Changes in the Oral Cavity Mucosal Surface under the Influence of Wearing Protective Face Masks—Nitric Oxide Concentration Analysis—Preliminary Report
Abstract
1. Introduction
2. Aim of the Study
3. Materials and Methods
3.1. Clinical Materials and Methods
3.2. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Lu, H.; Stratton, C.W.; Tang, Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Liu, W.; Tao, Z.-W.; Wang, L.; Yuan, M.-L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.-G.; et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020, 133, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.-l.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Calmes, D.; Graff, S.; Maes, N.; Frix, A.-N.; Thys, M.; Bonhomme, O.; Berg, J.; Debruche, M.; Gester, F.; Henket, M.; et al. Asthma and COPD Are Not Risk Factors for ICU Stay and Death in Case of SARS-CoV2 Infection. J. Allergy Clin. Immunol. Pract. 2021, 9, 160–169. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, G.; Tang, Y.; Peng, Z.; Pan, H. The coronavirus diseases 2019 (COVID-19) pneumonia with spontaneous pneumothorax: A case report. BMC Infect. Dis. 2020, 20, 662. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, C.; Wu, J.; Chen, M.; Wang, Z.; Luo, L.; Zhou, X.; Liu, X.; Huang, X.; Yuan, S.; et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef] [PubMed]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur. Respir. J. 2021, 57, 2003448. [Google Scholar] [CrossRef] [PubMed]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.-M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.-H.; et al. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2014564118. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.; Marinosci, A.; Agoritsas, T.; Calmy, A. Prophylaxis for COVID-19: A systematic review. Clin. Microbiol. Infect. 2021, 27, 532–537. [Google Scholar] [CrossRef]
- Hafeez, A.; Ahmad, S.; Siddqui, S.; Ahmad, M.; Mishra, S. A Review of COVID-19 (Coronavirus Disease-2019) Diagnosis, Treatments and Prevention. EJMO 2020, 4, 116–125. [Google Scholar]
- Otrisal, P.; Bungau, C.; Obsel, V.; Melicharik, Z.; Tont, G. Selected Respiratory Protective Devices: Respirators and Significance of Some Markings. Sustainability 2021, 13, 4988. [Google Scholar] [CrossRef]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pedi. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention, Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 21 July 2022).
- Bourouiba, L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA 2020, 323, 1837–1838. [Google Scholar] [CrossRef]
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.-L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.E.; Koh, D.; Fones, C.; Qian, F.; Ng, V.; Tan, B.H.; Wong, K.S.; Chew, W.M.; Tang, H.K.; Ng, W.; et al. Appropriate use of personal protective equipment among healthcare workers in public sector hospitals and primary healthcare polyclinics during the SARS outbreak in Singapore. Occup. Environ. Med. 2005, 62, 473. [Google Scholar] [CrossRef]
- Sokołowska, M.; Włodek, L. Dobre i złe strony tlenku azotu. Folia Cardiol. 2001, 8, 467–474. [Google Scholar]
- Available online: https://ksiegarnia.pwn.pl/Druga-twarz-tlenu,68720454,p.html (accessed on 21 July 2022).
- Beckman, J. Biochemistry of Nitric Oxide and Peroxynitrite. Kubes P wyd. Nitric Oxide: A modulator of Cell–Cell Interactions in the Microcirculation; Landes Company: Austin, TX, USA, 1995; pp. 1–18. [Google Scholar]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M. Nitric oxide metabolism and breakdown. Biochem. Biophys. Acta 1999, 1411, 273–289. [Google Scholar] [CrossRef]
- Marsh, N.; Marsh, A. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin. Experimen. Pharmacol. Physiol. 2000, 27, 313–319. [Google Scholar] [CrossRef]
- Kasperski, J.; Wyszyńska, M.; Kustra, S.; Czecior, E.; Misiołek, M.; Kasperska-Zając, A. Letter to the Editor: Does Helicobacter Pylori infection increase the levels of exhaled nitric oxide? Eur. J. Inflamm. 2013, 11, 279–282. [Google Scholar] [CrossRef]
- Kumor, M.; Przybyłowski, T.; Maskey-Warzęchowska, M.; Hildebrand, K.; Fangrat, A.; Bielecki, P.; Górska, K.; Kościuch, J.; Kucińska, J.; Chazan, R. Powtarzalność pomiaru stężenia tlenku azotu w powietrzu wydechowym (FENO) przeprowadzonego z wykorzystaniem zestawu NIOX u zdrowych osób. Pneumonol. Alergol. 2004, 72, 283–289. [Google Scholar] [CrossRef]
- Korn, S.; Wilk, M.; Voigt, S.; Weber, S.; Keller, T.; Buhl, R. Measurement of Fractional Exhaled Nitric Oxide: Comparison of Three Different Analysers. Respiration 2020, 99, 1–8. [Google Scholar] [CrossRef]
- Schiller, B.; Hammer, J.; Barben, J.; Trachsel, D. Comparability of a hand-held nitric oxide analyser with online and offline chemiluminescence-based nitric oxide measurement. Pediatr. Allergy Immunol. 2009, 20, 679–685. [Google Scholar] [CrossRef]
- Amaral, R.; Jácome, C.; Almeida, R.; SáSousa, A.; Pinho, B.; Guedes, R.; Jacinto, T.; Fonseca, J. Reproducibility of the Vivatmo pro measurements for exhaled nitric oxide values. Eur. Respir. J. 2019, 54, PA3909. [Google Scholar] [CrossRef]
- Matuschek, C.; Moll, F.; Fangerau, H.; Fischer, J.C.; Zänker, K.; van Griensven, M.; Schneider, M.; Kindgen-Milles, D.; Knoefel, W.T.; Lichtenberg, A.; et al. Face masks: Benefits and risks during the COVID-19 crisis. Eur. J. Med. Res. 2020, 25, 32. [Google Scholar] [CrossRef]
- Rosner, E. Adverse Effects of Prolonged Mask Use among Healthcare Professionals during COVID-19. Rosner. J. Infect. Dis. Epidemiol. 2020, 6, 130. [Google Scholar] [CrossRef]
- Foo, C.C.I.; Goon, A.T.J.; Leow, Y.-H.; Goh, C.-L. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—A descriptive study in Singapore. Contact Derm. 2006, 55, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Fan, J.; Li, X.; Gou, X.; Li, X.; Zhou, X. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine 2020, 99, 24. [Google Scholar] [CrossRef] [PubMed]
- Makowiec-Dąbrowska, T.; Gadzicka, E.; Bortkiewicz, A. Physiological Cost Of Wearing Protective Masks—A Narrative Review Of The Literature. Med. Pracy 2021, 72, 569–589. [Google Scholar] [CrossRef]
- Ong, J.; Bharatendu, C.; Goh, Y.; Tang, J.; Sooi, K.; Tan, Y.L.; Tan, B.; Teoh, H.L.; Ong, S.T.; Allen, D.M.; et al. Headaches Associated With Personal Protective Equipment—A Cross-Sectional Study Among Frontline Healthcare Workers During COVID-19. Headache 2020, 60, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tokura, H.; Guo, Y.P.; Wong, A.S.W.; Wong, T.; Chung, J.; Newton, E. Effects of wearing N95 and surgical facemasks on heart rate, thermal stress and subjective sensations. Int. Arch. Occup. Environ. Health 2005, 78, 501–509. [Google Scholar] [CrossRef]
- Kanzow, P.; Dylla, V.; Malina Mahler, A.; Hrasky, V.; Rödig, T.; Barre, F.; Scheithauer, S.; Wiegand, A. COVID-19 Pandemic: Effect of Different Face Masks on Self-Perceived Dry Mouth and Halitosis. Int. J. Environ. Res. Public Health 2021, 18, 9180. [Google Scholar] [CrossRef]
- Kisielinski, K.; Giboni, P.; Prescher, A.; Klosterhalfen, B.; Graessel, D.; Funken, S.; Kempski, O.; Hirsch, O. Is a mask that covers the mouth and nose free from undesirable side effects in everyday use and free of potential hazards? Int. J. Environ. Res. Public Health 2021, 18, 4344. [Google Scholar] [CrossRef]
- Johnson, A.T.; Scott, W.H.; Lausted, C.G.; Coyne, K.M.; Sahota, M.S.; Johnson, M.M. Effect of External Dead Volume on Performance While Wearing a Respirator. AIHAJ-Am. Ind. Hyg. Assoc. 2000, 61, 678–684. [Google Scholar] [CrossRef]
- Fikenzer, S.; Uhe, T.; Lavall, D.; Rudolph, U.; Falz, R.; Busse, M.; Hepp, P.; Laufs, U. Effects of Surgical and FFP2/N95 Face Masks on Cardiopulmonary Exercise Capacity. Clin. Res. Cardiol. 2020, 109, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Wang, D.Y. Objective Assessment of Increase in Breathing Resistance of N95 Respirators on Human Subjects. Ann. Occup. Hyg. 2011, 55, 917–921. [Google Scholar] [PubMed][Green Version]
- Roberge, R.; Bayer, E.; Powell, J.; Coca, A.; Roberge, M.; Benson, S. Effect of Exhaled Moisture on Breathing Resistance of N95 Filtering Facepiece Respirators. Ann. Occup. Hyg. 2010, 54, 671–677. [Google Scholar]
- Achanta, S.; Sasidharan, S.; Majji, D.; Uppala, D. “Mask Mouth” During COVID-19 Pandemic—A Myth or A Truth. Int. J. Med. Dent. Res. 2021, 1, 56–63. [Google Scholar]
- Muley, P. ‘Mask Mouth’—A Novel Threat to Oral Health in the COVID Era–Dr Pooja Muley. Dent. Trib. South Asia 2020. Available online: https://in.dental-tribune.com/news/mask-mouth-a-novel-threat-to-oral-health-in-the-covid-era/ (accessed on 12 November 2020).
- Liu, C.; Li, G.; He, Y.; Zhang, Z.; Ding, Y. Effects of Wearing Masks on Human Health and Comfort during the COVID-19 Pandemic. IOP Conf. Ser. Earth Environ. Sci. 2020, 531, 012034. [Google Scholar]
- Jamjoom, A.; Nikkar-Esfahani, A.; Fitzgerald, J. Operating Theatre Related Syncope in Medical Students: A Cross Sectional Study. BMC Med. Educ. 2009, 9, 14. [Google Scholar] [CrossRef]
- Liu, Z.; Chang, Y.; Chu, W.; Yan, M.; Mao, Y.; Zhu, Z.; Wu, H.; Zhao, J.; Dai, K.; Li, H.; et al. Surgical Masks as Source of Bacterial Contamination during Operative Procedures. J. Orthop. Transl. 2018, 14, 57–62. [Google Scholar]
- Klimek, L.; Huppertz, T.; Alali, A.; Spielhaupter, M.; Hörmann, K.; Matthias, C.; Hagemann, J. A New Form of Irritant Rhinitis to Filtering Facepiece Particle (FFP) Masks (FFP2/N95/KN95 Respirators) during COVID-19 Pandemic. World Allergy Organ. J. 2020, 13, 100474. [Google Scholar] [CrossRef]
- Reher, V.G.S.; Zenobio, E.G.; Costa, F.O.; Reher, P.; Soares, R.V. Nitric oxide levels in saliva increase with severity of chronic periodontitis. J. Oral Sci. 2007, 49, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.C.; Silva, T.A.; Chun, J.H.; Lara, V.S. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis. 2002, 8, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, M.; Iwase, M.; Nagumo, M. Elevated production of salivary nitric oxide in oral mucosal diseases. J. Oral Pathol. Med. 1999, 28, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.; Erbas, D.; Karabulut, A.A.; Ozturk, G.; Eksioglu, M. Behcet’s disease and nitric oxide production. Int. J. Dermatol. 2003, 42, 244–248. [Google Scholar] [CrossRef]
- Wyszyńska, M.; Czelakowska, A.; Rafał, R.; Zajac, M.; Mielnik, M.; Kasperski, J.; Skucha-Nowak, M. Measurement of the Level of Nitric Oxide in Exhaled Air in Patients Using Acrylic Complete Dentures and with Oral Pathologies. Coatings 2021, 11, 169. [Google Scholar] [CrossRef]
- Wyszyńska, M.; Rosak, P.; Czelakowska, A.; Białożyt-Bujak, E.; Kasperski, J.; Łopaciński, M.; Al Khatib, N.; Małgorzata Skucha-Nowak, M. Pilot Study of Use of Nitric Oxide in Monitoring Multiple Dental Foci in Oral Cavity—A Case Report. Healthcare 2022, 10, 195. [Google Scholar] [CrossRef]
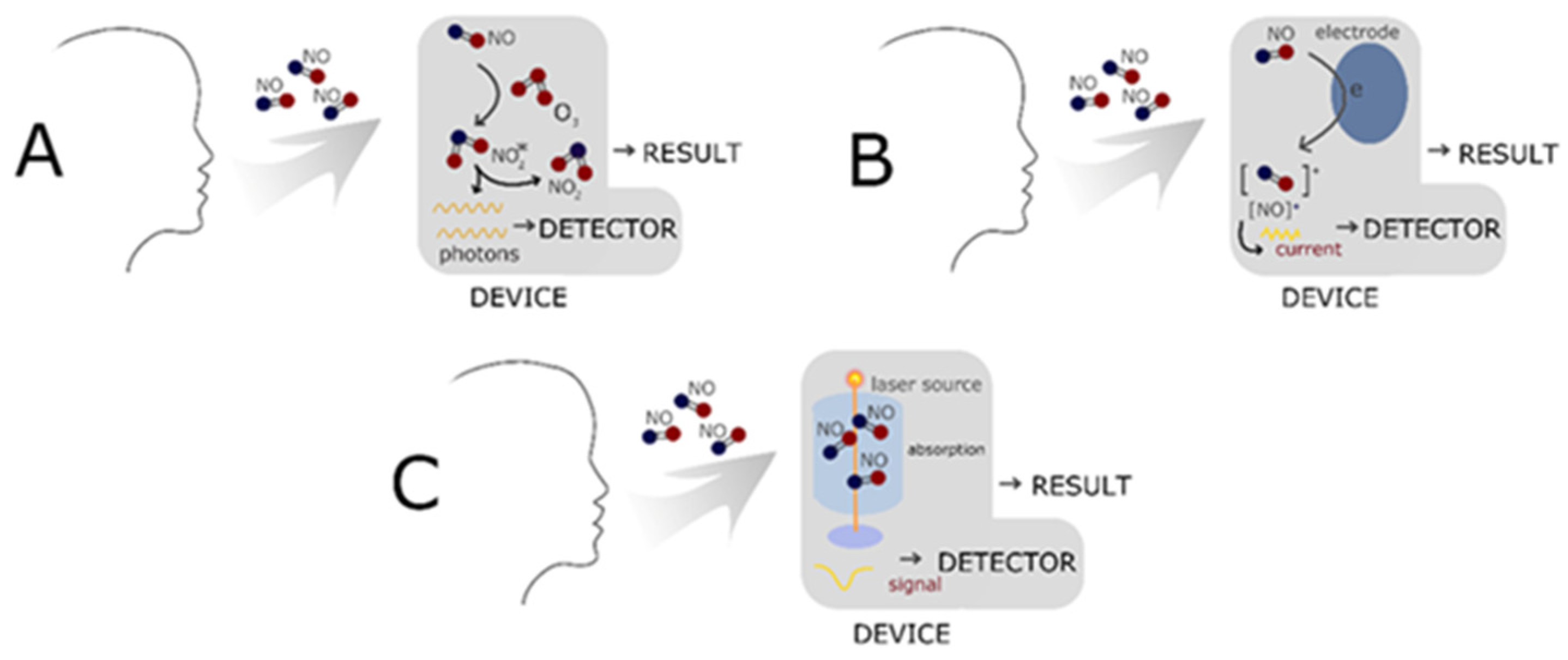
- Wyszyńska, M.; Nitsze-Wierzba, M.; Czelakowska, A.; Kasperski, J.; Żywiec, J.; Skucha-Nowak, M. An Evidence-Based Review of Application Devices for Nitric Oxide Concentration Determination from Exhaled Air in the Diagnosis of Inflammation and Treatment Monitoring. Molecules 2022, 27, 4279. [Google Scholar] [CrossRef]
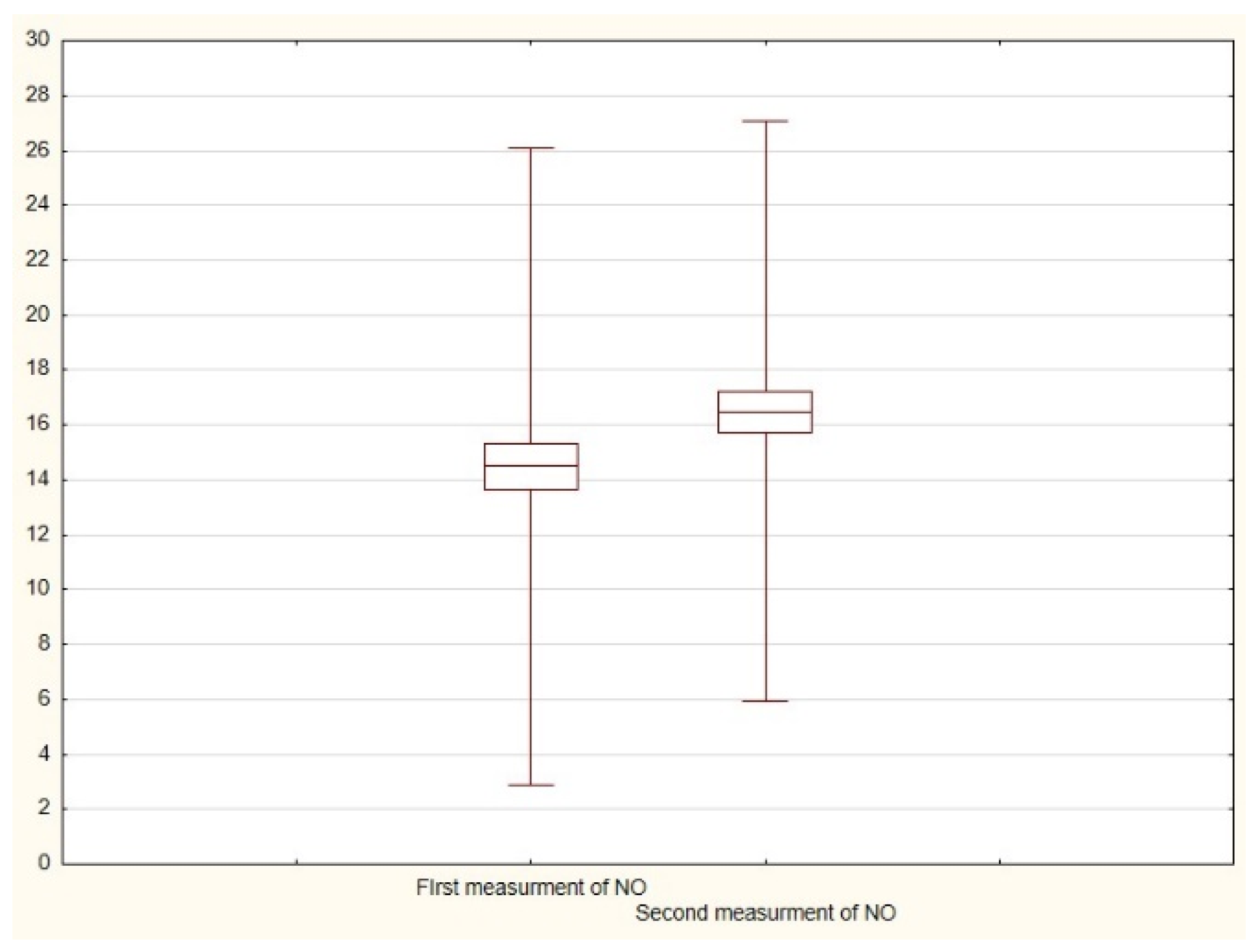
| N | Arithmetic Average | Median | Minimum | Maximum | Standard Deviation | |
|---|---|---|---|---|---|---|
| First measurement of exhaled NO * | 49 | 14.5 | 14.0 | 7.0 | 38.0 | 5.8 |
| Second measurement of exhaled NO ** | 49 | 16.5 | 16.0 | 8.0 | 41.0 | 5.3 |
| N | p-Value | |
|---|---|---|
| Difference between first and second measurements of exhaled NO | 42 | 0.000422 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszyńska, M.; Czelakowska, A.; Rosak, P.; Białożyt-Bujak, E.; Gruca, O.; Rosak-Szyrocka, J.; Kasperski, J.; Skucha-Nowak, M. Changes in the Oral Cavity Mucosal Surface under the Influence of Wearing Protective Face Masks—Nitric Oxide Concentration Analysis—Preliminary Report. Coatings 2022, 12, 1164. https://doi.org/10.3390/coatings12081164
Wyszyńska M, Czelakowska A, Rosak P, Białożyt-Bujak E, Gruca O, Rosak-Szyrocka J, Kasperski J, Skucha-Nowak M. Changes in the Oral Cavity Mucosal Surface under the Influence of Wearing Protective Face Masks—Nitric Oxide Concentration Analysis—Preliminary Report. Coatings. 2022; 12(8):1164. https://doi.org/10.3390/coatings12081164
Chicago/Turabian StyleWyszyńska, Magdalena, Aleksandra Czelakowska, Przemysław Rosak, Ewa Białożyt-Bujak, Olaf Gruca, Joanna Rosak-Szyrocka, Jacek Kasperski, and Małgorzata Skucha-Nowak. 2022. "Changes in the Oral Cavity Mucosal Surface under the Influence of Wearing Protective Face Masks—Nitric Oxide Concentration Analysis—Preliminary Report" Coatings 12, no. 8: 1164. https://doi.org/10.3390/coatings12081164
APA StyleWyszyńska, M., Czelakowska, A., Rosak, P., Białożyt-Bujak, E., Gruca, O., Rosak-Szyrocka, J., Kasperski, J., & Skucha-Nowak, M. (2022). Changes in the Oral Cavity Mucosal Surface under the Influence of Wearing Protective Face Masks—Nitric Oxide Concentration Analysis—Preliminary Report. Coatings, 12(8), 1164. https://doi.org/10.3390/coatings12081164








