Abstract
Solution-processable [1]benzothieno[3,2-b]benzothiophene (BTBT) derivatives with various end-capping groups, 2-(phenylethynyl)benzo[b]benzo[4,5]thieno[2,3-d]thiophene (Compound 1), 2-octyl-7-(5-(phenylethynyl)thiophen-2-yl)benzo[b]benzo[4,5]thieno[2,3-d]thiophene (Compound 2), and triisopropyl((5-(7-octylbenzo[b]benzo[4,5]thieno[2,3-d]thiophen-2-yl)thiophen-2-yl)ethynyl)silane (Compound 3), have been synthesized and characterized as active layers for organic field-effect transistors (OFETs). Thermal, optical, and electrochemical properties of the newly synthesized compounds were characterized using thermogravimetric analysis (TGA), a differential scanning calorimeter (DSC), UV–vis spectroscopy, and cyclic voltammetry (CV). Thin films of each compound were formed using the solution-shearing method and the thin film surface morphology and texture of the corresponding films were characterized using atomic force microscopy (AFM) and θ–2θ X-ray diffraction (XRD). All semiconductors exhibited p-channel characteristics in ambient and Compound 1 showed the highest electrical performance with a carrier mobility of ~0.03 cm2/Vs and current on/off ratio of ~106.
1. Introduction
Recently, research on solution-processable organic semiconductors (OSCs) has featured due to their intrinsic characteristics such as large-area processability, flexibility, and low cost [1,2,3,4,5,6,7,8,9]. The OSCs have been applied in various devices such as organic light-emitting diodes (OLED), sensors, radio-frequency identification (RFID) tags, and organic field-effect transistors (OFETs) [4,10,11,12,13,14,15,16]. For this purpose, considerable efforts have been made to develop new OSCs with air stability and high electrical performance. In particular, many researchers have paid more attention to π-conjugated small molecules than to polymeric molecules, because small molecular semiconductors are relatively easier-to-modify functional groups, more adaptable to solution process, and of higher purity levels than their counterparts [17,18,19,20]. The objective of the research performed for the development of small molecular OSCs is mainly to improve the π-orbital coupling in molecular crystals, hence the electronic transport of the corresponding compounds.
Among various chemical moieties employed in small molecular OSCs, [1]benzothieno[3,2-b]benzothiophene—(BTBT) is a favorable core structure used in OFETs with high electrical performance [21,22,23,24,25,26,27,28]. The BTBT core consists of four succeeding aromatic rings in a fused manner. Their extensive conjugated system contributes to the sufficient structural distribution for the highest occupied molecular orbital (HOMO) for efficient π-orbital overlap. In addition, unlike conventional thienoacene-based materials, BTBT derivatives generally exhibit ambient stability due to large band gaps (>3 eV). For example, Yuan et al. reported overwhelming field-effect mobility as high as 43 cm2/Vs for 2,7-dioctyl[1]benzothieno[3,2-b][1]benzothiophene (C8-BTBT) [29]. Moreover, 2-decyl-7-phenyl-[1]benzothieno[3,2-b][1]benzothiophene (Ph-BTBT C10) reported by Lino et al. showed mobility as high as 22.4 cm2/Vs based on thin films formed via spin coating [30,31]. In addition, C13-BTBT reported by Amin et al. showed mobility up to 17.2 cm2/Vs [26] and C12-Ph-BTBT with highly ordered liquid crystalline phases which exhibited mobility up to 8.7 cm2/Vs [22].
In recent years, many studies have reported that attaching appropriate end-capping groups to small molecular OSCs could enhance electrical performance as well as the solution processability of the corresponding compounds [32,33,34,35]. Furthermore, in some cases, they could prevent side reactions such as oxidation and polymerization, while affording ambient stability and promoting dense molecular packing [35]. Several researchers have also demonstrated that attaching olefinic or acetylenic linkage between core and functional groups to the molecules is beneficial for the extension of π-conjugation length and the optimization of planar structures [36,37,38,39,40,41,42,43,44,45]. In particular, the introduction of acetylenic linkage showed multiple advantages such as higher oxidation potential and lower highest occupied molecular orbital (HOMO) from the weak electron withdrawing nature of the corresponding group [38,43,46]. Furthermore, its cylinder-like π-electron density is more flexible to conformational and steric constraints [47,48]. For instance, Silvestri et al. demonstrated that an anthracene-based compound linked by acetylene exhibited three times greater mobility than that linked by olefin [43].
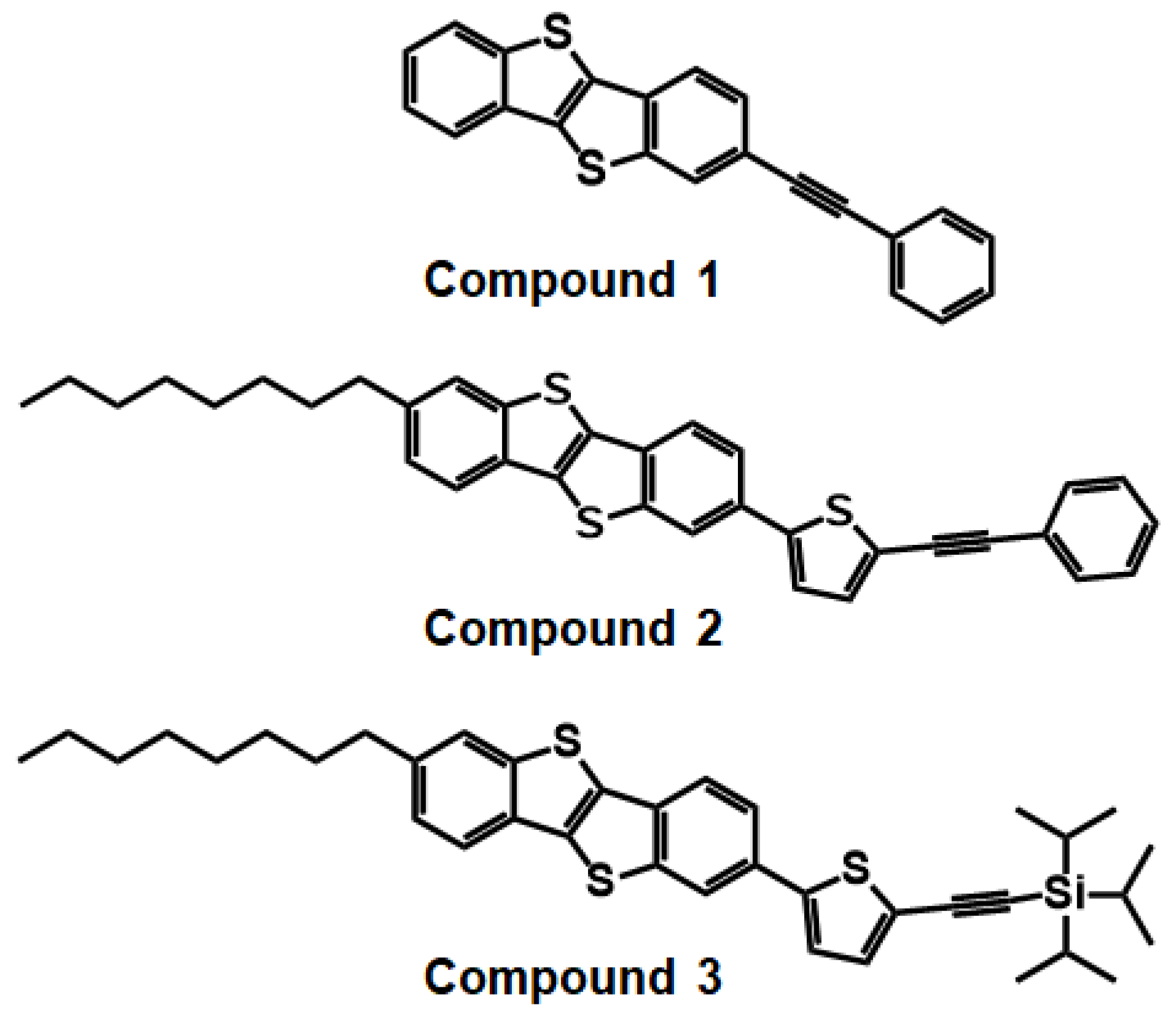
In this study, we have synthesized three BTBT derivatives, 2-(phenylethynyl)benzo[b]benzo[4,5]thieno[2,3-d]thiophene (Compound 1), 2-octyl-7-(5-(phenylethynyl)thiophen-2-yl)benzo[b]benzo[4,5]thieno[2,3-d]thiophene (Compound 2), and Triisopropyl((5-(7-octylbenzo[b]benzo[4,5]thieno[2,3-d]thiophen-2-yl)thiophen-2-yl)ethynyl)silane (Compound 3) (Figure 1). Physicochemical properties of the three synthesized compounds were investigated using thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), UV–vis spectroscopy, and cyclic voltammetry (CV). Theoretical calculation was performed to determine HOMO/LUMO energy levels. Then, atomic force microscopy (AFM) and X-ray diffraction (XRD) were utilized for the investigation of surface morphology and the texture of the thin films. Finally, electrical performance of all compounds was investigated by fabricating and characterizing OFETs based on solution-sheared OSC films. Compound 1, with the BTBT core and phenyl group linked by the acetylenic bond, showed the highest hole mobility up to ~0.03 cm2/Vs and current on/off ratio of over 106 with decent long-term ambient stability.
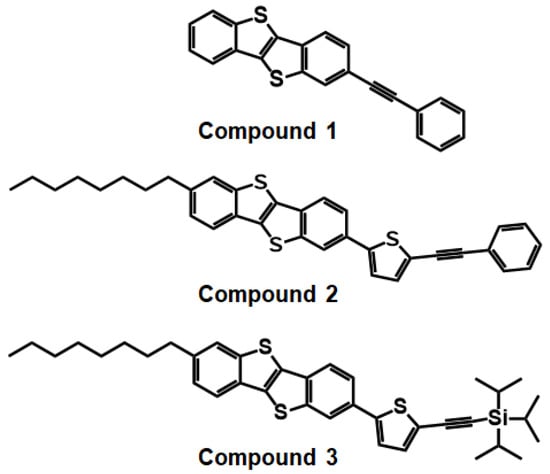
Figure 1.
Chemical structures of BTBT derivatives synthesized in this study.
2. Materials and Methods
2.1. Materials and Methods
All compounds came from commercial suppliers and were used without purification. Argon atmosphere, oven-dried glassware, and anhydrous solvents were used for air/moisture sensitive reactions. Unless otherwise noted, all compounds were purchased from commercial sources and used without further purification. 2-Iodobenzo[b]benzo[4,5]thieno[2,3-d]thiophene [49] and 2-(5-iodothiophen-2-yl)-7-octylbenzo[b]benzo[4,5]thieno[2,3-d]thiophene [50] were prepared following synthetic procedures in the literature. Solvents were freshly distilled or dried by passing through an alumina column. The synthesized compounds were characterized according to general analytical methods, as described in previous work [51].
2.2. Synthesis
2.2.1. Synthesis of 2-(Phenylethynyl)benzo[b]benzo[4,5]thieno[2,3-d]thiophene (1)
2-Iodobenzo[b]benzo[4,5]thieno[2,3-d]thiophene (100 mg, 0.273 mmol), ethynylbenzene (69.7 mg, 0.683 mmol), copper(I) iodide (1 mg, 5.25 mol), and Pd(PPh3)4 (15.8 mg, 0.0137 mmol) were dissolved in triethylamine (TEA, 20 mL). The mixture was stirred at 50 °C for 24 h under nitrogen. The reaction was quenched by water and extracted with CH2Cl2. The organic layer was dried over anhydrous MgSO4 and removed under reduced pressure. The resulting crude product was purified via flash column chromatography on silica gel using hexanes as the eluent to afford Compound 1 as a white solid (24.4 mg, 26%). 1H NMR (400 MHz, CDCl3): δ 8.08 (s, 1H), 7.92–7.82 (m, 3H), 7.60–7.54 (m, 3H), 7.47–7.34 (m, 5H). 13C NMR (100 MHz, CDCl3): δ 142.5, 142.3, 137.5, 134.8, 133.4, 133.0, 132.8, 131.7, 128.5, 128.4, 127.2, 125.4, 125.1, 124.2, 123.2, 121.8, 121.5, 120.0, 90.3, 89.5. HRMS-El(m/z): [M + H+] calcd. for C22H13S2, 341.0453; found, 341.0456.
2.2.2. Synthesis of 2-Octyl-7-(5-(phenylethynyl)thiophen-2-yl)benzo[b]benzo[4,5]thieno[2,3-d]thiophene (2)
2-(5-Iodothiophen-2-yl)-7-octylbenzo[b]benzo[4,5]thieno[2,3-d]thiophene (150 mg, 0.268 mmol), ethynylbenzene (69.7 mg, 0.683 mmol), copper(I) iodide (3 mg, 0.0158 mmol), and Pd(PPh3)4 (15 mg, 0.0130 mmol) were dissolved in TEA (20 mL). The mixture was stirred at 50 °C for 24 h under nitrogen. After cooling, the mixture was extracted with CH2Cl2 and dried using anhydrous MgSO4. The solvent was removed in vacuo and the residue was purified using flash column chromatography on silica gel to afford Compound 2 (110 mg, 77%). 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 1.4 Hz, 1H), 7.83 (d, J = 8.7 Hz, 1H), 7.77 (d, J = 8.2 Hz, 1H), 7.70 (s, 1H), 7.66 (dd, J = 8.5, 1.6 Hz, 1H), 7.54–7.51 (m, 2H), 7.37–7.25 (m, 6H), 2.76 (t, J = 7.8 Hz 2H), 1.74–1.67 (m, 2H), 1.39–1.28 (m, 10H), 0.88 (t, J = 6.9 Hz 3H). 13C NMR (101 MHz, CDCl3) δ 145.6, 143.1, 142.9, 140.7, 137.4, 134.3, 133.4, 133.1, 133.0, 132.5, 131.5, 131.1, 130.7, 128.4, 126.1, 123.4, 123.3, 123.1, 123.0, 121.7, 121.3, 121.0, 94.2, 82.9, 36.2, 31.9, 31.6, 29.5, 29.3, 29.2, 22.6, 14.0. HRMS-El(m/z): [M + H+] calcd. for C34H31S3, 535.1582; found, 535.1586.
2.2.3. Synthesis of Triisopropyl((5-(7-octylbenzo[b]benzo[4,5]thieno[2,3-d]thiophen-2-yl)thiophen-2-yl)ethynyl)silane (3)
2-(5-Iodothiophen-2-yl)-7-octylbenzo[b]benzo[4,5]thieno[2,3-d]thiophene (100 mg, 0.178 mmol), triisopropylsilylacetylene (0.1 mL, 0.445 mmol), copper(I) iodide (4 mg, 0.0178 mmol), and Pd(PPh3)4 (10.3 mg, 8.9 mol) were dissolved in TEA (15 mL). The mixture was stirred at 50 °C for 20 h under N2 atmosphere. The reaction was quenched by water and extracted with CH2Cl2. The organic layer was dried over anhydrous MgSO4 and removed under reduced pressure. The resulting crude product was purified via flash column chromatography on silica gel using hexanes to afford Compound 3 as a pale-yellow solid (90 mg, 82%). 1H NMR (400 MHz, CDCl3): δ 8.05 (d, J = 0.9 Hz, 1H), 7.80–7.78 (m, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.68 (d, J = 3.2 Hz, 1H), 7.61 (dd, J = 8.2, 1.4 Hz, 1H), 7.26 (dd, J = 8.2, 1.4 Hz, 1H), 7.21 (dd, J = 5.5, 4.1 Hz, 2H), 2.74 (t, J = 7.5 Hz, 2H), 1.71–1.64 (m, 2H), 1.43–1.26 (m, 10H), 1.14–1.13 (m, 21H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 145.3, 143.0, 142.8, 140.7, 134.2, 133.7, 132.8, 132.5, 131.0, 130.5, 126.1, 123.4, 123.1, 123.0, 123.0, 121.7, 121.4, 120.9, 99.6, 96.8, 36.2, 32.0, 31.8, 29.6, 29.5, 29.4, 22.8, 19.0, 18.8, 14.2, 11.4. HRMS-El(m/z): [M + H+] calcd. for C37H47S3Si, 615.2604; found, 615.2601.
2.3. Theoretical Calculation
Density functional theory (DFT) calculations for BTBT derivatives were performed to estimate optimized molecular structure and HOMO/LUMO energy of the corresponding compounds using the B3LYP method (Becke’s 3 parameters, the Lee–Yang–Parr) with 6–31G(d) basis sets as implemented in the Gaussian 09W program.
2.4. Device Fabrication
The OFETs were fabricated with a top-contact/bottom-gate (TC/BG) architecture. Highly n-doped silicon wafers with a 300-nm-thick thermally grown SiO2 gate dielectric layer (capacitance per unit area Ci = 11.4 nF/cm2) were used as substrates. The substrates were cleaned via sonication in acetone and isopropyl alcohol for 15 min each, followed by O2 plasma for 10 min (Harrick Plasma, 18 W). For the treatment of PS (polystyrene) brush, hydroxy-functionalized PS (Mw = 32 kg/mol) was used to form a hydrophobic surface of the substrates for the following film-forming process of the organic semiconductors. Then, the BTBT-based semiconductor films were formed via the solution-shearing (SS) method on the substrates with varying processing conditions (concentration of semiconductor solutions, solvents, substrate temperature, and shearing speed). After the optimized solution-shearing process, the substrates were annealed in a vacuum oven at 90 °C for 2 h to remove the residual solvent. The thickness of the semiconductor films (100–150 nm) was measured using atomic force microscopy (AFM). Finally, source and drain electrodes (gold; 30 nm) were evaporated through a shadow mask with a channel length/width of 100 μm/500 μm.
2.5. Device and Film Characterization
The device performance of OFETs was characterized using Keithley 4200 SCS equipped with a probe station. All electrical characterizations were performed in ambient environment. Charge carrier mobilities (μ) were calculated in the saturation regime by the following formula: µsat = (2IDSL)/[WCi(VG − Vth)2], where IDS is the current of source drain, and L and W stand for the channel length and width, respectively. Ci is the areal capacitance, VG is the gate voltage, and Vth is the threshold voltage. The device performance parameters were obtained from the average value of twelve devices [52,53]. The surface morphology and microstructure of semiconductor thin films were characterized using an atomic force microscope (AFM, Park System, XE 7, non-contact mode, Santa Clara, CA, USA) and X-ray diffractometer (XRD, Bruker, D8 Advance, Billerica, MA, USA), respectively.
3. Results
3.1. Thermal, Optical, and Electrochemical Properties
Thermal properties of BTBT-based compounds were characterized using thermogravimetric analysis (TGA) (Figure S1) and differential scanning calorimetry (DSC) (Figure S2). Compounds 1, 2, and 3 exhibited decomposition temperatures (5% weight loss) of 257, 369, and 336 °C, respectively (Table 1). Due to their extended organic molecular structures, all compounds showed high thermal stability. However, Compound 1 showed a slightly lower decomposition temperature than those of Compounds 2 and 3 due to the absence of the octyl side chain and thiophene ring that the others had [35,54]. In the DSC measurements, the BTBT derivatives showed sharp endotherms above 212, 214, and 158 °C, respectively (Table 1). Compound 3 exhibited relatively lower melting temperatures than Compounds 1 and 2, which could be ascribed to bulky functional groups attached to the BTBT core.

Table 1.
Thermal, optical, and electrochemical properties of BTBT derivatives.
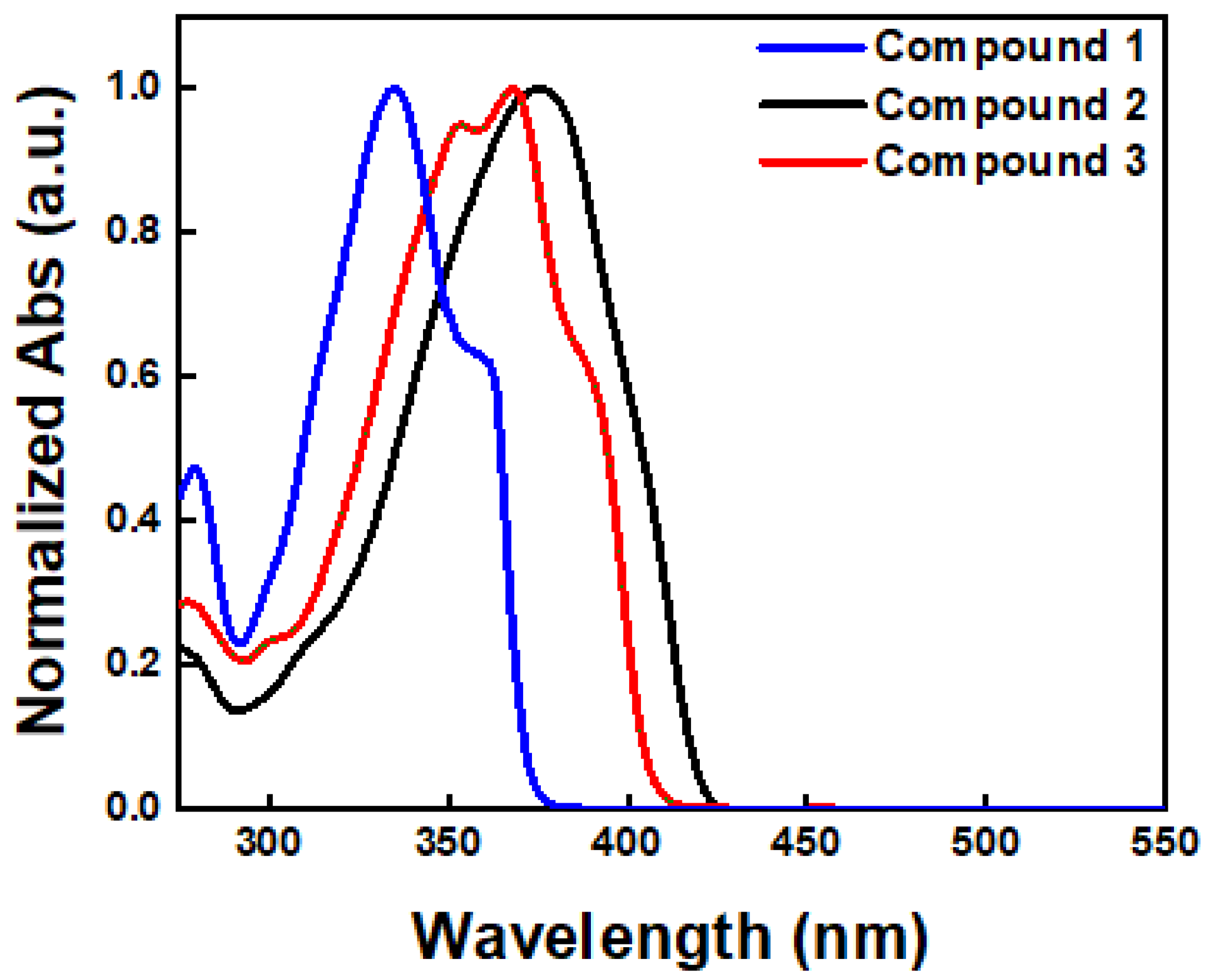
The optical properties of the developed compounds in diluted chloroform solution were measured using UV–Vis spectroscopy (Figure 2). The maximum absorption wavelengths for each compound were observed at 335, 375, and 368 nm, respectively. As shown in Figure 2, Compounds 2 and 3 exhibited red-shifted maximum absorption peaks compared to Compound 1, owing to the extended conjugation length attributed to the presence of thiophene rings (vide infra). Compound 2 particularly showed a slightly red-shifted maximum absorption peak compared to Compound 3 due to the existence of the phenyl group. Furthermore, the band gap energy (Egap) estimated from the onset wavelengths of each compound were 3.34, 2.96, and 3.06 eV, respectively (Table 1).
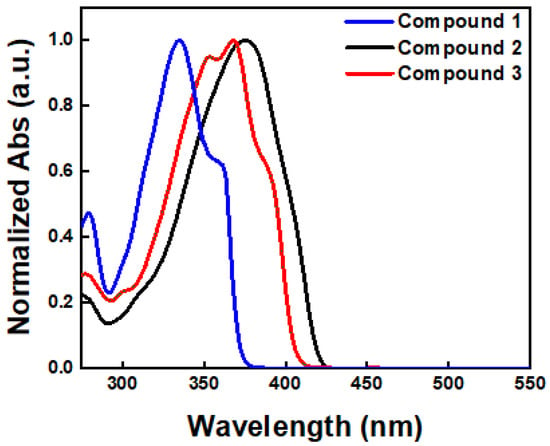
Figure 2.
Optical absorption spectra of Compounds 1–3.
As shown in Figure S3, the oxidation peaks for three compounds were 1.36, 0.82, and 1.14 V, respectively. Using the ferrocene/ferrocenium internal standard, experimental values of the highest occupied molecular orbital (HOMO) levels (EHOMO) could be obtained by using the ferrocene/ferrocenium internal standard with the regular formula:
EHOMO (eV) = −e(Eoxonset − EFc/Fc+onset) − 4.80 eV
In the formula, Eoxonset is the onset potential of the oxidation process. Experimental values of HOMO levels were estimated as −5.70, −5.16, and −5.48 eV for Compounds 1–3, respectively. Compounds 2 and 3 showed higher HOMO energy levels than Compound 1 because the added thiophene rings of Compounds 2 and 3 could act as electron donors [55,56]. The lowest unoccupied molecular orbital (LUMO) levels could be determined from HOMO levels and the band gap energy of each compound as −2.36, −2.20, and −2.42 eV for Compounds 1–3, respectively.
3.2. Theoretical Calculation
To characterize HOMO/LUMO energy distribution and the molecular structure of BTBT-based derivatives, a density functional theory (DFT, B3LYP) calculation was performed. As shown in Figure S4, HOMO and LUMO orbitals of Compounds 2 and 3 are dispersed on the main BTBT core and thiophene rings and barely distributed on the octyl side chain, indicating that the alkyl side chains of each compound had little influence on energy distribution [57,58]. The theoretically estimated HOMO/LUMO energy of Compounds 1–3 were −5.41/−1.71 eV for Compound 1, −5.18/−1.83 eV for Compound 2, and −5.30/−1.77 eV for Compound 3, respectively. The calculated band gap energies were 3.70, 3.35, and 3.53 eV for Compounds 1, 2, and 3, respectively. Although theoretically determined values, including HOMO and LUMO energy levels and the energy band gap, afforded a slight difference from experimental values, a similar trend was observed (vide supra).
3.3. Thin Film Microstructure and Morphology
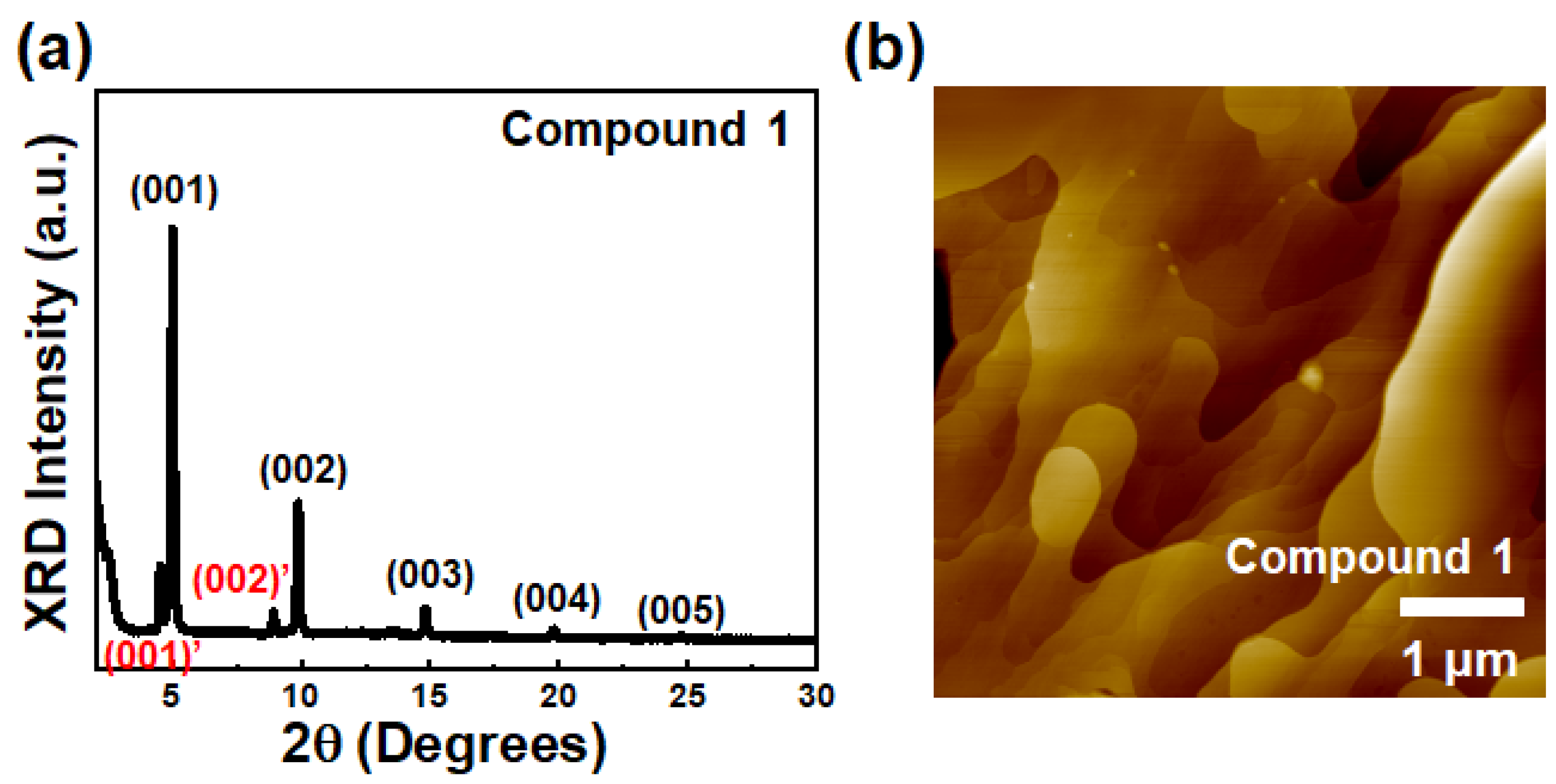
Wide-angle X-ray diffraction (XRD) and atomic force microscopy (AFM) were utilized to determine film texture and surface morphology of semiconductor films. As shown in Figure 3a, an XRD diffraction pattern of the thin films of Compound 1 exhibited several diffraction peaks with relatively high peak intensities, indicating that the thin films of Compound 1 have a relatively highly ordered microstructure. In contrast, the thin films of Compounds 2 and 3 showed weak (001) reflection without any other reflections, showing relatively poor film texture (Figure S5). The primary diffraction peaks (001) of Compounds 1–3 were observed at 2θ = 4.99° (d-spacing = 17.7 Å) for Compound 1, 2θ = 3.15° (d-spacing = 28.0 Å) for Compound 2, and 2θ = 3.49° (d-spacing = 25.0 Å) for Compound 3, respectively. The theoretically calculated molecular lengths obtained via DFT calculation were 18.0 Å for Compound 1, 31.5 Å for Compound 2, and 31.3 Å for Compound 3, respectively. The fact that the d-spacing value of Compound 1 is almost the same as for the molecular length of the corresponding compound indicates that molecules are almost vertically aligned on the surface along the substrate normal, affording enhanced molecular orbital overlap. On the contrary, d-spacing values of Compounds 2 and 3 were smaller than the calculated molecular lengths, showing an arrangement of corresponding molecules in the thin film with a tilted angle along the substrate normal.
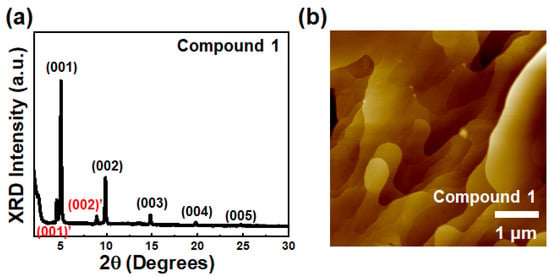
Figure 3.
(a) XRD graph and (b) AFM topographic image of solution-sheared film of Compound 1.
In the case of film surface analysis, non-contact mode AFM was performed to characterize the morphologies of the solution-sheared thin films for Compounds 1–3. As shown in Figure 3b, the thin film of Compound 1 exhibited a terrace-like morphology and larger grain size. On the other hand, the thin films of Compounds 2 and 3 showed relatively poor surface morphologies with large voids (Figure S5). Based on film microstructure and morphology analyses, thin films of Compound 1 with high film texture and large grains would perform better in OFETs compared to Compound 2 and 3 [59,60].
3.4. Thin Film Transistor Characterization
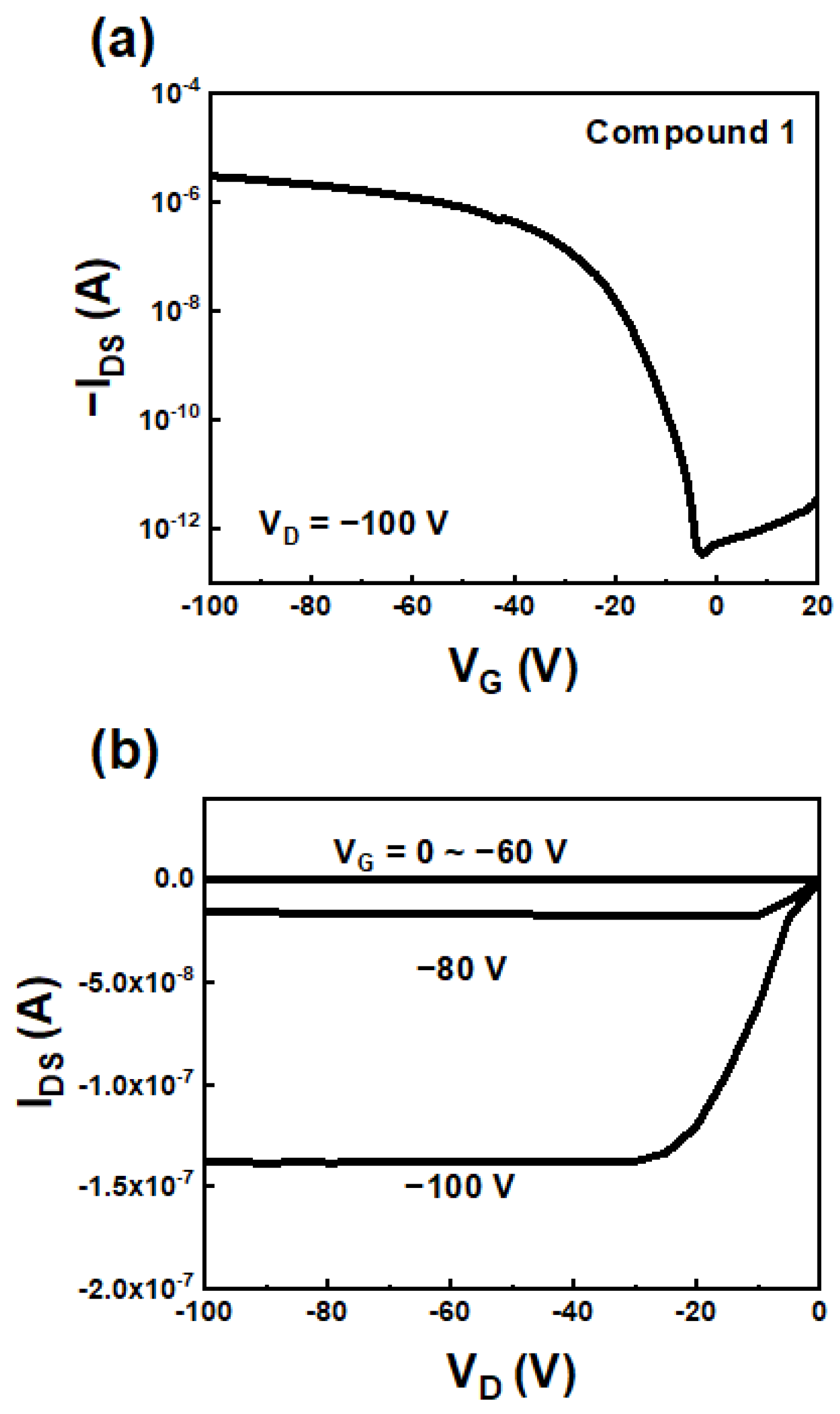
To estimate the charge carrier properties of the BTBT derivatives, top-contact/bottom-gate OFETs with BTBT derivatives were fabricated via the solution-shearing method on PS-brush-treated Si/SiO2 substrates. All the fabricated devices exhibited p-channel characteristics (Table 2). Among the three compounds, Compound 1 exhibited the highest electrical performance of ~0.03 cm2/Vs (Figure 4). Although Compounds 2 and 3 have more extended conjugation lengths than Compound 1, they exhibited lower electrical performance (0.006–0.007 cm2/Vs) than Compound 1 (Figure S6). Their lower electrical performance can be attributed to the poor film microstructure and morphology via reduced molecular packing and the intermolecular π-π interaction from steric hindrance around the thiophene rings and end-capping groups of Compounds 2 and 3. Finally, the fabricated device based on Compound 1 showed negligible degradability after storage for 30 days, demonstrating the long-term air stability of the corresponding compound (Figure S7).

Table 2.
OFET device performance based on the thin films of BTBT derivatives.
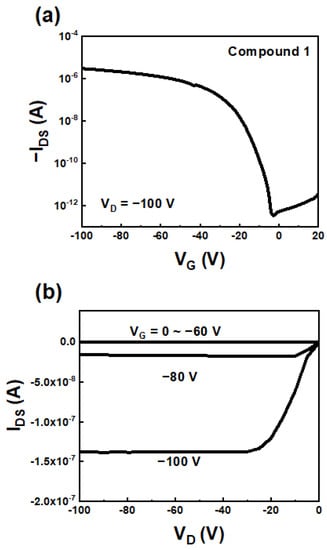
Figure 4.
(a) Transfer and (b) output characteristics of the OFETs based on Compound 1.
4. Conclusions
To sum up, we have developed and characterized three new [1]benzothieno[3,2-b]benzothiophene (BTBT)-based small molecular p-type organic semiconductors with various functional groups. All synthesized compounds exhibited decent solution processability, ambient stability, and transistor performance. In addition, XRD and AFM data for Compound 1 showed well-ordered microstructure and terrace-like thin film morphology with good inter-grain connectivity. OFET devices were fabricated using the solution-shearing method. Compound 1 in particular exhibited the highest electrical performance up to ~0.030 cm2/Vs and a current on/off ratio > 106 in an ambient condition. Hence, our study suggests that the BTBT core with proper end-capped groups could be a promising moiety for the further development of solution-processed OSCs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/coatings13010181/s1: Figure S1: Thermogravimetric analysis (TGA) of BTBT derivatives: (a) Compound 1, (b) Compound 2, and (c) Compound 3; Figure S2: Differential scanning calorimetry (DSC) analysis of BTBT derivatives: (a) Compound 1, (b) Compound 2, and (c) Compound 3; Figure S3: Cyclic voltammograms of Compounds 1–3; Figure S4: Molecular orbital surfaces of HOMO and LUMO by DFT calculation of Compounds (a) 1, (b) 2, and (c) 3; Figure S5: XRD profiles and AFM topographic images of solution-sheared film for (a) Compound 2 and (b) Compound 3; Figure S6: Representative transfer characteristics of OFETs based on (a) Compound 2 and (b) Compound 3; Figure S7: Transfer curve of OFETs based on Compound 1 after 30 days of device fabrication.
Author Contributions
Conceptualization, C.K. and S.S.; investigation, S.R. (Seunghyup Ryu), C.Y., S.R. (Soomin Ryu), J.A., C.K. and S.S.; data curation, S.R. (Seunghyup Ryu), C.Y., S.R. (Soomin Ryu) and J.A.; visualization, S.R. (Soomin Ryu) and C.Y.; writing―original draft, S.R. (Seunghyup Ryu) and C.Y.; supervision, C.K. and S.S.; validation, C.K. and S.S.; writing―review&editing, C.K. and S.S.; funding acquisition, C.K. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2021R1F1A1050933, RS-2022-00142063).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data that support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Youn, J.; Vegiraju, S.; Emery, J.D.; Leever, B.J.; Kewalramani, S.; Lou, S.J.; Zhang, S.; Prabakaran, K.; Ezhumalai, Y.; Kim, C. Diperfluorophenyl fused thiophene semiconductors for n-type organic thin film transistors (OTFTs). Adv. Electron. Mater. 2015, 1, 1500098. [Google Scholar] [CrossRef]
- Ho, D.; Lee, J.; Park, S.; Park, Y.; Cho, K.; Campana, F.; Lanari, D.; Facchetti, A.; Seo, S.; Kim, C.; et al. Green solvents for organic thin-film transistor processing. J. Mater. Chem. C 2020, 8, 5786–5794. [Google Scholar] [CrossRef]
- Park, Y.; Baeg, K.-J.; Kim, C. Solution-processed nonvolatile organic transistor memory based on semiconductor blends. ACS Appl. Mater. Interfaces 2019, 11, 8327–8336. [Google Scholar] [CrossRef] [PubMed]
- Vegiraju, S.; He, G.Y.; Kim, C.; Priyanka, P.; Chiu, Y.J.; Liu, C.W.; Huang, C.Y.; Ni, J.S.; Wu, Y.W.; Chen, Z. Solution-Processable Dithienothiophenoquinoid (DTTQ) Structures for Ambient-Stable n-Channel Organic Field Effect Transistors. Adv. Funct. Mater. 2017, 27, 1606761. [Google Scholar] [CrossRef]
- Lee, J.; Chen, H.-F.; Batagoda, T.; Coburn, C.; Djurovich, P.I.; Thompson, M.E.; Forrest, S.R. Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency. Nat. Mater. 2016, 15, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Kim, K.; Choi, B.D.; Roh, J.; Lee, C.; Noh, Y.Y.; Seo, S.; Kim, M.G.; Kim, C. Multifunctional Organic-Semiconductor Interfacial Layers for Solution-Processed Oxide-Semiconductor Thin-Film Transistor. Adv. Mater. 2017, 29, 1607055. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Ozdemir, R.; Kim, H.; Earmme, T.; Usta, H.; Kim, C. BODIPY-based semiconducting materials for organic bulk heterojunction photovoltaics and thin-film transistors. ChemPlusChem 2019, 84, 18–37. [Google Scholar] [CrossRef]
- Guo, X.; Quinn, J.; Chen, Z.; Usta, H.; Zheng, Y.; Xia, Y.; Hennek, J.W.; Ortiz, R.P.; Marks, T.J.; Facchetti, A. Dialkoxybithiazole: A new building block for head-to-head polymer semiconductors. J. Am. Chem. Soc. 2013, 135, 1986–1996. [Google Scholar] [CrossRef]
- Baude, P.; Ender, D.; Haase, M.; Kelley, T.; Muyres, D.; Theiss, S. Pentacene-based radio-frequency identification circuitry. Appl. Phys. Lett. 2003, 82, 3964–3966. [Google Scholar] [CrossRef]
- Campana, F.; Kim, C.; Marrocchi, A.; Vaccaro, L. Green solvent-processed organic electronic devices. J. Mater. Chem. C 2020, 8, 15027–15047. [Google Scholar] [CrossRef]
- Ho, D.; Jeon, M.; Kim, H.; Gidron, O.; Kim, C.; Seo, S. Solution-processable dithieno [3,2-b:2′,3′-d] thiophene derivatives for organic thin-film transistors and complementary-like inverters. Org. Electron. 2018, 52, 356–363. [Google Scholar] [CrossRef]
- Han, J.; Thirupathaiah, B.; Kwon, G.; Kim, C.; Seo, S. Synthesis and characterization of carbazole-and α-carboline-based thiophene derivatives as organic semiconductors for organic thin-film transistors. Dyes Pigm. 2015, 114, 78–84. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, W.; Duan, L. Recent progress in solution processable TADF materials for organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 5577–5596. [Google Scholar] [CrossRef]
- Qiao, Y.; Guo, Y.; Yu, C.; Zhang, F.; Xu, W.; Liu, Y.; Zhu, D. Diketopyrrolopyrrole-containing quinoidal small molecules for high-performance, air-stable, and solution-processable n-channel organic field-effect transistors. J. Am. Chem. Soc. 2012, 134, 4084–4087. [Google Scholar] [CrossRef]
- Quinn, J.T.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Vegiraju, S.; Hsieh, C.-M.; Huang, D.-Y.; Chen, Y.-C.; Priyanka, P.; Ni, J.-S.; Esya, F.A.; Kim, C.; Yau, S.L.; Chen, C.-P. Synthesis and characterization of solution-processable diketopyrrolopyrrole (DPP) and tetrathienothiophene (TTA)-based small molecules for organic thin film transistors and organic photovoltaic cells. Dyes Pigm. 2016, 133, 280–291. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Chen, L.-H.; Kim, C.; Chang, H.-C.; Liang, Y.-J.; Feng, C.-Y.; Yeh, C.-M.; Ho, J.-C.; Lee, C.-C.; Chen, M.-C. High-performance bottom-contact organic thin-film transistors based on benzo[d,d′]thieno[3,2-b;4,5-b′]dithiophenes (BTDTs) derivatives. ACS Appl. Mater. Interfaces 2012, 4, 6992–6998. [Google Scholar] [CrossRef]
- Gao, X.; Hu, Y. Development of n-type organic semiconductors for thin film transistors: A viewpoint of molecular design. J. Mater. Chem. C 2014, 2, 3099–3117. [Google Scholar] [CrossRef]
- Katz, H.E.; Bao, Z.; Gilat, S.L. Synthetic chemistry for ultrapure, processable, and high-mobility organic transistor semiconductors. Acc. Chem. Res. 2001, 34, 359–369. [Google Scholar] [CrossRef]
- Guo, X.; Ortiz, R.P.; Zheng, Y.; Kim, M.-G.; Zhang, S.; Hu, Y.; Lu, G.; Facchetti, A.; Marks, T.J. Thieno [3,4-c] pyrrole-4,6-dione-based polymer semiconductors: Toward high-performance, air-stable organic thin-film transistors. J. Am. Chem. Soc. 2011, 133, 13685–13697. [Google Scholar] [CrossRef]
- He, Y.; Xu, W.; Murtaza, I.; Zhang, D.; He, C.; Zhu, Y.; Meng, H. Molecular phase engineering of organic semiconductors based on a [1]benzothieno[3,2-b][1] benzothiophene core. RSC Adv. 2016, 6, 95149–95155. [Google Scholar] [CrossRef]
- He, Y.; Sezen, M.; Zhang, D.; Li, A.; Yan, L.; Yu, H.; He, C.; Goto, O.; Loo, Y.L.; Meng, H. High Performance OTFTs Fabricated Using a Calamitic Liquid Crystalline Material of 2-(4-Dodecyl phenyl)[1] benzothieno [3,2-b][1] benzothiophene. Adv. Electron. Mater. 2016, 2, 1600179. [Google Scholar] [CrossRef]
- Cho, J.-M.; Mori, T. Low-temperature band transport and impact of contact resistance in organic field-effect transistors based on single-crystal films of Ph-BTBT-C10. Phys. Rev. Appl. 2016, 5, 064017. [Google Scholar] [CrossRef]
- Takimiya, K.; Ebata, H.; Sakamoto, K.; Izawa, T.; Otsubo, T.; Kunugi, Y. 2, 7-Diphenyl[1]benzothieno [3,2-b]benzothiophene, a new organic semiconductor for air-stable organic field-effect transistors with mobilities up to 2.0 cm2V-1s-1. J. Am. Chem. Soc. 2006, 128, 12604–12605. [Google Scholar] [CrossRef]
- Schweicher, G.; Lemaur, V.; Niebel, C.; Ruzié, C.; Diao, Y.; Goto, O.; Lee, W.Y.; Kim, Y.; Arlin, J.B.; Karpinska, J. Bulky end-capped[1]benzothieno[3,2-b] benzothiophenes: Reaching high-mobility organic semiconductors by fine tuning of the crystalline solid-state order. Adv. Mater. 2015, 27, 3066–3072. [Google Scholar] [CrossRef]
- Amin, A.Y.; Khassanov, A.; Reuter, K.; Meyer-Friedrichsen, T.; Halik, M. Low-voltage organic field effect transistors with a 2-tridecyl[1] benzothieno[3,2-b][1]benzothiophene semiconductor layer. J. Am. Chem. Soc. 2012, 134, 16548–16550. [Google Scholar] [CrossRef]
- Ullah, M.; Wawrzinek, R.; Nagiri, R.C.; Lo, S.C.; Namdas, E.B. UV–deep blue–visible light-emitting organic field effect transistors with high charge carrier mobilities. Adv. Opt. Mater. 2017, 5, 1600973. [Google Scholar] [CrossRef]
- Colella, S.; Ruzié, C.; Schweicher, G.; Arlin, J.B.; Karpinska, J.; Geerts, Y.; Samorì, P. High Mobility in Solution-Processed 2,7-Dialkyl-[1]benzothieno[3,2-b][1] benzothiophene-Based Field-Effect Transistors Prepared with a Simplified Deposition Method. ChemPlusChem 2014, 79, 371–374. [Google Scholar] [CrossRef]
- Yuan, Y.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.; Chen, J.; Nordlund, D.; Toney, M.F.; Huang, J.; Bao, Z. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5, 3005. [Google Scholar] [CrossRef]
- Iino, H.; Hanna, J.-I. Liquid crystalline organic semiconductors for organic transistor applications. Polym. J. 2017, 49, 23–30. [Google Scholar] [CrossRef]
- Iino, H.; Usui, T.; Hanna, J.-I. Liquid crystals for organic thin-film transistors. Nat. Commun. 2015, 6, 6828. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate–Adsorbent Interactions. C 2018, 4, 62. [Google Scholar] [CrossRef]
- Niemczak, M.; Rzemieniecki, T.; Sobiech, Ł.; Skrzypczak, G.; Praczyk, T.; Pernak, J. Influence of the alkyl chain length on the physicochemical properties and biological activity in a homologous series of dichlorprop-based herbicidal ionic liquids. J. Mol. Liq. 2019, 276, 431–440. [Google Scholar] [CrossRef]
- Othman Zailani, N.H.Z.; Yunus, N.M.; Ab Rahim, A.H.; Bustam, M.A. Thermophysical properties of newly synthesized ammonium-based protic ionic liquids: Effect of temperature, anion and alkyl chain length. Processes 2020, 8, 742. [Google Scholar] [CrossRef]
- Tian, H.K.; Shi, J.W.; He, B.; Hu, N.H.; Dong, S.Q.; Yan, D.H.; Zhang, J.P.; Geng, Y.H.; Wang, F.S. Naphthyl and Thionaphthyl End-Capped Oligothiophenes as Organic Semiconductors: Effect of Chain Length and End-Capping Groups. Adv. Funct. Mater. 2007, 17, 1940–1951. [Google Scholar] [CrossRef]
- Ozdemir, M.; Choi, D.; Zorlu, Y.; Cosut, B.; Kim, H.; Kim, C.; Usta, H. A new rod-shaped BODIPY-acetylene molecule for solution-processed semiconducting microribbons in n-channel organic field-effect transistors. New J. Chem. 2017, 41, 6232–6240. [Google Scholar] [CrossRef]
- Diallo, A.K.; Videlot-Ackermann, C.; Marsal, P.; Brisset, H.; Fages, F.; Kumagai, A.; Yoshimoto, N.; Serein-Spirau, F.; Lère-Porte, J.-P. Acetylenic spacers in phenylene end-substituted oligothiophene core for highly air-stable organic field-effect transistors. Phys. Chem. Chem. Phys. 2010, 12, 3845–3851. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.; Wang, L.; Ji, C.; Wang, Y.; Tian, W.; Yang, X.; Yin, L. High performance asymmetrical push–pull small molecules end-capped with cyanophenyl for solution-processed solar cells. Chem. Commun. 2014, 50, 10251–10254. [Google Scholar] [CrossRef]
- Kang, H.; Jang, Y.; Ho, D.; Ryu, S.; Kim, C.; Seo, S. Development and Characterization of Solution-Processable Dithieno [3,2-b:2’,3’-d] thiophenes Derivatives with Various End-capped Groups for Organic Field-Effect Transistors. ChemPlusChem 2022, 87, e202200267. [Google Scholar] [CrossRef]
- Kim, H.; Reddy, M.R.; Kwon, G.; Choi, D.; Kim, C.; Seo, S. Synthesis and characterization of 2,7-diethynyl-benzo[b]benzo[4,5]thieno[2,3-d] thiophene derivative as organic semiconductors for organic thin-film transistors. Synth. Met. 2016, 220, 599–605. [Google Scholar] [CrossRef]
- Kim, Y.J.; Back, J.Y.; Kim, S.O.; Jeon, C.W.; Park, C.E.; Kim, Y.H. Efficient Diketopyrrolopyrrole-Based Small-Molecule Bulk-Heterojunction Solar Cells with Different Electron-Donating End-Groups. Chem. Asian J. 2014, 9, 2505–2513. [Google Scholar] [CrossRef]
- Roy, V.; Zhi, Y.G.; Xu, Z.X.; Yu, S.C.; Chan, P.W.H.; Che, C.M. Functionalized Arylacetylene Oligomers for Organic Thin-Film Transistors (OTFTs). Adv. Mater. 2005, 17, 1258–1261. [Google Scholar] [CrossRef]
- Silvestri, F.; Marrocchi, A.; Seri, M.; Kim, C.; Marks, T.J.; Facchetti, A.; Taticchi, A. Solution-processable low-molecular weight extended arylacetylenes: Versatile p-type semiconductors for field-effect transistors and bulk heterojunction solar cells. J. Am. Chem. Soc. 2010, 132, 6108–6123. [Google Scholar] [CrossRef]
- Wu, Z.; Li, A.; Fan, B.; Xue, F.; Adachi, C.; Ouyang, J. Phenanthrene-functionalized 3,6-dithiophen-2-yl-2,5-dihydropyrrolo[3,4–c]pyrrole-1,4-diones as donor molecules for solution-processed organic photovoltaic cells. Sol. Energy Mater. Sol. Cells 2011, 95, 2516–2523. [Google Scholar] [CrossRef]
- Yu, C.; He, C.; Yang, Y.; Cai, Z.; Luo, H.; Li, W.; Peng, Q.; Zhang, G.; Liu, Z.; Zhang, D. New Conjugated Molecules with Two and Three Dithienyldiketopyrrolopyrrole (DPP) Moieties Substituted at meta Positions of Benzene toward p-and n-Type Organic Photovoltaic Materials. Chem. Asian J. 2014, 9, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Marrocchi, A.; Silvestri, F.; Seri, M.; Facchetti, A.; Taticchi, A.; Marks, T.J. Conjugated anthracene derivatives as donor materials for bulk heterojunction solar cells: Olefinic versus acetylenic spacers. Chem. Commun. 2009, 1380–1382. [Google Scholar] [CrossRef] [PubMed]
- Braunecker, W.A.; Oosterhout, S.D.; Owczarczyk, Z.R.; Larsen, R.E.; Larson, B.W.; Ginley, D.S.; Boltalina, O.V.; Strauss, S.H.; Kopidakis, N.; Olson, D.C. Ethynylene-linked donor–acceptor alternating copolymers. Macromolecules 2013, 46, 3367–3375. [Google Scholar] [CrossRef]
- Broggi, A.; Tomasi, I.; Bianchi, L.; Marrocchi, A.; Vaccaro, L. Small Molecular Aryl Acetylenes: Chemically Tailoring High-Efficiency Organic Semiconductors for Solar Cells and Field-Effect Transistors. ChemPlusChem 2014, 79, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, W.; Tian, H.; Wang, H.; Yan, D.; Zhang, J.; Geng, Y.; Wang, F. Benzothienobenzothiophene-based conjugated oligomers as semiconductors for stable organic thin-film transistors. ACS Appl. Mater. Interfaces 2014, 6, 5255–5262. [Google Scholar] [CrossRef]
- Park, W.; Yun, C.; Yun, S.; Lee, J.-J.; Bae, S.; Ho, D.; Earmme, T.; Kim, C.; Seo, S. [1]Benzothieno[3,2-b][1] benzothiophene-based liquid crystalline organic semiconductor for solution-processed organic thin film transistors. J. Ind. Eng. Chem. 2022, 114, 161–170. [Google Scholar] [CrossRef]
- Kim, Y.; Yun, C.; Yun, S.; Ho, D.; Earmme, T.; Kim, C.; Seo, S. Modification of alkyl side chain on thiophene-containing benzothieno [3,2-b]benzothiophene-based organic semiconductors for organic field-effect transistors. Synth. Met. 2022, 291, 117173. [Google Scholar] [CrossRef]
- Choi, H.H.; Cho, K.; Frisbie, C.D.; Sirringhaus, H.; Podzorov, V. Critical assessment of charge mobility extraction in FETs. Nat. Mater. 2018, 17, 2–7. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, I.; Salleo, A.; Chabinyc, M. Avoid the kinks when measuring mobility. Science 2016, 352, 1521–1522. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.B.; Tantiwiwat, M.; Walker, B.; Nguyen, T.-Q. Design, synthesis, and self-assembly of oligothiophene derivatives with a diketopyrrolopyrrole core. J. Phys. Chem. C 2008, 112, 15543–15552. [Google Scholar] [CrossRef]
- Do, T.T.; Ha, Y.E.; Kim, J.H. Effect of the number of thiophene rings in polymers with 2,1,3-benzooxadiazole core on the photovoltaic properties. Org. Electron. 2013, 14, 2673–2681. [Google Scholar] [CrossRef]
- Kim, B.; Ma, B.; Donuru, V.R.; Liu, H.; Frechet, J.M. Bodipy-backboned polymers as electron donor in bulk heterojunction solar cells. Chem. Commun. 2010, 46, 4148–4150. [Google Scholar] [CrossRef]
- Seo, D.; Yoon, Y.; Yeo, H.M.; Lee, K.K.; Kim, B.; Kwak, K. Density Functional Theory Study of Silolodithiophene Thiophenepyrrolopyrroledione-based Small Molecules: The Effect of Alkyl Side Chain Length in Electron Donor Materials. Bull. Korean Chem. Soc. 2015, 36, 513–519. [Google Scholar]
- Ma, Z.; Geng, H.; Wang, D.; Shuai, Z. Influence of alkyl side-chain length on the carrier mobility in organic semiconductors: Herringbone vs. pi–pi stacking. J. Mater. Chem. C 2016, 4, 4546–4555. [Google Scholar] [CrossRef]
- Geiger, M.; Acharya, R.; Reutter, E.; Ferschke, T.; Zschieschang, U.; Weis, J.; Pflaum, J.; Klauk, H.; Weitz, R.T. Effect of the degree of the gate-dielectric surface roughness on the performance of bottom-gate organic thin-film transistors. Adv. Mater. Interfaces 2020, 7, 1902145. [Google Scholar] [CrossRef]
- Rumer, J.; Rossbauer, S.; Planells, M.; Watkins, S.; Anthopoulos, T.; McCulloch, I. Reduced roughness for improved mobility in benzodipyrrolidone-based, n-type OFETs. J. Mater. Chem. C 2014, 2, 8822–8828. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).