Realizing Efficient Photoelectrochemical Performance for Well-Designed CdS@ZnIn2S4 Heterostructure Photoanode with Directional Interfacial Charge Transfer Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photoanode Preparation
2.2.1. Preparation of CdS Nanorods
2.2.2. Preparation of CdS@ZIS
2.3. Characterization
2.3.1. Standard Characterization
2.3.2. Photoelectrochemical Characterization
3. Results and Discussion
3.1. Design and Construction of CdS@ZIS Heterostructure
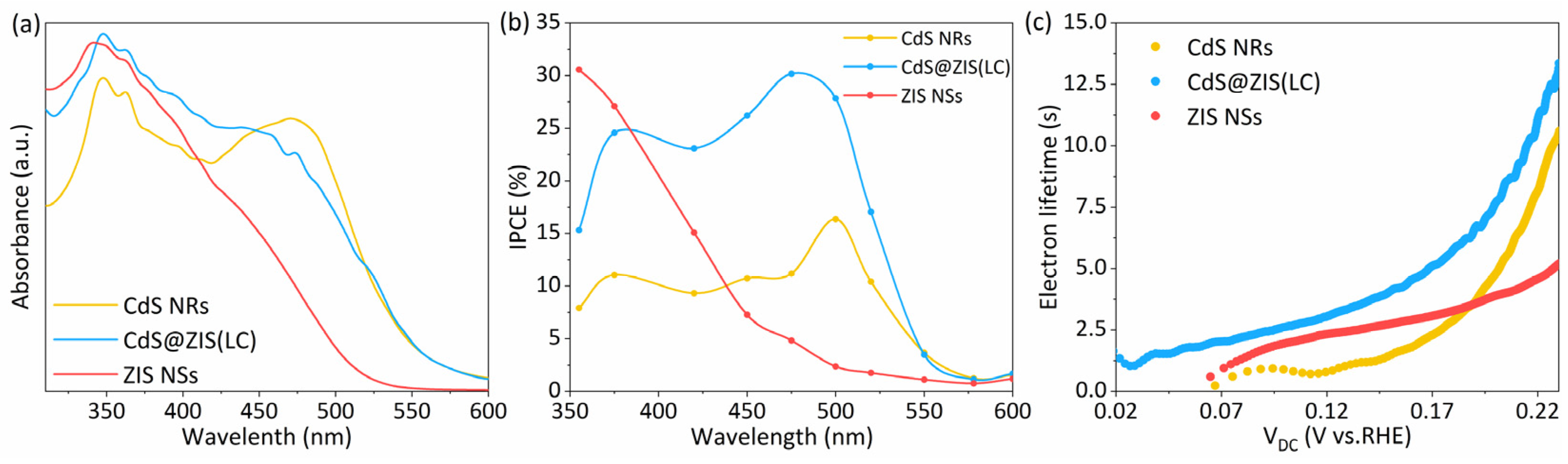
3.2. Microstructure and Composition Characterization
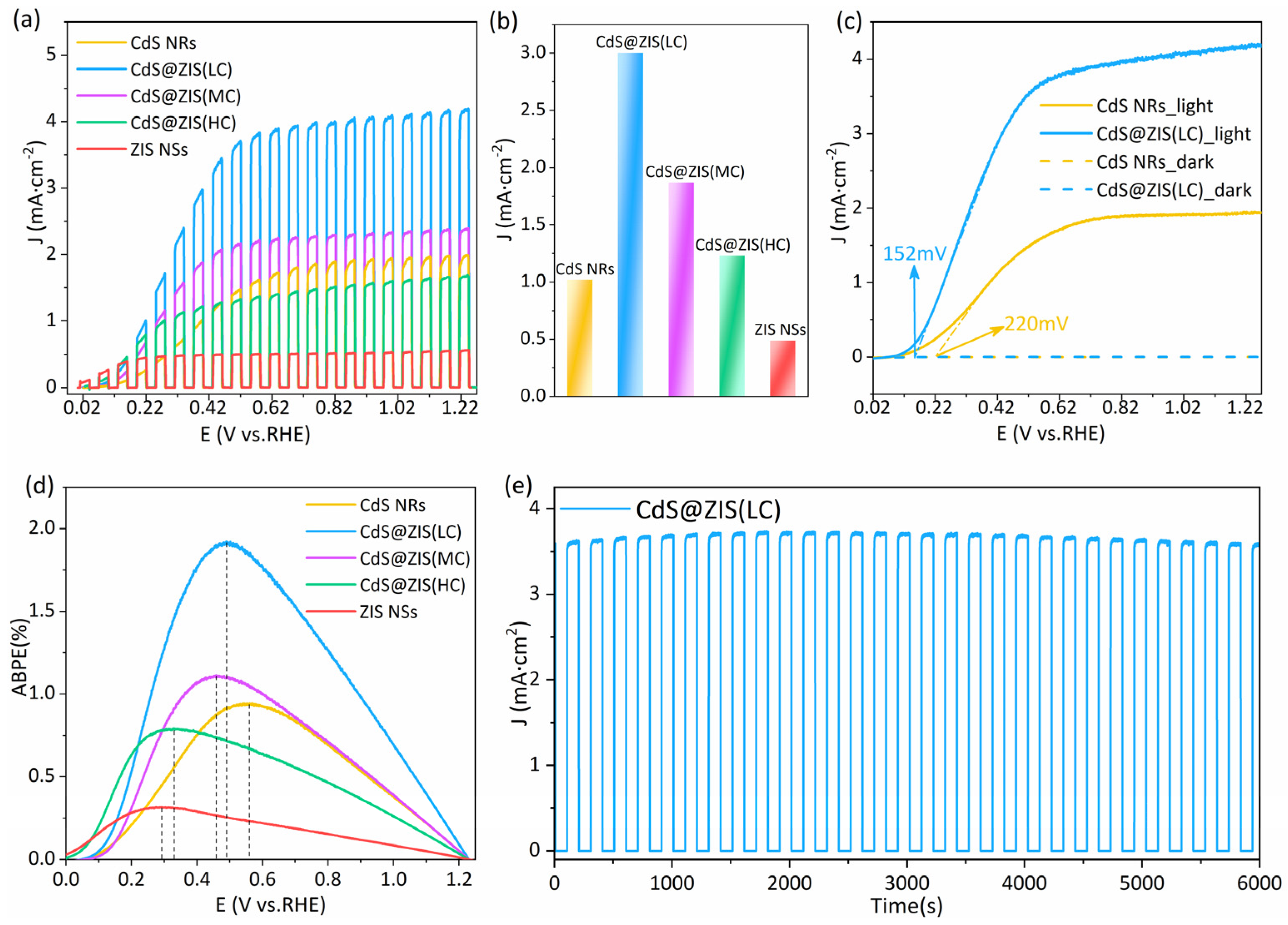
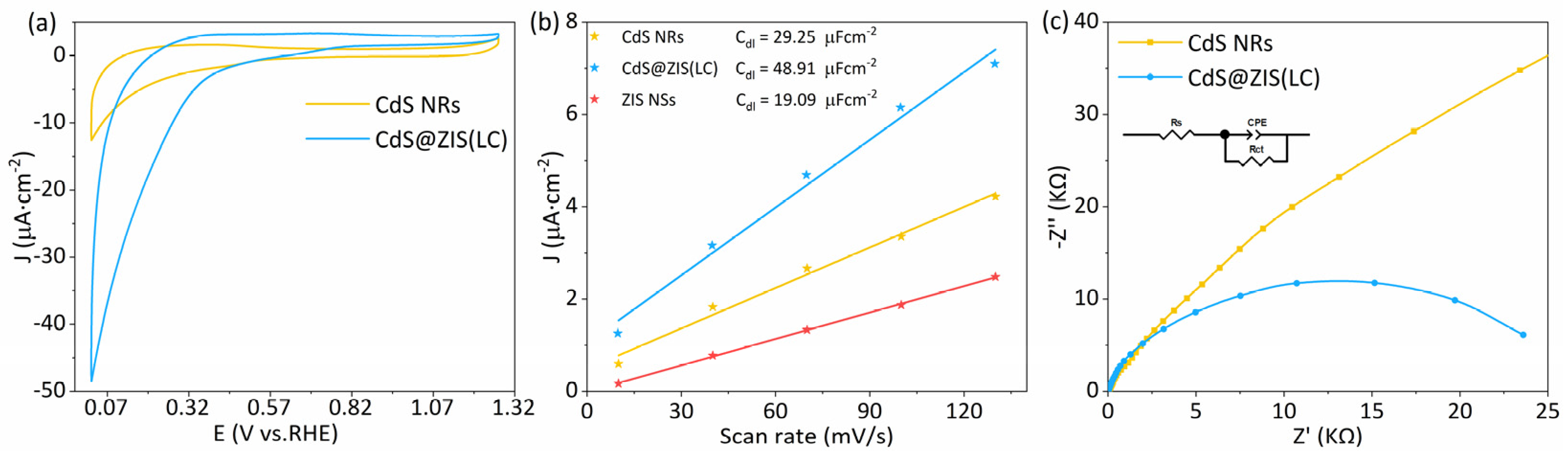
3.3. Photoelectrochemical Performance and Electrochemical Analysis
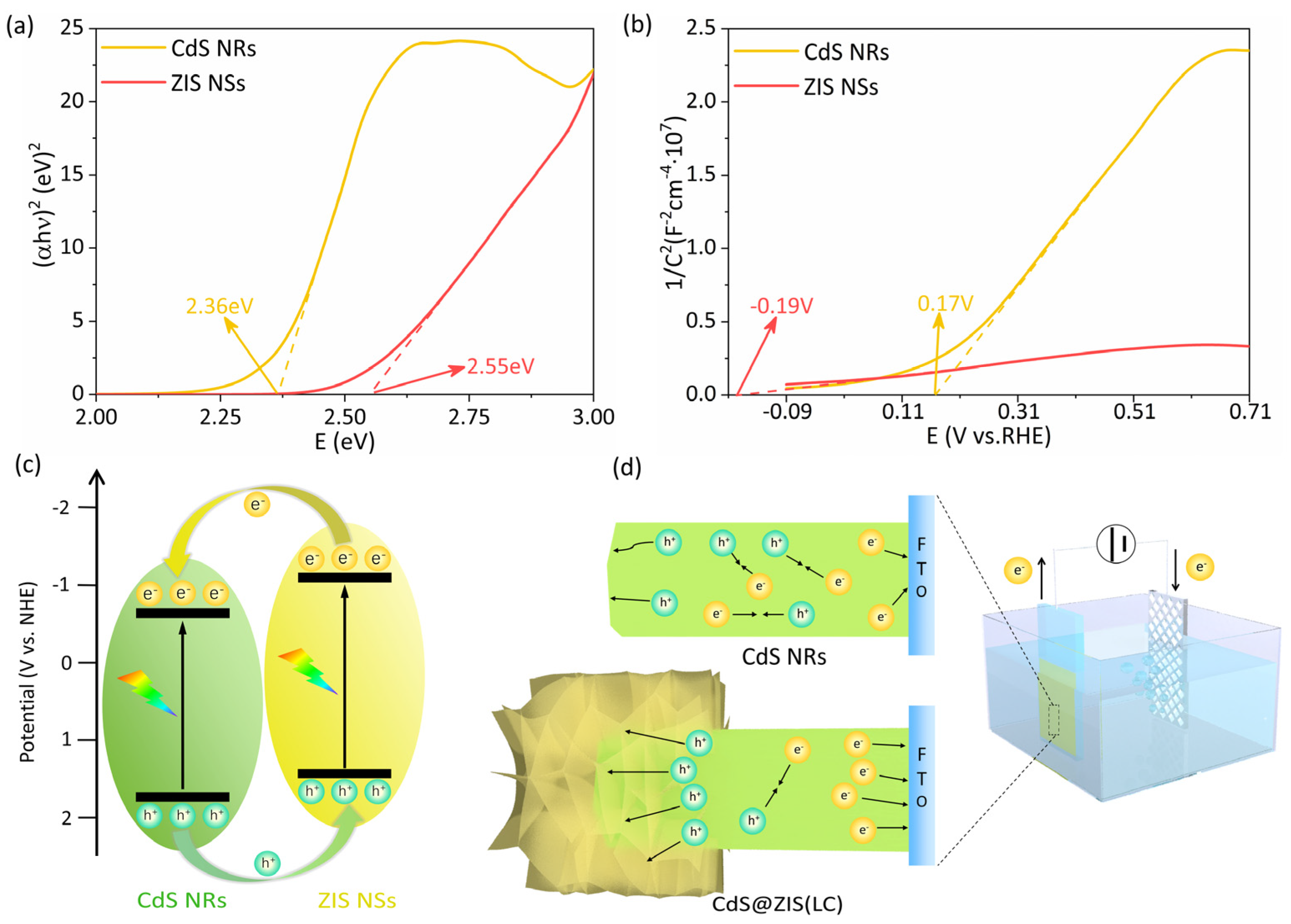
3.4. Highly Beneficial Photoinduced Charge Dynamics via Rational Microstructure Design
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braiek, Z.; Naceur, J.B.; Jrad, F.; Assaker, I.B.; Chtourou, R. Novel synthesis of graphene oxide/In2S3/TiO2 NRs heterojunction photoanode for enhanced photoelectrochemical (PEC) performance. Int. J. Hydrogen Energy 2022, 47, 3655–3666. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, M.; Li, J.; Guo, J.; Zhou, Q.; Zhao, X.; Wang, S.; Wang, L.; Wang, J.; Chen, Y. Atomic heterojunction-induced accelerated charge transfer for boosted photocatalytic hydrogen evolution over 1D CdS nanorod/2D ZnIn2S4 nanosheet composites. J. Colloid Interface Sci. 2021, 604, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Long, L.; Lv, G.; Han, Q.; Wu, X.; Qian, Y.; Wang, D.; Zhou, Y.; Zou, Z. Achieving Direct Z-Scheme Charge Transfer through Constructing 2D/2D α-Fe2O3/CdS Heterostructure for Efficient Photocatalytic CO2 Conversion. J. Phys. Chem. C 2021, 125, 23142–23152. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Huang, B.; Zhang, X.; Qin, X.; Li, H.; Dai, Y.; Li, Y. Anisotropic photoelectrochemical (PEC) performances of ZnO single-crystalline photoanode: Effect of internal electrostatic fields on the separation of photogenerated charge carriers during PEC water splitting. Chem. Mater. 2016, 28, 6613–6620. [Google Scholar] [CrossRef]
- Gill, R.; Ghosh, S.; Sharma, A.; Kumar, D.; Nguyen, V.-H.; Vo, D.-V.N.; Pham, T.-D.; Kumar, P. Vertically aligned ZnO nanorods for photoelectrochemical water splitting application. Mater. Lett. 2020, 277, 128295. [Google Scholar] [CrossRef]
- Long, L.; Wu, Q.; Chao, Q.; Dong, Y.; Wu, L.; Zhang, Q.; Zhou, Y.; Wang, D. Surface-state-mediated interfacial charge dynamics between carbon dots and ZnO toward highly promoting photocatalytic activity. J. Chem. Phys. 2020, 153, 044708. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Ai, G.; Li, H.; Liu, S.; Mo, R.; Zhong, J. Solar water splitting by TiO2/CdS/Co–Pi nanowire array photoanode enhanced with Co–Pi as hole transfer relay and CdS as light absorber. Adv. Funct. Mater. 2015, 25, 5706–5713. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Wang, Z.; Zheng, Z.; Wang, P.; Liu, Y.; Zhang, X.; Qin, X.; Dai, Y.; Huang, B. Fabrication of BiVO4 photoanode consisted of mesoporous nanoparticles with improved bulk charge separation efficiency. Appl. Catal. B Environ. 2018, 238, 586–591. [Google Scholar] [CrossRef]
- Bai, S.; Han, J.; Zhao, Y.; Chu, H.; Wei, S.; Sun, J.; Sun, L.; Luo, R.; Li, D.; Chen, A. rGO decorated BiVO4/Cu2O nn heterojunction photoanode for photoelectrochemical water splitting. Renew. Energy 2020, 148, 380–387. [Google Scholar] [CrossRef]
- Phuan, Y.W.; Ong, W.-J.; Chong, M.N.; Ocon, J.D. Prospects of electrochemically synthesized hematite photoanodes for photoelectrochemical water splitting: A review. J. Photochem. Photobiol. C 2017, 33, 54–82. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, H.; Kumbhar, V.S.; Choi, Y.-W.; Kim, J.; Lee, K. Enhancement of photoelectrochemical properties with α–Fe2O3 on surface modified FTO substrates. Ceram. Int. 2020, 46, 20012–20019. [Google Scholar] [CrossRef]
- Meng, L.; Rao, D.; Tian, W.; Cao, F.; Yan, X.; Li, L. Simultaneous manipulation of O-doping and metal vacancy in atomically thin Zn10In16S34 nanosheet arrays toward improved photoelectrochemical performance. Angew. Chem. Int. Ed. 2018, 57, 16882–16887. [Google Scholar] [CrossRef]
- He, Y.; Rao, H.; Song, K.; Li, J.; Yu, Y.; Lou, Y.; Li, C.; Han, Y.; Shi, Z.; Feng, S. 3D hierarchical ZnIn2S4 nanosheets with rich Zn vacancies boosting photocatalytic CO2 reduction. Adv. Funct. Mater. 2019, 29, 1905153. [Google Scholar] [CrossRef]
- Sun, J.; Sun, L.; Yang, X.; Bai, S.; Luo, R.; Li, D.; Chen, A. Photoanode of coupling semiconductor heterojunction and catalyst for solar PEC water splitting. Electrochim. Acta 2020, 331, 135282. [Google Scholar] [CrossRef]
- Yin, T.; Long, L.; Tang, X.; Qiu, M.; Liang, W.; Cao, R.; Zhang, Q.; Wang, D.; Zhang, H. Advancing Applications of Black Phosphorus and BP-Analog Materials in Photo/Electrocatalysis through Structure Engineering and Surface Modulation. Adv. Sci. 2020, 7, 2001431. [Google Scholar] [CrossRef]
- Ho, T.A.; Bae, C.; Joe, J.; Yang, H.; Kim, S.; Park, J.H.; Shin, H. Heterojunction photoanode of atomic-layer-deposited MoS2 on single-crystalline CdS nanorod arrays. ACS Appl. Mater. Inter. 2019, 11, 37586–37594. [Google Scholar] [CrossRef]
- Xu, H.; Fan, W.; Zhao, Y.; Chen, B.; Gao, Y.; Chen, X.; Xu, D.; Shi, W. Amorphous iron (III)-borate decorated electrochemically treated-BiVO4 photoanode for efficient photoelectrochemical water splitting. Chem. Eng. J. 2021, 411, 128480. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Guo, Z.; Miao, L.; Han, S.-T.; Wang, Z.; Zhang, X.; Zhang, H.; Peng, Z. Recent advances in black phosphorus-based photonics, electronics, sensors and energy devices. Mater. Horiz. 2017, 4, 997–1019. [Google Scholar] [CrossRef]
- Fu, Y.; Cao, F.; Wu, F.; Diao, Z.; Chen, J.; Shen, S.; Li, L. Phase-Modulated Band Alignment in CdS Nanorod/SnSx Nanosheet Hierarchical Heterojunctions toward Efficient Water Splitting. Adv. Funct. Mater. 2018, 28, 1706785. [Google Scholar] [CrossRef]
- Zhou, G.; Long, L.; Wang, P.; Hu, Y.; Zhang, Q.; Liu, C. Designing CuO/ZnO nanoforest device toward optimal photocatalytic performance through structure and facet engineering. Mater. Lett. 2020, 273, 127907. [Google Scholar] [CrossRef]
- Yang, C.; Liu, S.; Li, M.; Wang, X.; Zhu, J.; Chong, R.; Yang, D.; Zhang, W.-H.; Li, C. The role of glutathione on shape control and photoelectrical property of cadmium sulfide nanorod arrays. J. Colloid Interface Sci. 2013, 393, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Xiong, S.; Meng, M.; Liu, L.; Zhang, J.; Wu, X.; Chu, P.K. Enhancement of Ferromagnetism in Nonmagnetic Metal Oxide Nanoparticles by Facet Engineering. Small 2017, 13, 1602951. [Google Scholar] [CrossRef]
- Wang, B.; Peng, F.; Yang, S.; Cao, Y.; Wang, H.; Yu, H.; Zhang, S. Hydrogenated CdS nanorods arrays/FTO film: A highly stable photocatalyst for photocatalytic H2 production. Int. J. Hydrogen Energy 2018, 43, 17696–17707. [Google Scholar] [CrossRef]
- He, Z.; Zhang, J.; Li, X.; Guan, S.; Dai, M.; Wang, S. 1D/2D Heterostructured photocatalysts: From design and unique properties to their environmental applications. Small 2020, 16, 2005051. [Google Scholar] [CrossRef]
- Long, L.; Niu, X.; Yan, K.; Zhou, G.; Wang, J.; Wu, X.; Chu, P.K. Highly fluorescent and stable black phosphorus quantum dots in water. Small 2018, 14, 1803132. [Google Scholar] [CrossRef]
- David, S.; Mahadik, M.A.; An, G.W.; Ryu, J.; Kim, H.G.; Jang, J.S. Effect of directional light dependence on enhanced photoelectrochemical performance of ZnIn2S4/TiO2 binary heterostructure photoelectrodes. Electrochim. Acta 2018, 276, 223–232. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Wang, X.; Yang, P.; Zheng, L.; Zhao, Z.; Zheng, H. Facile construction TiO2/ZnIn2S4/Zn0. 4Ca0. 6In2S4 ternary hetero-structure photo-anode with enhanced photo-electrochemical water-splitting performance. Surf. Interfaces 2021, 26, 101323. [Google Scholar] [CrossRef]
- Chot, C.Y.; Chong, M.N.; Soh, A.K.; Tan, K.W.; Ocon, J.D.; Saint, C. Facile synthesis and characterisation of functional MoO3 photoanode with self-photorechargeability. J. Alloys Compd. 2020, 838, 155624. [Google Scholar] [CrossRef]
- Sun, Q.; Qi, L. Triple-layer ITO/BiVO4/Fe2TiO5 heterojunction photoanode coated with iron silicate for highly efficient solar water splitting. Chem. Eng. J. 2021, 426, 131290. [Google Scholar]
- She, H.; Yue, P.; Ma, X.; Huang, J.; Wang, L.; Wang, Q. Fabrication of BiVO4 photoanode cocatalyzed with NiCo-layered double hydroxide for enhanced photoactivity of water oxidation. Appl. Catal. B Environ. 2020, 263, 118280. [Google Scholar] [CrossRef]
- Shakir, I.; Shahid, M.; Cherevko, S.; Chung, C.-H.; Kang, D.J. Ultrahigh-energy and stable supercapacitors based on intertwined porous MoO3–MWCNT nanocomposites. Electrochim. Acta 2011, 58, 76–80. [Google Scholar] [CrossRef]
- Paudel, D.R.; Pan, U.N.; Singh, T.I.; Gudal, C.C.; Kim, N.H.; Lee, J.H. Fe and P doped 1T-phase enriched WS23D-dendritic nanostructures for efficient overall water splitting. Appl. Catal. B Environ. 2021, 286, 119897. [Google Scholar] [CrossRef]


| Name | Formula | Formula Weight | Purity |
|---|---|---|---|
| Cadmium nitrate tetrahydrate | CdN2O6·4H2O | 308.47 | AR, 99% |
| Thiourea | CH4N2S | 76.12 | AR, 99% |
| Reduced L-glutathione | C10H17N3O6S | 307.32 | AR, 98% |
| Zinc chloride | ZnCl2 | 136.3 | AR, 98% |
| Indium (III) chloride tetrahydrate | InCl3·4H2O | 293.24 | AR, ≥99% |
| Sodium sulfate anhydrous | Na2SO4 | 142.04 | AR, 99% |
| Sodium sulfite anhydrous | Na2SO3 | 126.04 | AR, 98% |
| Ethanol | C2H6O | 46.07 | AR, ≥99.7% |
| Acetone | C3H6O | 58.08 | AR, ≥99.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Qian, Y.; Lv, G.; Long, L.; Zhou, Y.; Wang, D. Realizing Efficient Photoelectrochemical Performance for Well-Designed CdS@ZnIn2S4 Heterostructure Photoanode with Directional Interfacial Charge Transfer Dynamics. Coatings 2022, 12, 1210. https://doi.org/10.3390/coatings12081210
Wu X, Qian Y, Lv G, Long L, Zhou Y, Wang D. Realizing Efficient Photoelectrochemical Performance for Well-Designed CdS@ZnIn2S4 Heterostructure Photoanode with Directional Interfacial Charge Transfer Dynamics. Coatings. 2022; 12(8):1210. https://doi.org/10.3390/coatings12081210
Chicago/Turabian StyleWu, Xianchen, Yu Qian, Gangyang Lv, Liyuan Long, Yong Zhou, and Dunhui Wang. 2022. "Realizing Efficient Photoelectrochemical Performance for Well-Designed CdS@ZnIn2S4 Heterostructure Photoanode with Directional Interfacial Charge Transfer Dynamics" Coatings 12, no. 8: 1210. https://doi.org/10.3390/coatings12081210
APA StyleWu, X., Qian, Y., Lv, G., Long, L., Zhou, Y., & Wang, D. (2022). Realizing Efficient Photoelectrochemical Performance for Well-Designed CdS@ZnIn2S4 Heterostructure Photoanode with Directional Interfacial Charge Transfer Dynamics. Coatings, 12(8), 1210. https://doi.org/10.3390/coatings12081210






