Synthesis, Characterization and Investigation of Anticorrosion Properties of an Innovative Metal–Organic Framework, ZnMOF-BTA, on Carbon Steel in HCl Solution
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of ZnMOF-BTA
2.3. Characterization of the Synthesized ZnMOF-BTA
2.4. X-ray Crystallography
2.5. BTA Release
2.6. Weight Loss Measurement
2.7. Electrochemical Tests
3. Results and Discussion
3.1. Characterization of ZnMOF-BTA
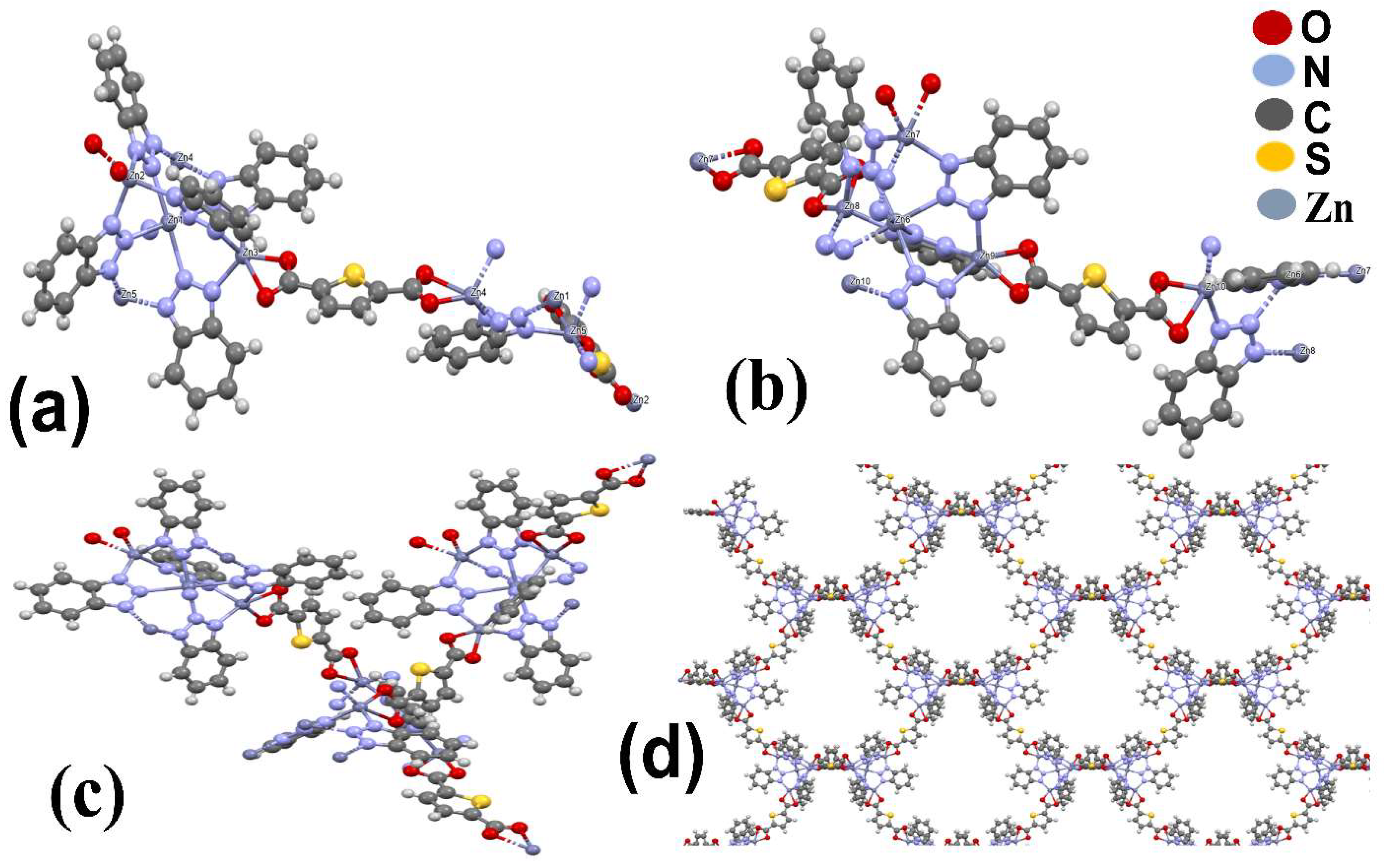
3.1.1. Crystal Structure
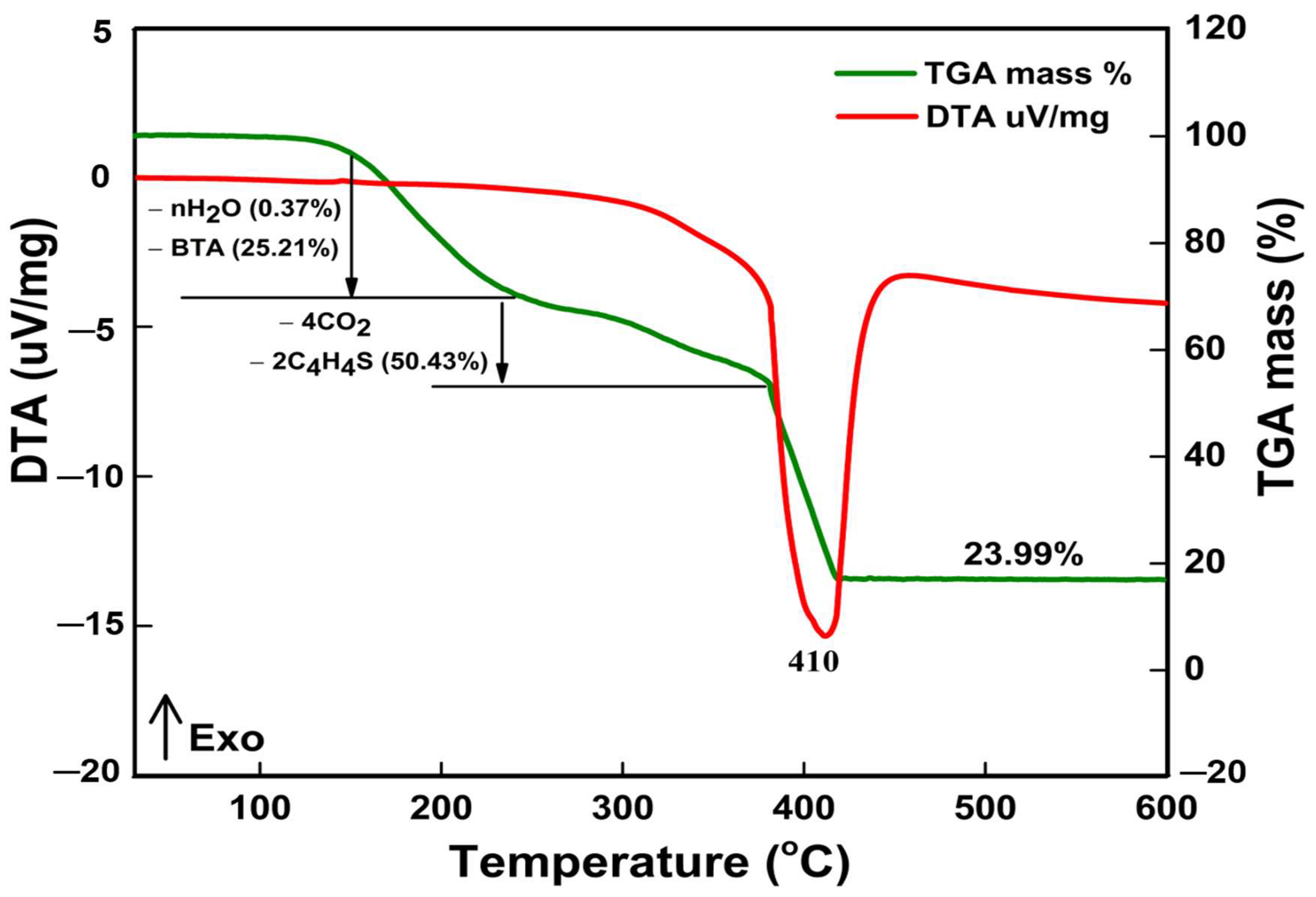
3.1.2. FTIR, XPS, and Thermal Stability Analyses
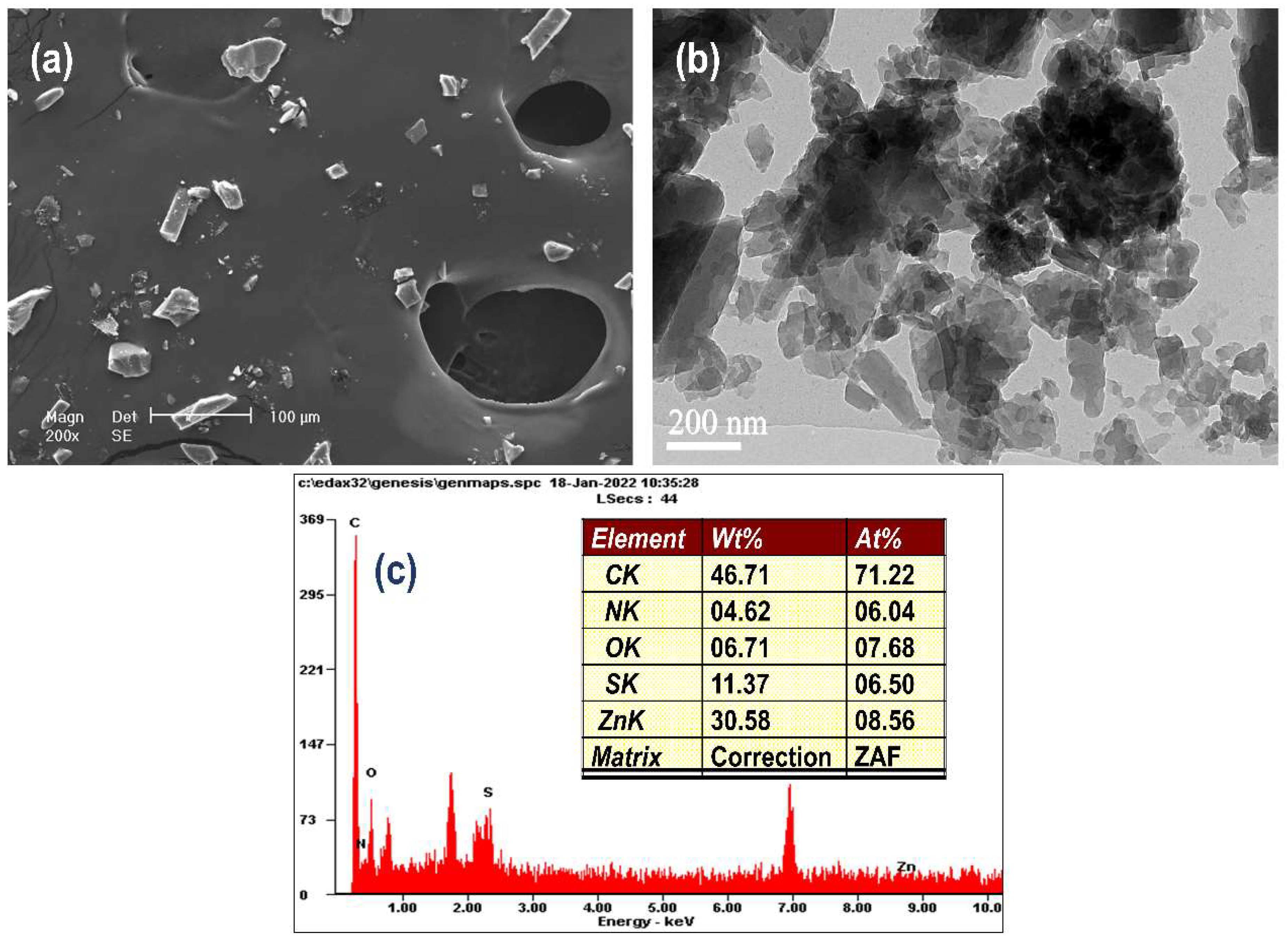
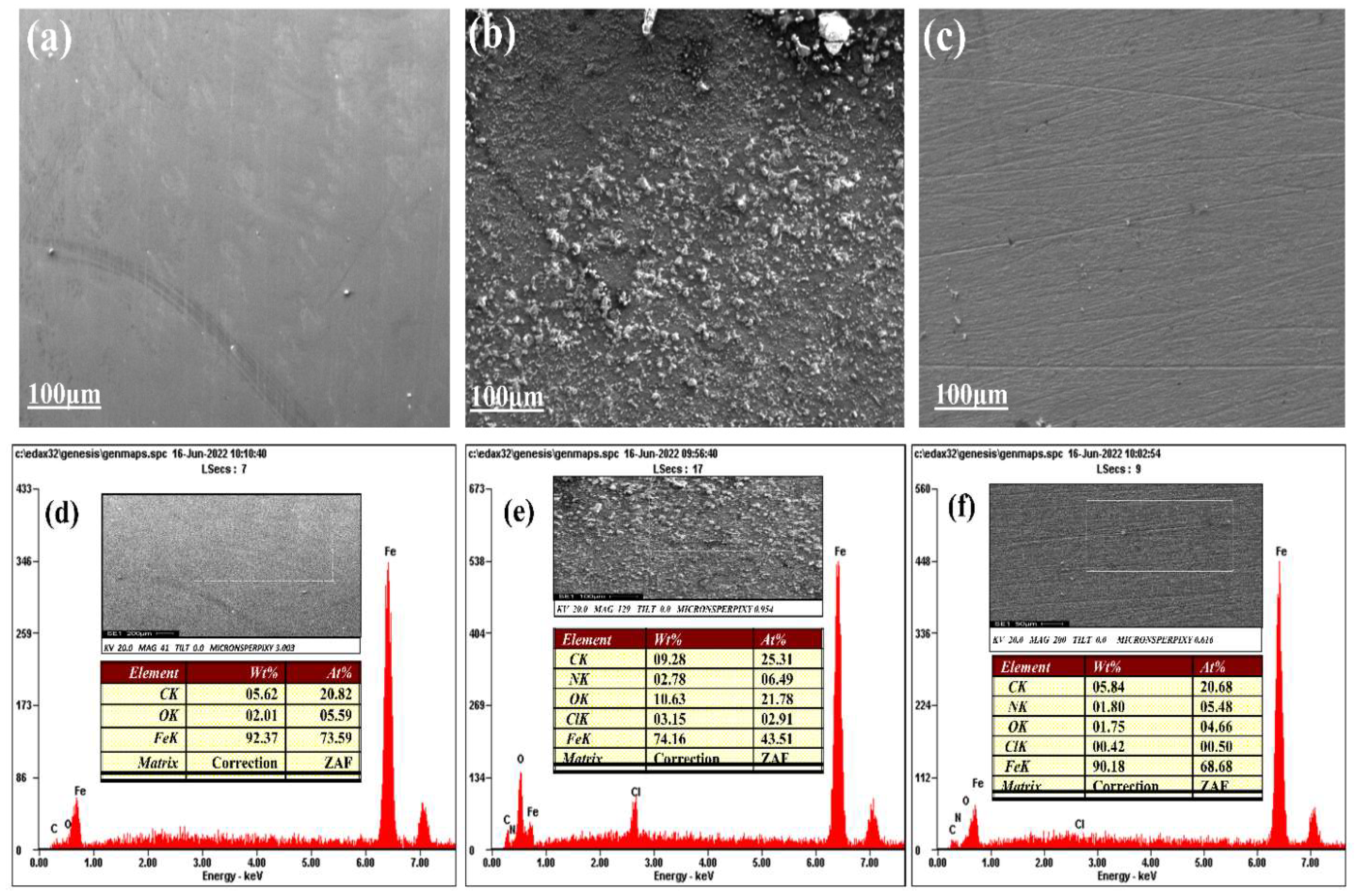
3.2. Crystal Surface Morphology Analysis
3.3. BTA Release from ZnMOF-BTA
3.4. Weight Loss Measurement
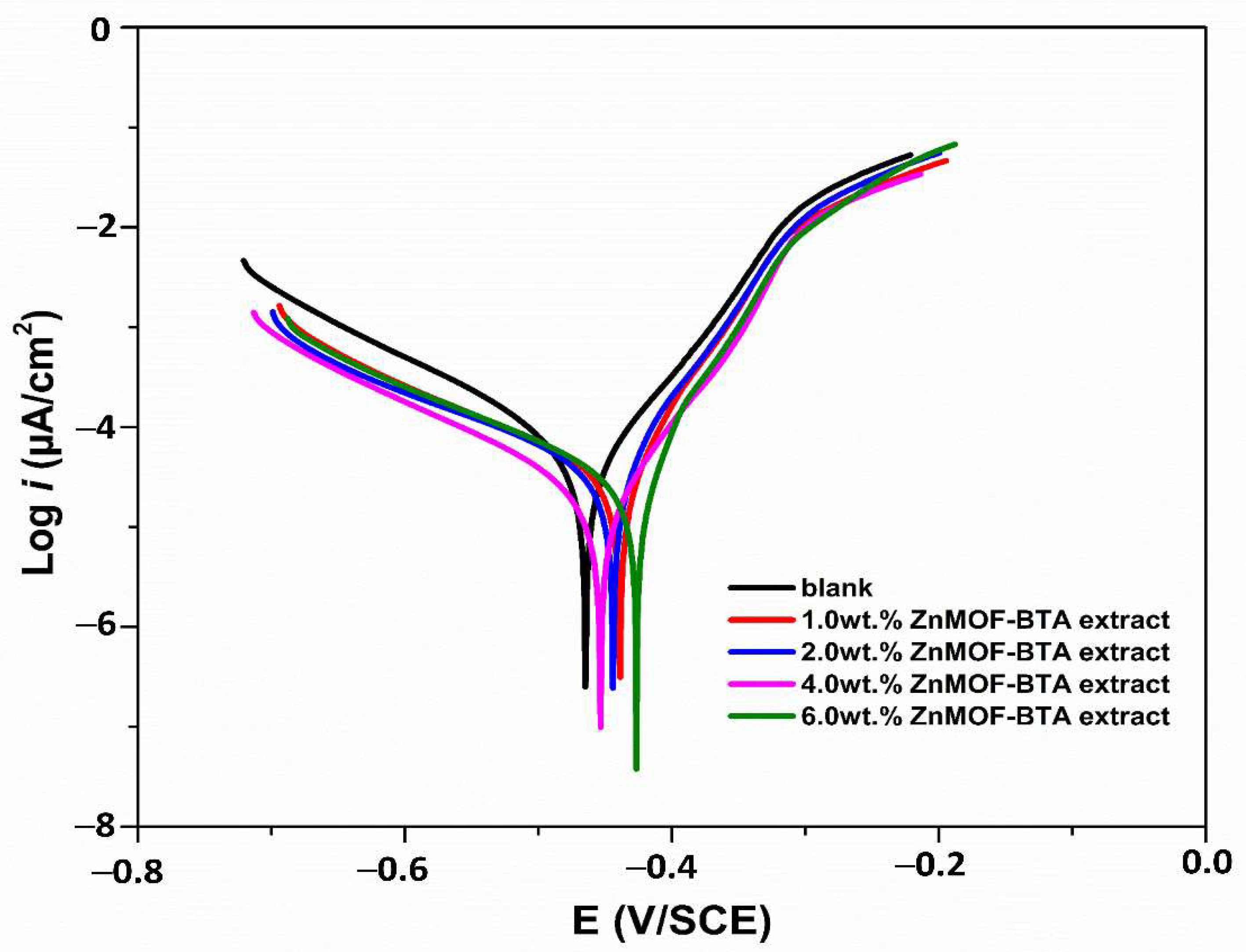
3.5. Potentiodynamic Polarization Test
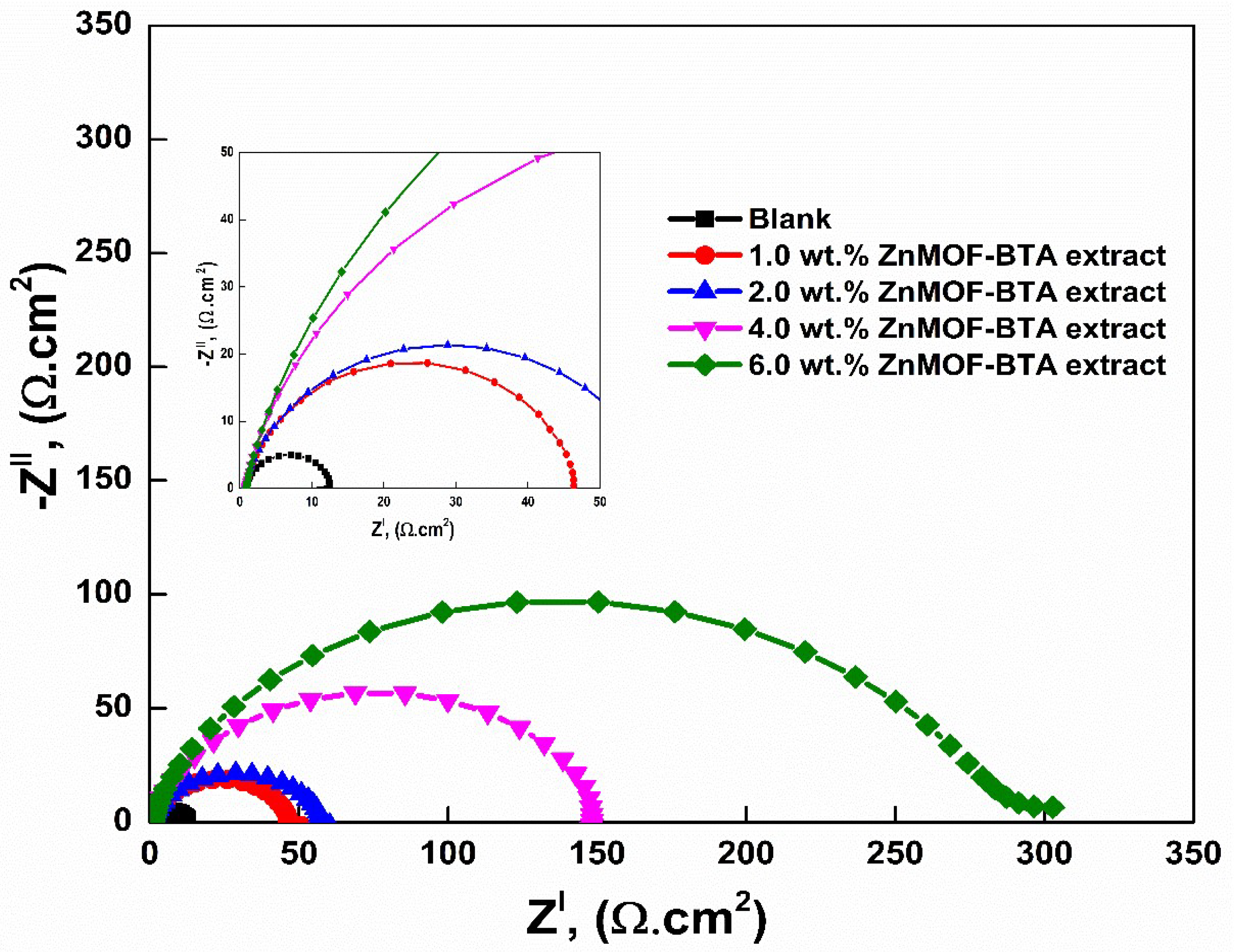
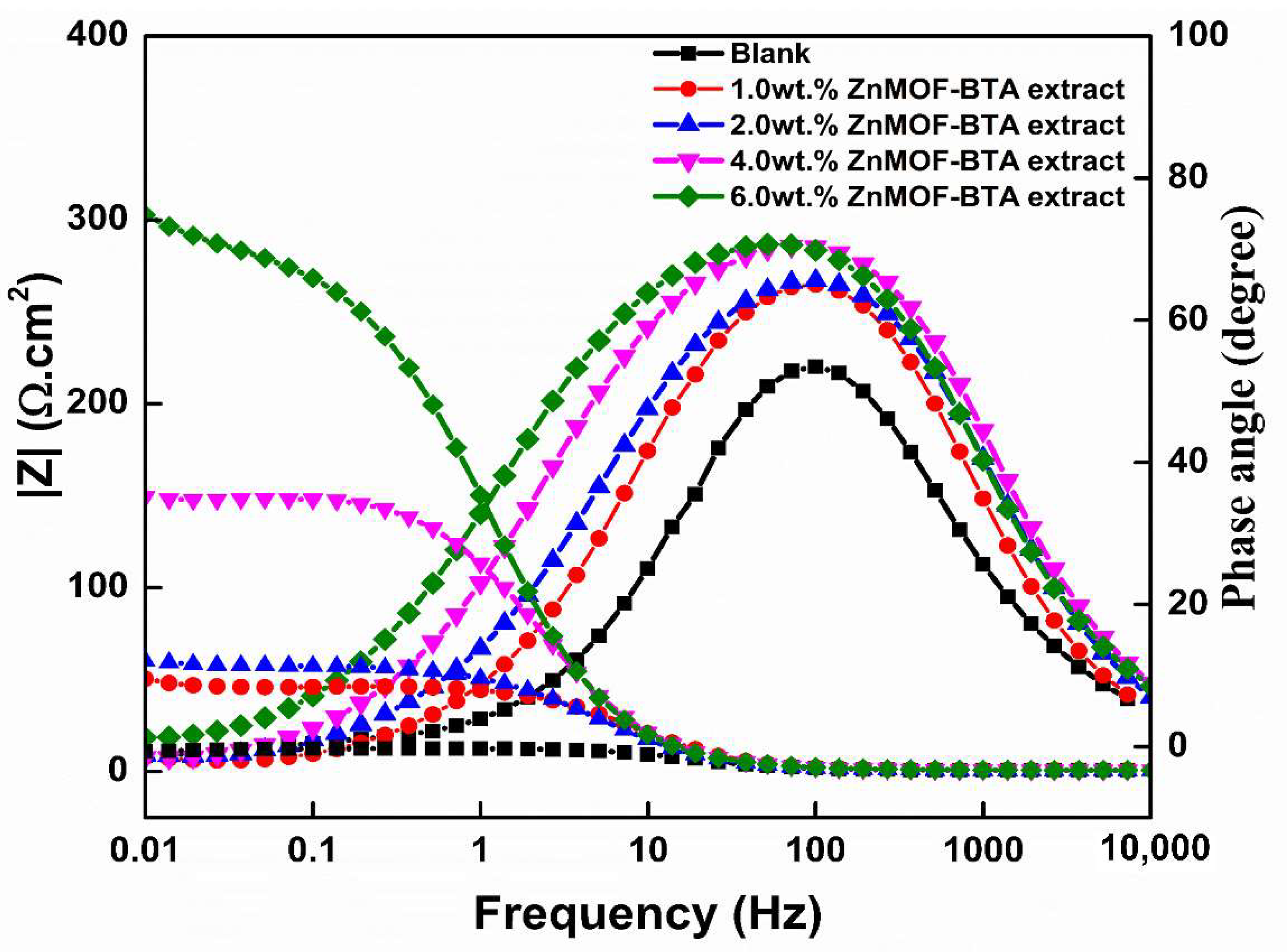
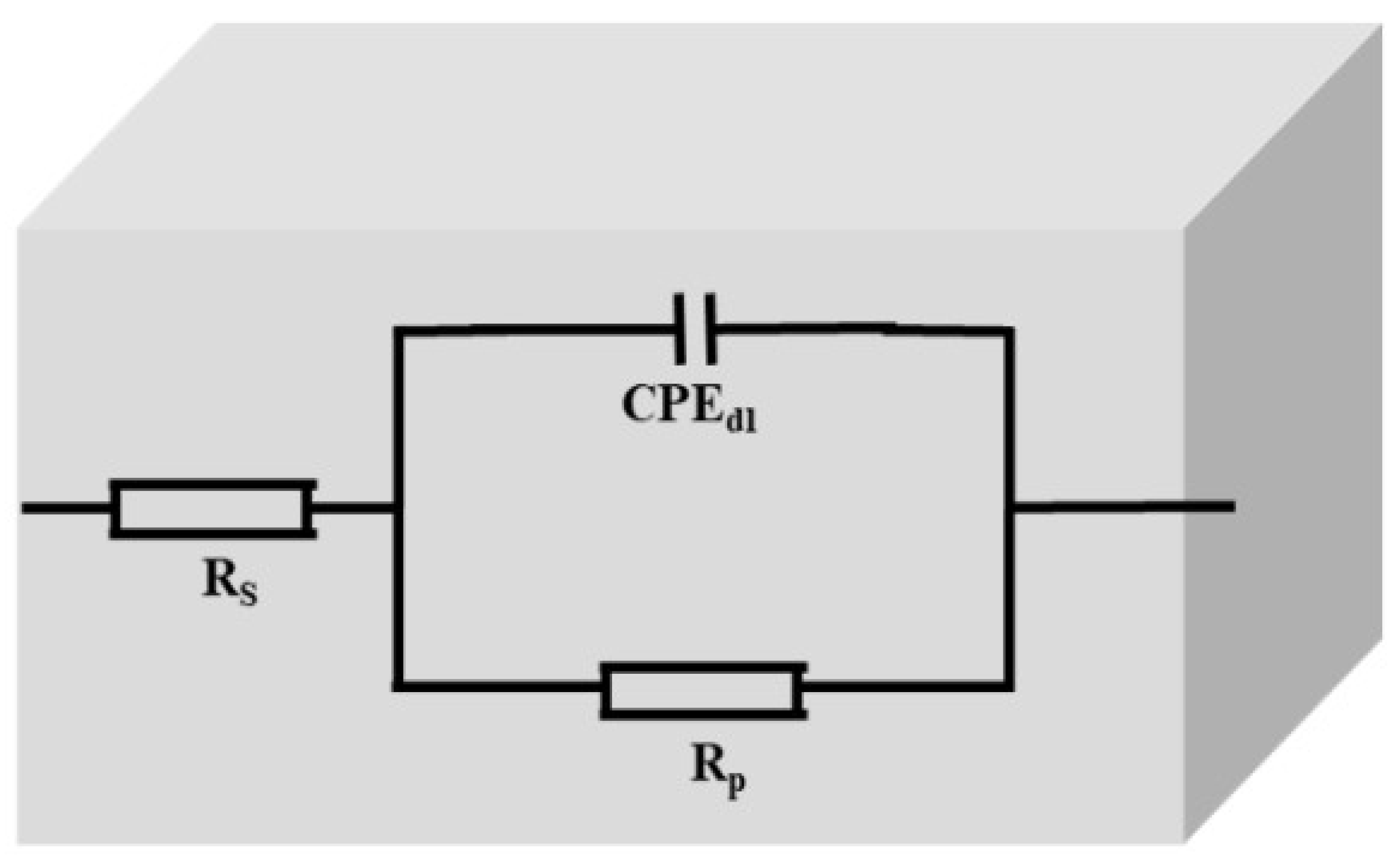
3.6. Electrochemical Impedance Spectroscopy (EIS)
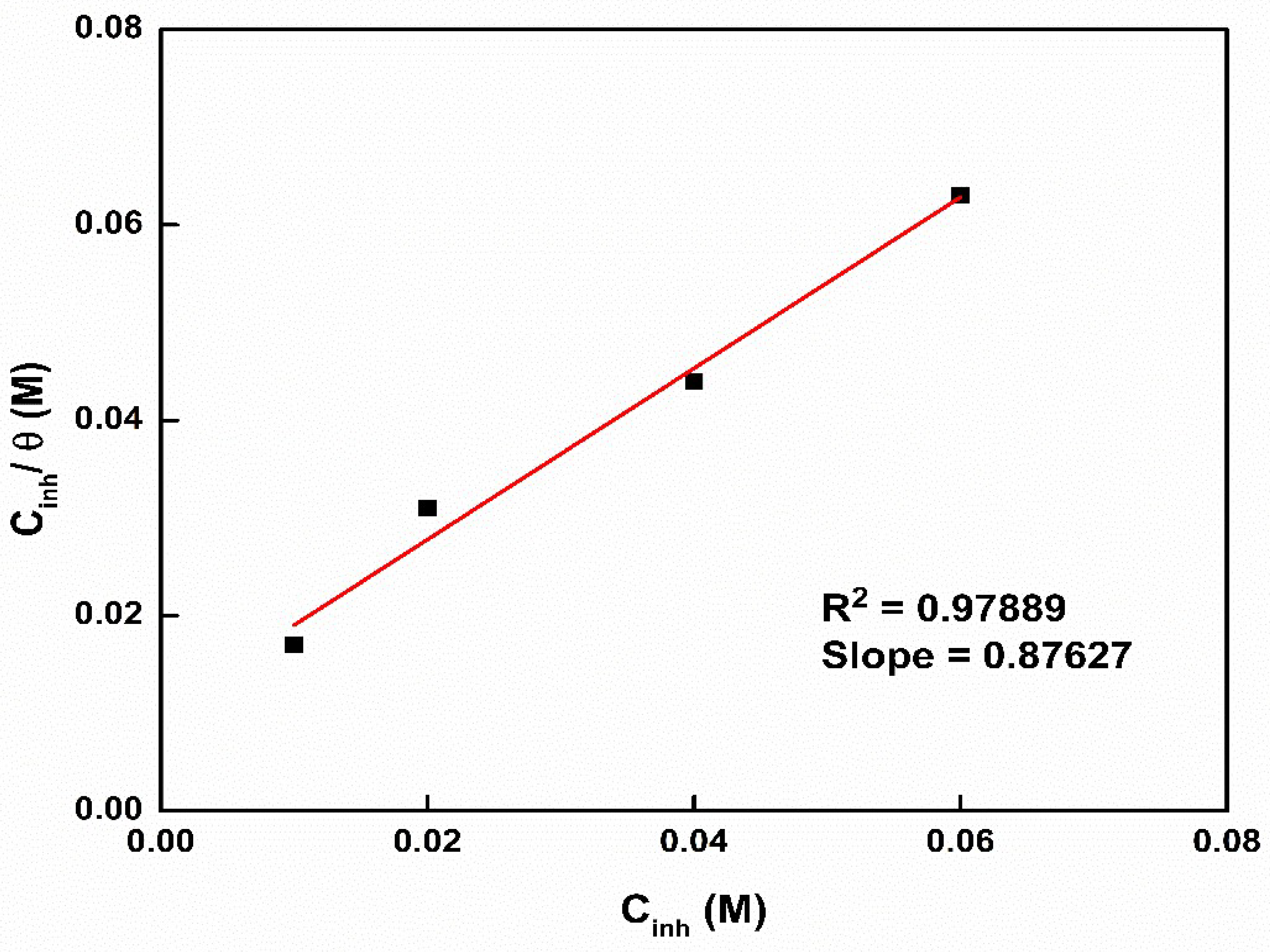
3.7. Adsorption Isotherm, Corrosion Morphology, and Mechanism of Inhibition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.K.; Quraishi, M.A. Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros. Sci. 2010, 52, 152–160. [Google Scholar] [CrossRef]
- Tang, F.; Chen, G.; Brow, R.K.; Koenigstein, M.L. Corrosion Resistance of a Sand Particle-Modified Enamel Coating Applied to Smooth Steel Bars. Materials 2014, 7, 6632–6645. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Rout, T.K.; Udayabhanu, G.; Narayan, R. Water-based & eco-friendly epoxy-silane hybrid coating for enhanced corrosion protection & adhesion on galvanized steel. Prog. Org. Coat. 2016, 101, 24–44. [Google Scholar]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Phenylimidazole Corrosion Inhibitor on Copper: An XPS and ToF-SIMS Surface Analytical Study. Coatings 2021, 11, 966. [Google Scholar] [CrossRef]
- Al Suhybani, A.A.; Sultan, Y.H.; Hamid, W.A. Corrosion of aluminum in alkaline solutions. Materwiss Werksttech 2012, 22, 301–307. [Google Scholar] [CrossRef]
- Zohdy, K.M. Surface Protection of Carbon Steel in Acidic Solution Using Ethylenediaminetetraacetic Disodium Salt. Int. J. Electrochem. Sci. 2015, 10, 414–431. [Google Scholar]
- Knack, D.H.; Marshall, J.L.; Harlow, G.P.; Dudzik, A.; Szaleniec, M.; Liu, S.; Heider, J. BN/CC isosteric compounds as enzyme inhibitors: N-and B-ethyl-1,2-azaborine inhibit ethylbenzene hydroxylation as nonconvertible substrate analogues. Angew. Chem. Int. Ed. Engl. 2013, 52, 2599–2601. [Google Scholar] [CrossRef]
- Yin, D.; Yu, Z.; Chen, L.; Cao, K. Enhancement of the Anti-Corrosion Performance of Composite Epoxy Coatings in Presence of BTA-loaded Copper-Based Metal-Organic Frameworks. Int. J. Electrochem. 2019, 14, 4240–4253. [Google Scholar] [CrossRef]
- Udoh, I.I.; Shi, H.; Daniel, E.F.; Li, J.; Gu, S.; Liu, F.; Han, E. Active anticorrosion and self-healing coatings: A review with focus on multi-action smart coating strategies. J. Mater. Sci. Technol. 2022, 116, 224–237. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Jain, P.; Patidar, B.; Bhawsar, J. Potential of Nanoparticles as a Corrosion Inhibitor: A Review. J. Bio. Tribo-Corros. 2020, 6, 43. [Google Scholar] [CrossRef]
- Kamburova, K.; Radeva, T. Polyelectrolyte-modified kaolinite nanocontainers for entrapment of corrosion inhibitor benzotriazole. Colloid Polym. Sci. 2018, 296, 1157–1164. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Taghizadeh, M.; Taghizadeh, A.; Hayati, B.; Shekarchi, A.A.; Vossoughi, M. Activated carbon/metal-organic framework nanocomposite: Preparation and photocatalytic dye degradation mathematical modeling from wastewater by least squares support vector machine. J. Environ. Manag. 2019, 233, 660–672. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Lu, W.; Gu, Z.; Luo, Z.; Zhou, H.; Zhou, H. Confinement of metal-organic polyhedra in silica nanopores. J. Am. Chem. Soc. 2012, 134, 15923–15928. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Ultrasound-assisted green synthesis and application of recyclable nanoporous chromium-based metal-organic framework. Korean J. Chem. Eng. 2018, 36, 287–298. [Google Scholar] [CrossRef]
- Zafari, S.; Shahrak, M.N.; Ghahramaninezhad, M. New MOF-Based Corrosion Inhibitor for Carbon Steel in Acidic Media. Met. Mater. Int. 2019, 26, 25–38. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Ahn, W. Synthesis of metal-organic frameworks: A mini-review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Sheldrick, G. Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. 2015, 71, 9–18. [Google Scholar]
- Ikeuba, A.I.; Ita, B.I.; Okafor, P.C.; Bassey, V.M.; Ugi, B.U.; Kporokpo, E.B. Green Corrosion Inhibitors for Mild Steel in H2SO4 Solution: Flavonoids of Gongronema latifolium. Prot. Met. Phys. Chem. Surf. 2015, 51, 1043–1049. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Okafor, P.C.; Ekpe, U.J.; Ebenso, E.E. Alkaloid and non-alkaloid ethanolic extracts from seeds of Garcinia kola as green corrosion inhibitors of mild steel in H2SO4 solution. Int. J. Electrochem. Sci. 2013, 8, 7455–7467. [Google Scholar]
- Ikeuba, A.I.; Okafor, P.C. Green corrosion protection for mild steel in acidic media: Saponins and crude extracts of Gongronema latifolium. Pigment. Resin Technol. 2018, 48, 57–64. [Google Scholar] [CrossRef]
- Abeng, F.E.; Ikpi, M.E.; Okafor, P.C.; Anadebe, V.C.; Uwakwe, K.J.; Ikeuba, A.I.; Okafor, N.A. Corrosion inhibition of API 5L X-52 steel in oilfield acidizing solution by Gentamicine and sulfamethoxazole: Experimental, plane-wave density functional theory (PWDFT) and the generalized-gradient approximation (GGA) simulations. J. Adhes. Sci. Technol. 2021, 35, 1–24. [Google Scholar] [CrossRef]
- Karimi, M. Investigation of the Stability of Oxadiasole and Their Analogs Using Quantum Mechanics Computation. Comput. Chem. 2016, 4, 11–16. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Abu-Dief, A.M.; Abdel-Rahman, L.H.; Sañudo, E.C.; Aliaga-Alcalde, N. Electrochemical and theoretical quantum approaches on the inhibition of C1018 carbon steel corrosion in acidic medium containing chloride using some newly synthesized phenolic Schiff bases compounds. J. Electroanal. 2015, 743, 120–133. [Google Scholar] [CrossRef]
- Praveen, B.M.; Alhadhrami, A.; Prasanna, B.M.; Hebbar, N.; Prabhu, R. Anti-Corrosion Behavior of Olmesartan for Soft-Cast Steel in 1 mol dm−3 HCl. Coatings 2021, 11, 965. [Google Scholar] [CrossRef]
- Sharma, D.; Jha, R. Analysis of structural, optical and magnetic properties of Fe/Co co-doped ZnO nanocrystals. Ceram. Int. 2017, 43, 8488–8496. [Google Scholar] [CrossRef]
- Phiri, I.; Bon, C.Y.; Kim, S.; Mwemezi, M.; Hamenu, L.; Madzvamuse, A.; Kim, S.H.; Ko, J.M. Effects of novel benzotriazole based zwitterionic salt as electrolyte additive for lithium ion batteries. Curr. Appl. Phys. 2020, 20, 122–131. [Google Scholar] [CrossRef]
- Stojković, D.L.; Jevtić, V.V.; Vuković, N.; Vukić, M.; Čanović, P.; Zarić, M.M.; Mišić, M.M.; Radovanović, D.M.; Baskić, D.; Trifunović, S.R. Synthesis, characterization, antimicrobial and antitumor reactivity of new palladium(II) complexes with methionine and tryptophane coumarine derivatives. J. Mol. Struct. 2018, 1157, 425–433. [Google Scholar] [CrossRef]
- Gu, S.; Shi, H.; Zhang, C.; Wang, W.; Liu, F.; Han, E. Mesoporous CeO2 containers in water-borne epoxy coatings for dual active corrosion protection of mild steel. Prog. Org. Coat. 2021, 158, 106376. [Google Scholar] [CrossRef]
- Ge, L.; Zuo, F.; Liu, J.; Ma, Q.; Wang, C.; Sun, D.; Bartels, L.; Feng, P. Synthesis and Efficient Visible Light Photocatalytic Hydrogen Evolution of Polymeric g-C3N4 Coupled with CdS Quantum Dots. J. Phys. Chem. C 2012, 116, 13708–13714. [Google Scholar] [CrossRef]
- Huang, B.B.; Liu, Y.C.; Xie, Z.L. Biomass derived 2D carbons via a hydrothermal carbonization method as efficient bifunctional ORR/HER electrocatalysts. J. Mater. Chem. A 2017, 5, 23481–23488. [Google Scholar] [CrossRef]
- Macedo, J.C.A.; Gontijo, E.S.J.; Herrera, S.G.; Rangel, E.C.; Komatsu, D.; Landers, R.; Rosa, A.H. Organosulphur-modified biochar: An effective green adsorbent for removing metal species in aquatic systems. Surf. Interfaces 2021, 22, 100822. [Google Scholar] [CrossRef]
- Min, H.; Dietrich, P.M.; Girard-Lauriault, P.L.; Gross, T.; Lippitz, A.; Unger, W.E.S. Ambient-ageing processes in amine self-assembled monolayers on microarray slides as studied by ToF-SIMS with principal component analysis, XPS, and NEXAFS spectroscopy. Anal. Bioanal. Chem. 2012, 403, 613–623. [Google Scholar] [CrossRef]
- Song, Z.Y.; Duan, H.; Li, L.C.; Zhu, D.Z.; Cao, T.C.; Lv, Y.K.; Xiong, W.; Wang, Z.W.; Liu, M.X.; Gan, L.H. High-energy flexible solid-state supercapacitors based on O, N, S-tridoped carbon electrodes and a 3.5 V gel-type electrolyte. Chem. Eng. J. 2019, 372, 1216–1225. [Google Scholar] [CrossRef]
- Wu, D.; Wang, C.; Wu, H.; Wang, S.; Wang, F.; Chen, Z.; Zhao, T.; Zhang, Z.; Zhang, L.Y. Synthesis of hollow Co3O4 nanocrystals in situ anchored on holey graphene for high rate lithium-ion batteries. Carbon 2020, 163, 137–144. [Google Scholar] [CrossRef]
- Dhanaraj, P.V.; Rajesh, N.P.; Sundar, J.K.; Natarajan, S.; Vinitha, G. Studies on growth, crystal structure and characterization of novel organic nicotinium trifluoroacetate single crystals. Mater. Chem. Phys. 2011, 129, 457–463. [Google Scholar] [CrossRef]
- Al-Awadi, N.A.; Shuaib, N.M.; Abbas, A.; El-Sherif, A.A.A.; El-Dissouky, A.; Al-Saleh, E.A. Synthesis, Characterization, and Biological Activity of -Methyl-2-(-1,2,3-Benzotriazol-1-y1)-3-Oxobutan- ethioamide Complexes with Some Divalent Metal (II) Ions. Bioinorg. Chem. Appl. 2008, 2008, 479897. [Google Scholar] [CrossRef]
- Yeşilel, O.Z.; İlker, I.; Büyükgüngör, O. Three copper(II) complexes of thiophene-2,5-dicarboxylic acid with dissimilar ligands: Synthesis, IR and UV–Vis spectra, thermal properties and structural characterizations. Polyhedron 2009, 28, 3010–3016. [Google Scholar] [CrossRef]
- Khaled, M.A.; Ismail, M.A.; El-Hossiany, A.A.; Fouda, A.E.-S. Novel pyrimidine-bichalcophene derivatives as corrosion inhibitors for copper in 1 M nitric acid solution. RSC Adv. 2021, 11, 25314–25333. [Google Scholar] [CrossRef]
- Belayachi, M.; Serrar, H.; Zarrok, H.; el Assyry, A.; Zarrouk, A.; Oudda, H.; Boukhris, S.; Hammouti, B.; Ebenso, E.; Guenbour, A. New pyrimidothiazine Derivative as Corrosion Inhibitor for Carbon Steel in Acidic Media. Int. J. Electrochem. 2015, 10, 3010–3025. [Google Scholar]
- Velazquez-Torres, N.; Porcayo-Calderon, J.; Martinez-Valencia, H.; Lopes-Cecenes, R.; Rosales-Cadena, I.; Sarmiento-Bustos, E.; Rocabruno-Valdés, C.I.; Gonzalez-Rodriguez, G.J. Corrosion Resistance of a Plasma-Oxidized Ti6Al4V Alloy for Dental Applications. Coatings 2021, 11, 1136. [Google Scholar] [CrossRef]
- Etaiw, S.E.; Fouda, A.; Amer, S.; El-bendary, M. Structure, Characterization and Anti-Corrosion Activity of the New Metal–Organic Framework [Ag(qox)(4-ab)]. J. Inorg. Organomet. Polym. Mater. 2011, 21, 327–335. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Gao, N.; Nwokolo, I.K.; Zhang, W.; Ma, L.; Liu, F.; Han, E.-H. Interface Characteristics and Anticorrosion Performances of Cold Galvanizing Coatings Incorporated with γ-chloropropyl Triethoxysilane Hot-Dip Galvanized Steel. Coatings 2021, 11, 402. [Google Scholar] [CrossRef]
- Rajeswari, V.; Devarayan, K.; Viswanathamurthi, P. Expired pharmaceutical compounds as potential inhibitors for cast iron corrosion in acidic medium. Res. Chem. Intermed. 2017, 43, 3893–3913. [Google Scholar] [CrossRef]
- Karimi, M.; Nabavi-Amri, S.A.; Soleymanpour, A. Study of Electrical Discharge Machining Parameters on Stainless Steel Using Copper Tool Electrode and Its Effect on the Structure and Electrochemical Properties. Acta Chim. Slov. 2021, 68, 279–288. [Google Scholar] [CrossRef]
- Song, L.; Zhou, Y.; Sheng, X.; Yao, Q.; Xi, X. MPEC-IMI as an effective green inhibitor to protect Q235 steel in 0.5 M HCl medium. Res. Chem. Intermed. 2018, 44, 5833–5855. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, W. Silk fibroin-Ti3C2TX hybrid nanofiller enhance corrosion protection for waterborne epoxy coatings under deep sea environment. Chem. Eng. J. 2021, 423, 130195. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corros. Sci. 2019, 146, 134–151. [Google Scholar] [CrossRef]
- Li, J.; Shi, H.; Liu, F.; Han, E. Self-healing epoxy coating based on tung oil-containing microcapsules for corrosion protection. Prog. Org. Coat. 2021, 156, 106236. [Google Scholar] [CrossRef]
- Hamed, E.; El-Rehim, S.S.A.; El-Shahat, M.F.; Shaltot, A.M. Corrosion inhibition of nickel in H2SO4 solution by alanine. Mater. Sci. Eng. B 2012, 177, 441–448. [Google Scholar] [CrossRef]
- Selvamani, R.; Singh, G.; Tiwari, V.S. Grain size effect on impedance and modulus properties of (Na0.5Bi0.5TiO3) (1−x) (BaZrO3) ceramics. Mater. Res. Express 2016, 3, 056301. [Google Scholar] [CrossRef]
- Soleymanibrojeni, M.; Shia, H.; Udoh, I.I.; Liu, F.; Han, E. Microcontainers with 3-amino-1,2,4-triazole-5-thiol for Enhancing Anticorrosion Waterborne Coatings for AA2024-T3. Prog. Org. Coat. 2019, 137, 105336. [Google Scholar] [CrossRef]
- Jafari, H.; Danaee, I.; Eskandari, H. Inhibitive Action of Novel Schiff Base Towards Corrosion of API 5L Carbon Steel in 1 M Hydrochloric Acid Solutions. Trans. Indian Inst. Met. 2015, 68, 729–739. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lgaz, H.; Lin, Y. Synthesis and investigation of pyran derivatives as acidizing corrosion inhibitors for N80 steel in hydrochloric acid: Theoretical and experimental approaches. J. Alloys Compd. 2018, 762, 347–362. [Google Scholar] [CrossRef]
- Madani, A.; Kaabi, I.; Sibous, L.; Bentouhami, E. Synthesis, characterization, and evaluation of the corrosion inhibition on mild steel of two new Schiff bases derived from 4,4’-diaminobiphenyl: Density functional theory investigation. J. Iran. Chem. Soc. 2021, 18, 3077–3095. [Google Scholar] [CrossRef]
- Madkour, L. Correlation between corrosion inhibitive effect and quantum molecular structure of Schiff bases for iron in acidic and alkaline media. Nature 2014, 2, 680–704. [Google Scholar]
- Olasunkanmi, L.O.; Moloto, B.P.; Obot, I.B.; Ebenso, E.E. Anticorrosion studies of some hydantoin derivatives for mild steel in 0.5M HCl solution: Experimental, quantum chemical, Monte Carlo simulations, and QSAR studies. J. Mol. Liq. 2018, 252, 62–74. [Google Scholar] [CrossRef]
- Chaouiki, A.; Lgaz, H.; Salghi, R.; Chafiq, M.; Gaonkar, S.L.; Bhat, K.S.; Subrahmanya, O.; Hassan, A.; Chung, I.-M.I.H. Inhibitory effect of a new isoniazid derivative as an effective inhibitor for mild steel corrosion in 1.0 M HCl: Combined experimental and computational study. Res. Chem. Intermed. 2020, 46, 2919–2950. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Alshabanat, M. Study the corrosion inhibition of carbon steel in 1 M HCl using extracts of date palm waste. Int. J. Electrochem. Sci. 2018, 13, 3777–3788. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Gupta, N.K. 2-(4-{[4-Methyl-6-(1-methyl-1H-1,3-benzodiazol-2-yl)-2-propyl-1H-1,3-benzodiazol-1-yl] methyl} phenyl) benzoic acid as green corrosion inhibitor for mild steel in 1 M hydrochloric acid. Ain Shams Eng. J. 2018, 9, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Rahiman, A.F.S.A.; Sethumanickam, S. Corrosion inhibition, adsorption and thermodynamic properties of poly (vinyl alcohol-cysteine) in molar HCl. Arab. J. Chem. 2017, 10, S3358–S3366. [Google Scholar] [CrossRef]
- Tan, B.; He, J.; Zhang, S.; Xu, C.; Chen, S.; Liu, H.; Li, W. Insight into anti-corrosion nature of Betel leaves water extracts as the novel and eco-friendly inhibitors. J. Colloid Interface Sci. 2021, 585, 287–301. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Li, N.; Xie, X. Inhibition effect of bamboo leaves extract on cold rolled steel in Cl3CCOOH solution. J. Mater. Res. Technol. 2017, 6, 158–170. [Google Scholar] [CrossRef] [Green Version]




| Fe | C | Si | Mn | P | S | |
|---|---|---|---|---|---|---|
| Q235 | Balance | 0.22 | 0.35 | 1.40 | 0.045 | 0.05 |
| Compound | ZnMOF-BTA |
|---|---|
| Moiety formula | 2(C24 H14 N9 O4 S Zn2.5) |
| Formula sum | C48 H28 N18 O8 S2 Zn5 |
| Formula weight | 1375.85 |
| Crystal description | Block/colorless |
| Crystal system | Monoclinic |
| Space group, Hall | C 1 2/c 1, -C 2yc |
| a (Å) | 35.2666(4) |
| b (Å) | 38.1974(2) |
| c (Å) | 26.9717(3) |
| α (°) | 90 |
| Β (°) | 127.718(2) |
| γ (°) | 90 |
| Volume (Å3) | 28740.8(8) |
| Radiation type | Cu Kα (λ = 1.54184) |
| T K | 150 |
| Z | 16 |
| Dx (g × cm−3) | 1.272 |
| μ mm−1 | 2.854 |
| F(000) | 11008.0 |
| θ range (°) | 3.9−75.4 |
| Crystal size mm | 0.15 × 0.13 × 0.12 |
| Tot. reflections | 59,616 |
| Uniq. Reflections, Rint | 28,131 |
| R1 indices (I > 2\(I)) | 0.0585 |
| wR2 indices (all data) | 0.1689 |
| Δρmin, Δρmax (e·Å−3) | −0.817, 1.337 |
| GOF | 1.038 |
| Bond | Distance (Å) | Bond | Distance (Å) | Bond | Bond Angles (Å) |
|---|---|---|---|---|---|
| Zn1−N2 i | 2.1996 (48) | Zn1−N11 i | 2.1552 (37) | N11−Zn1−N5 | 175.703 (144) |
| Zn1−N5 i | 2.1775 (38) | Zn1−N17 ii | 2.1904 (43) | N11−Zn1−N17 | 90.857 (151) |
| Zn2−N3 i | 2.0302 (42) | Zn2−O7 iv | 2.1142 (83) | N4−Zn2−N3 | 101.436 (194) |
| Zn2−N4 i | 2.0106 (56) | Zn2−O8 iv | 2.1464 (109) | N4−Zn2−O8 | 111.287 (270) |
| Zn3−N9 i | 2.0275 (51) | Zn3−O1 i | 2.5350 (30) | O2−Zn3−N13 | 128.942 (168) |
| Zn3−N12 i | 2.0063 (51) | Zn3−O2 i | 1.9401(30) | O2−Zn3−O1 | 75.783 (185) |
| Zn4−N1 iii | 2.0239 (49) | Zn4−O3 i | 1.9800 (38) | N16−Zn4−O4 | 93.501(182) |
| Zn4−N10 iii | 2.0420 (26) | Zn4−O4 i | 2.3117 (48) | O3−Zn4−O4 | 59.594 (166) |
| Zn5−N6 iii | 2.0533 (55) | Zn5−O5 i | 1.9427 (50) | N18−Zn6−N15 | 99.110 (181) |
| Zn5−N15 iii | 2.0402 (28) | Zn5−O6 i | 2.5700 (50) | O5−Zn5−O6 | 76.567 (191) |
| Zn6−N20 i | 2.1969 (48) | Zn6−N35 iii | 2.1905 (22) | ||
| Zn6−N26 i | 2.1905 (22) | Zn6−N32 iii | 2.1969 (49) | ||
| Zn7−N21 i | 2.0288 (35) | Zn7−O11 iv | 2.1141 (48) | ||
| Zn8−N25 i | 2.0177 (36) | Zn8−O9 i | 1.9713 (36) | ||
| Zn9−N19 i | 2.0207 (52) | Zn9−O13 i | 2.2496 (48) | ||
| Zn9−N27 i | 2.0515 (37) | Zn9−O14 i | 1.9666 (39) | ||
| Zn10−N30 ii | 2.0361 (31) | Zn10−O15 i | 2.4960 (30) | ||
| Zn10−N31 i | 2.0145 (40) | Zn10−O16 i | 1.9521(30) | ||
| C1−C2 i | 1.37 (1) | O1−C31 i | 1.233 (5) | ||
| C1−C6 i | 1.42 (1) | O3−C36 i | 1.244 (5) | ||
| N4−N5 i | 1.337 (7) | N3−C2 i | 1.36 (1) | ||
| N32−N33 i | 1.332 (7) | N24−C56 i | 1.367 (6) |
| Stage | Decomposition Steps | Temp Range (°C) | TG Weight Loss (%) | Decomposition Product | |
|---|---|---|---|---|---|
| Observed | Calculated | ||||
| 1st | [Zn5(BTA)6(TDC)2(H2O)n] | 95–118 | 0.37 | -nH2O | |
 | |||||
| [Zn5(BTA)6(TDC)2] | 119–220 | 25.21 | 25.40 | -6[C6H4N3] | |
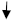 | |||||
| 2nd | [Zn5(TDC)2] | 230–400 | 50.43 | 50.80 | -4CO2 |
 | -2C4H4S | ||||
| 3rd | ZnO residue | 400–410 | 23.99 | 23.80 | 5ZnO |
| Sample | Weight % (g) | W (mg·cm−2·h−1) | I.Ew (%) | θ |
| Blank solution | - | 1.667 | - | - |
| ZnMOF-BTA | 1.0 | 0.703 | 57.83 | 0.5783 |
| 2.0 | 0.583 | 65.03 | 0.6503 | |
| 4.0 | 0.138 | 91.72 | 0.9172 | |
| 6.0 | 0.096 | 94.94 | 0.9494 |
| Sample | Weight % (g) | −Ecorr (mV/SCE) | βc (mV/dec) | βa (mV/dec) | icorr (μA/cm2) | I.Ep (%) | θ |
| Blank | - | 458.4 | 92.4 | 63.1 | 31.4 | - | - |
| ZnMOF-BTA | 1.0 | 438.5 | 87.4 | 40.4 | 18.2 | 42.0 | 0.420 |
| 2.0 | 444.0 | 65.1 | 33.9 | 13.8 | 56.1 | 0.561 | |
| 4.0 | 453.7 | 84.0 | 54.6 | 11.1 | 64.7 | 0.647 | |
| 6.0 | 425.9 | 50.2 | 26.0 | 09.1 | 71.0 | 0.710 |
| CPE | |||||||
|---|---|---|---|---|---|---|---|
| Inhibitor | Weight % (g) | Rs (Ω·cm2) | Rp (Ω·cm2) | YO (× 10−6 sn·Ω−1·cm−2) | n | Cdl (× 10−6 sn·Ω−1·cm−2) | I.EZ (%) |
| Blank | - | 0.53 | 11.71 ± 0.1 | 1239 | 0.87 | 658.22 | - |
| ZnMOF-BTA | 1.0 | 1.90 | 45.95 ± 0.3 | 904.0 | 0.88 | 586.13 | 73.3 |
| 2.0 | 2.31 | 56.98 ± 0.4 | 850.5 | 0.85 | 498.52 | 78.6 | |
| 4.0 | 4.22 | 147.6 ± 1.1 | 457.8 | 0.86 | 295.24 | 91.7 | |
| 6.0 | 7.25 | 276.3 ± 2.2 | 398.2 | 0.84 | 261.53 | 95.5 | |
| Inhibitor | Slope | Intercept | Kads(M−1) | R2 | ∆G°ads (KJ·mole−1) |
|---|---|---|---|---|---|
| ZnMOF-BTA | 0.87627 | 0.01027 | 1.141 | 0.97889 | −10.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwokolo, I.K.; Shi, H.; Ikeuba, A.I.; Gao, N.; Li, J.; Ahmed, S.; Liu, F. Synthesis, Characterization and Investigation of Anticorrosion Properties of an Innovative Metal–Organic Framework, ZnMOF-BTA, on Carbon Steel in HCl Solution. Coatings 2022, 12, 1288. https://doi.org/10.3390/coatings12091288
Nwokolo IK, Shi H, Ikeuba AI, Gao N, Li J, Ahmed S, Liu F. Synthesis, Characterization and Investigation of Anticorrosion Properties of an Innovative Metal–Organic Framework, ZnMOF-BTA, on Carbon Steel in HCl Solution. Coatings. 2022; 12(9):1288. https://doi.org/10.3390/coatings12091288
Chicago/Turabian StyleNwokolo, Izuchukwu K., Hongwei Shi, Alexander I. Ikeuba, Ningjie Gao, Jiwen Li, Sharjeel Ahmed, and Fuchun Liu. 2022. "Synthesis, Characterization and Investigation of Anticorrosion Properties of an Innovative Metal–Organic Framework, ZnMOF-BTA, on Carbon Steel in HCl Solution" Coatings 12, no. 9: 1288. https://doi.org/10.3390/coatings12091288
APA StyleNwokolo, I. K., Shi, H., Ikeuba, A. I., Gao, N., Li, J., Ahmed, S., & Liu, F. (2022). Synthesis, Characterization and Investigation of Anticorrosion Properties of an Innovative Metal–Organic Framework, ZnMOF-BTA, on Carbon Steel in HCl Solution. Coatings, 12(9), 1288. https://doi.org/10.3390/coatings12091288








