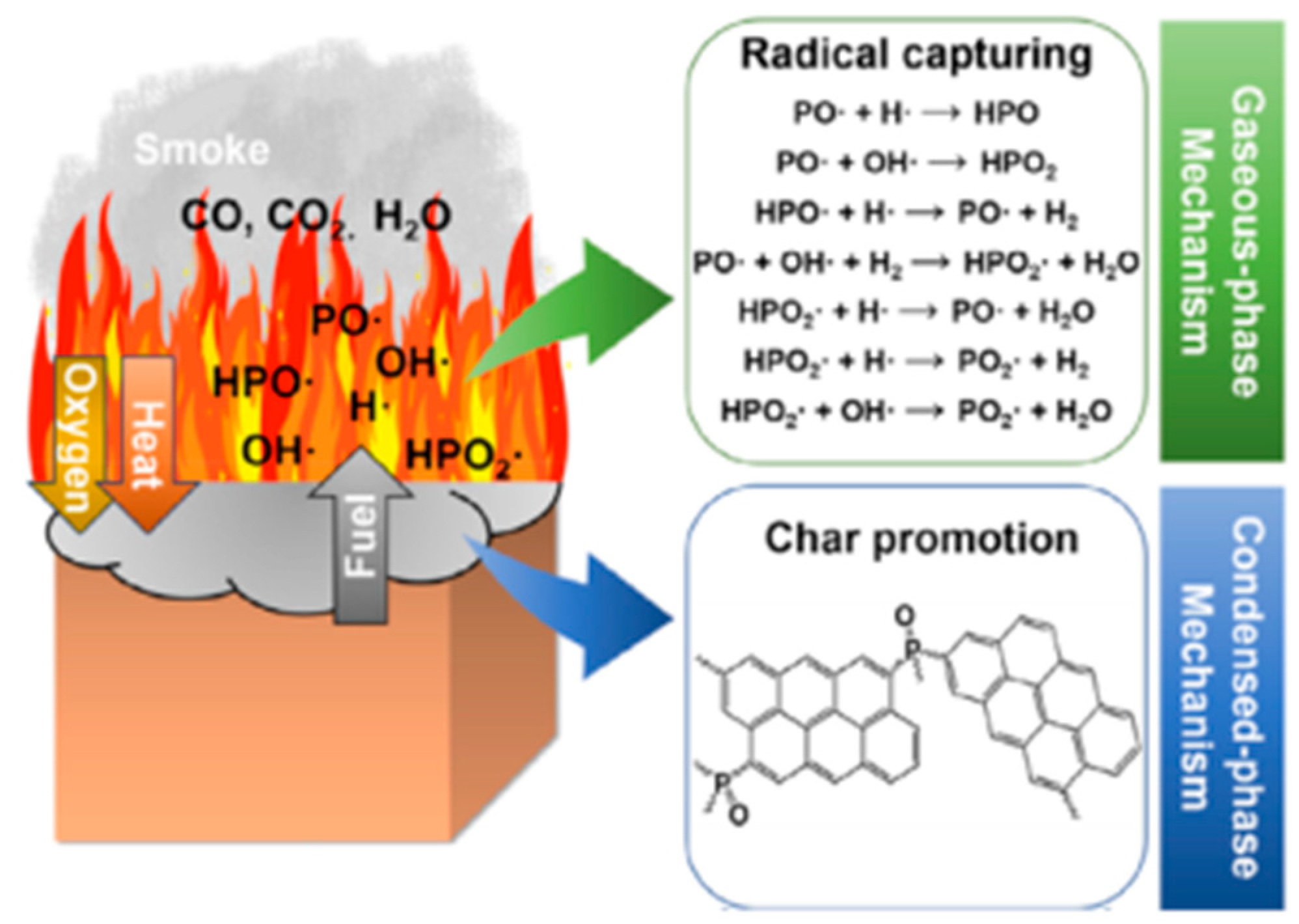
Phosphorus-based flame retardants are the most important halogen-free flame retardants used to improve the flame-retardant properties of EP. Common phosphorus-based flame retardants include phosphorus-containing derivatives (phosphazene, phosphaphenanthrene) [33,34,36], phosphate esters [63], and polyphosphates [35,64,65]. For the consideration of environmental protection, phosphorus-based flame retardants have more advantages than halogen-based flame retardants. The main flame-retardant mechanism involved is to suppress flame combustion and reduce heat release, control melt flow, and promote char layer formation. The free radicals (PO2•, PO•, and HPO•) generated by the decomposition of phosphorus-based flame retardants have a strong scavenging effect on H• and OH• in the gas phase, which effectively extinguishes the flame and reduces the reaction activity (See Figure 7) [66]. Furthermore, phosphoric acid and polyphosphoric acid produced by the decomposition of some phosphorus-containing compounds form a melt-adhesive layer that protects the polymer surface and prevents oxygen penetration. The combustion of nitrogen-based flame retardants not only promotes the formation of insulating layers but also releases inert gases to dilute the combustion atmosphere and often co-occurs with phosphorus-based flame retardants [67], which promotes the development of some phosphorus-nitrogen synergistic new flame retardants [37,39].
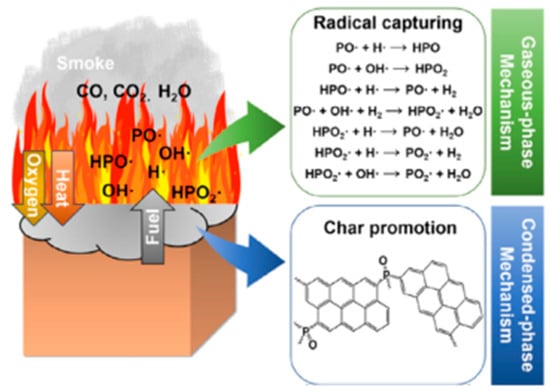
Figure 7.
Flame-retardant mechanism of phosphorus-containing flame retardants: radical capturing effect in gas phase and char promotion effect in condensed phase. (With permission from Ref. [66]; License number: 5617540136833, 2023, Elsevier).
9,10-dihydro-9-oxaze-10-phosphoxene-10-oxide (DOPO) and its derivatives have been widely used in EP systems due to their advantages of thermal stability, high flame-retardant efficiency, and strong design ability [13,68,69]. The new multi-component phosphorus-based flame retardant can be prepared by combining nitrogen, silicon, boron, sulfur, and other elements with the phosphorus phenanthene group as the reaction site for molecular design [70,71].
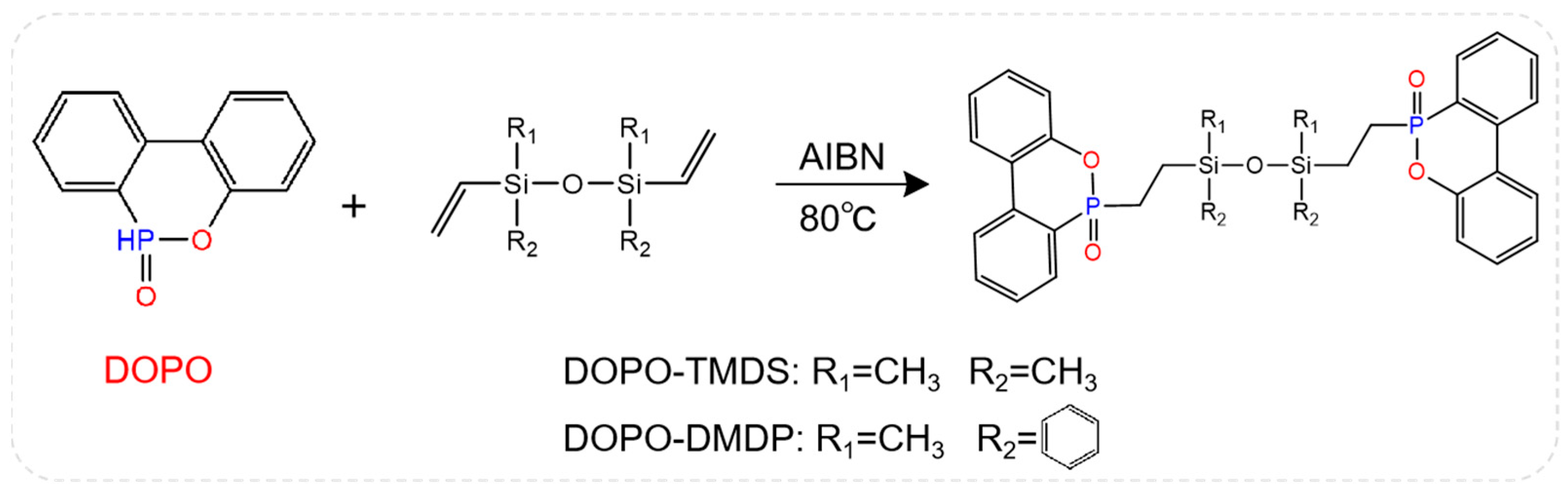
As mentioned above, the multi-element synergistic flame retardant formed by P and Si elements has an excellent flame-retardant effect. Figure 8 shows the structural formulas of two flame retardants (DOPO-TMDS and DOPO-DMDP) containing phosphorus phenanthene/siloxane groups, which are applied to the EP system [72]. When DOPO-TMDS content was 20 wt%, the LOI of flame-retardant epoxy resin was as high as 33% and passed the vertical burning test V-0 rating, and PHRR and THR were greatly reduced. In contrast, 24 wt% DOPO-DMDP was required to achieve a LOI value of 32% and obtain UL94 V-0 grade. In addition, although the addition of DOPO-TMDS and DOPO-DMDP enhanced the mechanical strength of the EP, a decrease in Tg was observed due to the presence of the siloxane structure.
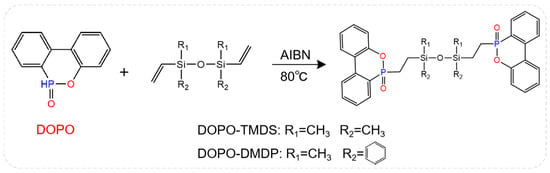
Figure 8.
Structures of DOPO-TMDS and DOPO-DMDP. (With permission from Ref. [72]; License number: 5617560166971, 2023, Springer Nature).
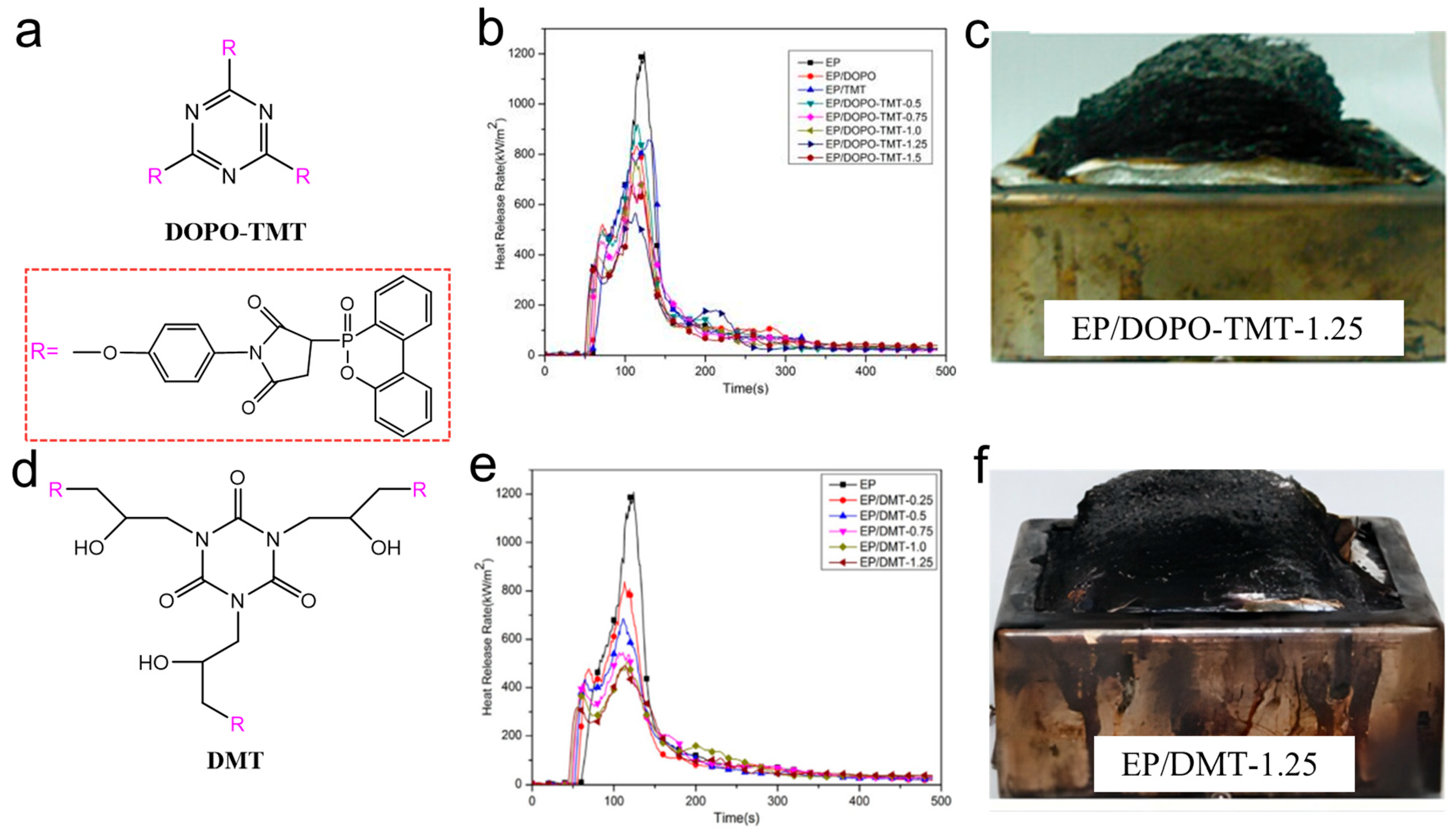
Huo et al. [73,74] designed two kinds of highly efficient flame-retardant additives, DOPO-TMT and DMT, containing DOPO and maleimide groups for EP systems (Figure 9a,d). In DOPO-TMT, phosphenanthrene and triazine were flame-retardant groups, and in DMT, phosphenanthrene and triazine-trione were flame-retardant groups. When the phosphorus concentration was only 1.25 wt%, the EP/DOPO-TMT-1.25 system passed UL94 V-0 grade with a LOI of 37.5%. The PHRR and THR values of EP/DOPO-TMT and EP/DMT samples gradually decreased with the increase in phosphorus content. Compared with EP, the PHRR of EP/DOPO-TMT-1.25 was reduced by 54%, while that of EP/DMT-1.25 was reduced by 59%, which shows a higher flame retardancy. DOPO acted with nitrogen-containing groups simultaneously in the gas and condensed phase. In the condensed phase, both DOPO and nitrogen-containing groups contributed to the formation of expanded and dense carbon residue, which acts as a fire barrier (Figure 9c,f). In the gas phase, DOPO groups produced free radical inhibitors to quench reactive free radicals, and the nitrogen-containing groups released inert gas to dilute the combustible gas by pyrolysis. This work provided strong evidence for the synergistic effect of phosphorus and nitrogen.
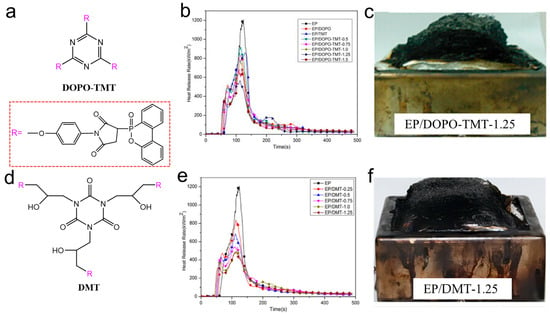
Figure 9.
(a) Structure of DOPO-TMT; (b) HRR curve for EP and EP/DOPO-TMT; (c) combustion residue of EP/DOPO-TMT-1.25; (d) structure of DMT; (e) HRR curve for EP and EP/DMT; (f) combustion residue of EP/DMT-1.25. (a–c) [73] and (d–f). (With permission from Ref. [74]; License number: 5617561446517, 2023, Elsevier).
The combination of phosphorus-containing flame retardants and other flame-retardant elements (nitrogen, boron, silicon) not only further improves the flame-retardant properties of flame retardants but also reduces the overall load of flame retardant in the material and maximizes the effectiveness under the same conditions [75]. Multi-component synergistic systems will become one of the possible development trends of phosphorus-based flame retardants in the future.
3.1.3. Carbon-Based Materials
The family of carbon-based nanomaterials, including carbon nanotubes (CNTs) [45,76], graphene [46,47,77], and expandable graphite (EG) [49,50,51], have been widely used in the preparation of polymer composites. The addition of carbon materials can not only enhance the mechanical properties of the substrate but also give the substrate excellent thermal stability, thermal or electrical conductivity, and fire resistance [78]. According to the reports, the flame-retardant mechanism of carbon-based materials mainly comes from strengthening the carbon layer and catalyzing the formation of carbon. The carbon layer formed on the heated surface of the matrix material blocked the contact between heat and oxygen, reduced the diffusion of active free radicals, and improved flame retardancy [79].
Graphene is a two-dimensional nanomaterial formed by a hexagonal network of sp2 hybrid carbon atoms with high mechanical strength and specific surface area [76]. Layered graphene can be dispersed in the polymer matrix, creating a “tortuous path” effect to slow down the heat diffusion rate and matrix decomposition rate [80]. However, graphene is prone to aggregation due to its special flake-like structure and the existence of π-π interactions and van der Waals force, and untreated graphene is difficult to disperse evenly in the polymer [81]. Therefore, in order to solve the problem of dispersion, many scholars often treat graphite to obtain graphene oxide (GO) to improve the interface binding force between graphene and epoxy resin first [45]. Then, GO is modified by chemical grafting or electrostatic adsorption of nanoparticles [82,83] to improve the dispersion and bring excellent flame retardancy to the graphene/epoxy resin system. For example, Li et al. [84] grafted 2-(diphenylphosphine) ethyl triethoxysilane (DPPES) onto the surface of graphene oxide nanosheets (GON) by condensation reaction. The addition of DPPES-GON (up to 10 wt%) to neat epoxy significantly increased its thermal stability and improved the LOI by 80%.
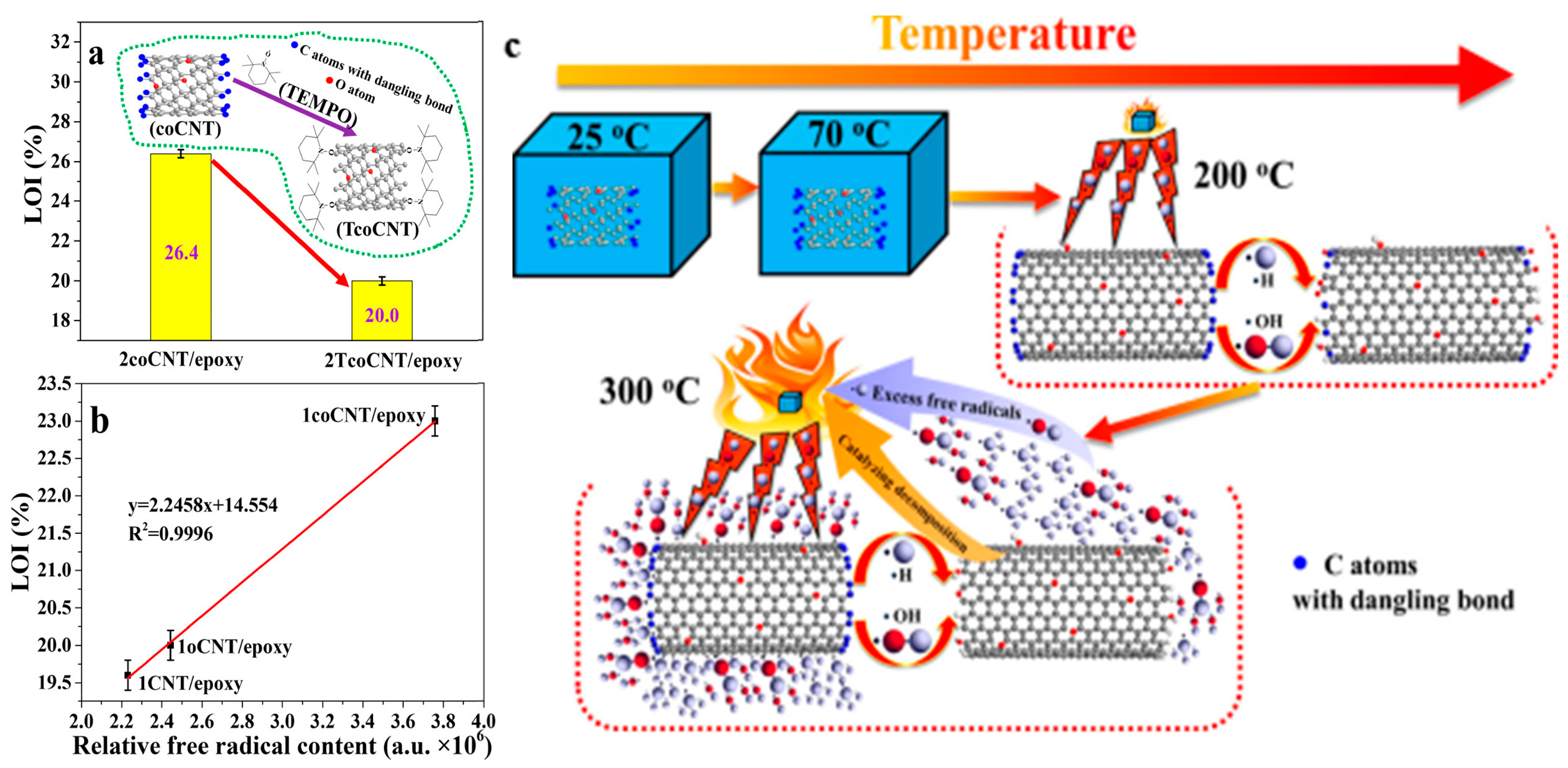
Based on their unique structure and properties, CNTs are widely used in nano-electronic devices, composite materials, electrical and thermal conductivity, nano-flame retardants, and other fields [85]. CNTs have the potential to improve the mechanical properties and fire resistance of epoxy resin matrices at the same time, but the difficulty of dispersion limits the load capacity of CNTs. Too low a load will lead to poor fire resistance, while too high a load will affect the mechanical properties of the composite [86]. Therefore, in order to balance the load contradiction and obtain CNTs/epoxy composites with superior mechanical properties and flame-retardant properties, reasonable modification of CNTs seems to be a feasible scheme. Wang et al. [86] performed concentrated acid oxidation and ball milling on pristine CNTs, which significantly increased the content of carbon-centered free radicals. The obtained carbon-centered free-radical-rich CNTs were used to try to improve the fire safety of a high-performance CNT/epoxy composite without sacrificing its mechanical properties. The test found that the overhanging bonds present in carbon-centered free-radical-rich CNTs had strong free radical scavenging capacity (as shown in Figure 10). This ability increased the loading of CNTs to 3 wt% with an LOI of 28.4% (LOI for pure EP was 21.9%) while enhancing mechanical properties.
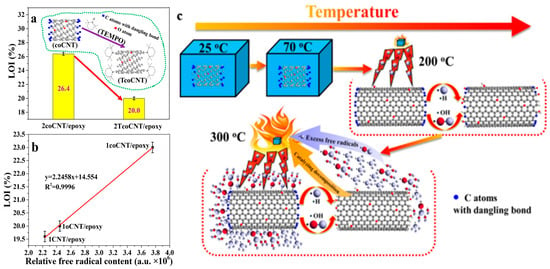
Figure 10.
LOI performances of the 2coCNT/epoxy and 2TcoCNT/epoxy composites (a); relationship between the relative free radical content of the filler and the LOI performance of the composite (b); free radical scavenging effect of the coCNTs during thermal decomposition of composites (c) [86].
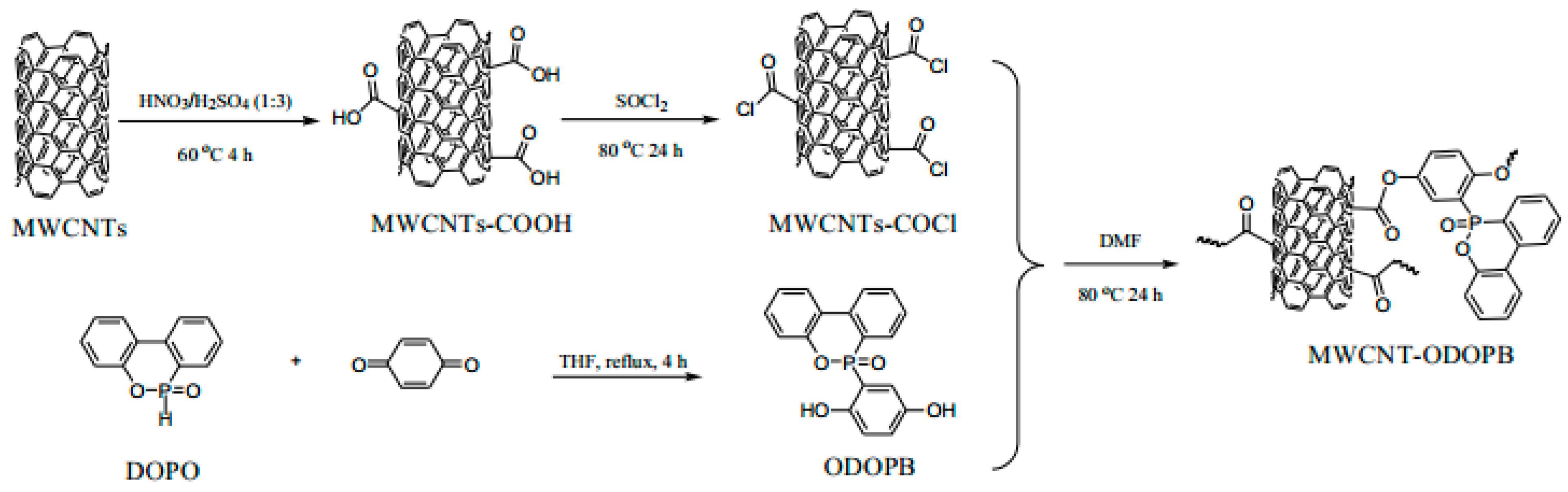
Moreover, functionalized grafting or inorganic filler loading of CNTs are also the modification approaches that have recently attracted much attention from researchers. It has been reported [87,88] that grafting functionalized siloxane (PDMA) onto CNTs can enhance the interfacial adhesion between CNTs and the polymer matrix, improve their dispersion in epoxy resins, and contribute to enhancing the flame-retardant properties of composites. Dopo-functionalized multiwalled carbon nanotubes (MWCNT) were obtained by grafting 10-(2,5-dihydroxyphenyl)-9,10-dihydro-9-oxygen-10-phospho-10-oxide (ODOPB) onto the outer surface of acylated multiwalled carbon nanotubes (MWCNT), as shown in Figure 11 [89]. MWCNT-ODOPB was co-introduced into the epoxy matrix with aluminum diethyl phosphate as a flame-retardant filler. When MWCNT-ODOPB content was only 1 wt%, the composite system achieved a LOI value of 39.5% and passed the UL94 V-0 grade. It is mainly due to the char formation of MWCNT-ODOPB and the synergistic effect of diethylaluminum phosphate. In a simpler method [90], phosphorus-nitrogen flame retardants were coated on CNTs and combined with epoxy resin to form composite materials. The synergistic effect of phosphorus-nitrogen flame retardants and nanomaterials endowed composites with excellent flame-retardant properties.
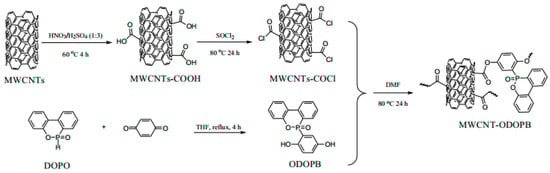
Figure 11.
Synthesis of MWCNT-ODOPB [89].
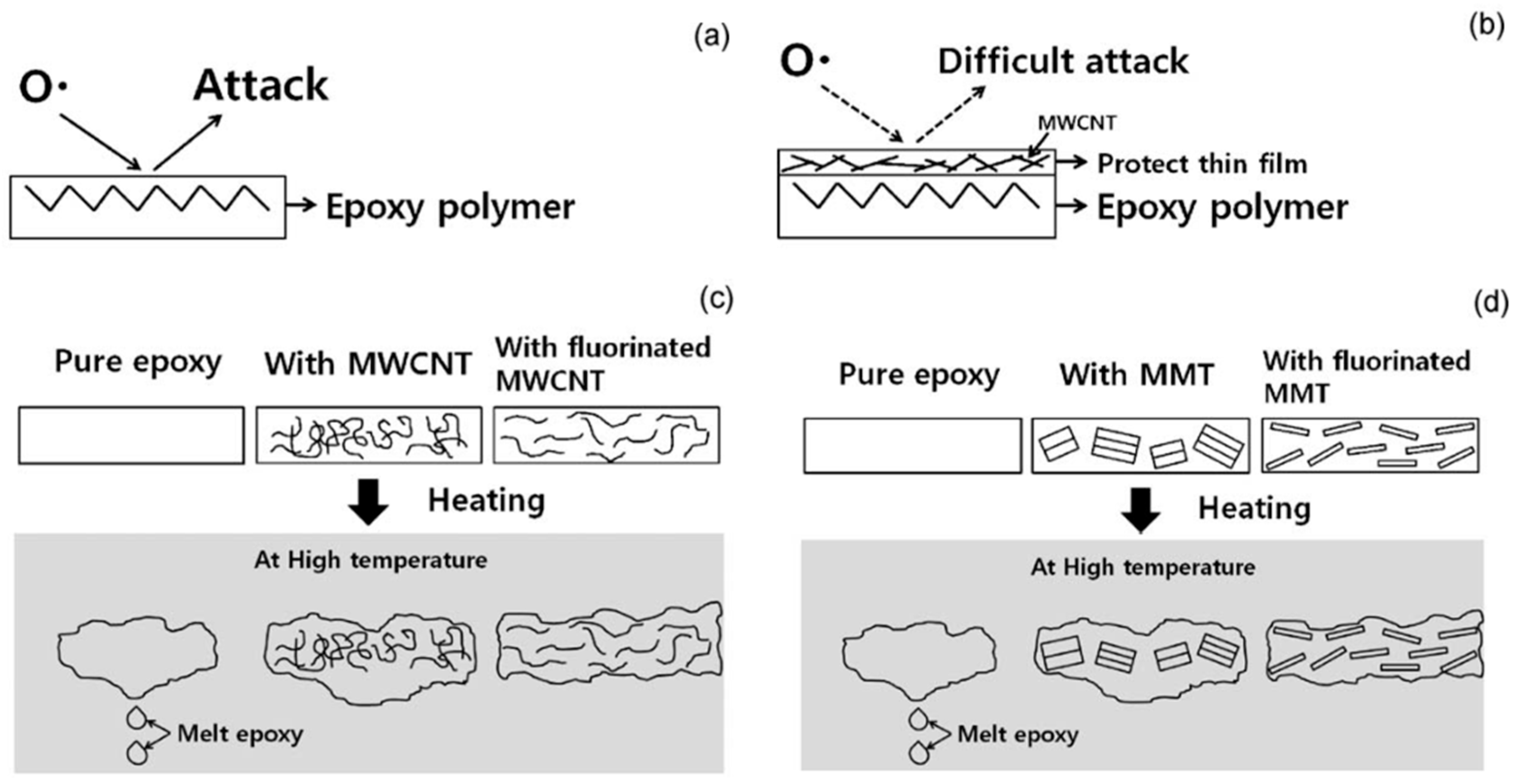
Typically, the fire performance of the epoxy resin was effectively improved by the organic combination of inorganic nanoclay and carbon nanotubes [91]. Lee et al. [92] studied the effect of adding fluoromontmorillonite-containing MMT/MWCNT additives on EP. The mechanism of flame retardants was analyzed from three aspects, and conclusions were drawn: ① Fluorinated MMT/MWCNT enhanced the dispersion in epoxy resin. ② The addition of MWCNT reduced the degradation rate of epoxy resin and increased carbon residue yield (increased from 9.1% to 15.4%). Meanwhile, due to the formation of gel epoxy resin during combustion and the flame retardation of MWCNTs, the fire was hindered and had difficulty spreading, as shown in Figure 12c. ③ The exfoliated MMT acted as a heat storage medium to inhibit the heat transfer inside the epoxy resin, as shown in Figure 12d. Under the joint action of the three aspects, the flame-retardant performance of the epoxy resin was significantly improved, and the LOI value was increased from 21% to 31%.
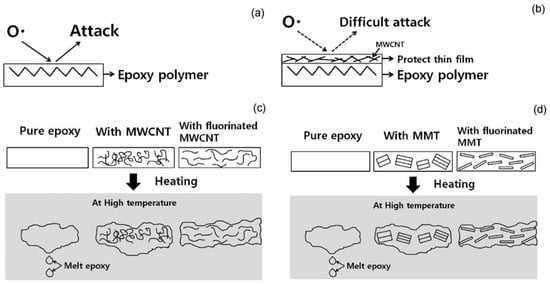
Figure 12.
The mechanism of flame retardant additives: (a) Oxygen free radicals initiate high temperature thermal oxidation of polymers; (b) Oxygen radicals was hindered by the formation of MWCNT/carbon char protective film; (c) Formation of internal network structure of MWCNT/EP composites; (d) Formation of internal network structure of MMT/EP composites. (With permission from Ref. [92]; License number: 5617570717974, 2023, Elsevier).
As another well-known carbon-based material, expandable graphite (EG) is a typical expansion flame retardant with an inherent self-expanding graphite flake [93]. When heated, it can expand and form a worm-like carbon layer that acts as a physical barrier to heat transfer, providing the polymer with fire-protection properties [50]. In addition, although EG contributes to the formation of carbon, the carbon structure is not tightly enough [49]. Therefore, EG will often be used in conjunction with other materials [23]. Yang et al. [94] reported the effect of DOPO or 6-phenoxy-cyclotriphosphonitrile (HPCP) and EG as flame retardants on the flame retardancy of epoxy resins. They found that the residual chars of EP/EG/DOPO and EP/EG/HPCP composites exhibited more compact and tough structures composed of worm-like graphite and a carbonized resin matrix.
3.2. Reactive Flame-Retardant Method
Although adding a flame retardant to improve the flame-retardant properties of polymer materials is a relatively mature method, the disadvantages of the additive system are obvious, including reduced mechanical properties of the resin and poor durability in long-term use. And what is worse is that as the material degrades, the flame retardant migrates, and there will be seepage, which will lead to the loss of flame retardancy and environmental pollution. Conversely, chemical reaction flame-retardant modification is achieved by functional modification of the epoxy molecular chain or curing agent. The flame-retardant elements are connected in the resin cross-linking network in the form of chemical bonds so that the flame-retardant elements have difficulty migrating and seeping, and the flame-retardant ability is more durable.
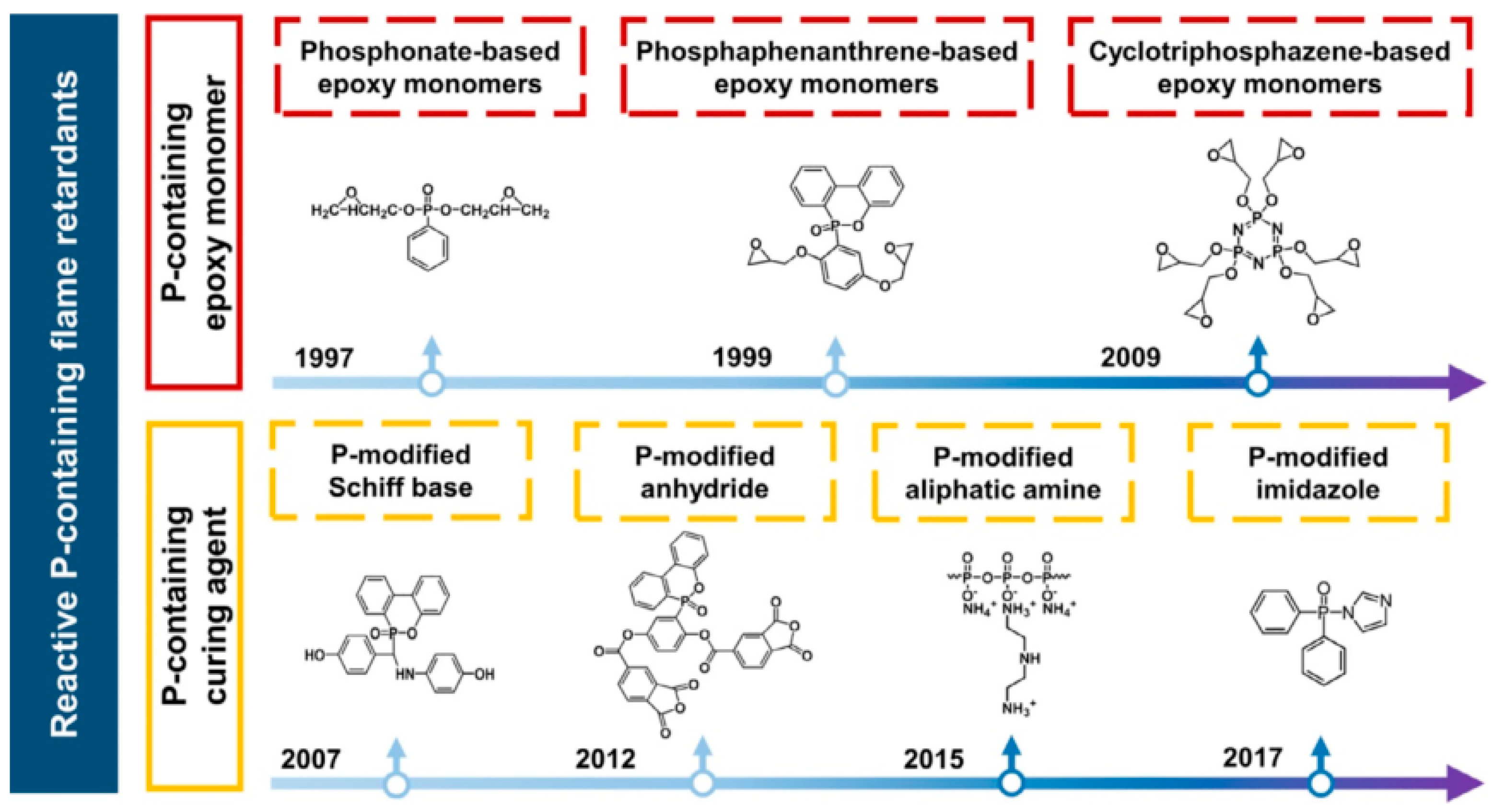
Given the above, phosphorus flame retardant is an important halogen-free flame retardant, which has a flame-retardant effect in both condensed and gaseous phases [95]. As early as 20 years ago, researchers proposed the idea of introducing phosphorus-containing groups in the main or side chains of epoxy resins to improve the flammability of the resin itself (as shown in Figure 13). By introducing a low amount of flame-retardant elements (such as phosphorus, nitrogen, silicon, etc.) into the epoxy resin chain, the LOI value, UL94 level, and thermal stability are improved, and the PHRR and THR are reduced without affecting the mechanical and physical properties. Huo [66], Dagdag [33], and Zhi [16] made detailed reviews on this aspect.
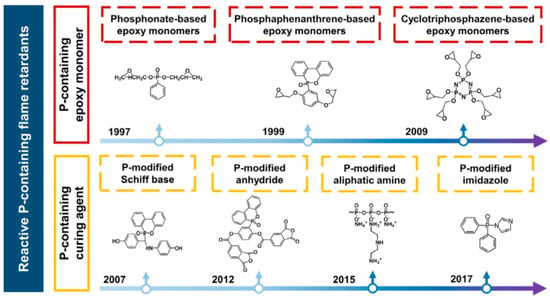
Figure 13.
Development of reactive phosphorous-containing flame retardants: representative phosphorous-containing epoxy resins and curing agents [66].
Currently, 90% of epoxy resins are bisphenol A (BPA) resins, and 67% of BPA resins are derived from petrochemical and non-renewable raw materials [96,97]. Moreover, BPA has a series of disadvantages, such as flammability, and the toxicity caused by material degradation not only destroys the ecological environment but also endangers organisms. For this reason, with the requirements of regulations and environmental protection, bio-based epoxy resin materials derived from sources such as vegetable oil [98,99], lignin [100,101], itaconic acid [102], and cardanol [103,104] have attracted attention because of the great availability of raw materials, and they have been applied in many fields such as toughening, flame retardation, and high-temperature resistance.
How to design and prepare a sustainable, biodegradable flame-retardant epoxy resin, which is expected to replace resource-limited petrochemical products, especially bisphenol A type epoxy resin (DGEBA), is one of the potential directions for the future development of constitutive flame-retardant epoxy resin. Ma et al. [105] synthesized a novel epoxy resin (MVE) from vanillin and melamine (See Figure 14). The epoxy equivalent of MVE is about 217 g/eq. After being cured by 4,4-diaminodiphenylmethane (DDM), MVE showed better mechanical properties than DGEBA resin, and the carbon residue yield increased to 41.77%. MVE has excellent intrinsic flame retardancy and achieved a LOI value of 39.5%; THR and smoke generation rate (SPR) were reduced by 67.44% and 64.69%, respectively, which was much better than DGEBA. In addition, under suitable conditions (THF:H2O = 6:4, 50 °C), the cured product of MVE/DDM can be completely degraded within 6 h.
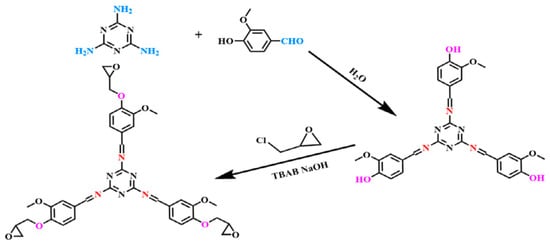
Figure 14.
Synthesis of intermediates of vanillin derivatives. (With permission from Ref. [105]; License number: 5617580564610, 2023, Elsevier).
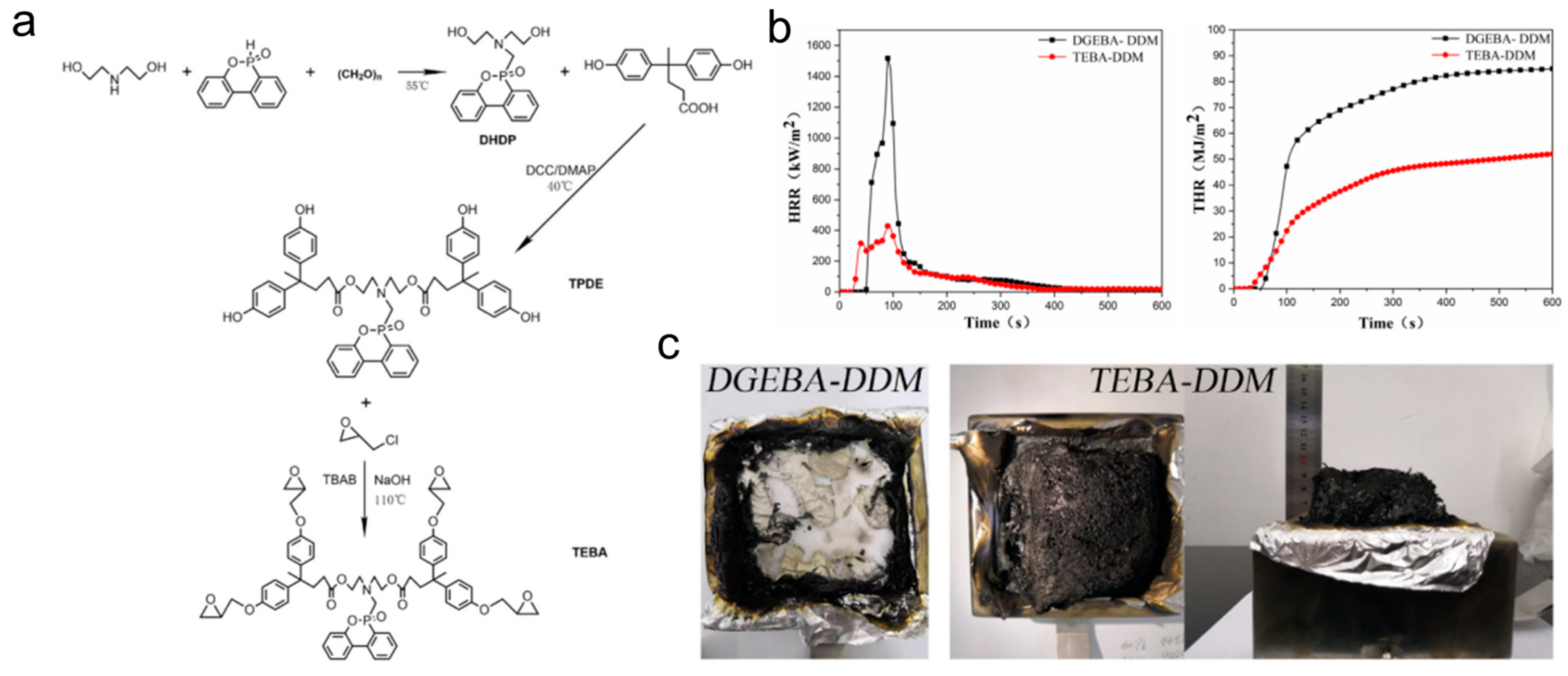
Diphenol acid (DPA), as a plant derivative, has been shown to be one of the sustainable alternatives to bisphenol A. A flame-retardant bio-based epoxy resin (TEBA) was synthesized from DOPO, diethanolamine, and DPA in three steps (Figure 15) [106]. Compared with the original resin, the TEBA passed UL94 V-0 grade with an LOI of 42.3%, and the PHRR, THR, and total smoke production (TSP) were decreased by 67%, 27%, and 35%, respectively. As a consequence, the presence of phosphorous and nitrogen flame-retardant elements in the molecular chain of the resin endowed TEBA-DDM with remarkable fire resistance.
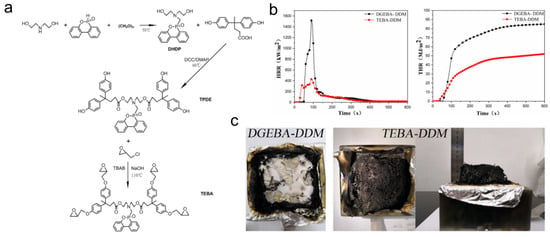
Figure 15.
(a) The synthesis of TEBA; (b) HRR and THR curves of the TBEA-DDM and DGEBA-DDM epoxy thermosets; (c) digital photos of epoxy thermosets char residues after the cone calorimeter test. (With permission from Ref. [106]; License number: 5617580950671, 2023, Elsevier).
4. Epoxy Fireproof Coating
Fireproof coatings are one of the important branches in the field of coatings, which are used to protect materials such as metals, polymers, textiles, and wooden structures [107]. EP is one of the most important binders in common coatings used in human production and life [108]. Generally, EP cross-links under the action of an amine curing agent to form a coating with high strength, high resistance to impact [109], and high chemical resistance, but the EP coating system also has defects such as easy combustion and poor toughness. Therefore, in order to improve upon the above problems, the existing solutions mainly include two aspects: on the one hand, starting from the molecular structure, the molecular structure of the monomer or curing agent can be optimized, and functional molecular chains at the end of the chain, such as a toughened polyurethane chain and flame-retardant silicone chain, can be inserted. On the other hand, directly filled organic or inorganic fillers can improve the mechanical, anti-corrosion, and flame-retardant properties of the EP coating.
After sorting out the reports on epoxy resin flame-retardant coatings in recent years (Table 3), it can be found that most of the research focuses on the preparation of new flame-retardant fillers, among which the modification methods include chemical reaction, organic–inorganic/inorganic–inorganic hybrids, assembly, coating, etc.

Table 3.
Flame retardancy of epoxy coatings containing new flame retardants.
However, with the deepening of research on fireproof materials, people now have higher and higher expectations for the service life of coatings, so the demand for multi-functional organic coatings has become more and more urgent [118]. When it is difficult for a single-performance coating to meet the application requirements in some harsh environments, flame-retardant coatings based on EP gradually developed into high-performance coatings with multiple functions such as flame retardancy and anti-corrosion or wear resistance qualities in order to extend the service life. Furthermore, the organic combination and synergy of the components may be the key to the successful preparation of multi-functional integrated fireproof coatings.
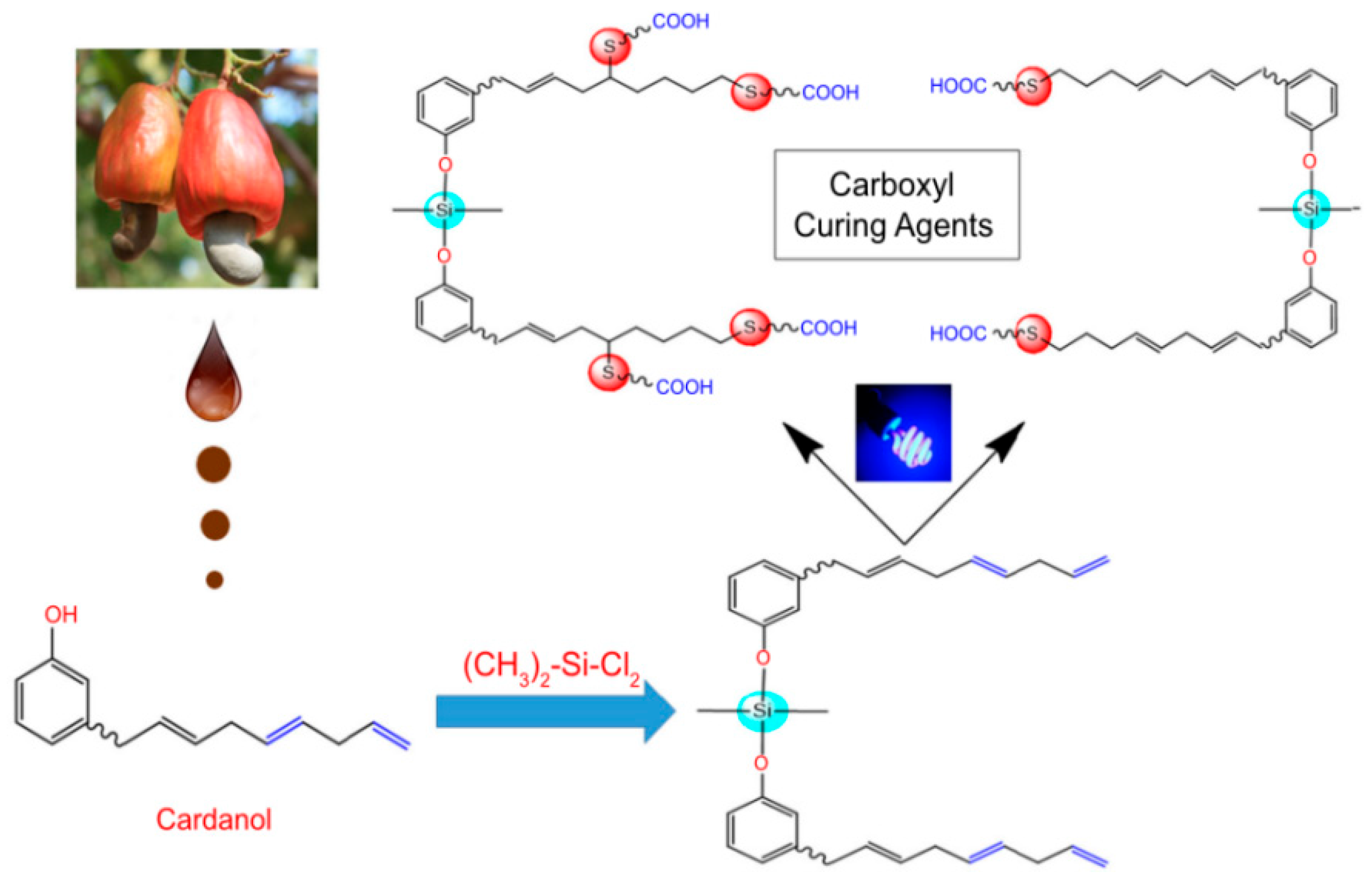
As shown in Figure 16, two kinds of silicon-sulfur multi-functional carboxyl curing agents (S-Ⅰ and S-II) were synthesized from cashew phenol as raw materials by mercapto-ene coupling method and applied to EP [119]. The curing agent is mixed with conventional epoxy resin instead of a traditional curing agent and coated on the steel plate to form a coating. The coatings cured by S-Ⅰ and S-II still maintain excellent corrosion resistance after 1000 h of exposure to a salt spray test. Compared with the S-I coating, the S-II-cured coating exhibits high impedance values and low corrosion rates and has a higher weight loss temperature and carbon residue yield (As shown in Table 4). This is because the presence of additional functional groups in S-II increased the cross-linking density of the resin and improved the thermal stability and other coating properties.
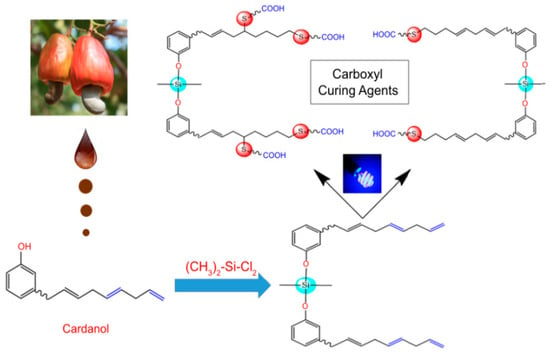
Figure 16.
The synthesis of multi-functional carboxyl curing agent. (With permission from Ref. [119]; License number: 5617630790172, 2023, Springer Nature).

Table 4.
TGA values and electrochemical parameters of coatings cured with S-I and S-II.
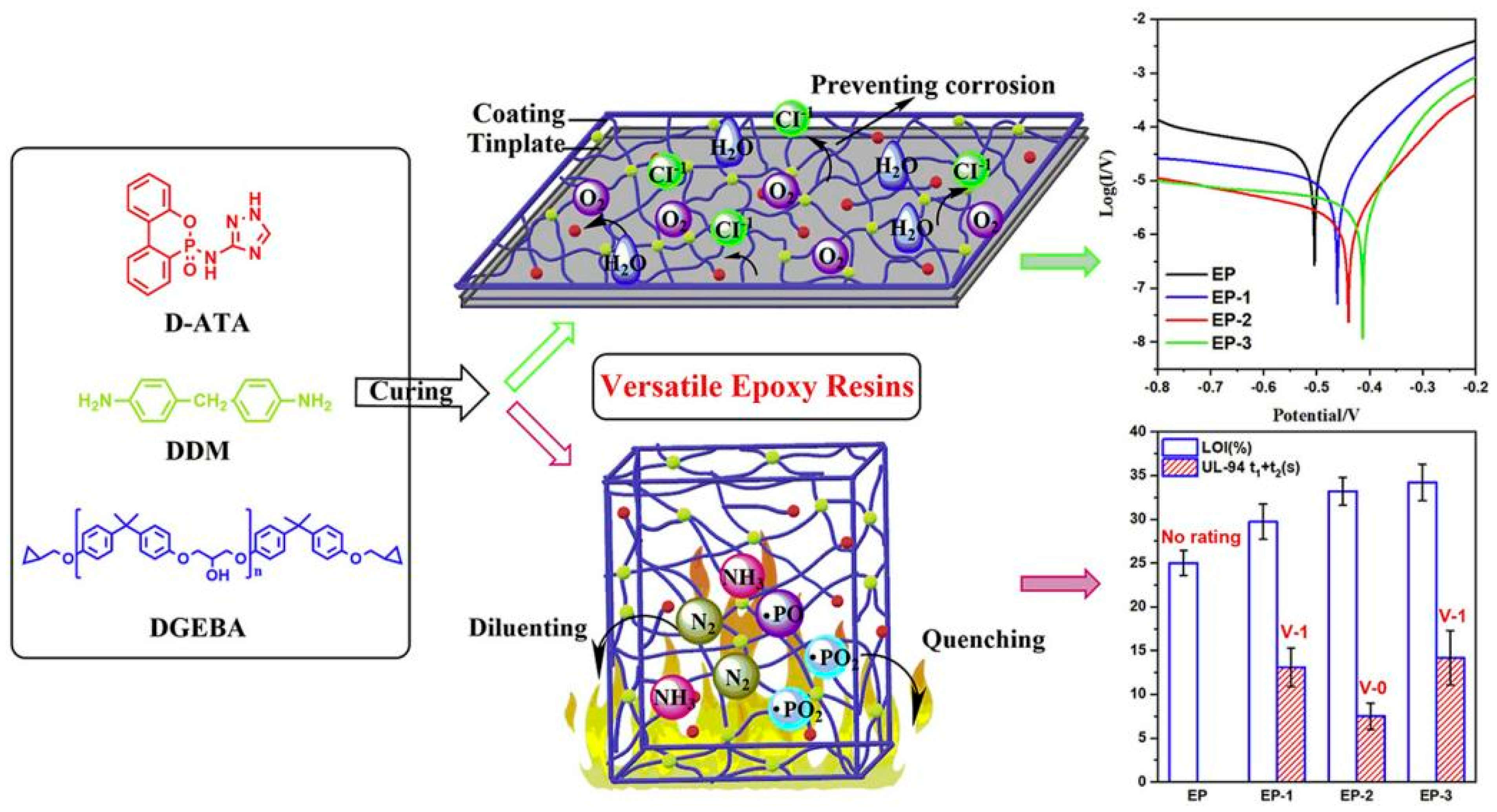
It has been reported that the introduction of bifunctional phosphate-containing triazole derivatives (D-ATA) into the epoxy resin system through curing reactions can simultaneously improve the flame retardancy and corrosion resistance of the resin (Figure 17) [120]. In this system, the structure of triazole phosphate not only contributes to flame retardation but also effectively inhibits electrochemical corrosion. When D-ATA content was only 5 wt%, the flame-retardant epoxy resin achieved an LOI value of 33.2%, passed the UL94 V-0 grade, and residual char yield reached 16.6%. Moreover, the anti-corrosion efficiency of EP/5 wt% D-ATA was increased by 95.3% compared with pure EP. At the same time, the mechanical properties of composite epoxy thermoset materials were also improved due to the existence of hydrogen bonds and π-π interaction in D-ATA.
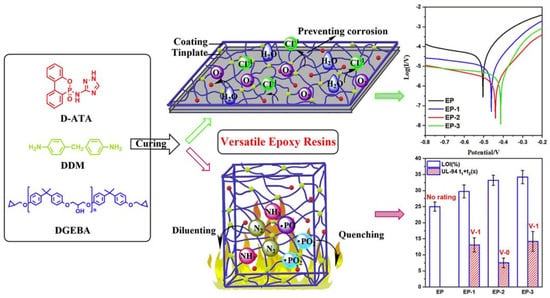
Figure 17.
Preparation of corrosive flame-retardant multi-functional epoxy resin coating. (With permission from Ref. [120]; License number: 5617631477525, 2023, Elsevier).
Porous coordination polymers (PCPs), commonly known as metal–organic frameworks (MOFs), are a new class of organic–inorganic porous structures in which metal cations serve as nodes and organic molecules serve as bridges [121]. Because of their controllable structure and easy surface functionalization, MOFs are often used to prepare MOFs/polymer nanocomposites. Additionally, MOFs have satisfactory compatibility with polymer substrates due to the presence of organic–inorganic hybrid structures [122]. The existing research reports indicate that the addition of MOFs to epoxy resins can affect various properties of epoxy resins, such as anti-corrosion [123,124], flame retardation [125,126,127], thermomechanical [128], and dielectric properties. Accordingly, MOFs/EP system has great potential in the preparation of multi-functional integrated coatings such as fire protection and anti-corrosion.
For example, it has been reported that an anti-corrosion coating (ZnG@ZIF-8/EP) was successfully prepared by adding MOF-based anti-corrosion materials loaded with corrosion inhibitors (ZnG@ZIF-8) to EP [129]. ZnG@ZIF-8 is uniformly dispersed in the coating system and exhibits better corrosion resistance than ZIF-8/EP, EP, and ZnG+ZIF-8/EP coatings. The mechanism study showed that the corrosion rate was delayed due to the defects, and the pores in the EP coating were fully populated by ZnG@ZIF-8. Organic ligands in the MOF structure not only help to improve compatibility but also provide flame-retardant elements or groups, including nitrogen-containing groups and aromatic structures [130]. The ligand of ZIF is composed of imidazole compounds rich in nitrogen atoms. Furthermore, it has recently been reported that nitrogen-rich MOF, when used as a flame retardant for EP, had a remarkable catalytic carbon formation ability to improve fire safety performance [131].
The multi-functional integrated flame-retardant coating is mostly used in special conditions; in addition to the necessary fire prevention ability, it also usually has other properties such as anti-fouling, antibacterial, anti-corrosion, and wear-resistant. For example, the fireproof coating applied to a tunnel surface needs both anti-fouling and water resistance [132]. This is because the environment inside the tunnel is closed, and the oil smoke generated by the car is not easy to dissipate and adsorb on the tunnel wall surface, so the fireproof coating on the surface should have the ability to resist the adhesion of oil smoke and withstand high-pressure water cleaning. For this reason, when designing the coating formulation, nano-silica, titanium dioxide, and organosilane were introduced into the epoxy resin expansion flame-retardant system to enhance the fire protection, anti-fouling, and waterproof properties of the coating.
5. Summary and Perspectives
As one of the most important polymer materials since it was discovered by human beings, epoxy resin is widely used in various fields due to its excellent performance. However, the high flammability caused by its structure is an urgent problem for researchers to explore and solve. This review first describes the combustion process of polymer and its flame-retardant mechanism. The research progress of flame-retardant epoxy resin in recent years is summarized from two modification methods: the physical additive flame-retardant method (including silicon-, phosphorus- nitrogen- and carbon-based flame retardants) and the reactive flame-retardant method, and their advantages and disadvantages and flame-retardant properties are briefly summarized. Finally, the new flame retardants used for epoxy resin flame-retardant coatings are summarized, and the development trend of epoxy flame-retardant coatings with multi-function integration of flame retardant/anti-corrosion is proposed. Although the flame-retardant chemical system has gradually matured after decades of development, the following issues still need to be further studied in the future:
- The flame-retardant life is short. With the degradation of the resin, the doped fillers will continue to overflow, losing the flame-retardant effect and easily causing environmental pollution. Therefore, green and efficient reactive flame retardants need to be further explored;
- The performance is not long-lasting, which makes it difficult to meet the application requirements of special environments, especially in the application of coating preparation. Generally, harsh environments (such as aviation, aerospace, plateau, and deep-sea) will accelerate the aging of materials, so there is an urgent need to develop a series of multi-functional epoxy flame-retardant materials that have long-term durability, low-temperature mechanical properties, antibacterial and corrosion resistance, etc.;
- The flame-retardant effect of multi-element coordination is much greater than that of a single element, but the high cost limits the development and application of multi-element flame retardants. Therefore, active flame retardants with cheap raw materials, simple preparation methods, and low overall costs need to be further explored. Ultimately, it will be an important research direction in the future to prepare reactive flame-retardant resins from abundant bio-based raw materials or to regulate the flame retardancy of resins through molecular structure design.
Author Contributions
Writing—original draft preparation, S.S.; Writing—review and editing, Supervision, Funding Acquisition, Q.Y. and B.Y.; Project Administration, Funding Acquisition, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by National Natural Science Foundation of China (52075524, U21A2046), Youth Innovation Promotion Association CAS (2022429), Gansu Province Science and Technology Plan (22JR5RA094, 20JR10RA060), Natural Science Foundation of Shandong Province (ZR2022ZD09), Gansu Province Basic Research Innovation Group Project (22JR5RA093), and LICP Cooperation Foundation for Young Scholars (HZJJ21-06).
Data Availability Statement
The data used to support the study can be available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, S.; Dhawan, R.; Shukla, S.K. Flame Retardant Polymer Nanocomposites: An Overview. Macromol. Symp. 2023, 407, 2200089. [Google Scholar] [CrossRef]
- Vahabi, H.; Sonnier, R.; Ferry, L. Effects of ageing on the fire behaviour of flame-retarded polymers: A review. Polym. Int. 2014, 64, 313–328. [Google Scholar] [CrossRef]
- Salasinska, K.; Mizera, K.; Celiński, M.; Kozikowski, P.; Borucka, M.; Gajek, A. Thermal properties and fire behavior of polyethylene with a mixture of copper phosphate and melamine phosphate as a novel flame retardant. Fire Saf. J. 2020, 115, 103137. [Google Scholar] [CrossRef]
- Zhou, S.; Tao, R.; Dai, P.; Luo, Z.; He, M. Two-step fabrication of lignin-based flame retardant for enhancing the thermal and fire retardancy properties of epoxy resin composites. Polym. Compos. 2020, 41, 2025–2035. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khosravi, F.; Mossayebi, Z.; Ardahaei, A.S.; Dehaghi, F.M.; Khorasani, M.; Neisiany, R.E.; Das, O.; Marani, A.; Mensah, R.A.; et al. The Flame Retardancy of Polyethylene Composites: From Fundamental Concepts to Nanocomposites. Molecules 2020, 25, 5157. [Google Scholar] [CrossRef] [PubMed]
- Majka, T.; Stachak, P. The studies on the production of polyethylene film with reduced flammability. In Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry, Santiago, Spain, 3 November 2017. [Google Scholar] [CrossRef]
- Giri, R.; Nayak, L.; Rahaman, M. Flame and fire retardancy of polymer-based composites. Mater. Res. Innov. 2020, 25, 104–132. [Google Scholar] [CrossRef]
- Huo, S.; Liu, Z.; Wang, J. Thermal properties and flame retardancy of an intumescent flame-retarded epoxy system containing phosphaphenanthrene, triazine-trione and piperidine. J. Therm. Anal. Calorim. 2019, 139, 1099–1110. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Huo, S.; Zhang, B.; Yang, S. Preparation and flame retardancy of DOPO-based epoxy resin containing bismaleimide. High Perform. Polym. 2016, 28, 1090–1095. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, M.; Zhang, Y.; Gao, X.; Zhang, Q. Preparation and performances of novel waterborne intumescent fire retardant coatings. Prog. Org. Coat. 2016, 95, 100–106. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Zhumabekova, A.; Serikbayeva, G.; Vikulova, M.; Svitkina, V. Effect of Phosphorus and Chlorine Containing Plasticizers on the Physicochemical and Mechanical Properties of Epoxy Composites. J. Compos. Sci. 2023, 7, 178. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H.; Zhan, T. Nitrogen/sulfur-containing DOPO based oligomer for highly efficient flame-retardant epoxy resin. Polym. Degrad. Stab. 2019, 171, 109023. [Google Scholar] [CrossRef]
- Wazarkar, K.; Kathalewar, M.; Sabnis, A. Reactive Modification of Thermoplastic and Thermoset Polymers using Flame Retardants: An Overview. Polym. Technol. Eng. 2015, 55, 71–91. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Qu, L.-Y.; Liu, Y.; Zhu, P. An overview of alginates as flame-retardant materials: Pyrolysis behaviors, flame retardancy, and applications. Carbohydr. Polym. 2021, 260, 117827. [Google Scholar] [CrossRef] [PubMed]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A comprehensive review of reactive flame-retardant epoxy resin: Fundamentals, recent developments, and perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Dorigato, A.; Fredi, G.; Fambri, L.; Lopez-Cuesta, J.-M.; Pegoretti, A. Polyethylene-based single polymer laminates: Synergistic effects of nanosilica and metal hydroxides. J. Reinf. Plast. Compos. 2018, 38, 62–73. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Lewin, M.; Weil, E.D. Mechanisms and modes of action in flame retardancy of polymers. Fire Retard. Mater. 2001, 1, 31–57. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Wang, X.; Wang, G.; Ding, D. The flame retardancy and smoke suppression effect of a hybrid containing CuMoO4 modified reduced graphene oxide/layered double hydroxide on epoxy resin. J. Hazard. Mater. 2018, 343, 364–375. [Google Scholar] [CrossRef]
- Liu, D.; Ji, P.; Zhang, T.; Lv, J.; Cui, Y. A bi-DOPO type of flame retardancy epoxy prepolymer: Synthesis, properties and flame-retardant mechanism. Polym. Degrad. Stab. 2021, 190, 109629. [Google Scholar] [CrossRef]
- Jiao, C.; Zhang, C.; Dong, J.; Chen, X.; Qian, Y.; Li, S. Combustion behavior and thermal pyrolysis kinetics of flame-retardant epoxy composites based on organic–inorganic intumescent flame retardant. J. Therm. Anal. Calorim. 2015, 119, 1759–1767. [Google Scholar] [CrossRef]
- Gao, M.; Yang, S. A novel intumescent flame-retardant epoxy resins system. J. Appl. Polym. Sci. 2010, 115, 2346–2351. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-Cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Zielecka, M.; Rabajczyk, A.; Pastuszka, Ł.; Jurecki, L. Flame Resistant Silicone-Containing Coating Materials. Coatings 2020, 10, 479. [Google Scholar] [CrossRef]
- Gérard, C.; Fontaine, G.; Bourbigot, S. New Trends in Reaction and Resistance to Fire of Fire-retardant Epoxies. Materials 2010, 3, 4476–4499. [Google Scholar] [CrossRef]
- Mishra, K.; Singh, R.P. Quantitative evaluation of the effect of dispersion techniques on the mechanical properties of polyhedral oligomeric silsesquioxane (POSS)–epoxy nanocomposites. Polym. Compos. 2018, 39, E2445–E2453. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Yang, W.; Niu, L.; Zhao, J.; Ma, B.; Kang, C. Influence of montmorillonite on the properties of halogen–antimony flame retardant polypropylene composites. Polym. Compos. 2019, 40, 1930–1938. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Tang, G.; Chen, J.; Huang, Z.-Q.; Hu, Y. Biobased polyelectrolyte multilayer-coated hollow mesoporous silica as a green flame retardant for epoxy resin. J. Hazard. Mater. 2018, 342, 689–697. [Google Scholar] [CrossRef]
- Shree, V.; Sen, A.K.; Basu, S.; Ratna, D. Use of a combination of phosphorous-containing epoxy resin and silica fillers for development of flame retardant thermoset polymer composites. J. Vinyl Addit. Technol. 2023, 29, 130–143. [Google Scholar] [CrossRef]
- Guo, W.; Nie, S.; Kalali, E.N.; Wang, X.; Wang, W.; Cai, W.; Song, L.; Hu, Y. Construction of SiO2@UiO-66 core–shell microarchitectures through covalent linkage as flame retardant and smoke suppressant for epoxy resins. Compos. Part B Eng. 2019, 176, 107261. [Google Scholar] [CrossRef]
- Dagdag, O.; El Bachiri, A.; Hamed, O.; Haldhar, R.; Verma, C.; Ebenso, E.; El Gouri, M. Dendrimeric Epoxy Resins Based on Hexachlorocyclotriphosphazene as a Reactive Flame Retardant Polymeric Materials: A Review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3240–3261. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, X.; Li, Z.; Wang, Y. Synthesis of new phosphorous-containing flame retardant and the properties of flame retardant epoxy resins. Pigment. Resin Technol. 2021, 50, 554–562. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Ma, S.; Yuan, W.; Li, Q.; Feng, J.; Zhu, J. Vanillin-derived phosphorus-containing compounds and ammonium polyphosphate as green fire-resistant systems for epoxy resins with balanced properties. Polym. Adv. Technol. 2018, 30, 264–278. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, M.; Qu, L.; Sun, G. Effect of phosphorus-containing flame retardants on flame retardancy and thermal stability of tetrafunctional epoxy resin. Polym. Adv. Technol. 2015, 26, 1531–1536. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Wang, C.; Chen, J.; Ding, A. Synthesis of an efficient phosphorus and triazine ring-containing reactive flame retardant for epoxy resin. Multidiscip. Model. Mater. Struct. 2023, 19, 587–603. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, K.; Jiang, J.; Li, J.; Liu, Y. Highly dispersed melamine cyanurate flame-retardant epoxy resin composites. Polym. Int. 2017, 66, 85–91. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Wang, X. Preparation and properties of epoxy resin modified with phosphorus and nitrogen flame retardants. Mater. Res. Express 2023, 10, 035303. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Fire Retardancy of Aluminum Hydroxide Reinforced Flame Retardant Modified Epoxy Resin Composite. Russ. J. Appl. Chem. 2018, 91, 680–686. [Google Scholar] [CrossRef]
- Liao, H.; Liu, Y.; Jiang, J.; Li, J.; Liu, Y. Synergism and antagonism of phosphorus-containing epoxy resin combined with different metal hydroxides. J. Fire Sci. 2016, 34, 3–12. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.; Zhu, J. Synergistic flame retardant effect of aluminum hydroxide and ammonium polyphosphate on epoxy resin. J. Appl. Polym. Sci. 2022, 139, e53168. [Google Scholar] [CrossRef]
- Budd, R.; Cree, D. Effect of fire retardants on mechanical properties of a green bio-epoxy composite. J. Appl. Polym. Sci. 2019, 136, 47398. [Google Scholar] [CrossRef]
- Jiajun, M.; Junxiao, Y.; Yawen, H.; Ke, C. Aluminum–organophosphorus hybrid nanorods for simultaneously enhancing the flame retardancy and mechanical properties of epoxy resin. J. Mater. Chem. 2011, 22, 2007–2017. [Google Scholar] [CrossRef]
- Toldy, A.; Szebényi, G.; Molnár, K.; Tóth, L.F.; Magyar, B.; Hliva, V.; Czigány, T.; Szolnoki, B. The Effect of Multilevel Carbon Reinforcements on the Fire Performance, Conductivity, and Mechanical Properties of Epoxy Composites. Polymers 2019, 11, 303. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Gao, S.; Yang, X.; Fan, R.; Zhi, M.; Fu, M. Recent advances in the flame retardancy role of graphene and its derivatives in epoxy resin materials. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106539. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X.; Deng, N.; Chu, Z. Preparation of a novel mono-component intumescent flame retardant for enhancing the flame retardancy and smoke suppression properties of epoxy resin. J. Therm. Anal. Calorim. 2018, 134, 1505–1519. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F.; Al-Sehemi, A.G.; Assiri, M.A.; Raza, M.R.; Irfan, A. Effect of expandable graphite and ammonium polyphosphate on the thermal degradation and weathering of intumescent fire-retardant coating. J. Appl. Polym. Sci. 2021, 138, 50310. [Google Scholar] [CrossRef]
- Yuan, Z.; Shu, Z.; Qi, L.; Cai, W.; Liu, W.; Wang, J.; Derradji, M.; Wang, Y. Curing behavior, mechanical, and flame-retardant properties of epoxy-based composites filled by expandable graphite and ammonium polyphosphate. J. Appl. Polym. Sci. 2023, 140, e53267. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, Y.; Liu, Z.; Peng, H.; Esmaeili, N. Synergistic action of expandable graphite on fire safety of a self-intumescent flame retardant epoxy resin. J. Appl. Polym. Sci. 2023, 140, e53425. [Google Scholar] [CrossRef]
- Liu, C.; Huang, K.; Liu, R.; Li, Y.; Dai, L.; Wang, W. A multi-element flame retardant on the basis of silicon-phosphorus-nitrogen for combustibility suppressing of epoxy. Polym. Test. 2022, 111, 107582. [Google Scholar] [CrossRef]
- Qu, L.; Sui, Y.; Zhang, C.; Li, P.; Dai, X.; Xu, B.; Fang, D. POSS-functionalized graphene oxide hybrids with improved dispersive and smoke-suppressive properties for epoxy flame-retardant application. Eur. Polym. J. 2020, 122, 109383. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Guo, X.; Yang, R.; Li, X. Effects of an Organic-Inorganic Hybrid Containing Allyl Benzoxazine and POSS on Thermal Properties and Flame Retardancy of Epoxy Resin. Polymers 2019, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Franchini, E.; Galy, J.; Gérard, J.-F.; Tabuani, D.; Medici, A. Influence of POSS structure on the fire retardant properties of epoxy hybrid networks. Polym. Degrad. Stab. 2009, 94, 1728–1736. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Zeng, B.; Chen, G.; Wu, Y.; Chen, T.; Dai, L. A high synergistic P/N/Si-containing additive with dandelion-shaped structure deriving from self-assembly for enhancing thermal and flame retardant property of epoxy resins. React. Funct. Polym. 2018, 131, 89–99. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Zheng, Y.; Guo, J.; Chen, B.; Pan, Z.; Hu, Y. A combination of POSS and polyphosphazene for reducing fire hazards of epoxy resin. Polym. Adv. Technol. 2018, 29, 1242–1254. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Wang, L.-L.; Wang, M.-Z.; Yuen, A.C.-Y.; Chen, T.B.-Y.; Yang, W.; Pan, T.-Z.; Zhi, Y.-R.; Lu, H.-D. Simultaneous enhancements in the mechanical, thermal stability, and flame retardant properties of poly(1,4-butylene terephthalate) nanocomposites with a novel phosphorus–nitrogen-containing polyhedral oligomeric silsesquioxane. RSC Adv. 2017, 7, 54021–54030. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, X.; Yu, B.; Feng, X.; Mu, X.; Yuen, R.K.; Hu, Y. Flame-retardant-wrapped polyphosphazene nanotubes: A novel strategy for enhancing the flame retardancy and smoke toxicity suppression of epoxy resins. J. Hazard. Mater. 2017, 325, 327–339. [Google Scholar] [CrossRef]
- Zhai, C.; Xin, F.; Cai, L.; Chen, Y.; Qian, L. Flame retardancy and pyrolysis behavior of an epoxy resin composite flame-retarded by diphenylphosphinyl-POSS. Polym. Eng. Sci. 2020, 60, 3024–3035. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.H.; Song, C.F.; Chang, Y.; Chen, G.R.; Xu, Y.T.; Dai, L.Z. Modification of epoxy resin through the self-assembly of a surfactant-like multi-element flame retardant. J. Mater. Chem. A 2016, 4, 3462–3470. [Google Scholar] [CrossRef]
- Ye, X.; Feng, Y.; Tian, P.; Li, Z.; Li, Y.; Wang, W.; Li, J.; Qiao, L.; Wang, K.; Zhang, W.; et al. Engineering two nitrogen-containing polyhedral oligomeric silsesquioxanes (N-POSSs) to enhance the fire safety of epoxy resin endowed with superior thermal stability. Polym. Degrad. Stab. 2022, 200, 109946. [Google Scholar] [CrossRef]
- Jiao, C.; Dong, J.; Zhang, C.; Zhuo, J.; Chen, X. Synthesis and properties of a phosphate ester as curing agent in an epoxy resin system. Iran. Polym. J. 2014, 23, 591–598. [Google Scholar] [CrossRef]
- Qiu, S.; Ma, C.; Wang, X.; Zhou, X.; Feng, X.; Yuen, R.K.; Hu, Y. Melamine-containing polyphosphazene wrapped ammonium polyphosphate: A novel multifunctional organic-inorganic hybrid flame retardant. J. Hazard. Mater. 2018, 344, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Shao, Z.-B.; Chen, X.-F.; Long, J.-W.; Chen, L.; Wang, Y.-Z. Novel Multifunctional Organic–Inorganic Hybrid Curing Agent with High Flame-Retardant Efficiency for Epoxy Resin. ACS Appl. Mater. Interfaces 2015, 7, 17919–17928. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Weil, E.D.; Choudhary, V. Flame-Retarding Plastics and Elastomers with Melamine. J. Fire Sci. 1995, 13, 104–126. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.-Y.; Jian, R.-K.; Liu, Z.; Huang, G. Chemical structure construction of DOPO-containing compounds for flame retardancy of epoxy resin: A review. Prog. Org. Coat. 2023, 175, 107316. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, Y.; Yu, B.; Song, J.; Tan, D.; Zhao, J.; Yan, S. Synthesis of a hyperbranched polyamide oligomer containing DOPO for simultaneously enhancing the flame retardance and glass transition temperature of epoxy resin. Polym. Adv. Technol. 2020, 32, 525–537. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Hu, Y. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stab. 2016, 133, 358–366. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Hu, Y. Preparation and investigation of flame-retardant epoxy resin modified with a novel halogen-free flame retardant containing phosphaphenanthrene, triazine-trione, and organoboron units. J. Appl. Polym. Sci. 2017, 134, 45291. [Google Scholar] [CrossRef]
- Ding, J.; Tao, Z.; Zuo, X.; Fan, L.; Yang, S. Preparation and properties of halogen-free flame retardant epoxy resins with phosphorus-containing siloxanes. Polym. Bull. 2009, 62, 829–841. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Cheng, L. Synthesis of a Phosphorus/Nitrogen-Containing Additive with Multifunctional Groups and Its Flame-Retardant Effect in Epoxy Resin. Ind. Eng. Chem. Res. 2015, 54, 7777–7786. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Yang, S.; Wang, J.; Zhang, B.; Zhang, B.; Chen, X.; Tang, Y. Synthesis of a novel phosphorus-nitrogen type flame retardant composed of maleimide, triazine-trione, and phosphaphenanthrene and its flame retardant effect on epoxy resin. Polym. Degrad. Stab. 2016, 131, 106–113. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Wang, J. Flame Retardancy and Dispersion of Functionalized Carbon Nanotubes in Thiol-Ene Nanocomposites. Polymers 2021, 13, 3308. [Google Scholar] [CrossRef]
- Mostovoy, A.; Yakovlev, A.; Lopukhova, M. Directional control of physico-chemical and mechanical properties of epoxide composites by the addition of graphite-graphene structures. Polym. Technol. Mater. 2019, 59, 874–883. [Google Scholar] [CrossRef]
- Yang, Y.; Palencia, J.L.D.; Wang, N.; Jiang, Y.; Wang, D.-Y. Nanocarbon-Based Flame Retardant Polymer Nanocomposites. Molecules 2021, 26, 4670. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.-T.; Wang, D.-Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Najafi-Shoa, S.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Incorporation of epoxy resin and graphene nanolayers into silica xerogel network: An insight into thermal improvement of resin. J. Sol-Gel Sci. Technol. 2016, 80, 362–377. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Wu, Y.; Li, W.; Chen, C. Functionalized graphene with Co-ZIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin. J. Hazard. Mater. 2018, 363, 138–151. [Google Scholar] [CrossRef]
- Wei, J.; Vo, T.; Inam, F. Epoxy/graphene nanocomposites—Processing and properties: A review. RSC Adv. 2015, 5, 73510–73524. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, W.; Liu, J.; Lartey, P.O.; Yang, Y. Effect of reduction methods and functionalization on the dispersion of graphene in epoxy. J. Dispers. Sci. Technol. 2019, 41, 297–306. [Google Scholar] [CrossRef]
- Li, K.-Y.; Kuan, C.-F.; Kuan, H.-C.; Chen, C.-H.; Shen, M.-Y.; Yang, J.-M.; Chiang, C.-L. Preparation and properties of novel epoxy/graphene oxide nanosheets (GON) composites functionalized with flame retardant containing phosphorus and silicon. Mater. Chem. Phys. 2014, 146, 354–362. [Google Scholar] [CrossRef]
- Shi, X.; Luo, S.; Du, X.; Li, Q.; Cheng, S. Improvement the Flame Retardancy and Thermal Conductivity of Epoxy Composites via Melamine Polyphosphate-Modified Carbon Nanotubes. Polymers 2022, 14, 3091. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Su, D.S.; Wang, D.-Y. Carbon Nanotube/Epoxy Composites for Improved Fire Safety. ACS Appl. Nano Mater. 2020, 3, 4253–4264. [Google Scholar] [CrossRef]
- Wang, J. Novel Polysilicone Flame-Retardant Functionalized Carbon Nanotubes: Synthesis, Characterization and Flame Retardancy as Used in Epoxy-Based Composites. J. Macromol. Sci. Part B 2020, 60, 88–98. [Google Scholar] [CrossRef]
- Bao, X.; Wu, F.; Wang, J. Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes. Polymers 2021, 13, 3332. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, C.; Qiu, J.; Yao, Y.; Sakai, E.; Yang, L. Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites. Polymers 2020, 12, 613. [Google Scholar] [CrossRef]
- Xin, F.; Zhai, C.; Guo, C.; Chen, Y.; Qian, L. Carbon nanotubes coated with phosphorus-nitrogen flame retardant and its application in epoxy thermosets. Polym. Technol. Mater. 2019, 58, 1889–1899. [Google Scholar] [CrossRef]
- Nguyen, T.A. Study on the Synergies of Nanoclay and MWCNTs to the Flame Retardant and Mechanical Properties of Epoxy Nanocomposites. J. Nanomater. 2021, 2021, 5536676. [Google Scholar] [CrossRef]
- Im, J.S.; Lee, S.K.; In, S.J.; Lee, Y.-S. Improved flame retardant properties of epoxy resin by fluorinated MMT/MWCNT additives. J. Anal. Appl. Pyrolysis 2010, 89, 225–232. [Google Scholar] [CrossRef]
- Mamani, A.; Ebrahimi, M.; Ataeefard, M. A study on mechanical, thermal and flame retardant properties of epoxy/expandable graphite composites. Pigment. Resin Technol. 2017, 46, 131–138. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Wang, J.; Zhang, B. Synergistic flame-retardant effect of expandable graphite and phosphorus-containing compounds for epoxy resin: Strong bonding of different carbon residues. Polym. Degrad. Stab. 2016, 128, 89–98. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, M.; Zheng, Y.; Tao, W.; Han, Y.; Li, W.; Song, P.; Wang, H. One-pot scalable fabrication of an oligomeric phosphoramide towards high-performance flame retardant polylactic acid with a submicron-grained structure. Compos. Part B Eng. 2020, 183, 107695. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Pourchet, S.; Sonnier, R.; Ben-Abdelkader, M.; Gaillard, Y.; Ruiz, Q.; Placet, V.; Plasseraud, L.; Boni, G. New Reactive Isoeugenol Based Phosphate Flame Retardant: Toward Green Epoxy Resins. ACS Sustain. Chem. Eng. 2019, 7, 14074–14088. [Google Scholar] [CrossRef]
- Czub, P. Synthesis of high-molecular-weight epoxy resins from modified natural oils and Bisphenol A or BisphenolA-based epoxy resins. Polym. Adv. Technol. 2009, 20, 194–208. [Google Scholar] [CrossRef]
- Roudsari, G.M.; Mohanty, A.K.; Misra, M. Study of the Curing Kinetics of Epoxy Resins with Biobased Hardener and Epoxidized Soybean Oil. ACS Sustain. Chem. Eng. 2014, 2, 2111–2116. [Google Scholar] [CrossRef]
- Delmas, G.-H.; Benjelloun-Mlayah, B.; Le Bigot, Y.; Delmas, M. Biolignin™ based epoxy resins. J. Appl. Polym. Sci. 2013, 127, 1863–1872. [Google Scholar] [CrossRef]
- Dai, J.; Peng, Y.; Teng, N.; Liu, Y.; Liu, C.; Shen, X.; Mahmud, S.; Zhu, J.; Liu, X. High-Performing and Fire-Resistant Biobased Epoxy Resin from Renewable Sources. ACS Sustain. Chem. Eng. 2018, 6, 7589–7599. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Jiang, Y.; Fan, L.; Feng, J.; Zhu, J. Synthesis and properties of phosphorus-containing bio-based epoxy resin from itaconic acid. Sci. China Chem. 2013, 57, 379–388. [Google Scholar] [CrossRef]
- Wu, H.; Liu, C.; Cheng, L.; Yu, Y.; Zhao, H.; Wang, L. Enhancing the mechanical and tribological properties of epoxy composites via incorporation of reactive bio-based epoxy functionalized graphene oxide. RSC Adv. 2020, 10, 40148–40156. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Hodan, H.A.; Hameed, R.S.A.; Ezzat, A.O. Preparation of green cardanol-based epoxy and hardener as primer coatings for petroleum and gas steel in marine environment. Prog. Org. Coat. 2017, 111, 283–293. [Google Scholar] [CrossRef]
- Ma, J.; Li, G.; Hua, X.; Liu, N.; Liu, Z.; Zhang, F.; Yu, L.; Chen, X.; Shang, L.; Ao, Y. Biodegradable epoxy resin from vanillin with excellent flame-retardant and outstanding mechanical properties. Polym. Degrad. Stab. 2022, 201, 109989. [Google Scholar] [CrossRef]
- Chi, Z.; Guo, Z.; Xu, Z.; Zhang, M.; Li, M.; Shang, L.; Ao, Y. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar] [CrossRef]
- Abuhimd, H.; Rao, T.N.; Song, J.-I.; Yarasani, P.; Ahmed, F.; Parvatamma, B.; Alothman, A.A.; Al-Anazy, M.M.; Ifseisi, A.A. Influence of Magnesium Aluminate Nanoparticles on Epoxy-Based Intumescent Flame Retardation Coating System. Coatings 2020, 10, 968. [Google Scholar] [CrossRef]
- Laoutid, F.; Jouyandeh, M.; Murariu, O.; Vahabi, H.; Saeb, M.R.; Brison, L.; Murariu, M.; Dubois, P. New Transparent Flame-Retardant (FR) Coatings Based on Epoxy-Aluminum Hypophosphite Nanocomposites. Coatings 2023, 13, 140. [Google Scholar] [CrossRef]
- Yan, X.; Chang, Y. Effect of MF-Coated Epoxy Resin Microcapsules on Properties of Waterborne Wood Coating on Basswood. Coatings 2020, 10, 785. [Google Scholar] [CrossRef]
- Ou, M.; Lian, R.; Zhu, J.; Li, R.; Cui, J.; Guan, H.; Liu, L.; Jiao, C.; Chen, X. Aromatic P/N/Co-containing microsphere flame retardant for enhancing fire safety and mechanical properties of epoxy coating with lower curing temperature. Prog. Org. Coat. 2023, 183, 107728. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, G.; Chen, C.; Chen, C.; Zhong, F.; Wang, M.; Zou, R. Mussel inspired polydopamine@KH560-linked hexagonal boron nitride and CNTs nanoflame retardants improve fire performance of composite coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127717. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Ruan, F.; Yang, W.; Fang, Z.; Zheng, D.; Li, X.; Li, N.; Qiao, M.; Liu, J. Synthesis of a Reactive Template-Induced Core–Shell PZS@ZIF-67 Composite Microspheres and Its Application in Epoxy Composites. Polymers 2021, 13, 2646. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ma, W.; Zhang, Z.; Liu, Y.; Lu, F.; Yu, B. Construction of layer-by-layer assembled green coating on titanate nanotubes to improve the flame retardancy of epoxy resin. J. Appl. Polym. Sci. 2020, 137, 49369. [Google Scholar] [CrossRef]
- Dong, S.; Xiao, G.; Chen, C.; Chen, C.; Yang, Z.; Zhong, F.; Wang, M.; Zou, R. Zn-Al layered double metal hydroxide anchored reduced graphene oxide for enhancing the fire performance of composite coatings. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127736. [Google Scholar] [CrossRef]
- Yan, W.; Xie, P.; Yang, Z.; Luo, G.; Huang, W.; Tian, Q.; Tu, C.; Zhang, C.; Yang, C.; Wang, K. Flame-retardant behaviors of aluminum phosphates coated sepiolite in epoxy resin. J. Fire Sci. 2020, 39, 3–18. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, G.; Zhong, F.; Dong, S.; Yang, Z.; Chen, C.; Wang, M.; Zou, R. Synergistic effect of carbon nanotubes bonded graphene oxide to enhance the flame retardant performance of waterborne intumescent epoxy coatings. Prog. Org. Coat. 2022, 162, 106598. [Google Scholar] [CrossRef]
- Zhong, F.; Chen, C.; Yang, X.; Zhou, J.; Zheng, W.; Huang, H.; Wang, P.; Li, W. Self-assembly of zinc hydroxystannate on polyethyleneimine supported boron nitride to improve the flame protection properties of waterborne epoxy coatings. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129557. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials 2020, 13, 2881. [Google Scholar] [CrossRef]
- Wazarkar, K.; Sabnis, A. Sustainable cardanol-based multifunctional carboxyl curing agents for epoxy coatings: Si–S synergism. J. Coat. Technol. Res. 2020, 17, 1217–1230. [Google Scholar] [CrossRef]
- Ai, Y.-F.; Xia, L.; Pang, F.-Q.; Xu, Y.-L.; Zhao, H.-B.; Jian, R.-K. Mechanically strong and flame-retardant epoxy resins with anti-corrosion performance. Compos. Part B Eng. 2020, 193, 108019. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks. Chem. Mater. 2016, 29, 26–39. [Google Scholar] [CrossRef]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef]
- Hussain, M.M.; Majeed, M.K.; Ma, H.; Wang, Y.; Saleem, A.; Lotfi, M. PTFE/EP Reinforced MOF/SiO 2 Composite as a Superior Mechanically Robust Superhydrophobic Agent towards Corrosion Protection, Self-Cleaning and Anti-Icing. Chem. A Eur. J. 2022, 28, e202103220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Su, J.; Yin, C.; Li, H.; Yao, Y.; Zhang, L.; Yao, X.; Zhang, X.; Liu, F.-Q. Constructing a robust ZIF-7 based superhydrophobic coating with the excellent performance in self-cleaning, anti-icing, anti-biofouling and anti-corrosion. Appl. Surf. Sci. 2023, 622, 156907. [Google Scholar] [CrossRef]
- Song, K.; Pan, Y.-T.; Zhang, J.; Song, P.; He, J.; Wang, D.-Y.; Yang, R. Metal–Organic Frameworks–Based Flame-Retardant System for Epoxy Resin: A Review and Prospect. Chem. Eng. J. 2023, 468, 143653. [Google Scholar] [CrossRef]
- Pan, Y.-T.; Zhang, Z.; Yang, R. The rise of MOFs and their derivatives for flame retardant polymeric materials: A critical review. Compos. Part B Eng. 2020, 199, 108265. [Google Scholar] [CrossRef]
- Yao, T.; Yang, R.; Sun, C.; Lin, Y.; Liu, R.; Yang, H.; Chen, J.; Gu, X. Pyrolysis Kinetics of Lignin-Based Flame Retardants Containing MOFs Structure for Epoxy Resins. Molecules 2023, 28, 2699. [Google Scholar] [CrossRef]
- Haeri, Z.; Ramezanzadeh, B.; Ramezanzadeh, M. Recent progress on the metal-organic frameworks decorated graphene oxide (MOFs-GO) nano-building application for epoxy coating mechanical-thermal/flame-retardant and anti-corrosion features improvement. Prog. Org. Coat. 2022, 163, 106645. [Google Scholar] [CrossRef]
- Ren, B.; Chen, Y.; Li, Y.; Li, W.; Gao, S.; Li, H.; Cao, R. Rational design of metallic anti-corrosion coatings based on zinc gluconate@ZIF-8. Chem. Eng. J. 2020, 384, 123389. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, Y.; Zhou, K. A novel exploration of metal–organic frameworks in flame-retardant epoxy composites. J. Therm. Anal. Calorim. 2019, 138, 905–914. [Google Scholar] [CrossRef]
- Sang, L.; Cheng, Y.; Yang, R.; Li, J.; Kong, Q.; Zhang, J. Polyphosphazene-wrapped Fe–MOF for improving flame retardancy and smoke suppression of epoxy resins. J. Therm. Anal. Calorim. 2020, 144, 51–59. [Google Scholar] [CrossRef]
- Hong-jun, J.I.; Shi-fu, C.U.; Tao, A.I.; Hong-wei, J.I. Properties of fireproofing coating with dirt resistance and water resistance on tunnel surface. J. Traffic Transp. Eng. 2017, 17, 50–60. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).