Multi-Elemental Coatings on Zirconium Alloy for Corrosion Resistance Improvement
Abstract
1. Introduction
- (i)
- For the cladding: steam oxidation, ballooning and burst, and internal oxidation;
- (ii)
2. Materials and Methods
2.1. Investigated Materials
2.2. Coating Method
2.3. Materials Characterization
- -
- The energy dispersive spectroscopy (EDS) method, using the Quantax 400 (Bruker, Billerica, MA, USA) system. The Quantax 400 library (database) was built for a high voltage (HV) of 15 kV. The system was calibrated for Cu reference, with a registered standard supplied by Micro-Analysis Consultant Ltd. (MAC, St. Ives, UK). The samples were subjected to an electron beam with HV 15 kV acceleration. The identification of elements was performed by an analysis of the energies of the X-ray emitted by excitation of specimen atoms with a focused electron beam. Emitted X-rays have an energy that is characteristic of excited elements. Area, point, and line scan analyses were applied.
- -
- The instrumental neutron activation analysis (INAA) method was used to determine the concentration of trace and major elements in matrices. A sample was subjected to a neutron flux; then, radioactive nuclides were produced. As these radioactive nuclides decay, they emitted gamma rays, whose energies were characteristic for each nuclide. Comparison of the intensity of these gamma rays with those emitted by a standard permits quantitative measurement of the concentrations of the various nuclides.
- -
- Tests: 700 °C, up to 5 h, with a 1-h interval air chamber furnace;
- -
- Thermal treatment: 800 and 1100 °C, 4h, argon, tube furnace;
- -
- Long-term corrosion tests were performed in standard conditions for a pressurized water reactor (PWR), which means: 360 °C/195 bar/water simulating water used in PWR for 21, 42, and 63 days, using an autoclave Parr 4653 with a volume of 1 dm3.
- -
- pH (quantitative measure of the acidity or basicity of aqueous or other liquid solutions), DO (dissolved oxygen), and conductivity measurements with a ProLab 2500 digital meter for IDS sensors (SI Analytics, Mainz, Germany);
- -
- The presence of ions (anions and cations) was determined using the inductively coupled plasma mass spectrometry (ICP-MS) method, with the following spectrometers: Elan DRC II (Perkin Elmer, Waltham, MA, USA) and Thermo Electron Corporation Solar M6-Mk II.
3. Results and Discussion
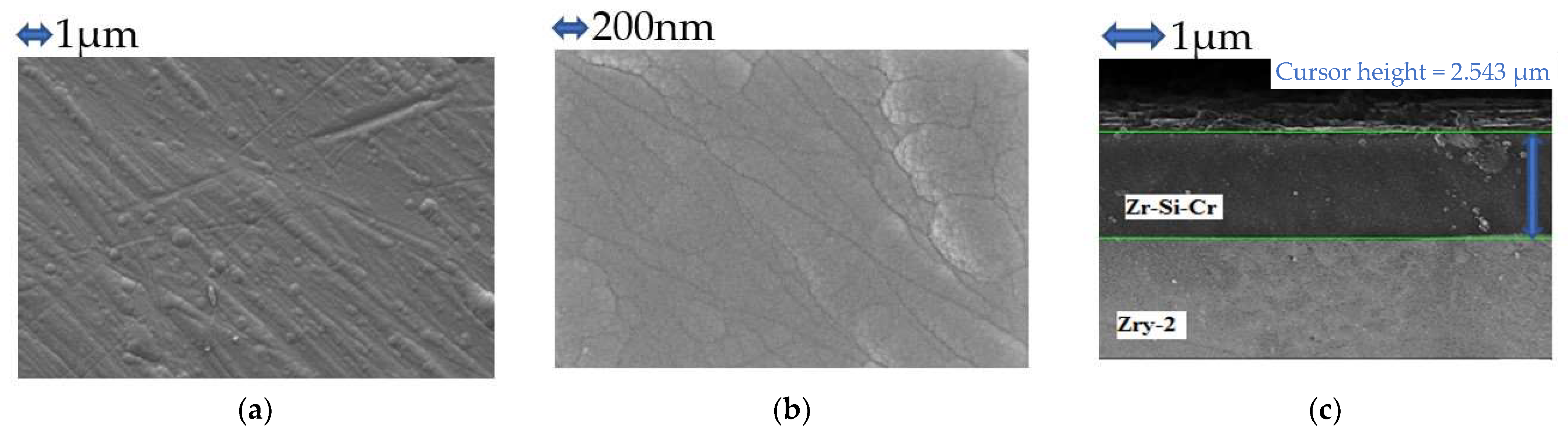
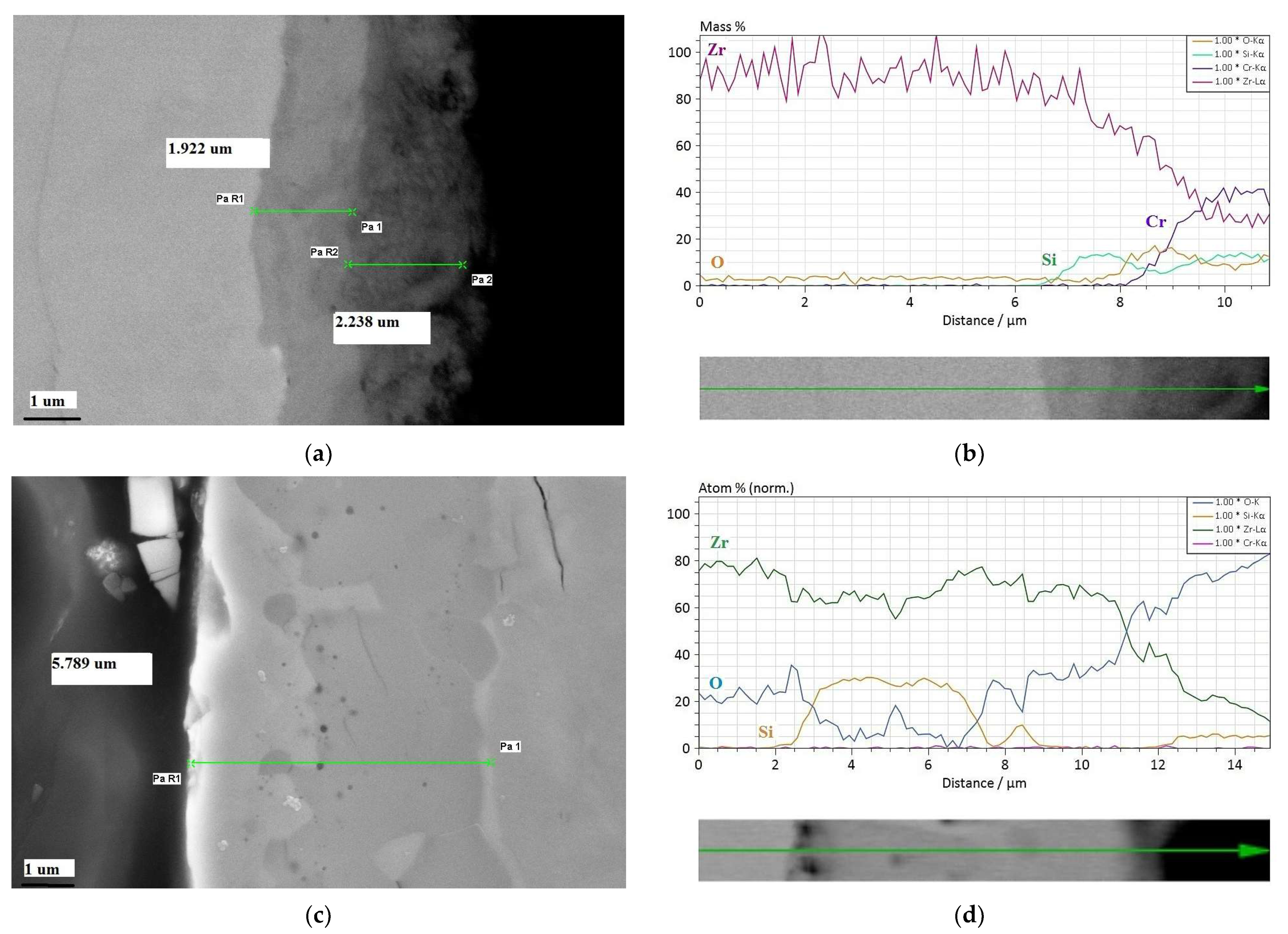
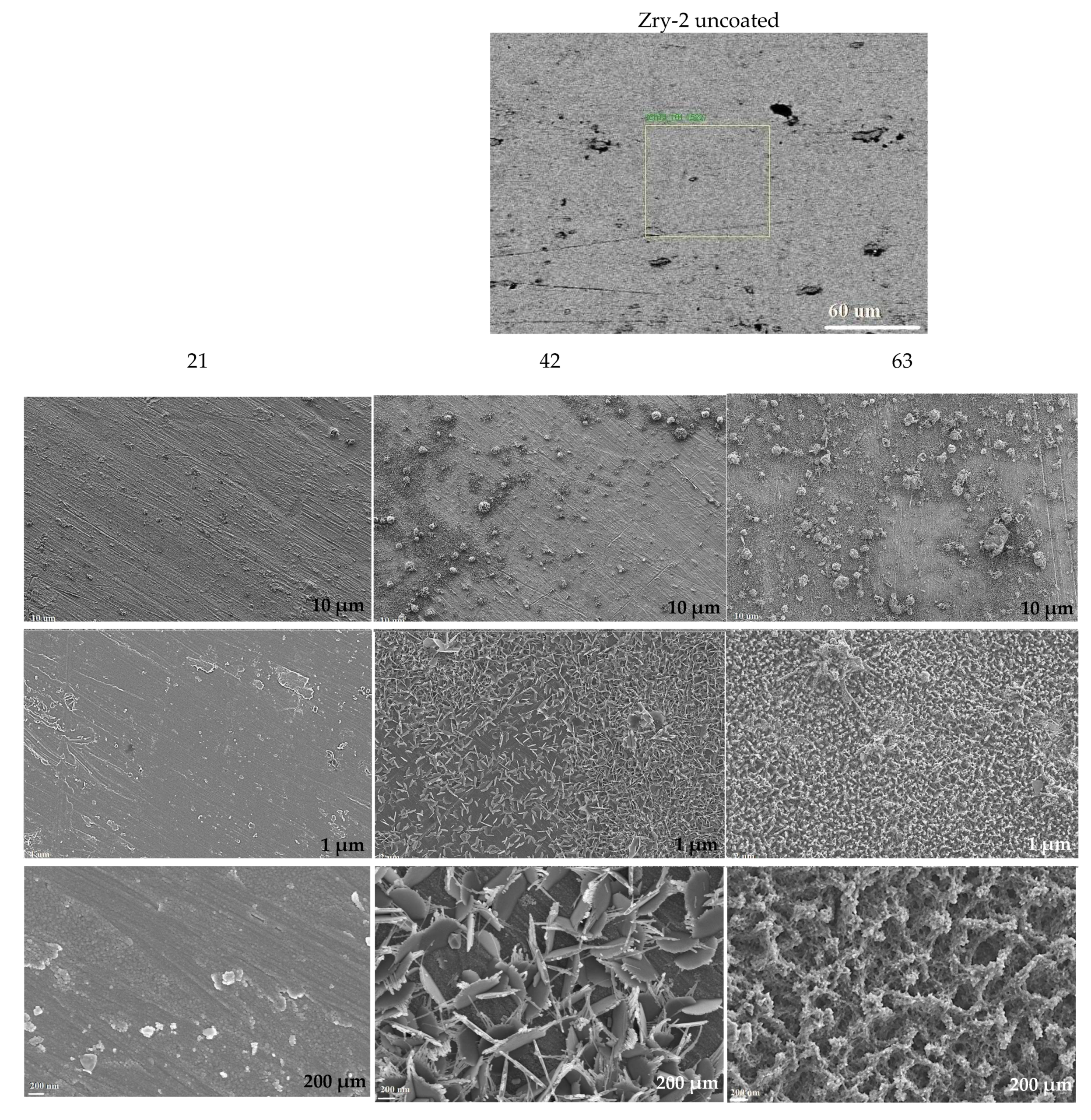
3.1. Formed Coatings/Surface Layers
3.2. Material Oxidation Behavior
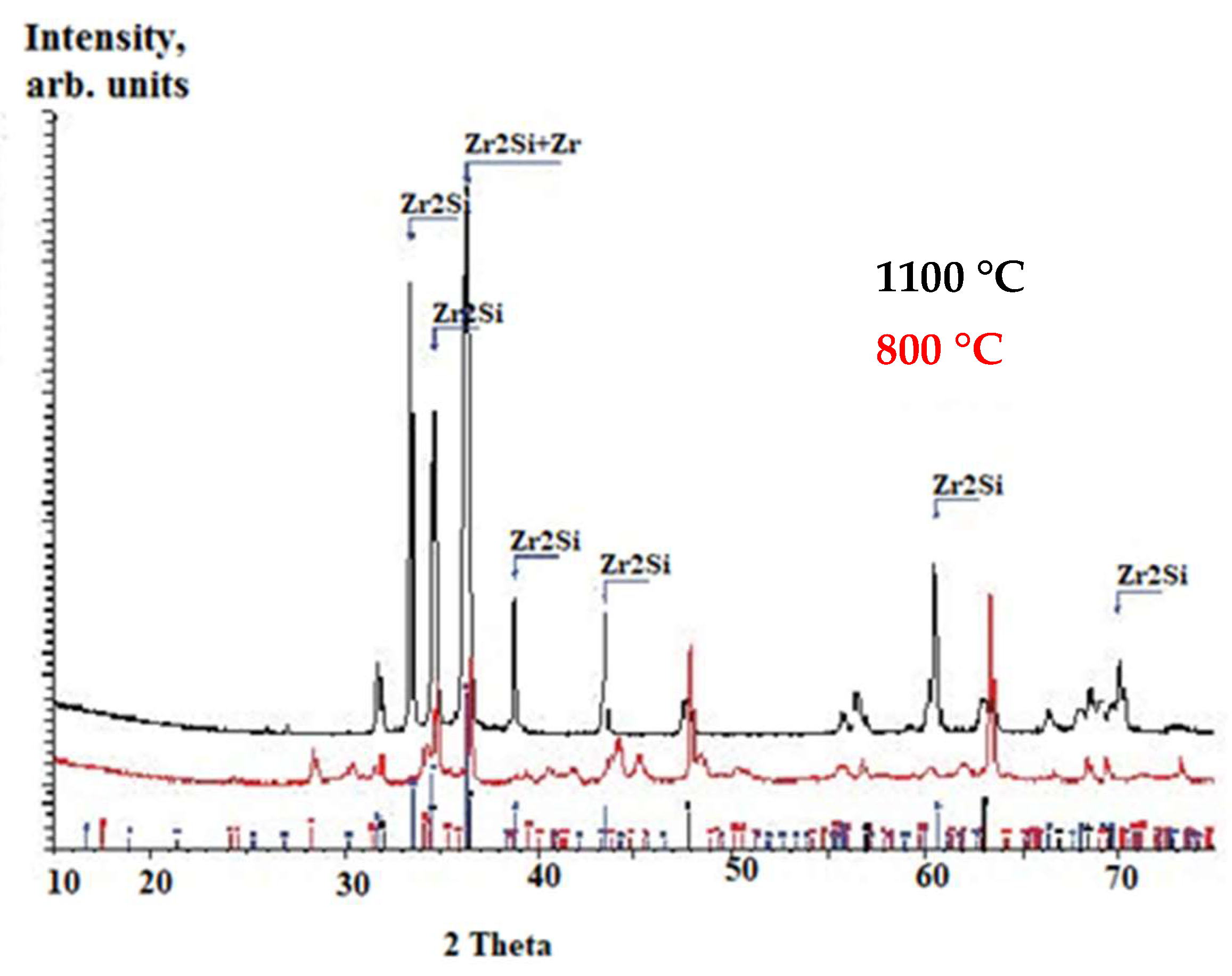
3.2.1. Thermal Treatment: 800 °C and 1100 °C, 4 h, Argon
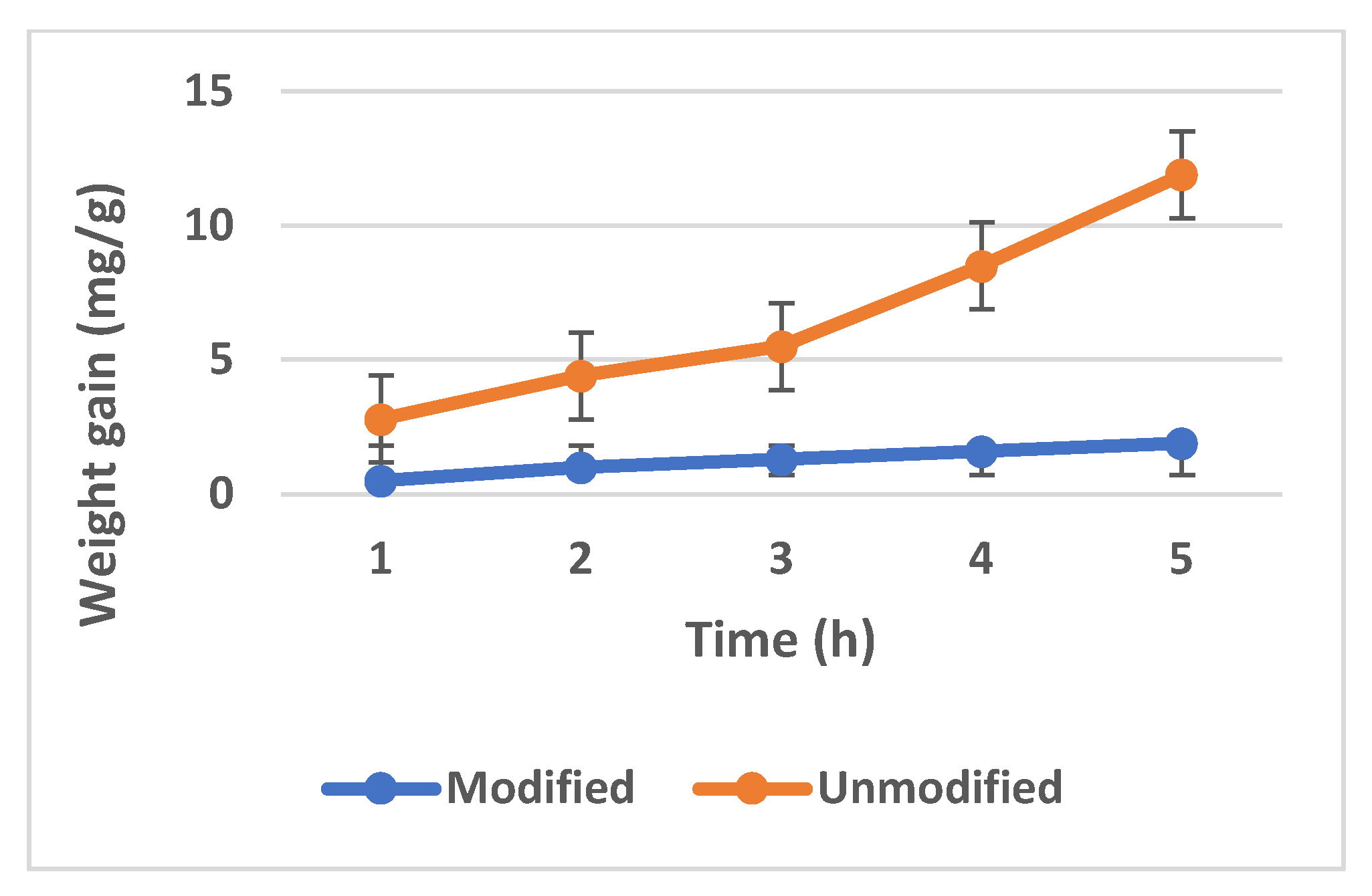
3.2.2. Oxidation Experiment: 700 °C/1, 2, 3, 4, and 5 h/air
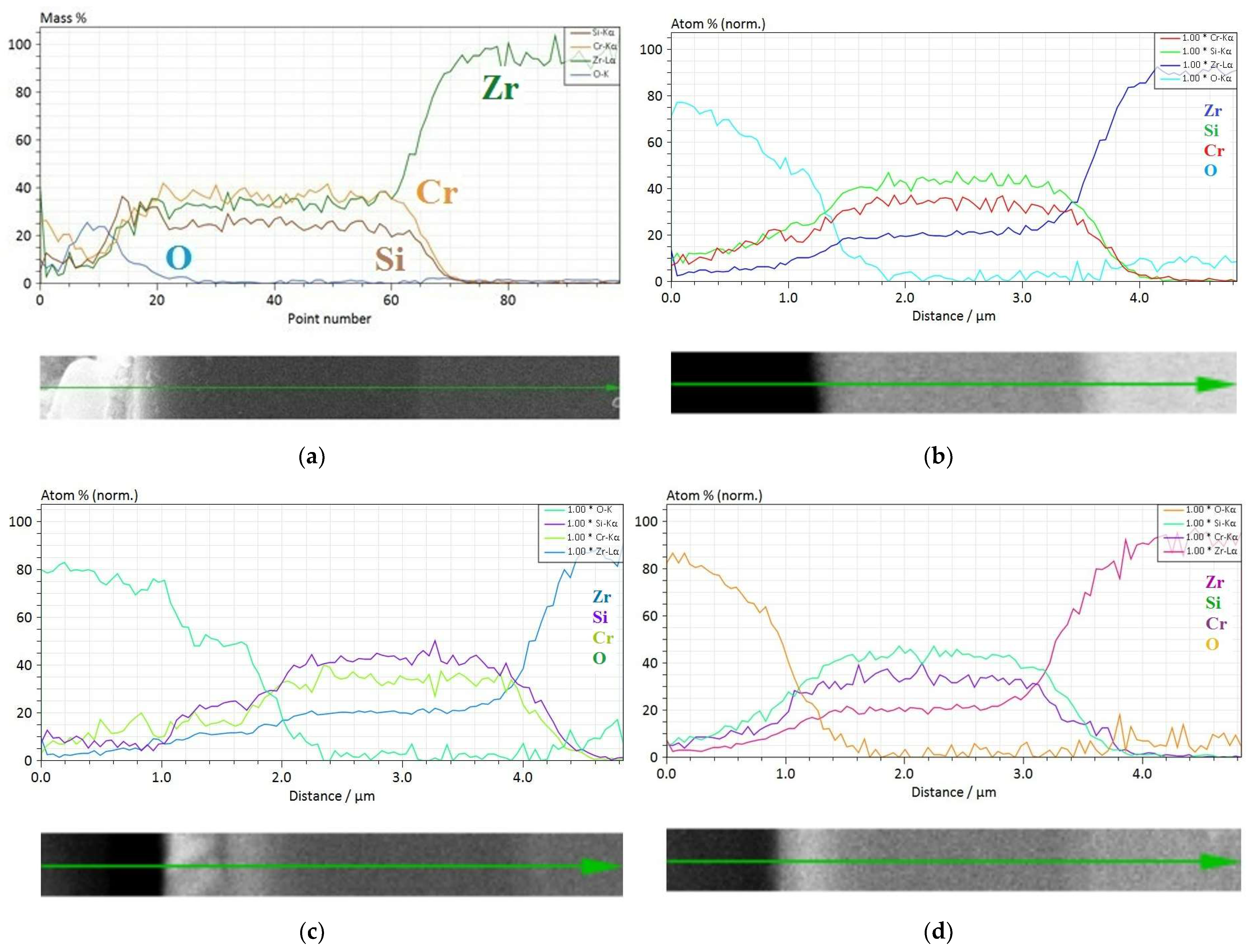
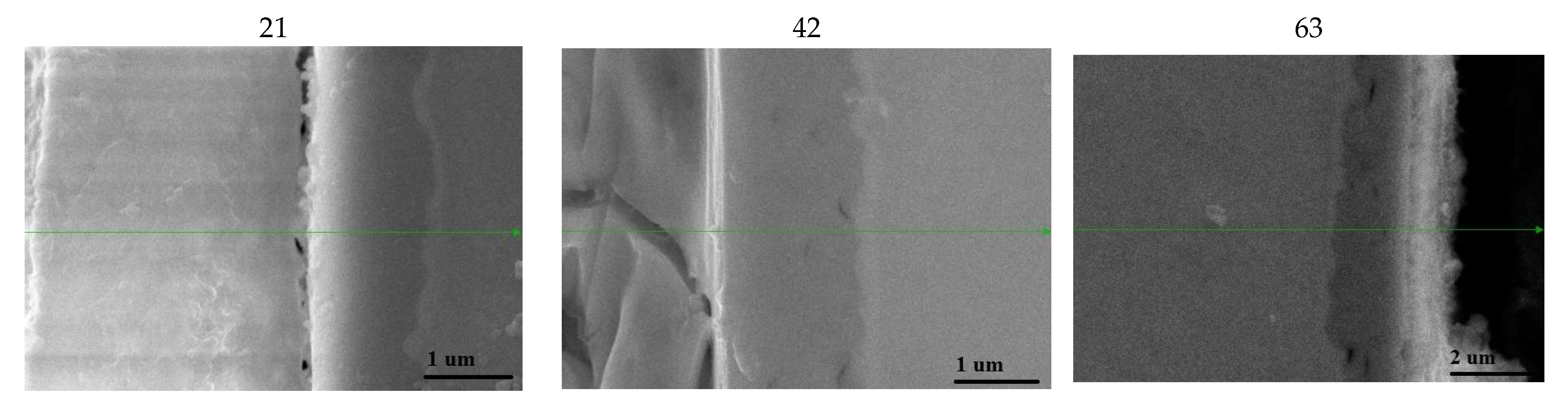
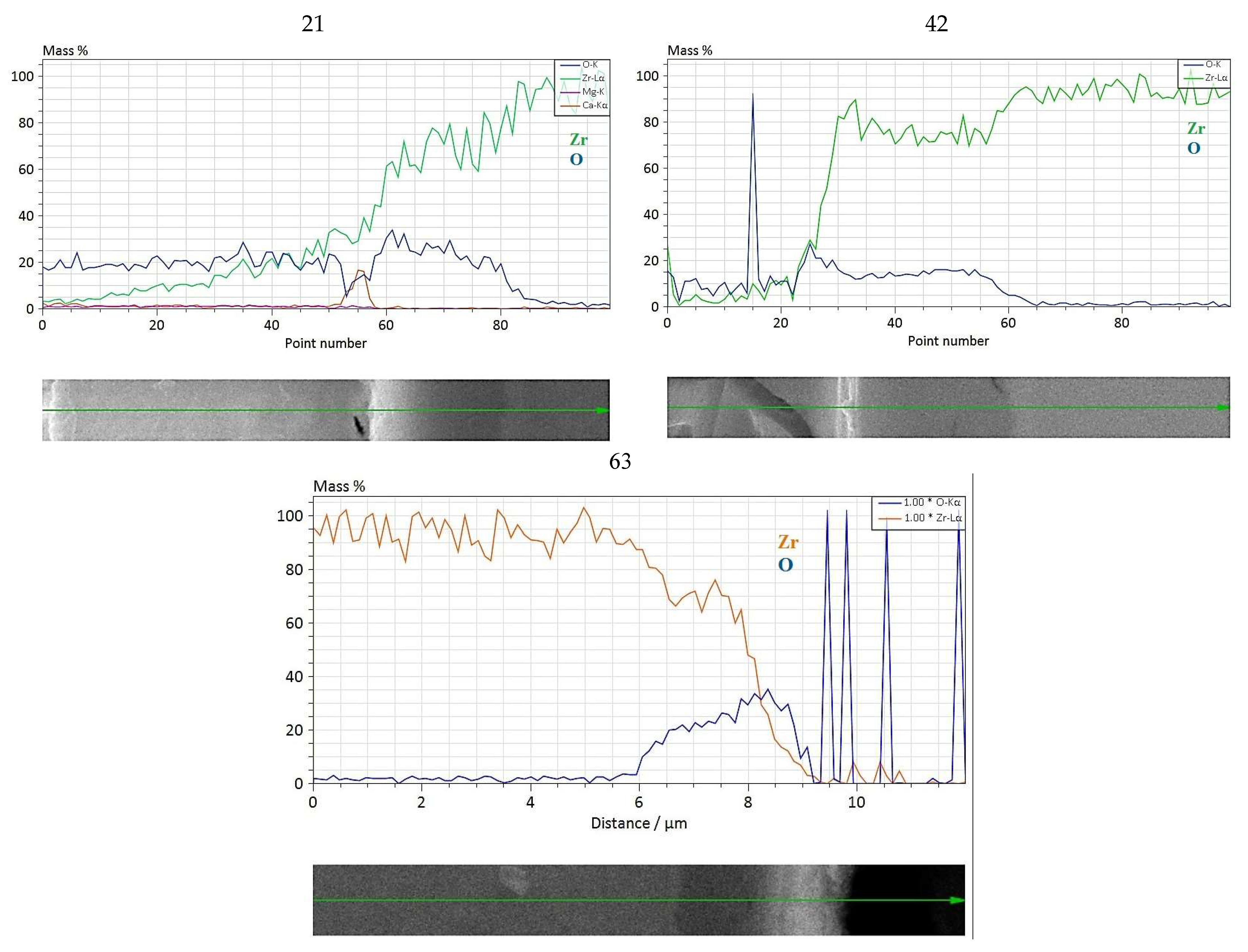
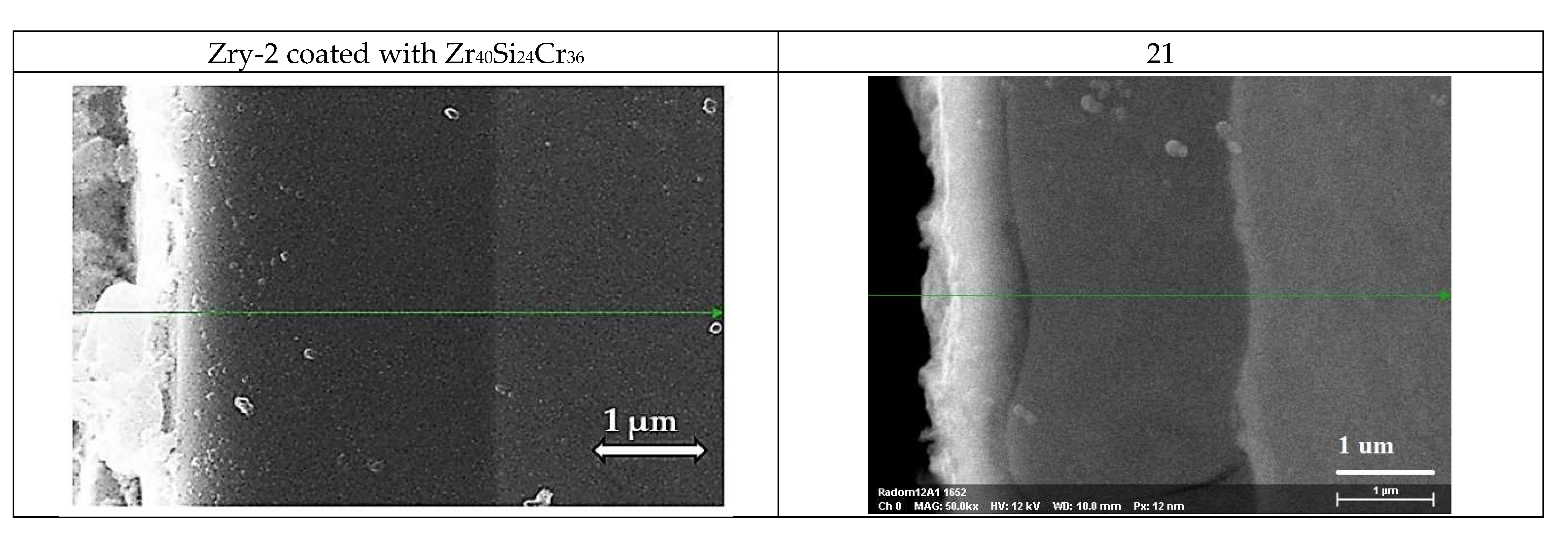
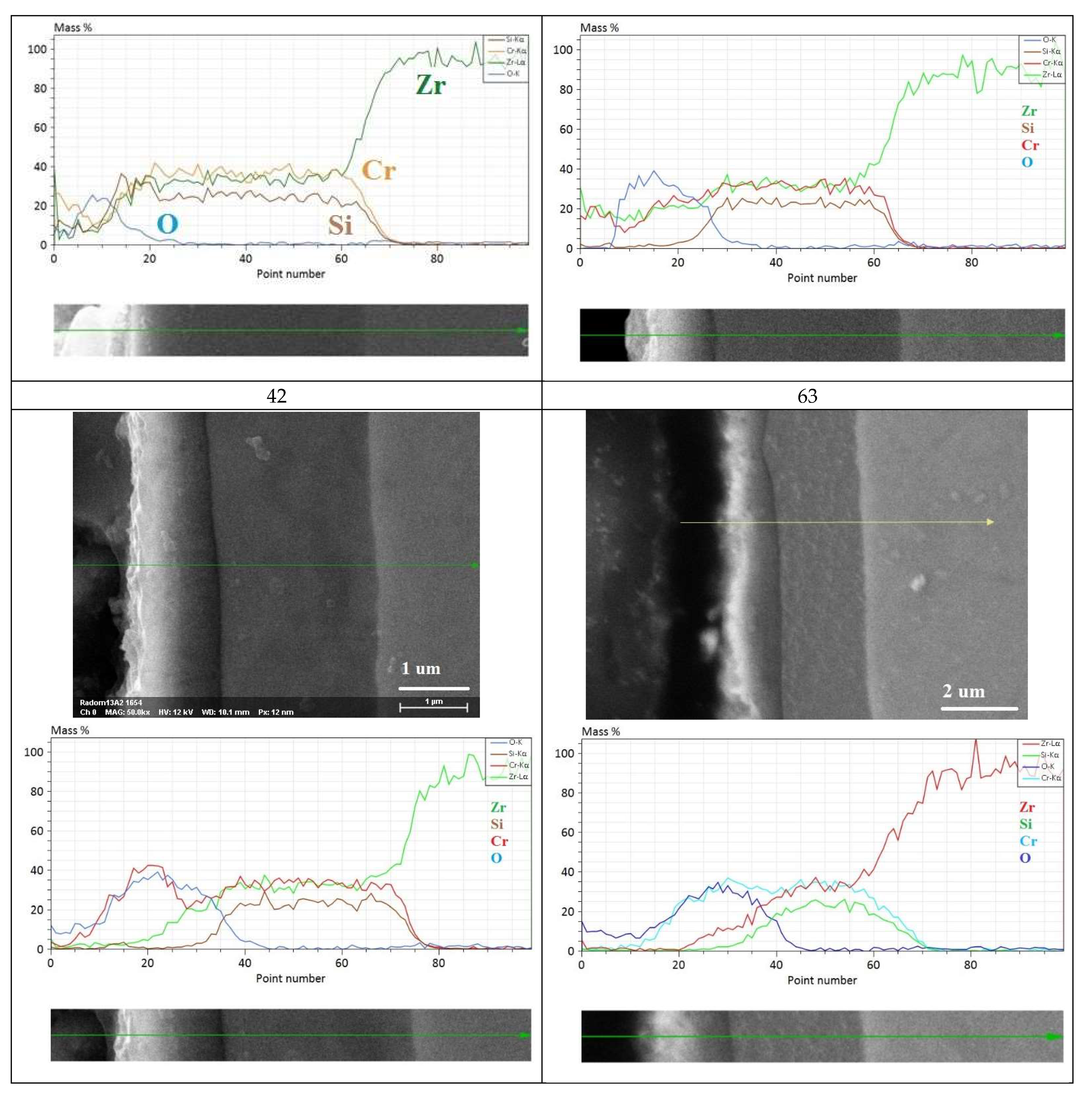
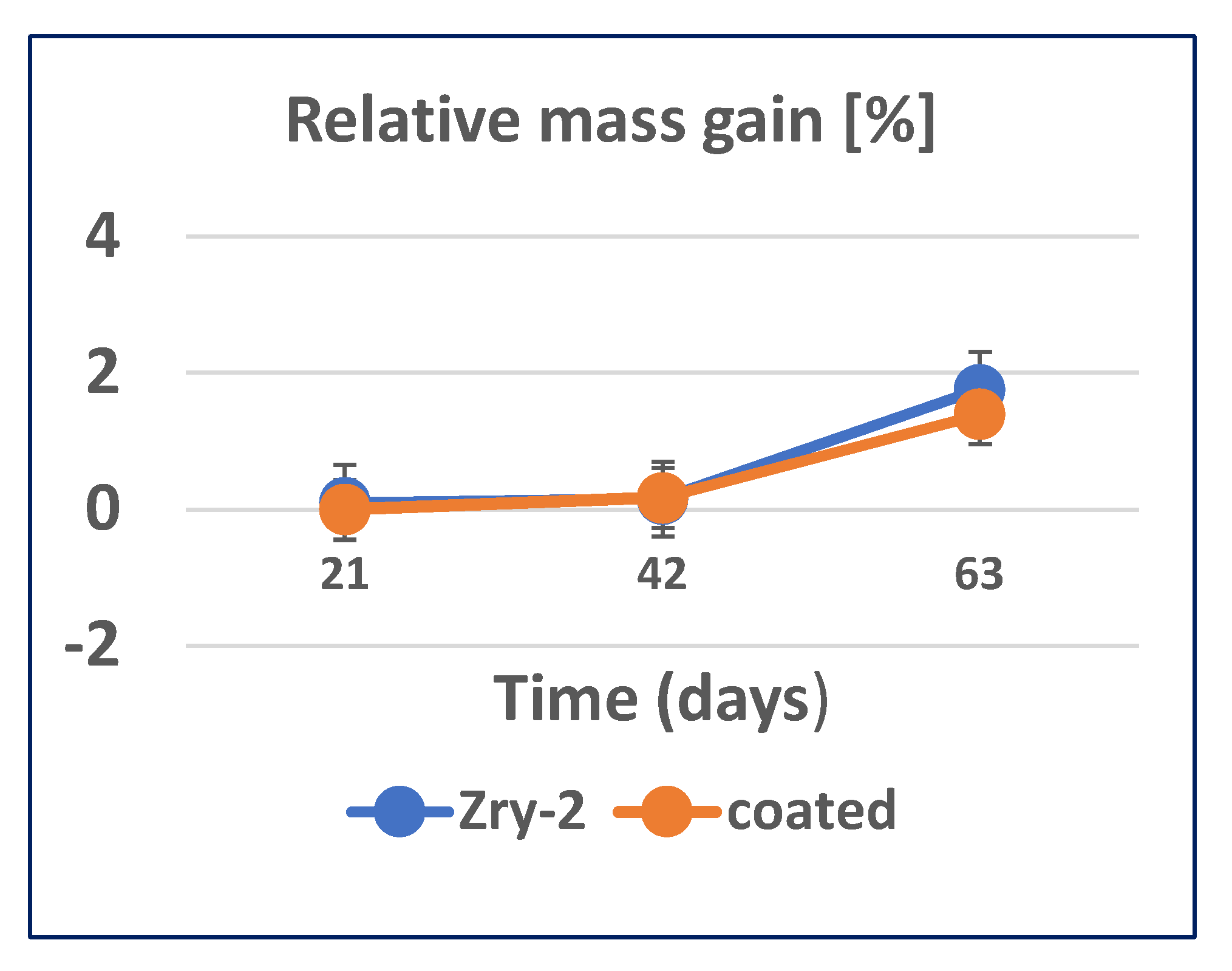
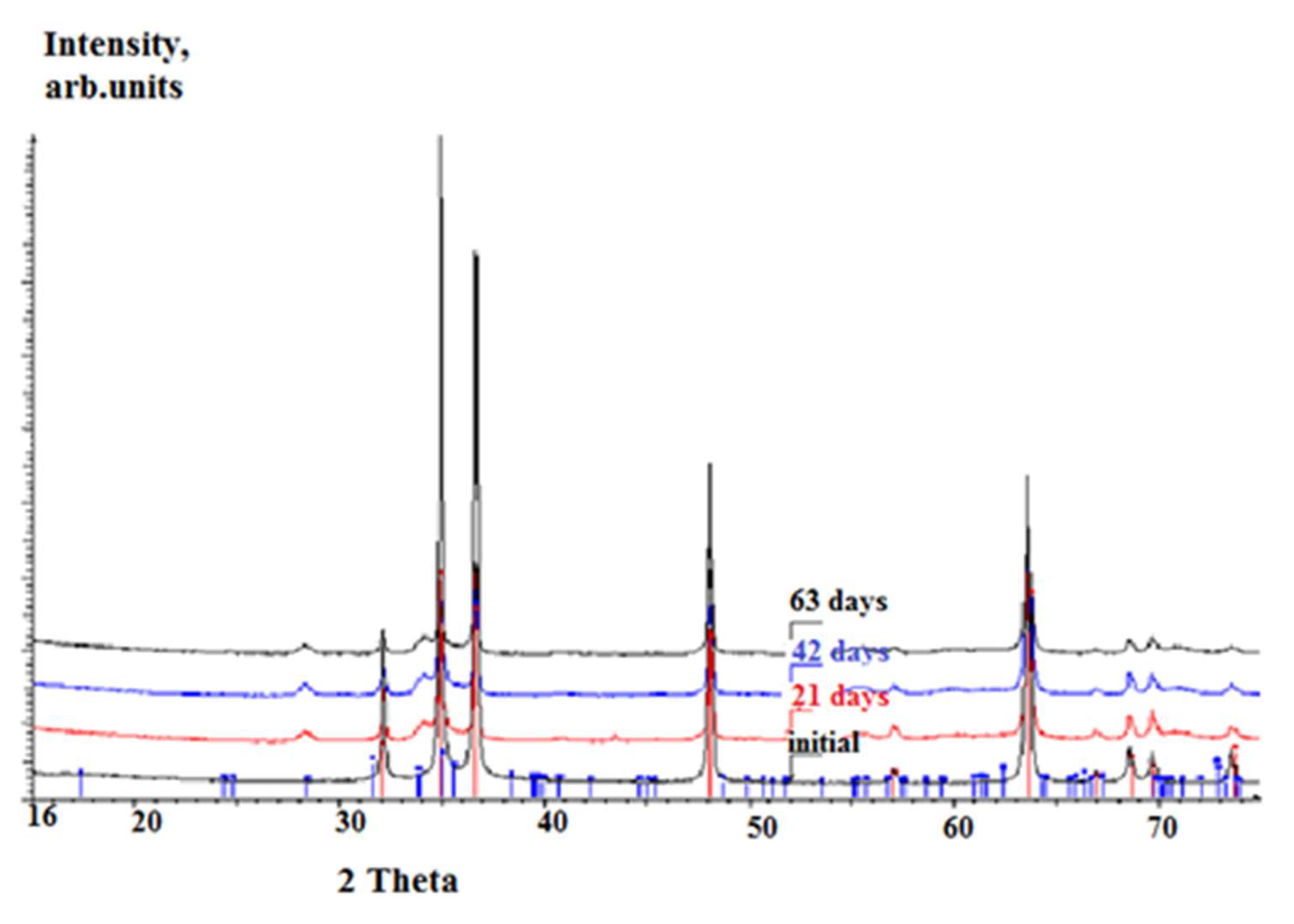
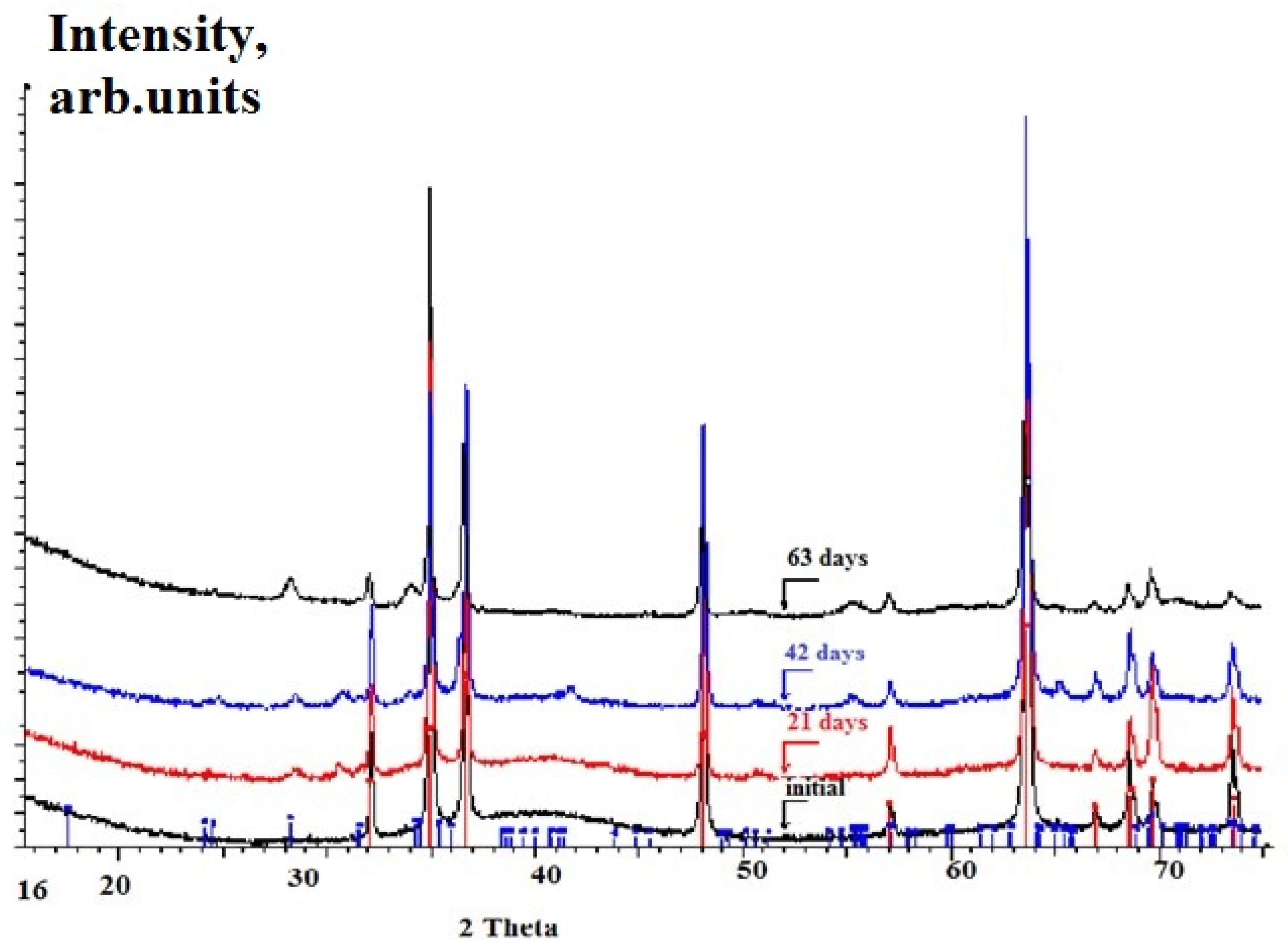
3.2.3. Long-Term Corrosion Tests
- (i)
- For Zry-2: 1.5488 (21 days), 2.074 (42 days), and 2.67 µm (63 days);
- (ii)
- For Zry-2 coated with Zr40Si24Cr36: 0.5166 (21 days), 1.090 (42 days), and 1.32 µm (63 days).
4. Conclusions
- (i)
- Thermal treatment: 800 °C and 1100 °C, 4 h, argon
- -
- Formation of the Zr2Si phase, stable up to 1950 °C.
- -
- Elemental diffusion: silicon towards the base material and chromium towards the surface.
- (ii)
- Oxidation experiment: 700 °C/1, 2, 3, 4, and 5 h/air
- -
- Significantly lower weight gain (mg/g), in the case of Zr40Si24Cr36 coated Zry-2, as compared with the unmodified material.
- -
- The formed coating was stable in the experiment conditions.
- (iii)
- Long-term corrosion tests: 360 °C/195 bar/water simulating water used in PWR reactors, for 21, 42, and 63 days
- -
- The oxide layer formed on the coated samples was thinner by 35% after the 21-days test, by 53% after the 42-days test, and by 51% after the 63-days test, as compared to the unmodified material.
- -
- The relative mass gain (%) displayed a lower oxidation rate of oxidized layer formation in the case of Zr40Si24Cr36-coated Zry-2, as compared to the unmodified material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, T.; Busby, J.; Meyer, M.; Petti, D. Materials challenges for nuclear systems. Mater. Today 2010, 13, 14–23. [Google Scholar] [CrossRef]
- Mariani, R.D.; Medvedev, P.; Porter, D.L.; Hayes, S.L.; Cole, J.I.; Bai, X.M. Novel accident-tolerant fuel meat and cladding. In Proceedings of the LWR Fuel Performance Meeting, Top Fuel 2013, Charlotte, NC, USA, 15–19 September 2013; Idaho National Laboratory: Idaho Falls, ID, USA, 2013; pp. 763–770. [Google Scholar]
- Allen, T.R.; Konings, R.J.M.; Motta, A.T. Corrosion of zirconium alloys. In Comprehensive Nuclear Materials; Konings, R.J.M., Ed.; Elsevier: Amsterdam, Austria, 2012; Volume 5, pp. 49–68. [Google Scholar]
- Tang, C.; Stueber, M.; Seifert, H.J.; Steinbrueck, M. Protective coatings on zirconium-based alloys as accident-tolerant fuel (ATF) claddings. Corros. Rev. 2017, 35, 141–165. [Google Scholar] [CrossRef]
- Idarraga-Trujillo, I.; Le Flem, M.; Brachet, J.-C.; Le Saux, M.; Hamon, D.; Muller, S.; Vanderberghe, V.; Tupin, M.; Papin, E.; Monsifrot, E.; et al. Assessment at CEA of Coated Nuclear Fuel Cladding for LWRs with Increased Margins in LOCA and beyond LOCA Conditions. In Proceedings of the Top Fuels 2013, Charlotte, NC, USA, 15–19 September 2013; Volume 2013, pp. 860–867. [Google Scholar]
- Zinkle, S.J.; Was, G.S. Materials challenge in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. TECDOC-1797, Accident tolerant fuel concepts for light water reactors. In Proceedings of the Technical Meeting, Oak Ridge, TN, USA, 13–16 October 2014; IAEA: Vienna, Austria, 2014. [Google Scholar]
- Bossis, P.; Pêcheur, D.; Hanifi, K.; Thomazet, J.; Blat, M. Comparison of the high burn-up corrosion on M5 and low tin Zircaloy-4. J. ASTM Int. 2006, 3, 494–525. [Google Scholar] [CrossRef]
- Lee, Y.H.; McKrell, T.; Kazimi, M.S. Safety of light water reactor fuel with silicon carbide cladding. In Advanced Nuclear Power Program; MIT-ANP-TR-150; Massachusetts Institute of Technology: Cambridge, MA, USA, 2014. [Google Scholar]
- Sawabe, T.; Sonoda, T.; Furuya, M.; Kitajima, S.; Kinoshita, M.; Tokiwai, M. Microstructure of oxide layers formed on zirconium alloy by air oxidation, uniform corrosion and fresh-green surface modification. J. Nucl. Mater. 2011, 419, 310–319. [Google Scholar] [CrossRef]
- Werner, Z.; Piekoszewski, J.; Szymczyk, W. Generation of high-intensity pulsed ion and plasma beams for material processing. Vacuum 2001, 63, 701–708. [Google Scholar] [CrossRef]
- Chernov, I.; Ivanova, S.V.; Krening, M.K.; Koval, N.; Larionov, V.V.; Lider, A.M.; Pushilina, N.S.; Stepanova, E.; Stepanova, O.; Cherdantsev, Y.P. Properties and structural state of the surface layer in a zirconium alloy modified by a pulsed electron beam and saturated by hydrogen. Tech. Phys. 2012, 57, 392–398. [Google Scholar] [CrossRef]
- Sartowska, B.; Starosta, W.; Barlak, M.; Waliś, L. Modification of zirconium alloy surface using intensity pulsed plasma beams. Arch. Mater. Sci. Eng. 2016, 77, 53. [Google Scholar] [CrossRef][Green Version]
- Bai, X.; Xu, J.; He, F.; Fan, Y. The air oxidation of yttrium ion implanted zircaloy-4 at 500 °C. Nucl. Instrum. Methods Phys. Res. B 2000, 160, 49–53. [Google Scholar] [CrossRef]
- Chaia, N.; Mathieu, S.; Rouillard, F.; Vilasi, M. The ability of silicide coating to delay the catastrophic oxidation of vanadium under severe conditions. J. Nucl. Mater. 2015, 457, 124–129. [Google Scholar] [CrossRef]
- Starosta, W.; Semina, V.K.; Smolik, J.; Waliś, L.; Rydzewski, M.; Sartowska, B. Studies on magnetron-sputtered zirconium-silicide coatings deposited on zirconium alloy for the enhancement of their high-temperature oxidation resistance. Nukleonika 2018, 63, 73–79. [Google Scholar] [CrossRef]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides; Wiley-VCH Verlag GmbH & KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Terrani, K.A.; Zinkle, S.J.; Snead, L.L. Advanced oxidation-resistant iron-based alloys for LWR fuel cladding. J. Nucl. Mater. 2014, 448, 420–435. [Google Scholar] [CrossRef]
- Strafford, K.N.; Smart, R.S.C.; Sare, I.; Subramanian, C. Surface Engineering, 1st ed.; Technomoc Publishing Company, Inc.: Lancaster, PA, USA, 1995; pp. xiii–xiv, 9–16. [Google Scholar]
- Dezfuli, S.; Sabzi, M. Deposition of ceramic nanocomposite coatings by electroplating pro-cess: A review of layer deposition mechanisms and effective parameters on the formation on the coating. Ceram. Int. 2019, 45, 21835–21842. [Google Scholar] [CrossRef]
- Sabzi, M.; Mousavi Anijdan, S.H. Microstructural analysis and optical properties evaluation of sol-gel heterostructured NiO-TiO2 film used for solar panels. Ceram. Int. 2019, 45, 3250–3255. [Google Scholar] [CrossRef]
- Ueno, S.; Ohji, T.; Lin, H.T. Corrosion and recession behavior of zircon in water vapor environment at high temperature. Corros. Sci. 2007, 49, 1162–1171. [Google Scholar] [CrossRef]
- Yeom, H.; Lockhart, C.; Mariani, R.; Xu, P.; Corradini, M.; Sridharan, K. Evaluation of steam corrosion and water quenching behavior of zirconium-silicide coated LWR fuel claddings. J. Nucl. Mater. 2018, 499, 256–267. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Mariani, R.; Bai, D.; Sridharan, K. Evolution of multi-layered scale structures during high temperature oxidation of ZrSi2. J. Mater. Res. 2016, 31, 3409–3419. [Google Scholar] [CrossRef]
- Kaiser, A.; Lobert, M.; Telle, R. Thermal stability of zircon (ZrSiO4). J. Eur. Ceram. Soc. 2008, 28, 2199–2211. [Google Scholar] [CrossRef]
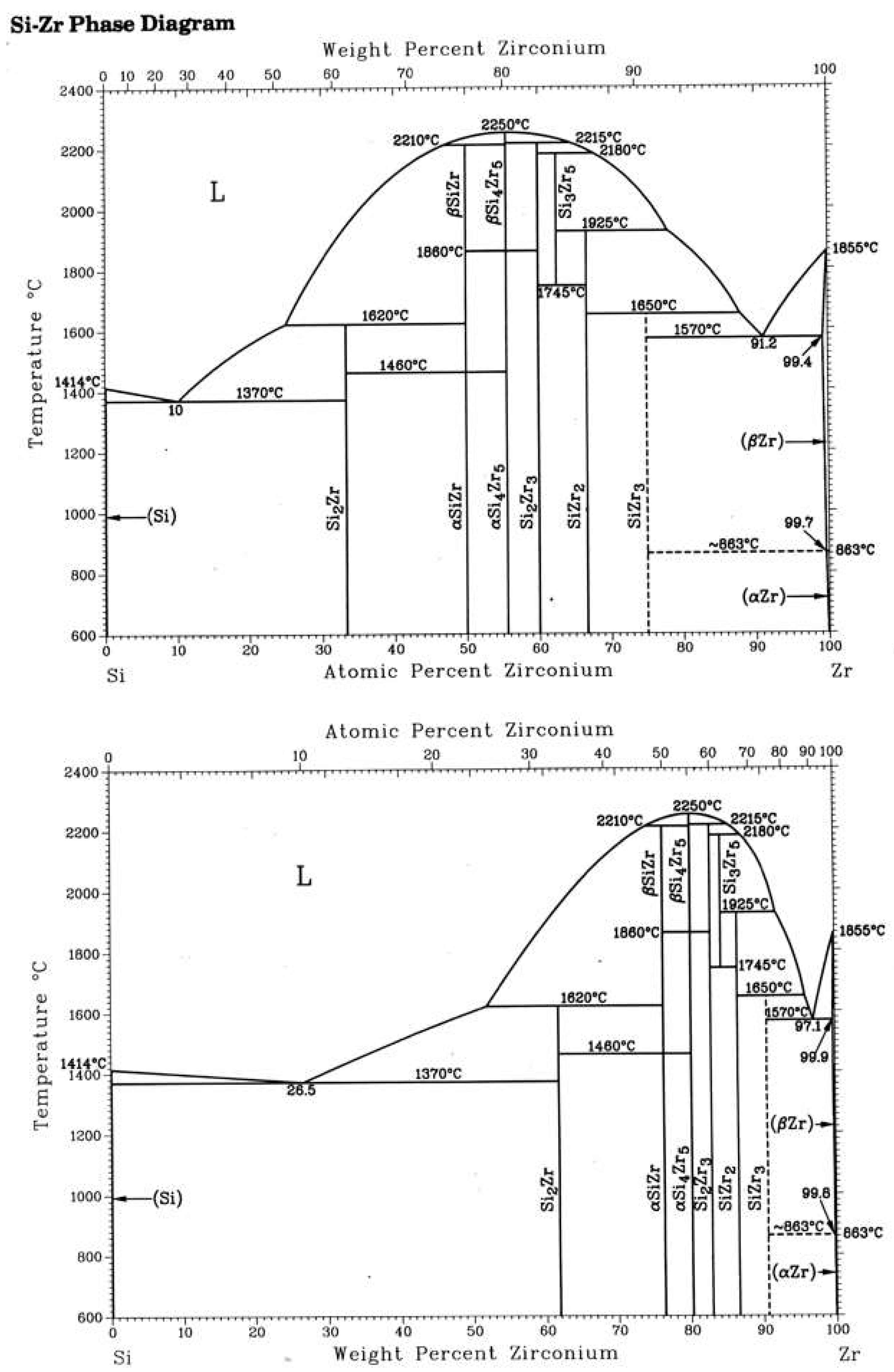
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. (Eds.) Binary Alloy Phase Diagrams 1990, 2nd ed.; ASM International: Almere, The Netherlands, 1990; Volume 1k, pp. 3383–3385. [Google Scholar]
- Han, X.; Xue, J.; Peng, S.; Zhang, H. An interesting oxidation phenomenon of Cr coatings on Zry-4 substrates in high temperature steam environment. Corros. Sci. 2019, 56, 117–124. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- PWR Primary Water Chemistry Guidelines: Revision 2; EPRI NP-7077; Electric Power Research Institute: Palo Alto, CA, USA, 1990.



| Alloy/Alloy Composition (wt.%) | Nb | Sn | Fe | Cr | Ni | O |
|---|---|---|---|---|---|---|
| Zircalloy-2 (Zry-2) | - | 1.50 | 0.15 | 0.1 | 0.05 | 0.1 |
| Zircalloy-4 (Zry-4) | - | 1.50 | 0.2 | 0.1 | - | - |
| M5 | 1.0 | - | - | - | - | 0.14 |
| ZIRLOTM | 1.0 | 1.0 | 0.1 | - | - | 0.1 |
| E365 | 1.0 | 1.2 | 0.35 | - | - | - |
| Element | Concentration [Wt.%] |
|---|---|
| Tin (Sn) | 1.3–1.6 |
| Iron (Fe) | 0.07–0.20 |
| Chromium (Cr) | 0.05–0.16 |
| Nickel (Ni) | 0.03–0.08 |
| Average (Fe + Cr + Ni) | 0.23–0.32 |
| IMPURITIES (no more) | [ppm] |
| Al | 50 |
| B | 0.5 |
| Cd | 0.5 |
| C | 500 |
| Co | 10 |
| Hf | 200 |
| Pb | 100 |
| Mg | 20 |
| Mn | 50 |
| Mo | 50 |
| Si | 100 |
| Ti | 50 |
| W | 50 |
| V | 50 |
| Element | Concentration [ppm] | Element | Concentration [ppm] |
|---|---|---|---|
| Ag | 0.86 | Mn | 99.09 |
| As | 1.3 | Mo | 1.1 |
| Au | 0.01 | Na | 176.46 |
| Ba | 69.12 | Nd | 30.69 |
| Br | 111.59 | Ni(Co-58) | 921.38 |
| Cd | 84 | U-238 | 2.15 |
| Ce | 2.72 | Th | 0.36 |
| Co | 1.71 | Pr | 3.61 |
| Cr | 619.06 | Rb | 16.48 |
| Cs | 1.87 | Sb | 3.99 |
| Cu | 318.77 | Sc | 0.31 |
| Dy | 0.72 | Se | 6.23 |
| Er | 5.57 | Si | 1630.4 |
| Eu | 0.18 | Sm | 0.18 |
| Fe | 10,292.27 | Sn | 12,489.51 |
| Ga | 83.12 | Ta | 0.61 |
| Gd | 0.89 | Tb | 0.7 |
| Hf | 40.96 | Tm | 0.15 |
| Hg | 2.74 | W | 0.3 |
| Ir | 0.0003 | Yb | 0.46 |
| K | 38.45 | Zn | 552.23 |
| La | 1.1 | Zr | 887,060.49 |
| Lu | 0.1 |
| 1A | 1B | 1C | Elements Concentration | ||
|---|---|---|---|---|---|
| 2A | 2B | 2C | |||
| 3A | 3B | 3C | wt.% | ||
| Sample Area | Zr | Si | Cr | ||
| 1A | 35.62 | 24.24 | 39.05 | ||
| 1B | 35.47 | 24.25 | 39.19 | ||
| 1C | 35.23 | 24.27 | 39.34 | ||
| 2A | 35.72 | 24.32 | 38.96 | ||
| 2B | 35.35 | 24.20 | 39.18 | ||
| 2C | 35.31 | 24.26 | 39.41 | ||
| 3A | 35.51 | 24.31 | 39.59 | ||
| 3B | 36.14 | 24.22 | 40.13 | ||
| 3C | 35.37 | 24.07 | 38.97 | ||
| Element | Concentration (wt.%) | Concentration (at. %) | Error (1 Sigma) |
|---|---|---|---|
| external part | |||
| Zirconium | 86.85 | 66.04 | 3.11 |
| Silicon | 12.31 | 30.39 | 0.52 |
| Chromium | 0.03 | 0.04 | 0.0 |
| internal part | |||
| Zirconium | 95.59 | 85.16 | 3.44 |
| Silicon | 1.76 | 5.11 | 0.10 |
| Chromium | 0.10 | 0.16 | 0.04 |
| Tin | 0.76 | 0.52 | 0.06 |
| Parameter Ions Concentrations [mg/L] | Initial | After 21 Days | After 42 Days |
|---|---|---|---|
| pH | 6.4 | 5.68 | 6.51 |
| DO [mg/L] | 8.88 | 7.41 | 7.32 |
| σ [μS/cm] | 11.90 | 62.3 | 66.1 |
| Cl− | 0.45 | 7.5 | 2.18 |
| NO3− | 0.033 | 1.38 | 0.183 |
| SO42− | 0.429 | 9.44 | 5.35 |
| Na+ | 4.4 | 3.52 | 2.14 |
| K+ | 0.4 | 0.46 | 0.95 |
| Ca2+ | 2.7 | 7.09 | 5.08 |
| Li+ | 0.3 | 2.08 | 1.674 |
| Mg2+ | 0.3 | 0.25 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartowska, B.; Starosta, W.; Waliś, L.; Smolik, J.; Pańczyk, E. Multi-Elemental Coatings on Zirconium Alloy for Corrosion Resistance Improvement. Coatings 2022, 12, 1112. https://doi.org/10.3390/coatings12081112
Sartowska B, Starosta W, Waliś L, Smolik J, Pańczyk E. Multi-Elemental Coatings on Zirconium Alloy for Corrosion Resistance Improvement. Coatings. 2022; 12(8):1112. https://doi.org/10.3390/coatings12081112
Chicago/Turabian StyleSartowska, Bożena, Wojciech Starosta, Lech Waliś, Jerzy Smolik, and Ewa Pańczyk. 2022. "Multi-Elemental Coatings on Zirconium Alloy for Corrosion Resistance Improvement" Coatings 12, no. 8: 1112. https://doi.org/10.3390/coatings12081112
APA StyleSartowska, B., Starosta, W., Waliś, L., Smolik, J., & Pańczyk, E. (2022). Multi-Elemental Coatings on Zirconium Alloy for Corrosion Resistance Improvement. Coatings, 12(8), 1112. https://doi.org/10.3390/coatings12081112






