Biocompatibility Analysis of GelMa Hydrogel and Silastic RTV 9161 Elastomer for Encapsulation of Electronic Devices for Subdermal Implantable Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GelMA and Photoinitiation
2.2. Preparation of Medical Silicone
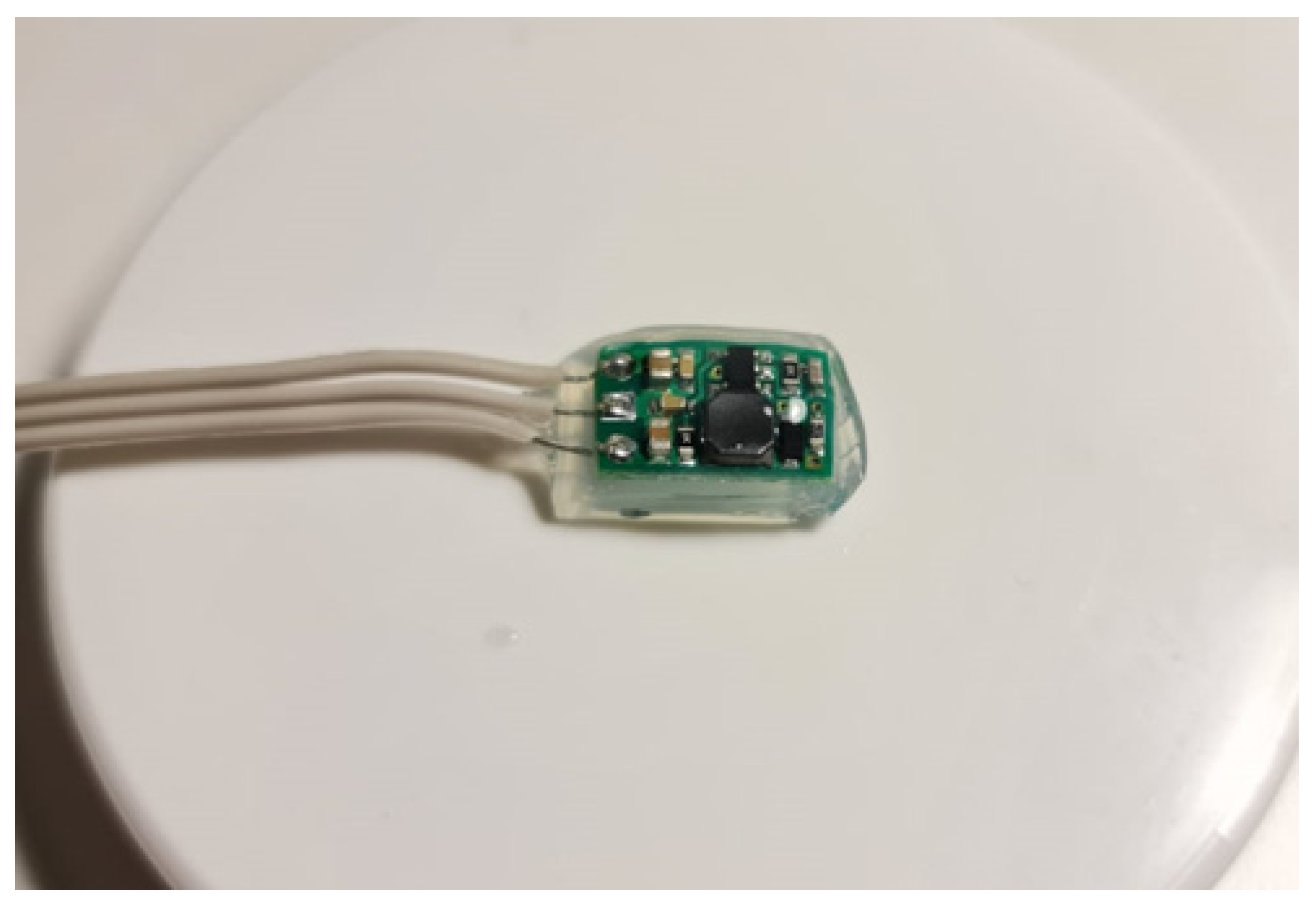
2.3. Electronic Device
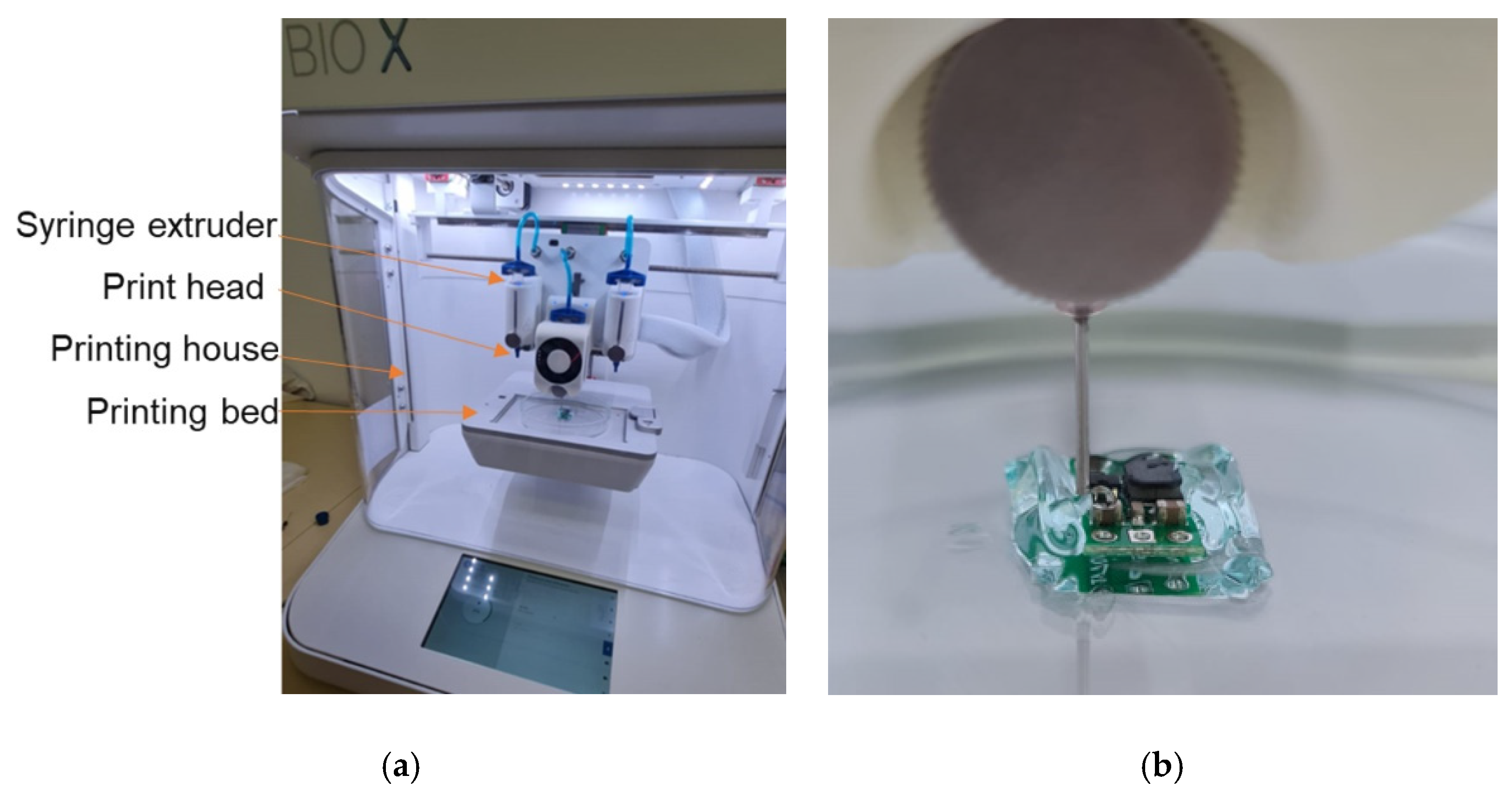
2.4. Bioprinter
2.5. GelMa Hydrogel Encapsulation
2.6. Silastic RTV 9161 Medical-Grade Silicone Encapsulation
2.7. In Vitro Experiment
2.8. In Vivo Experiment
3. Results
3.1. Biofunctional Experiment
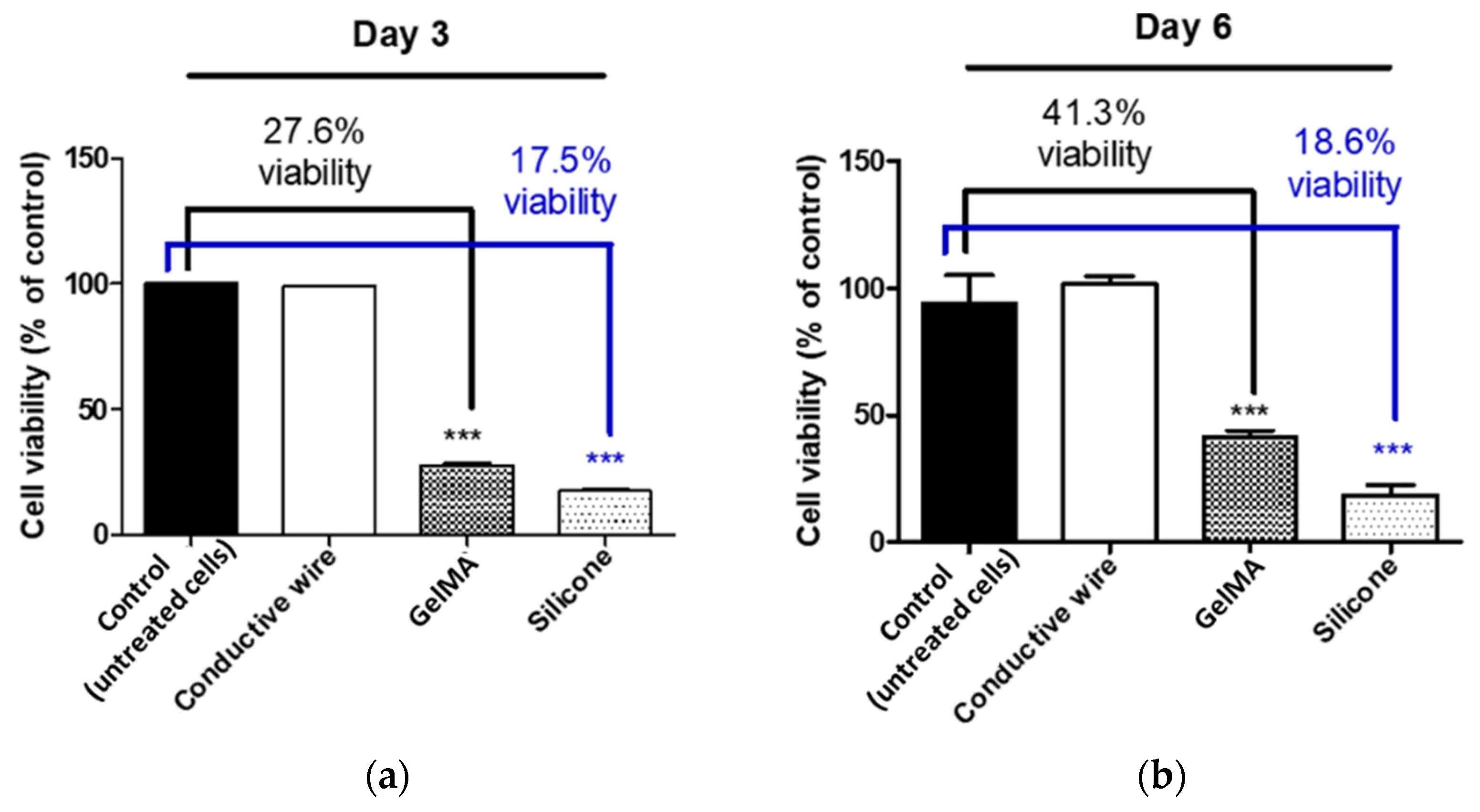
3.2. Biocompatibility In Vitro Results
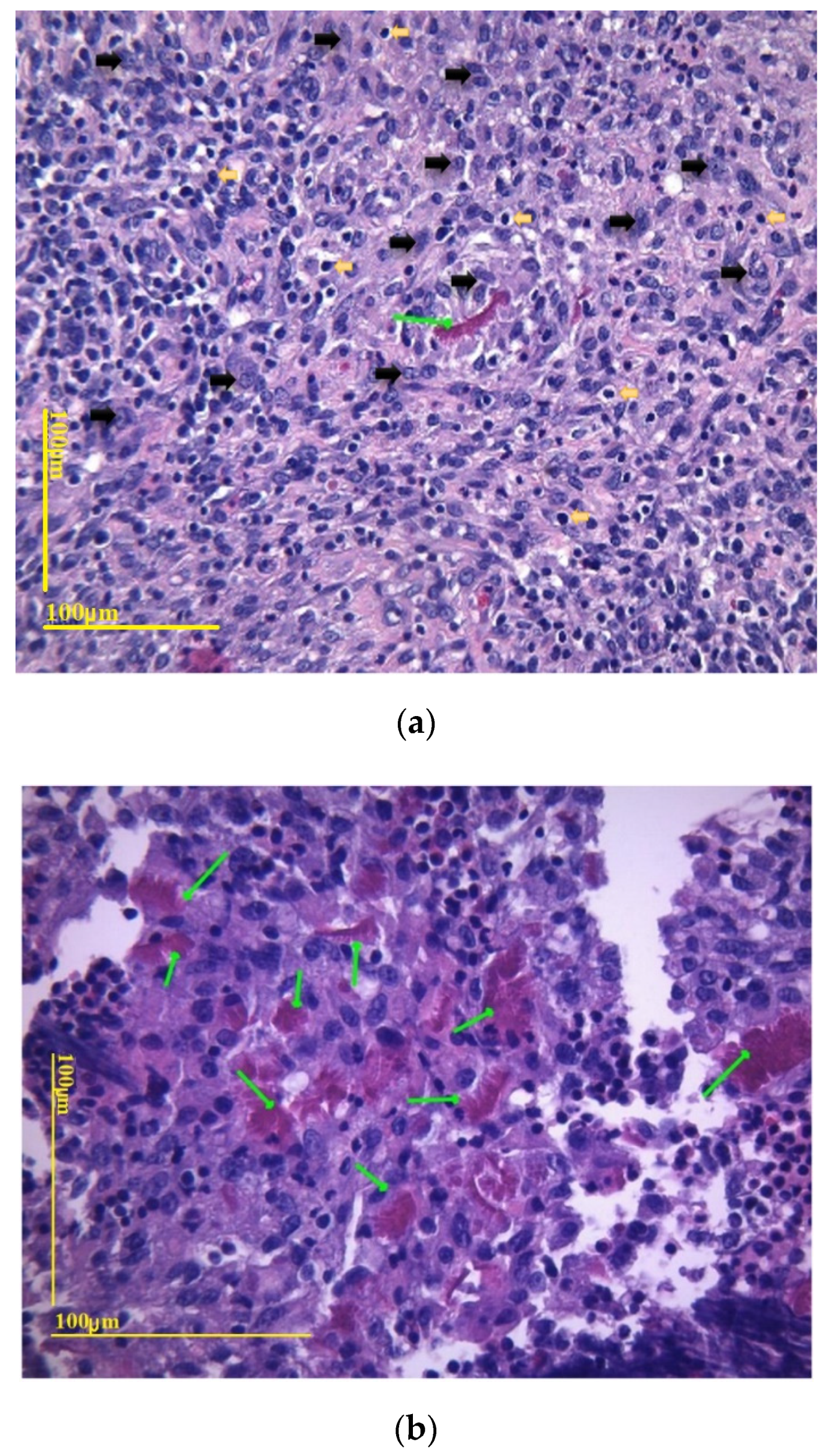
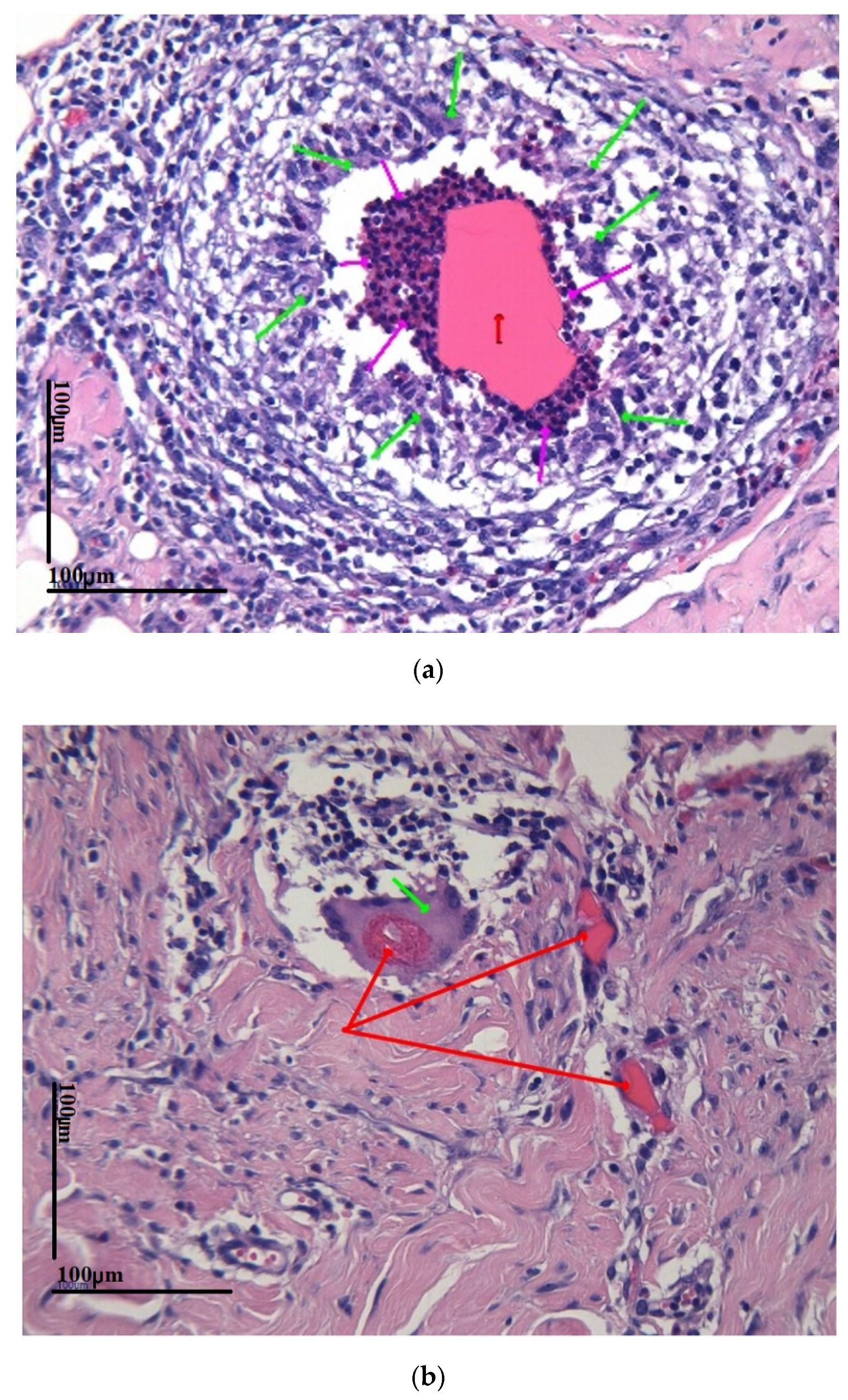
3.3. Biocompatibility In Vivo Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef]
- Prevost, T.P.; Balakrishnan, A.; Suresh, S.; Socrate, S. Biomechanics of brain tissue. Acta Biomater. 2011, 7, 83–95. [Google Scholar] [CrossRef]
- Albano, F.; Chung, M.D.; Blaauw, D.; Sylvester, D.M.; Wise, K.D.; Sastry, A.M. Design of an implantable power supply for an intraocular sensor, using POWER (power optimization for wireless energy requirements). J. Power Sources 2007, 170, 216–224. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Murphy, S.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- CELLINK, Crosslinking Agent. Available online: https://www.cellink.com/product/crosslinking-agent/?country=DZ (accessed on 7 December 2022).
- Slaoui, M.; Fiette, L. Histopathology procedures: From tissue sampling to histopathological evaluation. Methods Mol. Biol. 2011, 691, 69–82. [Google Scholar]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.Y.; Purcell, E.K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2017, 1, 862–877. [Google Scholar] [CrossRef]
- Hamerli, P.; Weigel, T.; Groth, T.; Paul, D. Surface properties of and cell adhesion onto allylamine-plasma-coated polyethylenterephtalat membranes. Biomaterials 2023, 24, 3989–3999. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Rajan, S.D.; Muthuswamy, J. Long-term changes in the material properties of brain tissue at the implant-tissue interface. J. Neural Eng. 2013, 10, 066001. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Wang, H.; Fenno, L.; Deisseroth, K.; Malliaras, G.G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 2017, 3, e1601649. [Google Scholar] [CrossRef] [PubMed]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Sani, E.S.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, I.I.I.; et al. In vitro and in vivo analysis of visible light cross-linkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Heltmann-Meyer, S.; Steiner, D.; Müller, C.; Schneidereit, D.; Friedrich, O.; Salehi, S.; Engel, F.B.; Arkudas, A.; Horch, R.E. Gelatin methacryloyl is a slow degrading material allowing vascularization and long-term usein vivo. Biomed. Mater. 2021, 16, 065004. [Google Scholar] [CrossRef]
- Aregueta-Robles, U.A.; Woolley, A.J.; Poole-Warren, L.A.; Lovell, N.H.; Green, R.A. Organic electrode coatings for next-generation neural interfaces. Front. Neuroeng. 2014, 7, 1–18. [Google Scholar] [CrossRef]
- Leach, J.B.; Achyuta, A.K.H.; Murthy, S.K. Bridging the divide between neuroprosthetic design, tissue engineering and neurobiology. Front. Neuroeng. 2010, 2, 1–19. [Google Scholar] [CrossRef]
- Rao, S.S.; Winter, J.O. Adhesion molecule-modified biomaterials for neural tissue engineering. Front. Neuroeng. 2009, 2, 1–14. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef]
- Yu, L.M.Y.; Leipzig, N.D.; Shoichet, M.S. Promoting neuron. Advances 2008, 11, 36–43. [Google Scholar]
- Lee, S.; Sani, E.S.; Spencer, A.R.; Guan, Y.; Weiss, A.S.; Annabi, N. Human-Recombinant-Elastin-Based Bioinks for 3D Bioprinting of Vascularized Soft Tissues. Adv. Mater. 2020, 2003915, 1–10. [Google Scholar]
- Wei, D.; Zhu, J.; Luo, L.; Huang, H.; Li, L.; Yu, X. Ultra-stretchable, fast self-healing, conductive hydrogels for writing circuits and magnetic sensors. Polym. Int. 2021, 9, 23–34. [Google Scholar] [CrossRef]
- Scimeca, J.C.; Verron, E. Nano-engineered biomaterials: Safety matters and toxicity evaluation. Mater. Today Adv. 2022, 15, 100260. [Google Scholar] [CrossRef]
- Tringides, C.; Mooney, D.J. Viscoelastic conductive hydrogel, U.S. Provisional Application Serial No. 63/090,960. 10 December 2022. [Google Scholar]
- Sommakia, S.; Gaire, J.; Rickus, J.L.; Otto, K.J. Resistive and reactive changes to the impedance of intracortical microelectrodes can be mitigated with polyethylene glycol under acute in vitro and in vivo settings. Front. Neuroeng. 2014, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragomir, D.C.; Carbunaru, V.; Moldovan, C.A.; Lascar, I.; Dontu, O.; Ristoiu, V.; Gheorghe, R.; Oproiu, A.M.; Firtat, B.; Franti, E.; et al. Biocompatibility Analysis of GelMa Hydrogel and Silastic RTV 9161 Elastomer for Encapsulation of Electronic Devices for Subdermal Implantable Devices. Coatings 2023, 13, 19. https://doi.org/10.3390/coatings13010019
Dragomir DC, Carbunaru V, Moldovan CA, Lascar I, Dontu O, Ristoiu V, Gheorghe R, Oproiu AM, Firtat B, Franti E, et al. Biocompatibility Analysis of GelMa Hydrogel and Silastic RTV 9161 Elastomer for Encapsulation of Electronic Devices for Subdermal Implantable Devices. Coatings. 2023; 13(1):19. https://doi.org/10.3390/coatings13010019
Chicago/Turabian StyleDragomir, David Catalin, Vlad Carbunaru, Carmen Aura Moldovan, Ioan Lascar, Octavian Dontu, Violeta Ristoiu, Roxana Gheorghe, Ana Maria Oproiu, Bogdan Firtat, Eduard Franti, and et al. 2023. "Biocompatibility Analysis of GelMa Hydrogel and Silastic RTV 9161 Elastomer for Encapsulation of Electronic Devices for Subdermal Implantable Devices" Coatings 13, no. 1: 19. https://doi.org/10.3390/coatings13010019
APA StyleDragomir, D. C., Carbunaru, V., Moldovan, C. A., Lascar, I., Dontu, O., Ristoiu, V., Gheorghe, R., Oproiu, A. M., Firtat, B., Franti, E., Dascalu, M., Neagu, T. P., Enescu, D. M., Ionescu, O., Ion, M., Mihailescu, C., Costea, R., Gonciarov, M., Ionescu, G., ... Teleanu, D. M. (2023). Biocompatibility Analysis of GelMa Hydrogel and Silastic RTV 9161 Elastomer for Encapsulation of Electronic Devices for Subdermal Implantable Devices. Coatings, 13(1), 19. https://doi.org/10.3390/coatings13010019







