Abstract
Dispersion coating may represent an alternative technology to extrusion coating, currently dominating the market of coated paper-based packaging. Being processed as inks, dispersion coatings can be applied with conventional equipment, achieving lower dry coat grammages. In this work, two styrene-butadiene-based (Tg1 ≅ 0 °C; Tg2 ≅ 15 °C) dispersion coatings filled with different amounts of kaolin were developed and rod-coated on two different paper substrates. The samples were tested for water, moisture, and grease barrier properties. Kaolin-containing formulations showed moisture barrier as low as 15 g/(m2∙day), as well as grease barrier higher than 24 h. The best formulation involved 20% by weight of kaolin, whereas higher amounts were detrimental for water barrier, beneficial for moisture barrier, and slightly detrimental for grease barrier properties. Benchmarked to two commercial grades, kaolin-filled coatings strongly improved grease barrier, yet achieving similar moisture barrier.
1. Introduction
Recently, cellulosic substrates gained much interest from the packaging industry as they are regarded to be much more sustainable solutions compared with polymeric solutions. However, cellulosic substrates cannot guarantee barrier properties that are comparable to polymeric counterparts without any functionalization. In particular, due to the highly hydrophilic nature of cellulose, water barrier properties represent a crucial limit [1]. Barrier improvement can be achieved in different ways, such as internal sizing, surface sizing, and surface coating [2,3,4], yet numerous packaging solutions on the market are surface-coated. Extrusion coating, lamination, and dispersion coatings represent three main surface coating technologies, despite that only the first two are dominating the market [5].
Dispersion coatings can be defined as (water-based) colloidal dispersions of polymeric particles, plus other additives, such as defoamers, dispersants, and possible functional fillers [6,7]. Early studies and applications date back to the 1940s [8]; despite being widely used for printing paper grades, it was only recently that dispersion coatings gained much interest in the industry for barrier purposes, boosted by the transition from plastic to paper-based packaging. Additionally, such technology was boosted by stringent directives increasingly asking for the reduction of noncellulosic content in the packaging to fulfill design guidelines for improved recyclability [9,10,11].
Several companies are producing on an industrial scale polymeric latices used in coating formulations. Such products are generally based on styrene-butadiene and styrene-acrylate latices, as reported in the literature [12]. Previous literature [13,14,15,16,17,18,19] widely focused on DOW’s styrene-butadiene latices, for example, on DL 966 grade; however, such specific grade is no more available on the market.
Moreover, previous studies focused on the addition of functional inorganic fillers, which are already widely used in the paper-based packaging industry [13,14,15,19,20,21,22] as a top coat to improve printability and glossiness or to improve moisture barrier properties of the coated substrates.
On the contrary, recent academic studies focused their attention on biobased polymers and additives, such as polysaccharides, proteins, and fatty acids [22,23,24,25,26,27]. However, at present, such solutions are produced mostly at a laboratory scale, with some EU-granted projects trying to scale them up, for example, R3PACK (https://www.r3pack.eu/ (accessed on 1 December 2022)), CelluWiz (http://www.celluwiz.eu/ (accessed on 5 June 2022)), and Ecofunco (https://www.ecofunco.eu/ (accessed on 5 June 2022)). Current issues may be related to feedstock availability, extraction process, or its yield, as well as—generally—higher cost.
Focusing on the readily available solutions for a large-scale application—that is, based on already-industrialized latices and fillers—this paper aims to formulate and test new experimental formulations involving styrene-butadiene latices filled with kaolin as a functional filler to improve barrier properties; at the same time, the authors benchmark such performance against current formulations available commercially.
2. Materials and Methods
2.1. Substrates
In this work, two virgin kraft fiber substrates were considered:
- Mondi Group (Weybridge, UK) ProVantage Komiwhite (125 g/m2), a white top kraft liner (KB);
- MetsäBoard (Espoo, Finland) MetsäBoard Pro WKL (145 g/m2), a double-coated white top kraft liner (KP).
Such paper grades are already used in paper- and corrugated-board-based packaging and represent solutions for both flexible packaging and rigid packaging, if processed as top liners for corrugated board. The main difference between the two papers is in the porosity of the side to be coated, that is, lower porosity for KP due to the double top coating.
2.2. Commercial Coatings and Experimental Coatings Formulation
In order to compare performances with those of present commercial products, two commercial barrier-coating grades were considered in this work:
- SB-B: styrene-butadiene dispersion coating with a dry solid content of 50% (on a weight basis); the formulation does not involve inorganic fillers.
- SA-B: styrene acrylate dispersion coating with a dry solid content of 46% (on a weight basis); the formulation does not involve inorganic fillers.
Both commercial coatings were used as provided. Stirring with a magnetic anchor at 500 rpm occurred for 30 min before the coating application. Fourier-transform infrared spectroscopy (FTIR) analyses using a Thermo Scientific (Waltham, MA, USA) Nicolet iS 10, and differential scanning calorimetry and thermogravimetric analyses (DSC-TGA) using a TA Instruments (New Castle, DE, USA) SDT Q600 are reported in Appendix A.
Regarding the experimental formulation, two styrene-butadiene latices were kindly provided by Trinseo (Horgen, Switzerland):
- HPH 39: styrene-butadiene latex, Tg ≅ 0 °C, dry solid content 54%.
- HPH 40: styrene-butadiene latex, Tg ≅ 15 °C, dry solid content 53%. This grade is reported by Trinseo to be similar to DL 966, the grade widely reported in previous literature [14,15].
Kaolin was used as a filler in experimental formulations. The specific grade was CamCoat 80 from Amberger Kaolinwerke (Hirschau, Germany), provided as pellets. Particle distribution is reported in Table 1. EXOlat C40 sodium polyacrylate, kindly provided by PCC Exol SA (Brzeg Dolny, Poland), was used as a dispersant (0.16% dry on dry pigment). The experimental formulations were developed at a constant dry solid content of 50%.

Table 1.
CamCoat 80 particle size distribution.
For both HPH 39 and HPH 40 latices, four formulations were considered, as reported in Table 2.

Table 2.
Experimental coating formulations for HPH 39 and HPH 40 styrene-butadiene latex.
Before any use, and similar to the commercial grades, the experimental formulations were stirred with a magnetic anchor for at least 30 min at 500 rpm.
2.3. Sample Preparation
Paper substrates were coated with a Meyer K-Bar (12 μm wet film thickness). The coating process was carried out manually at a constant speed of 40 mm/s.
After the coating, the wet-coated paper was placed in a frame (open on both the top and bottom) to reduce possible curling yet unaffecting heat access to the surface, and dried in an oven at 120 °C for 90 s. After cooling down at room conditions, squared samples (80 mm per side) were cut and conditioned at 23 ± 1 °C and 50 ± 2% relative humidity for at least 24 h prior to any characterization. A total of three test samples were prepared for each test. Uncoated (UC) samples were used as a reference to detect the effect of the addition of a coating layer. The thickness of each coated sample was calculated from the composition of the formulations—the density of the latex was to ρ = 1.05 g/cm3, and the kaolin one to ρ = 2.6 g/cm3.
2.4. Water Absorption (Cobb Test)
The water barrier property was evaluated with a Cobb 1800 test. The test aims to evaluate the water absorptiveness of the sample after a given time under 10 mm deep water, to be calculated as weight difference before and right after the test (Equation (1)):
where is the final sample mass (in grams), is the initial mass (in grams), and is the test area (in m2).
As defined in BS EN ISO 535:2014 [28], the samples underwent a 30 min test time at a temperature of 23 ± 1 °C and a relative humidity of 50 ± 2%. Due to the specimen dimension, the test area was set to almost 40 cm2.
2.5. Moisture Permeability (Water Vapor Transmission Rate)
Water vapor transmission rate (WVTR) evaluates the moisture transmission throughout a given sample in time. In the gravimetric method, the mass gain is due to a desiccant absorbing humidity while generating a water vapor pressure differential. WVTR was calculated from the steady slope of mass gain in time of the cup, as described in Equation (2):
where is the steady slope (in g/h), is the test area (in m2), and 24 is the conversion factor to achieve a WVTR measured in g/(m2∙day).
WVTR was measured using cups filled with 35.0 ± 0.1 g silica gel—according to BS ISO 2528:2017 [29]—for 48–96 h, depending on the barrier performance. The coated side of the samples faced the inner side of the cups. The test area of the samples was almost 20 cm2. Environment conditions were 23 ± 1 °C and 50 ± 2%, as in previous tests.
2.6. Grease Permeability
The grease permeability test allows us to determine the resistance of a paper—or coated paper—to grease permeation through the substrate or substrate-coat system. In the test, a disk of grease (3 mm thick) was laid on the surface, and a defined weight applied onto it. The test was carried out with palm kernel oil in accordance with BS ISO 16532-1:2008 [30] to assess the show-through time. The maximum test time was set to 24 h, as defined in the previously mentioned standard.
Contrary to Cobb 1800 and WVTR, higher grease permeability values represent higher barrier properties.
2.7. Scanning Electron Microscope
Coated and uncoated samples were gold-sputtered and analyzed by scanning electron microscopy (SEM) using a ZEISS EVO 50 (Zeiss, Wetzlar, Germany) electron microscope. To retrieve surface morphology, 100×, 250×, and 500× magnifications were used, while magnifications ranging between 1000× and 5000× were used to evaluate the cross section of the films. Images were analyzed using ImageJ v1.53e.
2.8. Spectrophotometry
All the samples were analyzed with a portable Konica Minolta CM-2500d (Konica Minolta, Chiyoda, Tokyo, Japan) spectrophotometer with D65/10° color space to retrieve the specular component excluded (SCE) CIE L*a*b* color coordinates as well as 8° gloss. ΔE* (CIEDE2000) color difference against uncoated samples was calculated and evaluated as described in [1].
2.9. Normalization
The results in this work were normalized to a 7 μm dry coat thickness, calculated over an exponential curve that passes through the uncoated substrate experimental data (Figure 1).
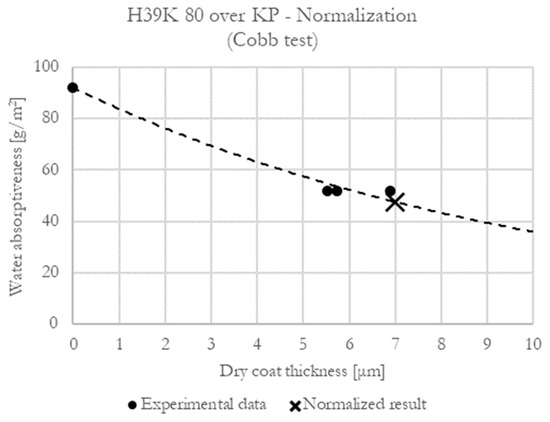
Figure 1.
Representative normalization curve (H39K 80 over KP substrate—Cobb test).
The normalization is due to kaolin, whose density (almost 2.5 times higher than one of the commercial dispersion coatings) strongly influences the coating thickness at an equal dry coat grammage. If neat polymeric dispersion coatings were exclusively used, normalization on the dry coat grammage would have worked as well.
3. Results
3.1. Sample Preparation
The coated samples achieved an average dry coat thickness of 7.2 ± 2.5 μm (representative SEM micrographs are reported in Appendix B). The high standard deviation is due to the presence of kaolin in the experimental formulations. Whereas kaolin ratio and dry coat density are directly proportional, coat thickness is inversely proportional to kaolin content. Indeed, at a given dry solid content in the experimental formulations, a higher kaolin content means a lower dry volume deposited, hence a reduced coat thickness.
All formulations were successfully applied to the substrates. However, some issues were encountered while applying H39K 100 and H40K 100 to KP. As represented in Figure 2, the observed issues were related to visible defects.
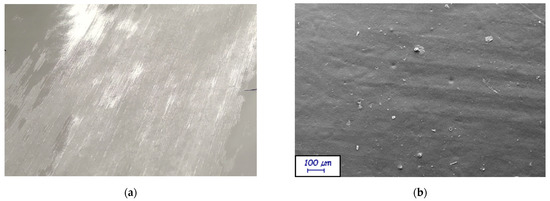
Figure 2.
(a) H39K 100 coating over KP substrate after drying: grooves affect the aesthetical and perceptive properties of the coated sample; (b) SEM micrograph (magnification: 250×) showing the grooves at a larger scale. ImageJ processing shows peak distance of around 150 μm.
Specifically, stripes were attributed to the wire wound on the K-Bar, as confirmed by SEM micrographs. Indeed, Figure 2b was analyzed with ImageJ in 15 different points, measuring the average peak distance of the grooves. The results report a 150 ± 6 μm average distance, which corresponds to the diameter of the wire wound on the K-Bar. Such phenomenon disappears as kaolin is added to the formulations, showing how it is related to the pure latex. It must be noted how, in general, H39K 100 and H40K 100 coatings are sticky—regardless of their Tg—possibly leading to blocking when processed in an industrial plant.
3.2. Cobb Test
The Cobb 1800 test results are reported in Figure 3. As clearly visible, the commercial coating grades (i.e., SB-B and SA-B) seem to better perform on both considered substrates, despite SB-B performing similarly to experimental formulations on the more porous substrate, namely, KB.
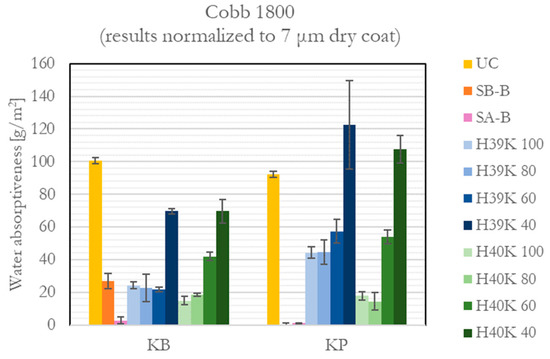
Figure 3.
Water absorptiveness (Cobb 1800) values for the uncoated (UC), commercial (SA-B and SB-B), and experimental dispersion coatings.
On the other hand, experimental formulations proved the role of the kaolin filler to be detrimental to water barrier properties. Indeed, the higher the filler content, the worse the barrier. There can be at least two explanations to such behavior. A first motivation can be related to the higher kaolin content that determines a formulation closer to the critical pigment volume concentration (CPVC), which is reported to be around 43% [31]—meaning a critical pigment mass concentration of around 65%, given a kaolin density of 2.6 g/cm3. Therefore, increasing the filler content to 60% by weight implies a higher coating porosity, leading to a higher roughness, as in Figure 4, thus a lower film continuity. An additional reason can be the higher surface wettability of the experimental coatings compared with the commercial ones (see Figure 5). This is attributed to the more hydrophilic behavior of the experimental formulations, which is due to the hydrophilic surfaces of kaolin [32]. Consequently, kaolin decreased the water barrier of the coatings containing up to 40% by weight of kaolin by attracting and possibly providing passage to water molecules through the coating itself. Instead, for the formulations containing 60% by weight of kaolin, the factor playing a major role in worse Cobb 1800 values seemed to be the entrapment of water molecules in the coating pores.
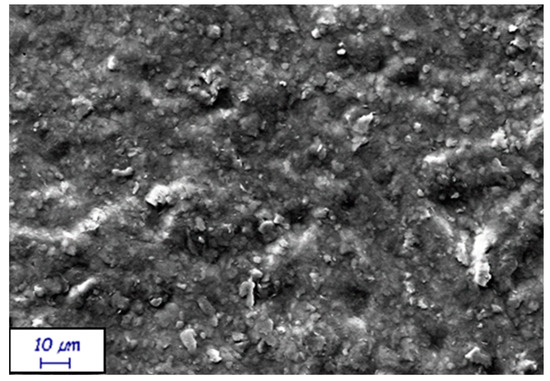
Figure 4.
Surface morphology of KB H39K 40 (magnification: 2000×). The sample results are rough due to the high filler concentration.
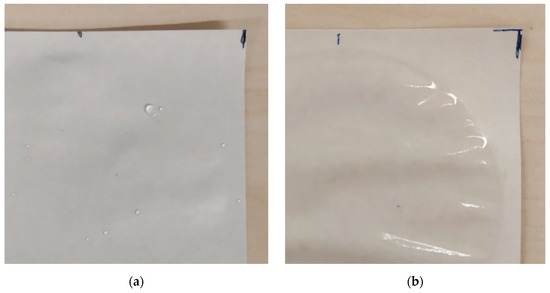
Figure 5.
Different coating wettability. (a) SA-B over KB: water droplets are clearly visible; (b) H39K 80 over KB: water is evenly spread across the tested surface.
For the experimental coatings, it is possible to observe from Figure 3 how, in general, Cobb 1800 values reach a minimum with a kaolin mass content of 20%, whereas higher concentrations lead to worse properties, which was attributed to a lower latex continuity throughout the coating. Indeed, Cobb 1800 results limit the fields of application for the experimental formulations, excluding packaging for liquids and very wet content, for example, soups and porridge.
Comparing the effect of the substrate, the experimental formulations generally showed worse performance on the KP substrate. Although a lower substrate porosity generally allows better film homogeneity—hence a higher barrier—latex intrinsic stickiness made its application over such a smooth substrate challenging.
3.3. WVTR
The moisture barrier property showed to strongly improve compared with uncoated paper substrates, as represented in Figure 6. From almost 350 g/(m2∙day) WVTR, the coatings reached values strictly lower than half of UC ones. Moreover, excluding H39K 100 and H40K 100—which still showed good performance—the coatings reduced the WVTR by more than 85%, compared with UC.
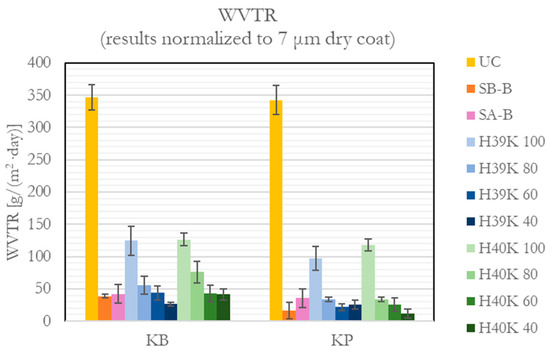
Figure 6.
WVTR values for the uncoated (UC), commercial (SA-B and SB-B), and experimental dispersion coatings.
Despite its double original coating—which contains mineral fillers—the Cobb 1800 and WVTR barrier properties of UC KP are close to UC KB ones. On the contrary, the addition of experimental formulations significantly reduced the WVTR, achieving values down to 10–20 g/(m2∙day) for the less porous paper. The presence of kaolin leads to improved performance, compared with pure latices; still, the results in Figure 6 do not show a significant difference among the different filler ratios for KP. Additionally, the presence of kaolin platelets allows us to achieve values similar to commercial coatings (i.e., SB-B and SA-B), strongly improving the WVTR of pure latices. Finally, the results in Figure 6 clearly show that a lower substrate porosity is essential to achieve better barrier properties due to lower coating defects and higher thickness homogeneity.
3.4. Grease Permeability
Grease permeability was successfully improved compared with UC samples, where KB has a resistance of a few seconds and KP of up to 7 min. The results are reported in Figure 7. It is possible to observe differences as high as two orders of magnitude, depending on whether the KB or KP substrate is considered.
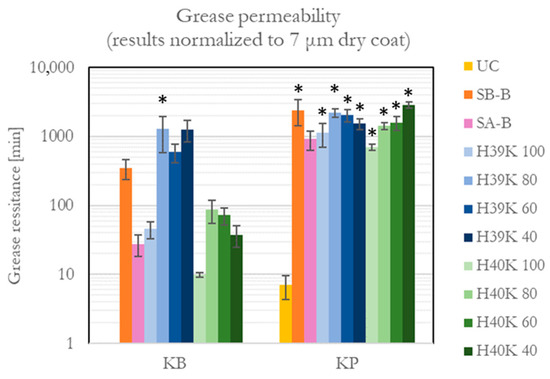
Figure 7.
Grease permeability values for the uncoated (UC), commercial (SA-B and SB-B), and experimental dispersion coatings. An asterisk (*) represents the samples that reached the end of the test.
According to the considered standard [30], the test ends after 24 h. In this work, it was observed how several sample conditions resisted the ultimate test time, especially if coated on KP. Therefore, actual resistance is higher than 1 day. Such conditions are highlighted with an asterisk in Figure 7.
In general, kaolin-filled experimental dispersion coatings showed higher grease barrier performance compared with H39K 100 and H40K 100. Moreover, kaolin-filled H39K formulations reached similar values on KB and KP substrates. Such behavior confirms, as for the WVTR performance, the beneficial presence of kaolin in the formulation. Despite some high standard deviations (even a small defect that allows grease permeation forces the test end), it generally seems how the best-performing experimental formulation contains around 20% of kaolin (dry mass weight).
3.5. Spectrophotometry
CIE L*a*b* coordinates for KB and KP substrates are reported in Figure 8. As clearly visible, the addition of kaolin slightly decreases a* coordinate and increases b* one, moving towards green-yellow hues. Lightness, generally, is reduced for every coated sample compared with UC samples.
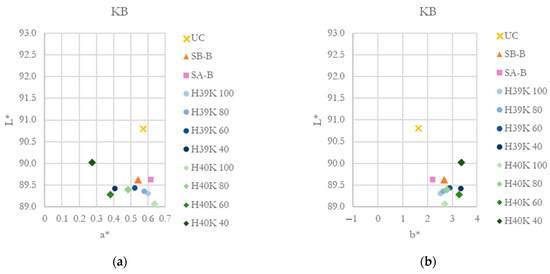
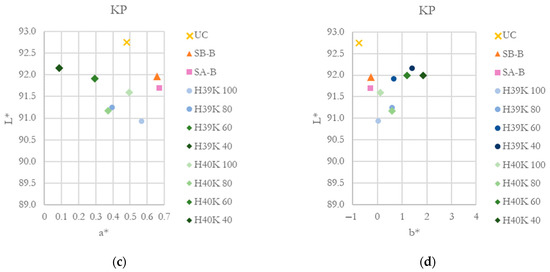
Figure 8.
CIE L*a*b* coordinates for the investigated substrates and coatings. (a) and (b) L*-a* and L*-b* coordinated for the KB substrate. (c) and (d) L*-a* and L*-b* coordinated for the KP substrate.
Nevertheless, considering ΔE* (CIE2000) values (Table 3), values below 2 are generally considered not perceivable by the human eye. Only highly filled experimental coatings involving the HPH 40 latex showed color variations slightly higher than 2 for both substrates considered in this work. Additionally, commercial coatings are characterized by the lowest color variation.

Table 3.
Experimental coating formulations for the HPH 39 and HPH 40 styrene-butadiene latex.
The most interesting value, however, is related to the glossiness of the surface (Figure 9). Indeed, regardless the coating, the substrate generally achieved higher glossiness values. It can be observed how increasing kaolin amounts—for almost every experimental coating—significantly reduces the glossiness of the coating. This is not true for H40K 100, where the coating defects (as of Figure 2) strongly influenced the measurements, as highlighted by the high standard deviation.
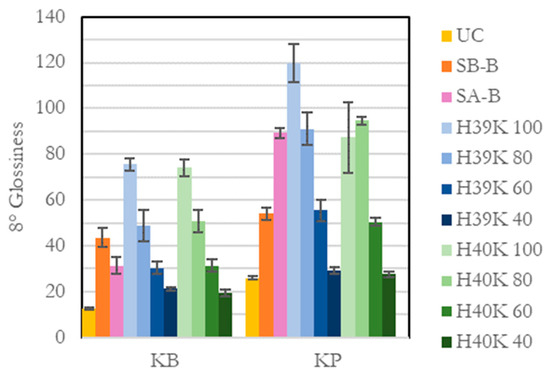
Figure 9.
8° glossiness for each substrate and coating involved in this study.
4. Discussion
Over KB, generally, HPH 39-based formulations performed better compared with HPH 40; however, over KP, it was the opposite. Since the KB substrate is of higher industrial interest due to its lower price, HPH 39 should be preferred as the latex in the developed dispersion coatings. Lower Tg implies a softer polymer at a given temperature, hence a higher chain mobility; moreover, lower Tg is reported to be beneficial to spread over kaolin [33]. Despite lower Tg also meaning a tendency to blocking [19], the addition of kaolin—or pigments, more in general—is reported to be beneficial, reducing such behavior.
The water barrier (Cobb 1800) properties proved to be better compared with a previous extensive work [34], especially considering a similar pigment ratio and a despite lower aspect ratio. Unfortunately, the water barrier property is not commonly evaluated in similar works, where WVTR was preferred. Water barrier is quite limited compared with commercial grades (Figure 3); however—as it is the case for this work—the Cobb test time is the most restrictive among the ones reported in the respective standard.
Comparing WVTR results with other works [14,35,36], it seems that the mass fraction of kaolin in the coating formulation does not widely affect WVTR, given that kaolin addition in the coating formulation is beneficial to moisture barrier properties. The results are coherent despite a higher kaolin aspect ratio in [14,34], which should be beneficial to obtain better moisture barrier properties; additionally, WVTR values are coherent with other works using a different filler [21].
The grease barrier properties of filled experimental formulations improved compared with unfilled formulations (i.e., H39K 100 and H40K 100), showing the positive effect of kaolin. A previous work [37] showed that kaolin did not influence grease barrier properties; however, it must be considered that polyvinyl alcohol, which was used in the formulation, already showed high grease barrier properties. Although the TAPPI T 559 (kit test) and ISO 16532-1:2008 [30] methodologies are different, similar latex and pigment formulations [34] coherently report how grease barrier properties are improved by kaolin addition.
The commercial grades involved in this study as a reference, namely, SB-B and SA-B, have interesting properties. It seems that, compared with SA-B, SB-B provides better grease yet worse water barrier properties. Despite good WVTR, grease barrier is the main drawback for the commercial grades. The addition of kaolin is then a simple yet effective way to overcome this.
As initially mentioned, the presented results were normalized over the coating thickness instead of dry coat grammage due to the increasing kaolin content of the experimental formulations, as kaolin-containing formulations are characterized by up to almost double coating density. Unfortunately, a higher coating density is negative when the noncellulosic content of the packaging is calculated. Indeed, comparing two coatings with the same thickness and equivalent barrier properties, kaolin-containing solutions would increase the noncellulosic content ratio of the packaging material, which is calculated by mass. Considering recyclability issues, it is widely reported how the fiber content of the paper-coated material should be maximized in content to ease the recycling yield and recyclability of the substrate [9,10,38]. A higher nonfibrous content may be acceptable if the content is preserved and its shelf life guaranteed, since the content itself may have a higher relative impact than the packaging itself [39]. Nevertheless, in the 4evergreen alliance document [40], all of the substances used in this context (styrene-butadiene, styrene acrylate, and kaolin) are reported to be fully compatible with standard recycling paper mills.
The addition of kaolin changed the aesthetic characteristics of the coated samples, especially in terms of reflectance. In Figure 10, it is possible to appreciate the aesthetic difference among experimental coating formulations.
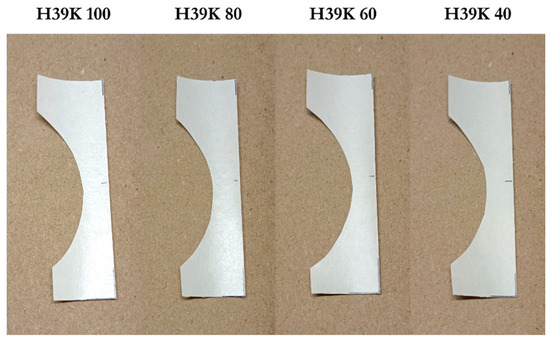
Figure 10.
Different qualitative glossiness for H39K 100, H39K 80, H39K 60, and H39K 40 coatings over KB—pictures taken in the same light conditions. As the kaolin content increases, the coated surface reduces its glossiness—as visible in the lower half of the samples—giving a soft touch aesthetic aspect.
Future works may investigate the consumer perception and aesthetic sensorial properties with a view to packaging end-of-life, understanding whether such properties can influence the consumer behavior or suggest the right practice alongside the environmental label, strictly required for packaging.
Finally, barrier properties can be influenced by the substrate porosity, as shown in this work and previously reported [19]. Lower substrate porosity allowed higher moisture and grease barrier properties for commercial coating, despite worse Cobb values, but only for experimental formulations. Both the effect of hydrophilic surfaces of kaolin and the obstacle in coating pure experimental latices influenced such behavior. A possible polymeric precoating over KB could be investigated to allow for a reduced substrate porosity, yet it is considered that kaolin would still negatively influence to some extent the water barrier properties.
5. Conclusions
Experimental formulations filled with an increasing kaolin content were successfully obtained, applied, and tested. Due to the nature of kaolin, water barrier properties were limited, yet moisture and grease barrier properties were very good. Experimental coatings were benchmarked with commercial coatings, showing how moisture barrier was similar, whereas the grease one achieved even better results, up to double resistance. The optimal results were obtained with formulations filled with 40% by weight of kaolin. The substrate strongly affected the overall barrier performance, having a white top double-coated substrate showing the best results. In terms of application, however, the white top liner is of higher interest.
Further studies may involve recyclability tests and the role of the final consumer, trying to understand whether such solutions truly represent viable alternatives to extrusion-coated or laminated substrates for a circular approach to paper-based packaging.
Author Contributions
Conceptualization, A.M. and B.D.C.; methodology, A.M. and M.V.D.; investigation, A.M.; data curation, A.M.; writing—original draft preparation, A.M. and M.V.D.; writing—review and editing, A.M. and M.V.D.; visualization, A.M. and M.V.D.; supervision, M.P. and B.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request. The data presented in this study are available on request from the corresponding author. Most data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
SB-B and SA-B were FTIR analyzed. The resulting spectra are reported in Figure A1 and Figure A2. Observing the characteristic peaks, it is easily observable how Figure A1 refers to a poly(styrene-butadiene) copolymer, whereas Figure A2 refers to a poly(styrene-acrylate) copolymer.
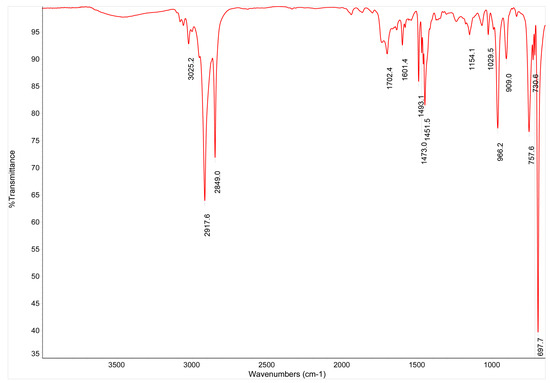
Figure A1.
FTIR spectrum for the SB-B commercial coating.
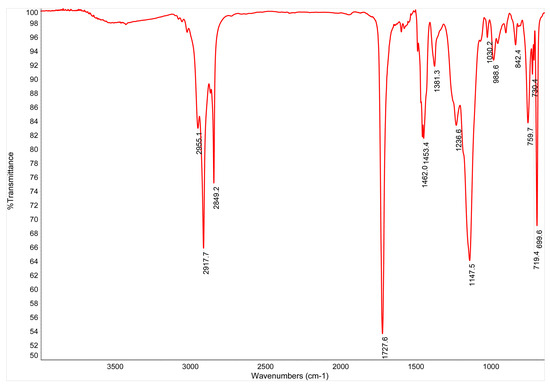
Figure A2.
FTIR spectrum for the SA-B commercial coating.
Additionally, the same dispersion coatings were DSC-TGA analyzed. The solid phase was obtained by evaporation of the solvent and then tested.
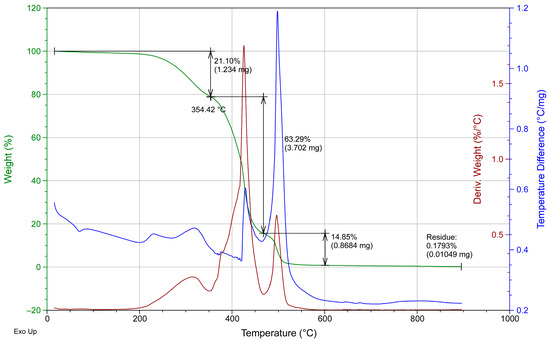
Figure A3.
DSC-TGA for the SB-B commercial coating.
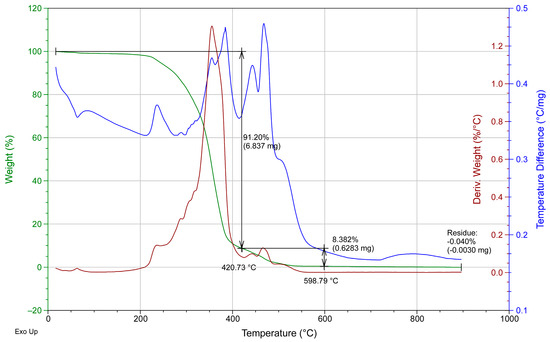
Figure A4.
DSC-TGA for the SA-B commercial coating.
Appendix B
The samples were analyzed at the SEM to ensure coherence between the calculated film thickness and the actual value. Figure A5 shows two representative micrographs.
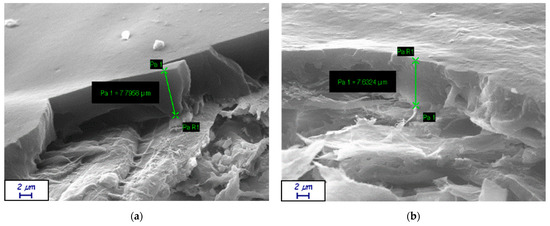
Figure A5.
Representative SEM micrographs of coated samples; green arrows represent the measured coat thickness. (a) H39K 100 over KB: thickness of almost 7.8 μm; (b) SB-B over KB: thickness around 7.6 μm.
References
- Marinelli, A.; Diamanti, M.V.; Lucotti, A.; Pedeferri, M.P.; Del Curto, B. Evaluation of Coatings to Improve the Durability and Water-Barrier Properties of Corrugated Cardboard. Coatings 2022, 12, 10. [Google Scholar] [CrossRef]
- Sangl, R.; Werner, A.; Kogler, W.; Tietz, M. Surface Sizing and Coating. In Handbook of Paper and Board; Holik, H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 745–784. ISBN 978-3-527-33184-0. [Google Scholar]
- Samyn, P. Wetting and Hydrophobic Modification of Cellulose Surfaces for Paper Applications. J. Mater. Sci. 2013, 48, 6455–6498. [Google Scholar] [CrossRef]
- Glenn, G.; Shogren, R.; Jin, X.; Orts, W.; Hart-Cooper, W.; Olson, L. Per- and Polyfluoroalkyl Substances and Their Alternatives in Paper Food Packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2596–2625. [Google Scholar] [CrossRef]
- Marinelli, A.; Sossini, L.; Santi, R.; Del Curto, B. Imballaggio Cellulosico Con Proprietà Barriera: Stato Dell’arte e Innovazione Dei Materiali, 1st ed.; Edizioni Dativo Srl: Milan, Italy, 2022; ISBN 9788894310931. [Google Scholar]
- Brander, J.; Thorn, I. Surface Application of Paper Chemicals; Blackie Academic and Professional: London, UK, 1997; ISBN 978-0-7514-0370-1. [Google Scholar]
- Keddie, J.L. Film Formation of Latex. Mater. Sci. Eng. R Rep. 1997, 21, 101–170. [Google Scholar] [CrossRef]
- Blackley, D.C. Latex and Paper. In Polymer Latices; Springer: Dordrecht, The Netherlands, 1997; Volume 3, pp. 434–473. [Google Scholar]
- Cepi. Paper-Based Packaging Recyclability Guidelines; Cepi: London, UK, 2019. [Google Scholar]
- CPI. Paper and Board Packaging Recyclability Guidelines; CPI: Swindon, UK, 2020. [Google Scholar]
- Marinelli, A.; Santi, R.; Del Curto, B. Linee Guida per La Facilitazione Delle Attività Di Riciclo Degli Imballaggi a Prevalenza Cellulosica, 1st ed.; CONAI: Milan, Italy, 2020; ISBN 9788894270020. [Google Scholar]
- Kimpimäki, T.; Savolainen, A.V. Barrier Dispersion Coating of Paper and Board. In Surface Application of Paper Chemicals; Brander, J., Thron, I., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 208–228. ISBN 978-94-010-7151-2. [Google Scholar]
- Martinez-Hermosilla, G.A.; Mesic, B.; Bronlund, J.E. Relative Permeability of Barrier Dispersion Coatings Applied on Paper-Based Materials; Mathematical Modeling and Experimental Validation. J. Coatings Technol. Res. 2022, 19, 543–558. [Google Scholar] [CrossRef]
- Kugge, C.; Johnson, B. Improved Barrier Properties of Double Dispersion Coated Liner. Prog. Org. Coatings 2008, 62, 430–435. [Google Scholar] [CrossRef]
- Mesic, B.; Cairns, M.; Järnstrom, L.; Joo Le Guen, M.; Parr, R. Film Formation and Barrier Performance of Latex Based Coating: Impact of Drying Temperature in a Flexographic Process. Prog. Org. Coatings 2019, 129, 43–51. [Google Scholar] [CrossRef]
- Schuman, T.; Wikström, M.; Rigdahl, M. Dispersion Coating with Carboxylated and Cross-Linked Styrene–Butadiene Latices. 1. Effect of Some Polymer Characteristics on Film Properties. Prog. Org. Coatings 2004, 51, 220–227. [Google Scholar] [CrossRef]
- Schuman, T.; Wikström, M.; Rigdahl, M. Dispersion Coating with Carboxylated and Cross-Linked Styrene–Butadiene Latices: 2. Effects of Substrate and Polymer Characteristics on the Properties of Coated Paperboard. Prog. Org. Coatings 2004, 51, 228–237. [Google Scholar] [CrossRef]
- Schuman, T.; Adolfsson, B.; Wikström, M.; Rigdahl, M. Surface Treatment and Printing Properties of Dispersion-Coated Paperboard. Prog. Org. Coatings 2005, 54, 188–197. [Google Scholar] [CrossRef]
- Schuman, T.; Karlsson, A.; Larsson, J.; Wikström, M.; Rigdahl, M. Characteristics of Pigment-Filled Polymer Coatings on Paperboard. Prog. Org. Coatings 2005, 54, 360–371. [Google Scholar] [CrossRef]
- Bollström, R.; Tuominen, M.; Määttänen, A.; Peltonen, J.; Toivakka, M. Top Layer Coatability on Barrier Coatings. Prog. Org. Coatings 2012, 73, 26–32. [Google Scholar] [CrossRef]
- Rissa, K.; Vähä-Nissi, M.; Lepistö, T.; Savolainen, A. Talc-Filled Water-Based Barrier Coatings. Pap. Timber 2022, 84, 467–472. [Google Scholar]
- Gutierrez, J.N.; Agate, S.; Venditti, R.A.; Pal, L. Study of Tobacco-Derived Proteins in Paper Coatings. Biopolymers 2021, 112, e23425. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.K.; Samyn, P. Bio-Based Coatings for Paper Applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 81–123. [Google Scholar]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the Art in the Development and Properties of Protein-Based Films and Coatings and Their Applicability to Cellulose Based Products: An Extensive Review. Coatings 2015, 6, 1. [Google Scholar] [CrossRef]
- BS EN ISO 535:2014; Paper and Board. Determination of Water Absorptiveness. Cobb Method. Available online: https://bsol.bsigroup.com/Bibliographic/BibliographicInfoData/000000000030259603 (accessed on 5 January 2021).
- BS ISO 2528:2017; Sheet Materials. Determination of Water Vapour Transmission Rate (WVTR). Gravimetric (Dish) Method. Available online: https://bsol.bsigroup.com/Bibliographic/BibliographicInfoData/000000000030349864 (accessed on 3 March 2022).
- BS ISO 16532-1:2008; Paper and Board. Determination of Grease Resistance. Permeability Test. Available online: https://bsol.bsigroup.com/Bibliographic/BibliographicInfoData/000000000030164327 (accessed on 2 February 2022).
- Asbeck, W.K.; Van Loo, M. Critical Pigment Volume Relationships. Ind. Eng. Chem. 1949, 41, 1470–1475. [Google Scholar] [CrossRef]
- Lyons, A.; Reed, G. Pigmented Aqueous Barrier Coatings. TAPPI J. 2020, 19, 551–558. [Google Scholar] [CrossRef]
- Bacquet, G.; Isoard, J.-C. Synthetic Latex Binders for Paper Manufacture. In Surface Application of Paper Chemicals; Brander, J., Thorn, I., Eds.; Blackie Academic and Professional: London, UK, 1997; pp. 48–68. ISBN 978-94-010-7151-2. [Google Scholar]
- Zou, Y.; Hsieh, J.S.; Mehnert, E.; Kokoszka, J. The Effect of Pigments and Latices on the Properties of Coated Paper. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 40–45. [Google Scholar] [CrossRef]
- Saroha, V.; Khan, H.; Raghuvanshi, S.; Dutt, D. Preparation and Characterization of PVOH/Kaolin and PVOH/Talc Coating Dispersion by One-Step Process. J. Coatings Technol. Res. 2022, 19, 1171–1186. [Google Scholar] [CrossRef]
- Zhu, Y.; Bousfield, D.; Gramlich, W.M. The Influence of Pigment Type and Loading on Water Vapor Barrier Properties of Paper Coatings before and after Folding. Prog. Org. Coatings 2019, 132, 201–210. [Google Scholar] [CrossRef]
- Andersson, C. New Ways to Enhance the Functionality of Paperboard by Surface Treatment—A Review. Packag. Technol. Sci. 2008, 21, 339–373. [Google Scholar] [CrossRef]
- CONAI Linee Guida per La Facilitazione Delle Attività Di Riciclo Degli Imballaggi a Prevalenza Cellulosica. Available online: http://www.progettarericiclo.com/docs/linee-guida-la-facilitazione-delle-attivita-di-riciclo-degli-imballaggi-prevalenza-cellulosica (accessed on 18 January 2021).
- Licciardello, F. Packaging, Blessing in Disguise. Review on Its Diverse Contribution to Food Sustainability. Trends Food Sci. Technol. 2017, 65, 32–39. [Google Scholar] [CrossRef]
- 4evergreen. Circularity by Design Guideline for Fibre-Based Packaging; 4evergreen: Brussels, Belgium, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).