Recent Progress in Research of Solid Tritium Breeder Materials Li2TiO3: A Review
Abstract
:1. Introduction
| Ceramic Breeder | Li Density (g/cm3) | Crystalline Phase | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|---|
| Li2O | 0.91 | Single phase | High-density Li High thermal conductivity Low activation | Reacting with water Radiation damage Li volatile | [24] |
| LiAlO2 | 0.28 | Stable phase | Good chemical stability Excellent irradiation stability | Low-density Li Poor tritium release | [25] |
| Li2ZrO3 | 0.38 (Monoclinic) 0.33 (Tetragonal) | Stable blow 1100 °C Stable above 1100 °C | Low tritium release temperature | High activation | [26] |
| Li2TiO3 | 0.25(α) 0.43(β) 0.32(γ) | Instability Stable blow 1150 °C Stable above 1150 °C | Low tritium release temperature Water insensitive | ~ | [27] |
| Li4SiO4 | 0.55(α) 0.51(γ) | Stable blow 650 °C Stable above 650 °C | High-density Li Good chemical stability | Water absorption | [28] |
2. Synthesis Methods of Li2TiO3 Powder
2.1. Solid-State Methods
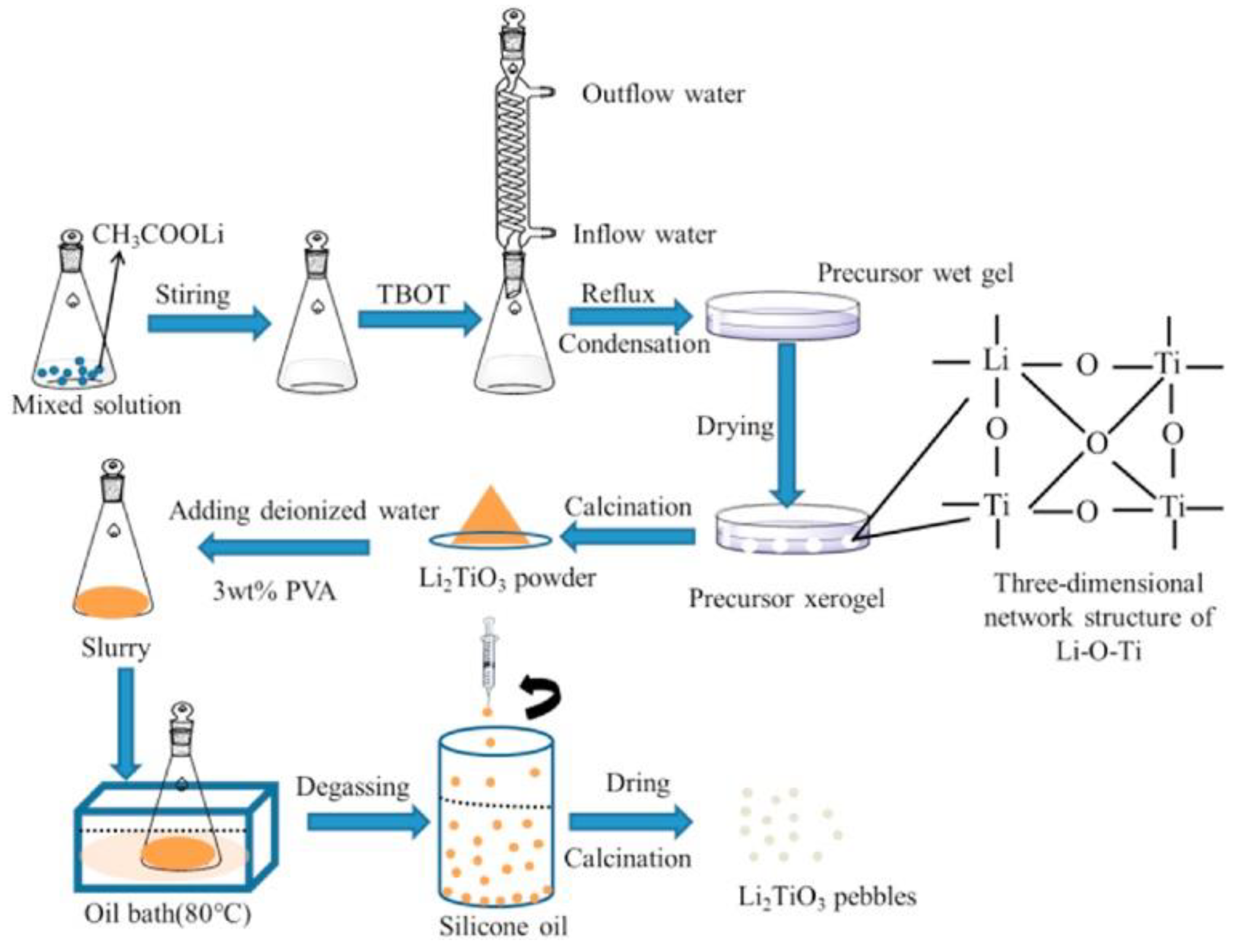
2.2. Sol–Gel Methods
2.3. Hydrothermal Methods
2.4. Solution Combustion Synthesis
2.5. Co-Precipitation Methods
3. Fabrication of Li2TiO3 Pebbles with Enhanced Li Density
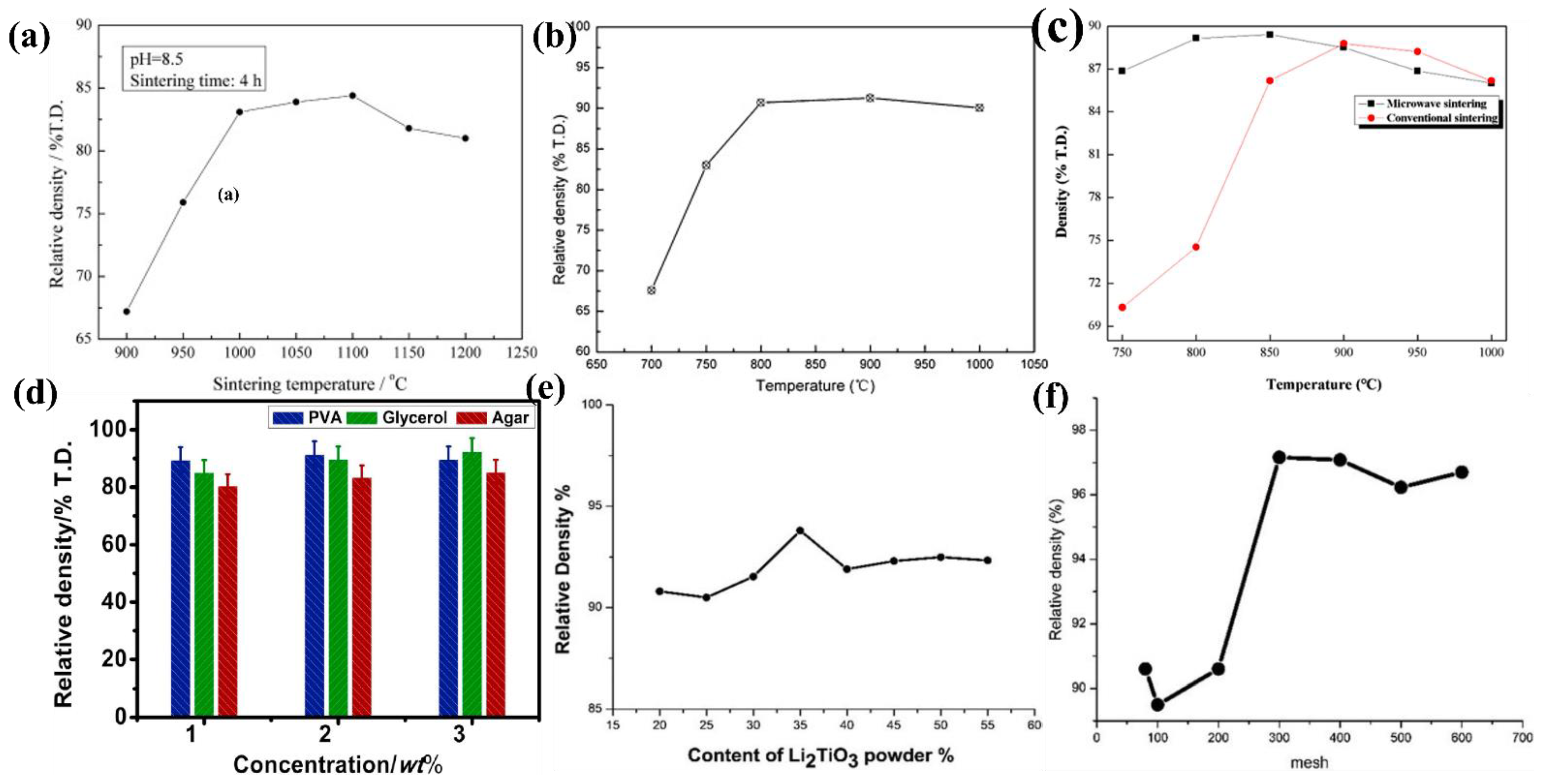
3.1. Improving Pellet Sintering Density
3.2. Adding Li2O Phase to Pebbles
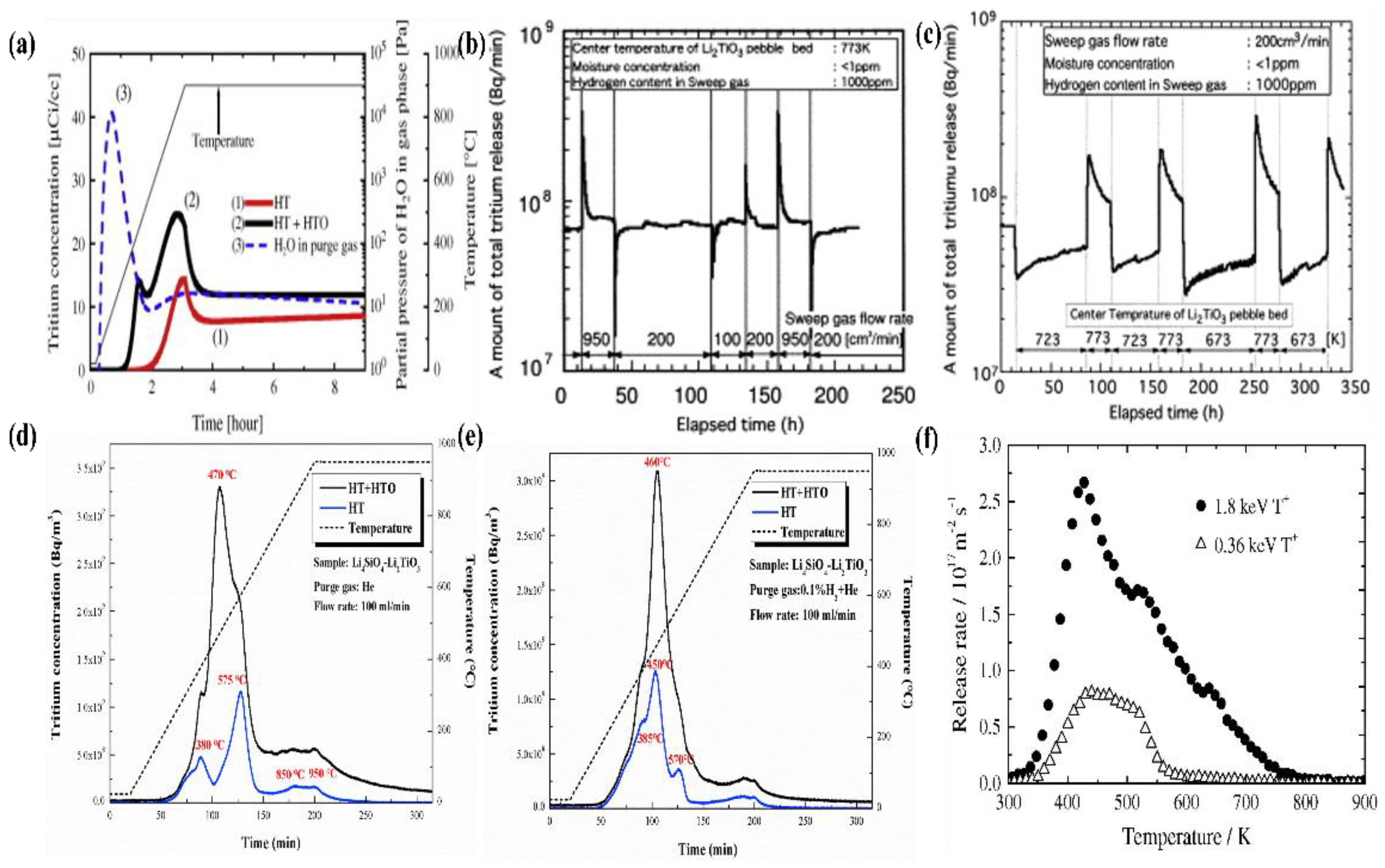
4. Tritium Release Property
4.1. Tritium Release Mechanism
4.2. Tritium Release Properties
5. Application of Deuterium in Spectroscopy
5.1. Deuterium Release Behavior
5.2. Deuterium Irradiation
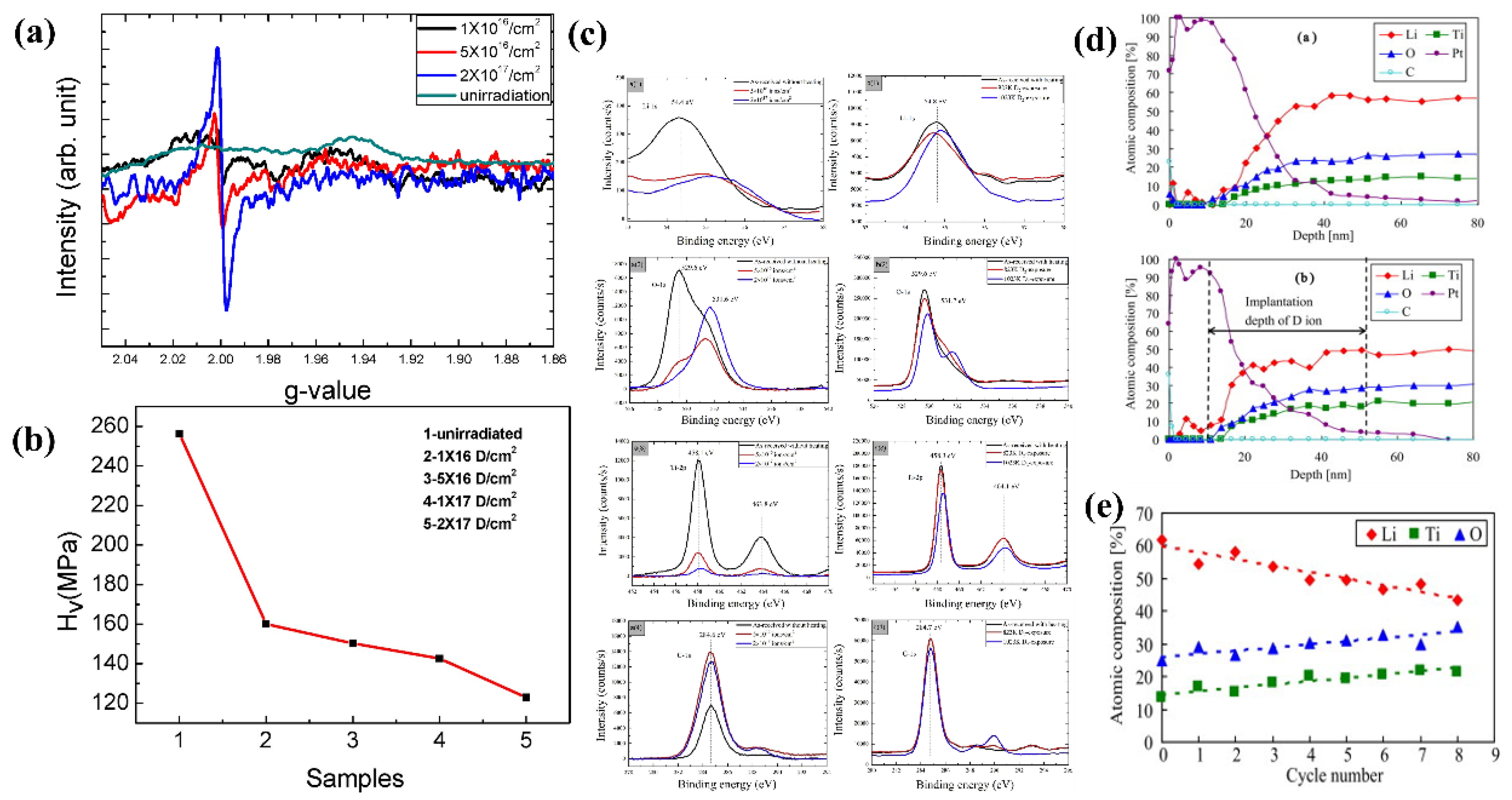
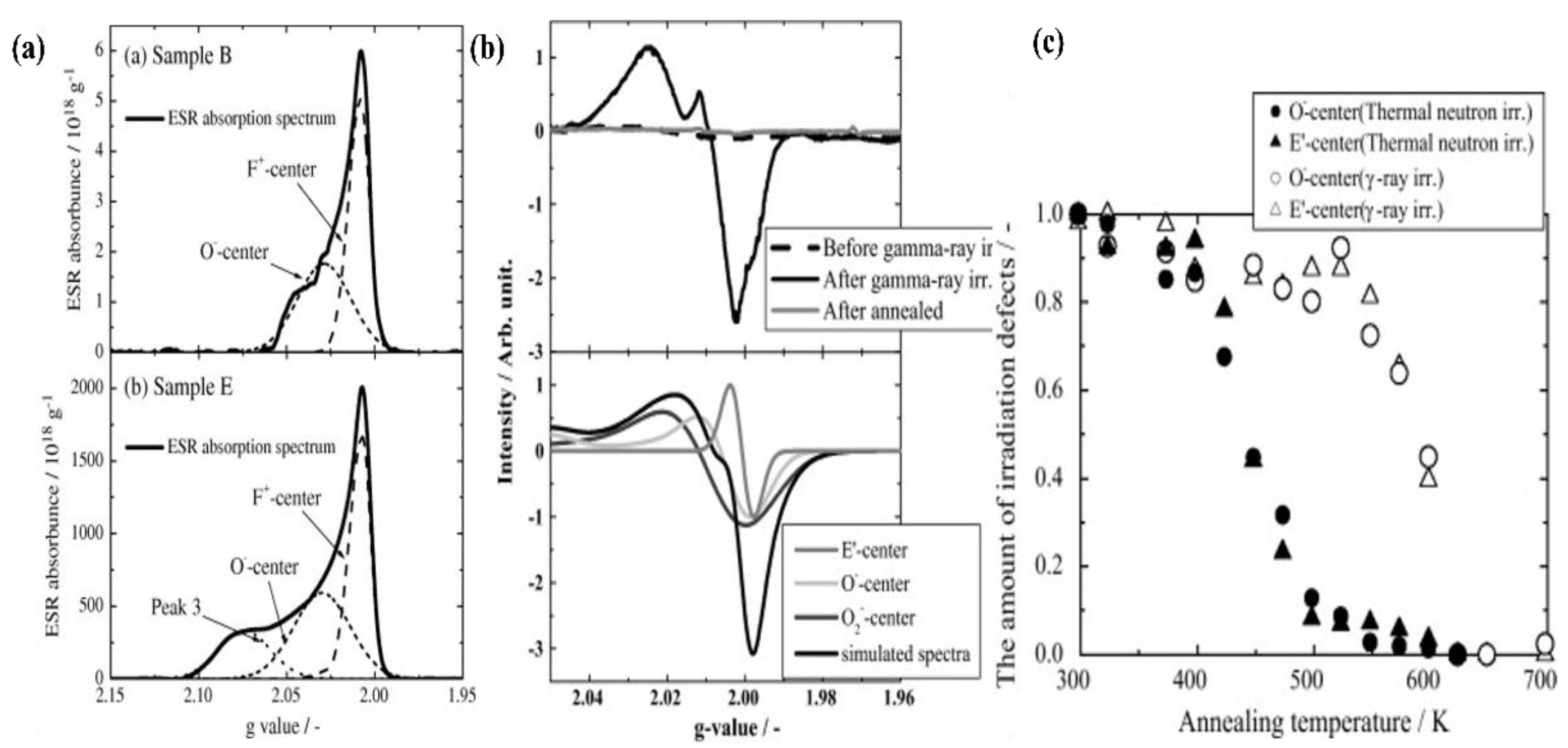
6. Irradiation Performance
6.1. Neutron Irradiation
6.2. Ion Irradiation
7. Modification
7.1. Phase Transformation
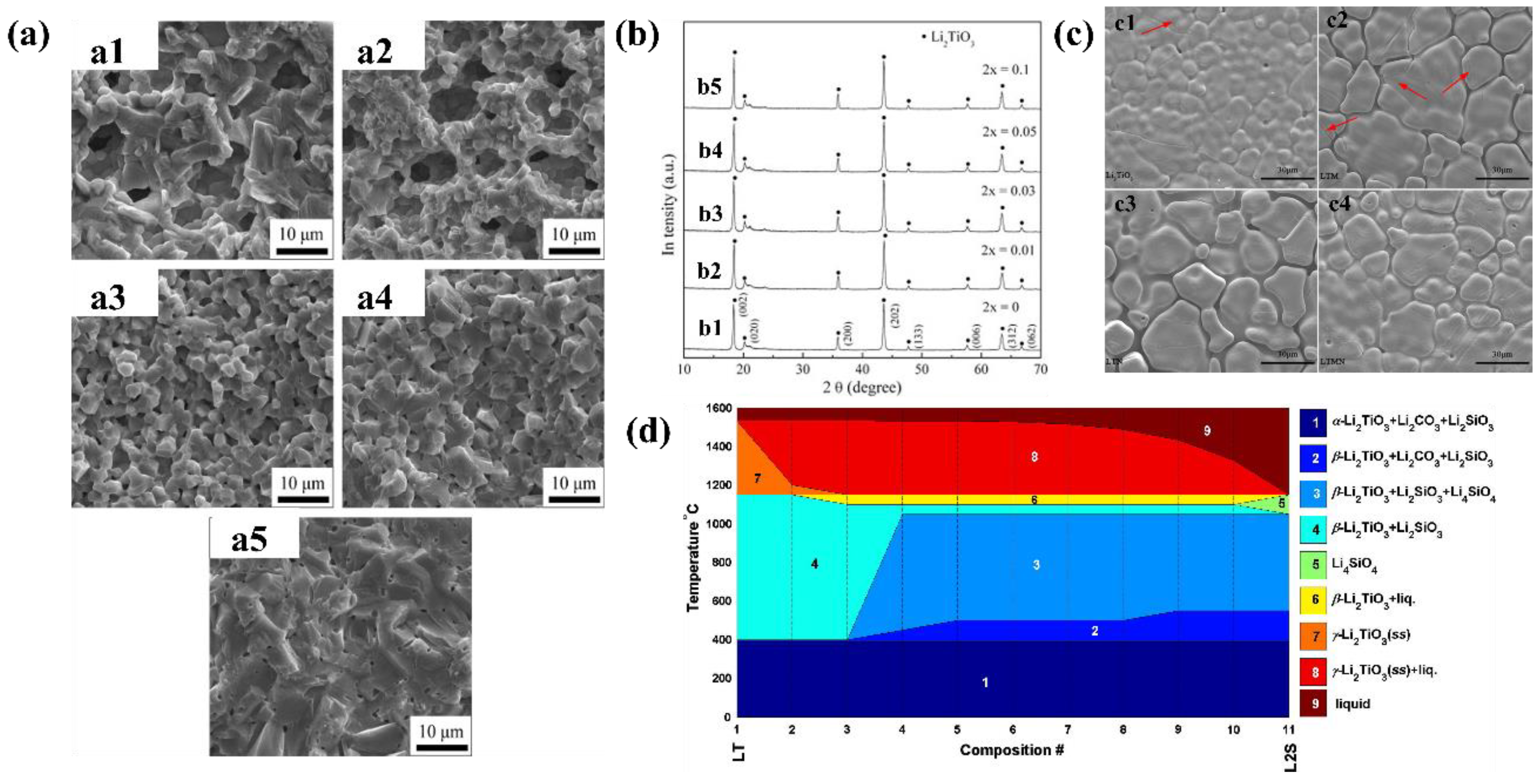
7.2. Preparation of Li2TiO3 Biphasic Ceramic Pebbles
8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ongena, J.; Oost, G. Van Energy for Future Centuries: Prospects for Fusion Power as a Future Energy Source. Fusion Sci. Technol. 2017, 1055, 3–16. [Google Scholar]
- Nowotny, J.; Hoshino, T.; Dodson, J.; Atanacio, A.J.; Ionescu, M.; Peterson, V.; Prince, K.E.; Yamawaki, M.; Bak, T.; Sigmund, W.; et al. ScienceDirect Towards Sustainable Energy. Generation of Hydrogen Fuel Using Nuclear Energy. Int. J. Hydrogen Energy 2016, 41, 12812–12825. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Wu, Y.C.; Li, J.G.; Wan, F.R.; Chen, J.L.; Luo, G.N.; Liu, X.; Chen, J.M.; Xu, Z.Y.; Zhou, X.G.; et al. Status and Strategy of Fusion Materials Development in China. J. Nucl. Mater. 2009, 386, 400–404. [Google Scholar] [CrossRef]
- Muroga, T.; Pint, B.A. Progress in the Development of Insulator Coating for Liquid Lithium Blankets. Fusion Eng. Des. 2010, 85, 1301–1306. [Google Scholar] [CrossRef]
- Abdou, M.; Morley, N.B.; Smolentsev, S.; Ying, A.; Malang, S.; Rowcliffe, A.; Ulrickson, M. Blanket/First Wall Challenges and Required R & D on the Pathway to DEMO. Fusion Eng. Des. 2015, 100, 2–43. [Google Scholar]
- Ganguly, S.; Sarkar, S.; Kumar, T.; Mishra, M. Thermally Developing Combined Electroosmotic and Pressure-Driven Flow of Nano Fluids in a Microchannel under the Effect of Magnetic Field. Chem. Eng. Sci. J. 2015, 126, 10–21. [Google Scholar] [CrossRef]
- Li, N. Lead-Alloy Coolant Technology and Materials e Technology Readiness Level Evaluation. Prog. Nucl. Energy. 2008, 50, 140–151. [Google Scholar] [CrossRef]
- Kali, R.; Mukhopadhyay, A. Spark Plasma Sintered / Synthesized Dense and Nanostructured Materials for Solid-State Li-Ion Batteries: Overview and Perspective. J. Power Sources 2014, 247, 920–931. [Google Scholar] [CrossRef]
- Roux, N.; Hollenberg, G.; Johnson, C.; Noda, K.; Verrall, R. Summary of Experimental Results for Ceramic Breeder Materials. Fusion Eng. Des. 1995, 27, 154–166. [Google Scholar] [CrossRef]
- Abdou, M.; Sze, D.; Wong, C.; Sawan, M.; Ying, A.; Morley, N.B.; Malang, S.; Sze, D.; Wong, C.; Sawan, M.; et al. U.S. Plans and Strategy for ITER Blanket Testing. Fusion Sci. Technol. 2017, 1055, 474–487. [Google Scholar] [CrossRef]
- Roux, N.; Tanaka, S.; Johnson, C.; Verrall, R. Ceramic Breeder Material Development. Fusion Eng. Des. 1998, 41, 31–38. [Google Scholar] [CrossRef]
- González De Vicente, S.M.; Hodgson, E.R.; Shikama, T. Functional Materials for Tokamak In-Vessel Systems—Status and Developments. Nucl. Fusion 2017, 57, 092009. [Google Scholar] [CrossRef]
- Raffray, A.R.; Akiba, M.; Chuyanov, V.; Giancarli, L.; Malang, S. Breeding Blanket Concepts for Fusion and Materials Requirements. J. Nucl. Mater. 2002, 307, 21–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, T.; Cui, K.; Shen, F.; Wang, J.; Yu, L.; Mao, H. Evolution of Surface Morphology, Roughness and Texture of Tungsten Disilicide Coatings on Tungsten Substrate. Vacuum 2021, 191, 110297. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Fu, T.; Wang, J.; Shen, F.; Cui, K.; Wang, H. Microstructure and Oxidation Resistance of Si-MoSi2 Ceramic Coating on TZM (Mo-0.5Ti-0.1Zr-0.02C) Alloy at 1500 °C. Surf. Coat. Technol. 2022, 431, 128037. [Google Scholar] [CrossRef]
- Li, X.X.; Zhang, M.; Zhou, W.Y.; Chang, Z.Q.; Liu, C.K.; Meng, D.Q. Fabrication of the Li2TiO3 Tritium Breeder Pebbles by a Capillary-Based Microfluidic Wet Process. J. Nucl. Sci. Technol. 2016, 53, 250–257. [Google Scholar] [CrossRef]
- Chen, R.; Shi, Q.; Yang, M.; Shi, Y.; Wang, H.; Dang, C.; Qi, J.; Liao, Z.; Lu, T. Microstructure and Phase Evolution of Li4TiO4 Ceramics Pebbles Prepared from a Nanostructured Precursor Powder Synthesized by Hydrothermal Method. J. Nucl. Mater. 2018, 508, 434–439. [Google Scholar] [CrossRef]
- Liao, Z.; Id, O.; Chen, R.; Qi, J.; Shi, Q.; Shi, Y.; Wang, H.; Guo, H.; Yang, M.; Liao, Z.; et al. Highly Efficient Preparation of Li2O Breeder Materials with Core-Shell Structure by Oil-Based Granulation Route. J. Am. Ceram Soc. 2020, 103, 5612–5623. [Google Scholar]
- Li, K.; Yang, W.; Wang, W.; Li, Y. First Principles Study of Tritium Diffusion in Li2TiO3 Crystal with Lithium Vacancy. Materials 2018, 11, 2383. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Yang, W.; Wang, W.; Li, Y. Tritium Adsorption in the Lithium Vacancy of Li2ZrO3:A First Principles Study. Int. J. Mod. Phys. C. 2018, 29, 1850086. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, X.; Yang, M.; Wei, J.; Shi, Y.; Huang, Z. A Facile Approach to Fabricate Li4 SiO4 Ceramic Pebbles as Tritium Breeding Materials. Mater. Lett. 2015, 159, 245–248. [Google Scholar] [CrossRef]
- Kinjyo, T.; Nishikawa, M.; Enoeda, M.; Fukada, S. Tritium Diffusivity in Crystal Grain of Li2TiO3 and Tritium Release Behavior under Several Purge Gas Conditions. Fusion Eng. Des. 2008, 83, 580–587. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Liu, H.; Xiang, M.; Zhou, H.; Zhang, Y.; Luo, G.N.; Qi, Q. Influence of Ion Irradiations on the Microstructure in the Tritium Breeder Material Li2TiO3. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2019, 450, 189–194. [Google Scholar] [CrossRef]
- Ogawa, S.; Masuko, Y.; Kato, H.; Yuyama, H.; Sakai, Y.; Niwa, E.; Hashimoto, T.; Mukai, K.; Hosino, T.; Sasaki, K. Li Vaporization Property of Two-Phase Material of Li2TiO3 and Li2SiO3 for Tritium Breeder. Fusion Eng. Des. 2015, 98–99, 1859–1863. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kinjyo, T.; Ishizaka, T.; Beloglazov, S.; Takeishi, T.; Enoeda, M.; Tanifuji, T. Release Behavior of Bred Tritium from LiAlO2. J. Nucl. Mater. 2004, 335, 70–76. [Google Scholar] [CrossRef]
- van der Laan, J.G.; Fedorov, A.V.; van Til, S.; Reimann, J. Ceramic Breeder Materials. Compr. Nucl. Mater. 2012, 4, 463–510. [Google Scholar]
- Mctague, J.P. Magnetoviscosity of Magnetic Colloids. J. Chem. Phys. 1969, 133, 10–14. [Google Scholar] [CrossRef]
- Klix, A.; Ochiai, K.; Terada, Y.; Morimoto, Y.; Hori, J.; Nishitani, T. Tritium Measurements for Li-Enriched Li2TiO3 Breeding Blanket Experiments with D-T Neutrons. Fusion Sci. Technol. 2017, 1055, 2–6. [Google Scholar]
- Kirillov, I.R.; Reed, C.B.; Barleon, L.; Miyazaki, K. Present Understanding of MHD and Heat Transfer Phenomena for Liquid Metal Blankets. Fusion Eng. Des. 1995, 27, 553–569. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.M.; Hamilton, H.B.; Sullivan, J.D. Testing of Lithium Titanate as an Alternate Blanket Material. J. Nucl. Mater. 1994, 212, 877–880. [Google Scholar] [CrossRef]
- Wu, X.; Wen, Z.; Lin, B.; Xu, X. Sol-Gel Synthesis and Sintering of Nano-Size Li2TiO3 Powder. Mater. Lett. 2008, 62, 837–839. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Wang, X.; Li, L.; Kong, L. Synthesis of Li2TiO3 Ceramic Breeder Powders by In-Situ Hydrolysis and Its Characterization. Mater. Lett. 2012, 89, 25–27. [Google Scholar] [CrossRef]
- Jung, C.H. Sintering Characterization of Li2TiO3 Ceramic Breeder Powders Prepared by the Solution Combustion Synthesis Process. J. Nucl. Mater. 2005, 341, 148–152. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Mohanty, T.; Prakash, D.; Tyagi, A.K.; Sinha, P.K. Monoclinic b -Li2TiO3 Nanocrystalline Particles Employing Novel Urea Assisted Solid State Route: Synthesis, Characterization and Sintering Behavior. J. Nucl. Mater. 2017, 490, 167–173. [Google Scholar] [CrossRef]
- Kinjyo, T.; Nishikawa, M. Tritium Release Behavior from Li4SiO4. Fusion Sci. Technol. 2004, 46, 561–569. [Google Scholar] [CrossRef]
- Mandal, D.; Shenoi, M.R.K.; Ghosh, S.K. Synthesis & Fabrication of Lithium-Titanate Pebbles for ITER Breeding Blanket by Solid State Reaction & Spherodization. Fusion Eng. Des. 2010, 85, 819–823. [Google Scholar]
- Park, Y.; Min, K.; Cho, S.; Ahn, M.; Lee, Y. Li2TiO3 Powder Synthesis by Solid-State Reaction and Pebble Fabrication for Tritium Breeding Material. Fusion Eng. Des. 2017, 124, 730–734. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.; Chen, R.; Huang, Z.; Gong, Y.; Zeng, Y.; Jiang, Y.; Qi, J.; Shi, Q.; Lu, T. Low-Cost Fabrication of Li2TiO3 Tritium Breeding Ceramic Pebbles via Low-Temperature Solid-State Precursor Method. Ceram. Int. 2019, 45, 17114–17119. [Google Scholar] [CrossRef]
- Si, W.; Liao, Q.; Hou, W.; Chen, L.; Li, X.; Zhang, Z.; Sun, M.; Song, Y.; Qin, L. Low-Frequency Broadband Absorbing Coatings Based on MOFs: Design, Fabrication, Microstructure and Properties. Coatings 2022, 12, 766. [Google Scholar] [CrossRef]
- Lulewicz, J.D.; Roux, N. Fabrication of Li2TiO3 Pebbles by the Extrusion –Spheronisation-Sintering Process. J. Nucl. Mater. 2002, 311, 803–806. [Google Scholar] [CrossRef]
- Liang, J.; Lu, W.Z.; Wu, J.M.; Guan, J.G. Microwave Dielectric Properties of Li2TiO3 Ceramics Sintered at Low Temperatures. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 99–102. [Google Scholar] [CrossRef]
- Guizard, C. Chapter 7 Sol-Gel Chemistry and Its Application to Porous Membrane Processing. Membr. Sci. Technol. 1996, 4, 227–258. [Google Scholar]
- Zhang, Y.; Yu, L.; Fu, T.; Wang, J.; Shen, F.; Cui, K. Microstructure Evolution and Growth Mechanism of Si-MoSi2 Composite Coatings on TZM (Mo-0.5Ti-0.1Zr-0.02 C) Alloy. J. Alloys Compd. 2022, 894, 162403. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, T.; Yu, L.; Shen, F.; Wang, J.; Cui, K. Improving Oxidation Resistance of TZM Alloy by Deposited Si–MoSi2 Composite Coating with High Silicon Concentration. Ceram. Int. 2022, 48, 20895–20904. [Google Scholar] [CrossRef]
- Wu, X.; Wen, Z.; Han, J.; Xu, X.; Lin, B. Fabrication of Li2TiO3 Pebbles by Water-Based Sol-Gel Method. Fusion Eng. Des. 2008, 83, 112–116. [Google Scholar] [CrossRef]
- Wu, X.; Wen, Z.; Xu, X.; Han, J.; Lin, B. Fabrication and Improvement of the Density of Li2TiO3 Pebbles by the Optimization of a Sol-Gel Method. J. Nucl. Mater. 2009, 393, 186–191. [Google Scholar] [CrossRef]
- Vittal Rao, T.V.; Bamankar, Y.R.; Mukerjee, S.K.; Aggarwal, S.K. Preparation and Characterization of Li2TiO3 Pebbles by Internal Gelation Sol-Gel Process. J. Nucl. Mater. 2012, 426, 102–108. [Google Scholar] [CrossRef]
- Deptuła, A.; Brykała, M.; Łada, W.; Olczak, T.; Sartowska, B.; Chmielewski, A.G.; Wawszczak, D.; Alvani, C. Preparation of Spherical Particles of Li2TiO3 (with Diameters below 100 Μm) by Sol-Gel Process. Fusion Eng. Des. 2009, 84, 681–684. [Google Scholar] [CrossRef]
- Cedex, F.-M. Materials. Chem. Mater. 1992, 4, 1990–1992. [Google Scholar]
- Tan, G.; Hu, X.; Wang, S.; Yang, X.; Li, Y.; Yang, Z.; Zhang, Y. A Process for Fabrication of Li2TiO3 Ceramic Pebbles with High Mechanical Properties via Non-Hydrolytic Sol-Gel Method. Ceram. Int. 2020, 46, 27686–27694. [Google Scholar] [CrossRef]
- Liu, H.S.; Chin, T.S.; Lai, L.S.; Chiu, S.Y.; Chung, K.H.; Changb, C.S.; Luib, M.T. Hydroxyapatite Synthesized by a Simplified Hydrothermal Method. Ceram. Int. 1997, 8842, 19–25. [Google Scholar] [CrossRef]
- Laumann, A.; Fehr, K.T.; Wachsmann, M.; Holzapfel, M.; Iversen, B.B. Metastable Formation of Low Temperature Cubic Li2TiO3 under Hydrothermal Conditions—Its Stability and Structural Properties. Solid State Ionics 2010, 181, 1525–1529. [Google Scholar] [CrossRef]
- Liu, W.; Di, J.; Zhang, W.; Xue, L.; Yan, Y. Influence of Titanium Sources on the Microstructures and Properties of Li2TiO3 Ceramics Prepared by Hydrothermal Method. Fusion Eng. Des. 2019, 138, 364–371. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Q.; Xue, L.; Yan, Y. Fabrication of Li2TiO3 Pebbles with Small Grain Size via Hydrothermal and Improved Dry-Rolling Methods. J. Nucl. Mater. 2015, 464, 389–393. [Google Scholar] [CrossRef]
- Yu, C.L.; Yanagisawa, K.; Kamiya, S.; Kozawa, T.; Ueda, T. Monoclinic Li2TiO3 Nano-Particles via Hydrothermal Reaction: Processing and Structure. Ceram. Int. 2014, 40, 1901–1908. [Google Scholar] [CrossRef]
- Tan, G.; Xu, D.; Tu, W.; Gu, H.; Ren, Y.; Hu, X.; Cai, L.; Song, S.; Li, Y.; Shen, Z.; et al. Low-Temperature Synthesis of Li2TiO3 Tritium Breeder Ceramic Pebbles by Water Controlled-Release Solvothermal Process. Adv. Powder Technol. 2021, 32, 1983–1991. [Google Scholar] [CrossRef]
- Wang, H.; Yang, M.; Gong, Y.; Feng, L.; Dang, C.; Shi, Y.; Shi, Q.; Wei, J.; Liao, Z.; Lu, T. Fabrication of Nanostructured Li2TiO3 Ceramic Pebbles as Tritium Breeders Using Powder Particles Synthesised via a CTAB-Assisted Method. Ceram. Int. 2017, 43, 5680–5686. [Google Scholar] [CrossRef]
- Yang, M.; Wang, H.; Chen, R.; Gong, Y.; Huang, Z.; Ran, G.; Chen, X.; Lu, T.; Xiao, C. Comparison of the Microwave and Conventional Sintering of Li2TiO3 Ceramic Pebbles. Ceram. Int. 2018, 44, 19672–19677. [Google Scholar] [CrossRef]
- Taylor, P.; Ghosh, S.K.; Datta, S.; Roy, S.K.; Ghosh, S.K.; Datta, S.; Roy, S.K. Solution Combustion Synthesis of Calcium Hydroxyapatite Nanoparticles. Trans. Indian Ceram. Soc. 2004, 63, 37–41. [Google Scholar]
- Zhou, Q.; Xue, L.; Wang, Y.; Li, H.; Chikada, T.; Oya, Y.; Yan, Y. Preparation of Li2TiO3 Ceramic with Nano-Sized Pores by Ultrasonic-Assisted Solution Combustion. J. Eur. Ceram. Soc. 2017, 37, 3595–3602. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, L.; Gao, Y.; Xue, L.; Yan, Y. Flash Synthesis of Li2TiO3 Powder by Microwave-Induced Solution Combustion. J. Nucl. Mater. 2014, 455, 101–105. [Google Scholar] [CrossRef]
- Jung, C.H.; Park, J.Y.; Kim, W.J.; Ryu, W.S.; Lee, S.J. Characterizations of Li2TiO3 Prepared by a Solution Combustion Synthesis and Fabrication of Spherical Particles by Dry-Rolling Granulation Process. Fusion Eng. Des. 2006, 81, 1039–1044. [Google Scholar] [CrossRef]
- Zhou, Q.; Mou, Y.; Ma, X.; Xue, L.; Yan, Y. Effect of Fuel-to-Oxidizer Ratios on Combustion Mode and Microstructure of Li2TiO3 Nanoscale Powders. J. Eur. Ceram. Soc. 2014, 34, 801–807. [Google Scholar] [CrossRef]
- Jung, C.H.; Park, J.Y.; Oh, S.J.; Park, H.K.; Kim, Y.S.; Kim, D.K.; Kim, J.H. Synthesis of Li2TiO3 Ceramic Breeder Powders by the Combustion Process. J. Nucl. Mater. 1998, 253, 203–212. [Google Scholar] [CrossRef]
- Guo, H.; Shi, Y.; Wang, H. Fabrication of Fine-Grained Li2TiO3 Ceramic Pebbles With Enhanced Crush Load By Rolling Method: Optimization of Li/Ti Ratio and Sintering Procedure. J. Nucl. Mater. 2022, 563, 153657. [Google Scholar] [CrossRef]
- Yin, J.; Sun, P.; Yang, H.; Wei, Y.; Hou, P.; Xu, X. A Carbon-Free Li2TiO3 /Li2 MTi3O8 (M═Zn 1/3 Co 2/3 ) Nanocomposite as High-Rate and Long-Life Anode for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1800960. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Li, B.; Fu, T.; Xie, D.; Cai, J.; Zhao, J. Improving the Electrochemical Properties of LiNi0.5Co0.2Mn0.3O2 at 4.6 v Cutoff Potential by Surface Coating with Li2TiO3 for Lithium-Ion Batteries. Phys. Chem. Chem. Phys. 2015, 17, 32033–32043. [Google Scholar] [CrossRef]
- Xiang, M.; Zhang, Y.; Zhang, Y.; Wang, C.; Liu, W.; Yu, Y. Effects of Grain Size and Porosity on Strength of Li2TiO3 Tritium Breeding Pebbles and Its Grain Growth Behavior. J. Nucl. Mater. 2016, 482, 163–169. [Google Scholar] [CrossRef]
- Hoshino, T.; Oikawa, F. Trial Fabrication Tests of Advanced Tritium Breeder Pebbles Using Sol-Gel Method. Fusion Eng. Des. 2011, 86, 2172–2175. [Google Scholar] [CrossRef]
- Hoshino, T. Development of Fabrication Technologies for Advanced Tritium Breeder Pebbles by the Sol-Gel Method. Fusion Eng. Des. 2013, 88, 2264–2267. [Google Scholar] [CrossRef]
- Katayama, K.; Someya, Y.; Tobita, K.; Fukada, S.; Hatano, Y.; Chikada, T. Influence of Hydrogen Addition to a Sweep Gas on Tritium Behavior in a Blanket Module Containing Li2TiO3 Pebbles. Fusion Eng. Des. 2016, 113, 221–226. [Google Scholar] [CrossRef]
- Casadio, S.; Van Der Laan, J.G.; Alvani, C.; Magielsen, A.J.; Stijkel, M.P. Tritium Release Kinetics from Li2TiO3 Pebbles as Prepared by Soft-Wet-Chemistry. J. Nucl. Mater. 2004, 329–333, 1252–1255. [Google Scholar] [CrossRef]
- Tanifuji, T.; Yamaki, D.; Nasu, S.; Noda, K. Tritium Release Behavior from Neutron-Irradiated Li2TiO3 Single Crystal. J. Nucl. Mater. 1998, 258–263, 543–548. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Yu, M. Building Homogenous Li2TiO3 Coating Layer on Primary Particles to Stabilize Li-Rich Mn-Based Cathode Materials. Small 2022, 18, 2106337. [Google Scholar] [CrossRef]
- Grisæ, V.; Kumada, T.; Tanifuji, T.; Nakazawa, T. ESR Spectroscopy of γ -Irradiated Li2TiO3 Ceramics. Radiat. Phys. Chem. 2000, 58, 113–117. [Google Scholar]
- Chikhray, Y.; Shestakov, V.; Maksimkin, O.; Turubarova, L.; Osipov, I.; Kulsartov, T.; Kuykabayeba, A.; Tazhibayeva, I.; Kawamura, H.; Tsuchiya, K. Study of Li2TiO3+5 Mol% TiO2 Lithium Ceramics after Long-Term Neutron Irradiation. J. Nucl. Mater. 2009, 386, 286–289. [Google Scholar] [CrossRef]
- Shlimas, D.I.; Zdorovets, M.V.; Kozlovskiy, A.L. Synthesis and Resistance to Helium Swelling of Li2TiO3 Ceramics. J. Mater Sci-Mater El. 2020, 31, 12903–12912. [Google Scholar] [CrossRef]
- Yu, C.L.; Liu, W.; Yang, L.T.; Wang, D.Y.; Wu, K.; Zhang, Z.P.; Wang, X.F.; Yanagisawa, K. Additives Affecting Properties of β-Li2TiO3 Pebbles in a Modified Indirect Wet Chemistry Process. J. Nucl. Mater. 2016, 480, 310–313. [Google Scholar] [CrossRef]
- Lai, Y.; Hong, C.; Jin, L.; Tang, X.; Zhang, H.; Huang, X.; Li, J.; Su, H. Temperature Stability and High-Qf of Low Temperature Firing Mg2SiO4–Li2TiO3 Microwave Dielectric Ceramics. Ceram. Int. 2017, 43, 16167–16173. [Google Scholar] [CrossRef]
- Hoshino, T. Pebble Fabrication of Super Advanced Tritium Breeders Using a Solid Solution of Li2+xTiO3+y with Li2ZrO3. Nucl. Mater. Energy 2016, 9, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Tan, G.; Cai, L.; Song, S.; Wu, W.; Feng, B.; Qin, Z.; Liu, R.; Shen, Z.; Zhang, Y. A Novel Process for Fully Automatic Mass-Production of Li2TiO3 Ceramic Pebbles with Uniform Structure and Size. Ceram. Int. 2022, 48, 6393–6401. [Google Scholar] [CrossRef]
- Mukai, K.; Sasaki, K.; Hashimoto, T.; Suzuki, A.; Hoshino, T.; Terai, T. Effect of Li/Ti Ratio on Microstructure and Thermal Diffusivity of Lithium Titanate for Solid Breeding Material. Fusion Eng. Des. 2011, 86, 2643–2646. [Google Scholar] [CrossRef]
- Engineering, F.; Karlsruhe, F. Phase Equilibria in the Li-Ti-O System and Physical Properties of Li2TiO3. Fusion Eng. Des. 2002, 62, 361–366. [Google Scholar]
- Hong, M.; Zhang, Y.; Mi, Y.; Xiang, M.; Zhang, Y. Fabrication and Characterization of Li2TiO3 Core-Shell Pebbles with Enhanced Lithium Density. J. Nucl. Mater. 2014, 445, 111–116. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawasaki, K.; Fujishima, T.; Miyahara, Y.; Oya, Y.; Okuno, K. Release Kinetics of Tritium Generated in Lithium-Enriched Li2+xTiO3 by Thermal Neutron Irradiation. Fusion Eng. Des. 2012, 87, 471–475. [Google Scholar] [CrossRef]
- Wang, H.; Xing, W.; Chen, S.; Song, C.; Dickey, M.D.; Deng, T. Liquid Metal Composites with Enhanced Thermal Conductivity and Stability Using Molecular Thermal Linker. Adv. Mater. 2021, 33, 2103104. [Google Scholar] [CrossRef]
- Lässer, R.; Bell, A.C.; Brennan, D.; Ciattaglia, S.; Coad, J.P.; Forrest, R.A.; Loughlin, M.J.; Newbert, G.; Patel, B.; Rolfe, A.; et al. Fusion Nuclear Technology Issues Studied on the JET Facilities. Fusion Eng. Des. 2002, 63, 35–46. [Google Scholar] [CrossRef]
- Coleman, M.; Hörstensmeyer, Y.; Cismondi, F. DEMO Tritium Fuel Cycle: Performance, Parameter Explorations, and Design Space Constraints. Fusion Eng. Des. 2019, 141, 79–90. [Google Scholar] [CrossRef]
- Johnson, C.E.; Kondo, T.; Roux, N.; Tanaka, S.; Vollath, D. Fabrication, Properties, and Tritium Recovery from Solid Breeder Materials. Fusion Eng. Des. 1991, 16, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Katayama, K.; Ipponsugi, A.; Hoshino, T. Influence of Lithium Mass Transfer on Tritium Behavior in Pebbles of Li2TiO3 with Excess Lithium. Fusion Eng. Des. 2020, 161, 112011. [Google Scholar] [CrossRef]
- Hanada, T.; Nishikawa, M.; Kanazawa, T.; Yamasaki, H.; Yamashita, N.; Fukada, S. Effect of Surface Water on Tritium Release Behavior from Li2TiO3. J. Nucl. Mater. 2011, 417, 735–738. [Google Scholar] [CrossRef]
- Kulsartov, T.; Shaimerdenov, A.; Zaurbekova, Z.; Kenzhina, I.; Chikhray, Y.; Kizane, G.; Blynskiy, P.; Akhanov, A.; Ponkratov, Y. Features of the In-Situ Experiments on Studying of Tritium Release from Lithium Ceramic Li2TiO3 Using Vacuum Extraction Method. Fusion Eng. Des. 2021, 172, 112703. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Fenyvesi, F.; Lekli, I.; Béreshova, M.; Bak, I.; Czagány, M.; Vasvári, G.; Bácskay, I.; Tóth, J.; Budai, I. Preparation of Acyclovir-Containing Solid Foam by Ultrasonic Batch Technology. Pharmaceutics 2021, 13, 1571. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Kikukawa, A.; Yamaki, D.; Nakamichi, M.; Enoeda, M. In-Situ Tritium Release Behavior from Li2TiO3 Pebble-Bed. Fusion Eng. Des. 2001, 59, 679–682. [Google Scholar] [CrossRef]
- Yang, M.; Ran, G.; Wang, H.; Dang, C.; Huang, Z.; Chen, X.; Lu, T.; Xiao, C. Fabrication and Tritium Release Property of Li2TiO3-Li4SiO4 Biphasic Ceramics. J. Nucl. Mater. 2018, 503, 151–156. [Google Scholar] [CrossRef]
- Kobayashi, M.; Uchimura, H.; Toda, K.; Oya, Y. Effects of Helium and Ambient Water Vapor on Tritium Release from Li2TiO. J. Nucl. Mater. 2014, 455, 735–738. [Google Scholar] [CrossRef]
- Zhu, D.; Oda, T.; Shono, Y.; Tanaka, S. Release Behavior of Hydrogen Isotopes Thermally Sorbed in Li2TiO3 Single Crystal. J. Nucl. Mater. 2013, 442, 437–441. [Google Scholar] [CrossRef]
- Hino, T.; Shibata, H.; Yamauchi, Y.; Nobuta, Y.; Suzuki, S.; Akiba, M. Deuterium Ion Irradiation for Tritium Breeding Material and Evaluation for Tritium Inventories in Test Blanket Module and Blanket of a Demonstration Reactor. J. Nucl. Mater. 2011, 417, 713–717. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, Y.; Liu, F.; An, Z.; Lu, T.; Xiang, M.; Zhou, H.; Li, X.; Zhao, M.; Zhang, Y.; et al. Release Behavior of Hydrogen Isotopes in Deuterium-Irradiated Li2TiO3. Fusion Eng. Des. 2016, 113, 318–323. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, M.; Xu, Y.; Liu, F.; Lu, T.; Liu, H.; Zhou, H.; Luo, G.N.; Qi, Q. The Influences of Deuterium Irradiation Defects on Mechanical Properties for Ceramic Breeder Material Li2TiO3. Fusion Eng. Des. 2017, 121, 182–187. [Google Scholar] [CrossRef]
- Gu, S.; Ji, B.; Qi, Q.; Wang, J.; Zhou, H.; Zhang, Y.; Luo, G.N. The Effects of Irradiation and High Temperature on Chemical States in Li2TiO3. Int. J. Hydrogen Energy 2019, 44, 32151–32157. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Yamauchi, Y.; Nobuta, Y.; Hino, T.; Akiba, M.; Enoeda, M. Influence of Surface Condition on Deuterium Release from Li2TiO3 Pebble. Fusion Eng. Des. 2014, 89, 1280–1283. [Google Scholar] [CrossRef]
- Nakazawa, T. High Energy Heavy Ion Induced Structural Disorder in Li2TiO3. J. Nucl. Mater. 2007, 370, 1398–1403. [Google Scholar] [CrossRef]
- Fukushima, H.; Shimomura, Y. Damage Structure Due to Displacement Cascades in 14 MeV Neutron-Irradiated Metals Studied by Positron Lifetime and TEM Techniques. J. Nucl. Mater. 1993, 205, 59–67. [Google Scholar] [CrossRef]
- Osuo, J.; Kobayashi, M.; Kurata, R.; Hamada, A.; Wang, W.; Fujii, T.; Yamana, H.; Luo, T.; Feng, K.; Oya, Y.; et al. Dependence of Gamma-Ray Dose on Annihilation Processes of Irradiation Defects in Li2TiO. Fusion Eng. Des. 2011, 86, 2362–2364. [Google Scholar]
- Kobayashi, M.; Toda, K.; Oya, Y.; Okuno, K. Dependency of Irradiation Damage Density on Tritium Migration Behaviors in Li2TiO3. J. Nucl. Mater. 2014, 447, 1–8. [Google Scholar] [CrossRef]
- Tio, L.; Suzuki, S.; Kobayashi, M.; Kurata, R.; Wang, W.; Fujii, T.; Yamana, H.; Feng, K.; Oya, Y.; Okuno, K. Elucidation of Annihilation Processes of Defects Induced by γ-Irradiation in Li2TiO3. Fusion Eng. Des. 2010, 85, 2331–2333. [Google Scholar]
- Kobayashi, Y.; Sawamura, M.; Kondo, S. Activation and Stabilization Mechanisms of Anionic Redox for Li Storage Applications: Joint Experimental and Theoretical Study on Li2TiO3–Limno2 Binary System. Mater. Today. 2020, 37, 43–55. [Google Scholar] [CrossRef]
- Ipponsugi, A.; Katayama, K.; Hoshino, T. The Influence of the Long-Term Heating under H2 Atmosphere on the Tritium Release Behavior from the Neutron-Irradiated Li2TiO3. Fusion Eng. Des. 2021, 170, 112495. [Google Scholar] [CrossRef]
- Nakazawa, T.; Grismanovs, V.; Yamaki, D.; Katano, Y.; Aruga, T. Disordering in Li2TiO3 Irradiated with High Energy Ions. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 206, 166–170. [Google Scholar] [CrossRef]
- Zhou, Q.; Oya, Y.; Chikada, T.; Zhang, W.; Xue, L.; Yan, Y. Preparation of Li2TiO3 by Hydrothermal Synthesis and Its Structure Evolution under High Energy Ar+ Irradiation. J. Eur. Ceram. Soc. 2017, 37, 4955–4961. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Xue, L.; Li, H.; Yan, Y. Synthesis of the Biphasic Mixture of Li2TiO3-Li4SiO4 and Its Irradiation Performance. J. Eur. Ceram. Soc. 2016, 36, 4107–4113. [Google Scholar] [CrossRef]
- Shimwell, J.; Kovari, M.; Lilley, S.; Zheng, S.; Morgan, L.W.G.; Packer, L.W.; Mcmillan, J. A Parameter Study of Time-Varying Tritium Production in Solid-Type Breeder Blankets. Fusion Eng. Des. 2016, 104, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Munakata, K.; Yokoyama, Y.; Baba, A. Tritium Release from Catalytic Breeder Materials. Fusion Eng. Des. 2001, 59, 683–687. [Google Scholar] [CrossRef]
- Roux, N.; Johnson, C.; Noda, K. Properties and Performance of Tritium Breeding Ceramics. J. Nucl. Mater. 1992, 194, 15–22. [Google Scholar]
- Cho, S.; Park, Y.; Chun, Y.; Min, K.; Ahn, M. Chemical Compatibility between ARAA Alloy and Lithium Meta-Titanate Breeder Material. Fusion Eng. Des. 2017, 124, 1052–1058. [Google Scholar] [CrossRef]
- Sathiyamoorthy, D.; Ghanwat, S.J.; Tripathi, B.M.; Danani, C. Novel Mixed-Oxide Ceramic for Neutron Multiplication and Tritium Generation. J. Nucl. Mater. 2011, 417, 775–779. [Google Scholar] [CrossRef]
- Dang, C.; Yang, M.; Gong, Y.; Feng, L.; Wang, H. A Promising Tritium Breeding Material: Nanostructured 2Li2TiO3-Li4SiO4 Biphasic Ceramic Pebbles. J. Nucl. Mater. 2018, 500, 265–269. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, Y.; Su, J. Grain Boundary Engineering Enabled High-Performance Garnet-Type Electrolyte for Lithium Dendrite Free Lithium Metal Batteries. Small Methods. 2022, 7, 2200667. [Google Scholar] [CrossRef]
- Knitter, R.; Kolb, M.H.H.; Kaufmann, U.; Goraieb, A.A. Fabrication of Modified Lithium Orthosilicate Pebbles by Addition of Titania. J. Nucl. Mater. 2013, 442, 433–436. [Google Scholar] [CrossRef]
- Cruz, D.; Bulbulian, S. Synthesis of Lithium Silicate Tritium Breeder Powders by a Modified Combustion Method. J. Nucl. Mater. 2003, 312, 262–265. [Google Scholar] [CrossRef]
- Urakawa, T.; Matsuura, H.; Kawamoto, Y.; Konishi, S. In Fluence of Modified Neutron Emission Spectrum on Tritium Production Performance in Blanket Systems with NBI-Heated Deuterium Plasma. Fusion Eng. Des. 2018, 136, 678–682. [Google Scholar] [CrossRef]
- Wu, X.; Wen, Z.; Xu, X.; Han, J. Synthesis and Ionic Conductivity of Mg-Doped Li2TIO3. Solid State Ionics 2008, 179, 1779–1782. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Yu, S.; Sun, Z.; Qiao, J. High-Q Microwave Ceramics of Li2TiO3 Co-Doped with Magnesium and Niobium. J. Am. Ceram. Soc. 2018, 101, 4066–4075. [Google Scholar] [CrossRef]
- Chen, G.H.; Xu, H.R.; Yuan, C.L. Microstructure and Microwave Dielectric Properties of Li2Ti1−x(Zn1/3Nb2/3)XO3 Ceramics. Ceram. Int. 2013, 39, 4887–4892. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, R.; Zhang, J. Structure, Microwave Dielectric Properties, and Low-Temperature Sintering of Acceptor/Donor Codoped Li2Ti1−x(Al0.5Nb0.5)XO3 Ceramics. J. Am. Ceram. Soc. 2016, 99, 825–832. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Kolb, M.H.H.; Gan, Y.; Kamlah, M.; Knitter, R. Solution Based Synthesis of Mixed-Phase Materials in the Li2TiO3-Li4SiO4 System. J. Nucl. Mater. 2015, 456, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, T.; Kato, K.; Natori, Y.; Oikawa, F.; Nakano, N.; Nakamura, M.; Sasaki, K.; Suzuki, A.; Terai, T.; Tatenuma, K. Development of Advanced Tritium Breeding Material with Added Lithium for ITER-TBM. J. Nucl. Mater. 2011, 417, 684–687. [Google Scholar] [CrossRef]
- Wang, J.; Kang, J.; Zhang, Y.; Huang, X. Viscosity Monitoring and Control on Oil-Film Bearing Lubrication with Ferrofluids. Tribol. Int. 2014, 75, 61–68. [Google Scholar]
- Qi, Q.; Wang, J.; Zhou, Q.; Zhang, Y.; Zhao, M.; Gu, S.; Nakata, M.; Zhou, H.; Oya, Y.; Luo, G. Comparison of Tritium Release Behavior in Li2TiO3 and Promising Core-Shell Li2TiO3-Li4SiO4 Biphasic Ceramic Pebbles. J. Nucl. Mater. 2020, 539, 152330. [Google Scholar] [CrossRef]
- Leys, O.; Odemer, C.; Maciejewski, U.; Kolb, M.H.; Knitter, R. Microstructure Analysis of Melt-Based Lithium Orthosilicate/Metatitanate Pebbles. Prakt. Metallogr. 2013, 50, 196–204. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, L.; Ran, G.; Gong, Y.; Wang, H.; Peng, S.; Xiao, C.; Chen, X.; Lu, T. Tritium Release Behavior of Li2TiO3 and 2Li2TiO3-Li4SiO4 Biphasic Ceramic Pebbles Fabricated by Microwave Sintering. Fusion Eng. Des. 2021, 168, 112390. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, R.; Yang, M.; Wang, H.; Guo, H.; Shi, Y.; Huang, Z.; Qi, J.; Shi, Q.; Lu, T. Fast Fabrication of High Quality Li2TiO3-Li4SiO4 Biphasic Ceramic Pebbles by Microwave Sintering: In Comparison with Conventional Sintering. Ceram. Int. 2019, 45, 19022–19026. [Google Scholar] [CrossRef]
- Pham, T.D.; Le, T.M.A.; Pham, T.M.Q.; Dang, V.H.; Vu, K.L.; Tran, T.K.; Hoang, T.H. Synthesis and Characterization of Novel Hybridized CeO2@SiO2Nanoparticles Based on Rice Husk and Their Application in Antibiotic Removal. Langmuir 2021, 37, 2963–2973. [Google Scholar] [CrossRef]
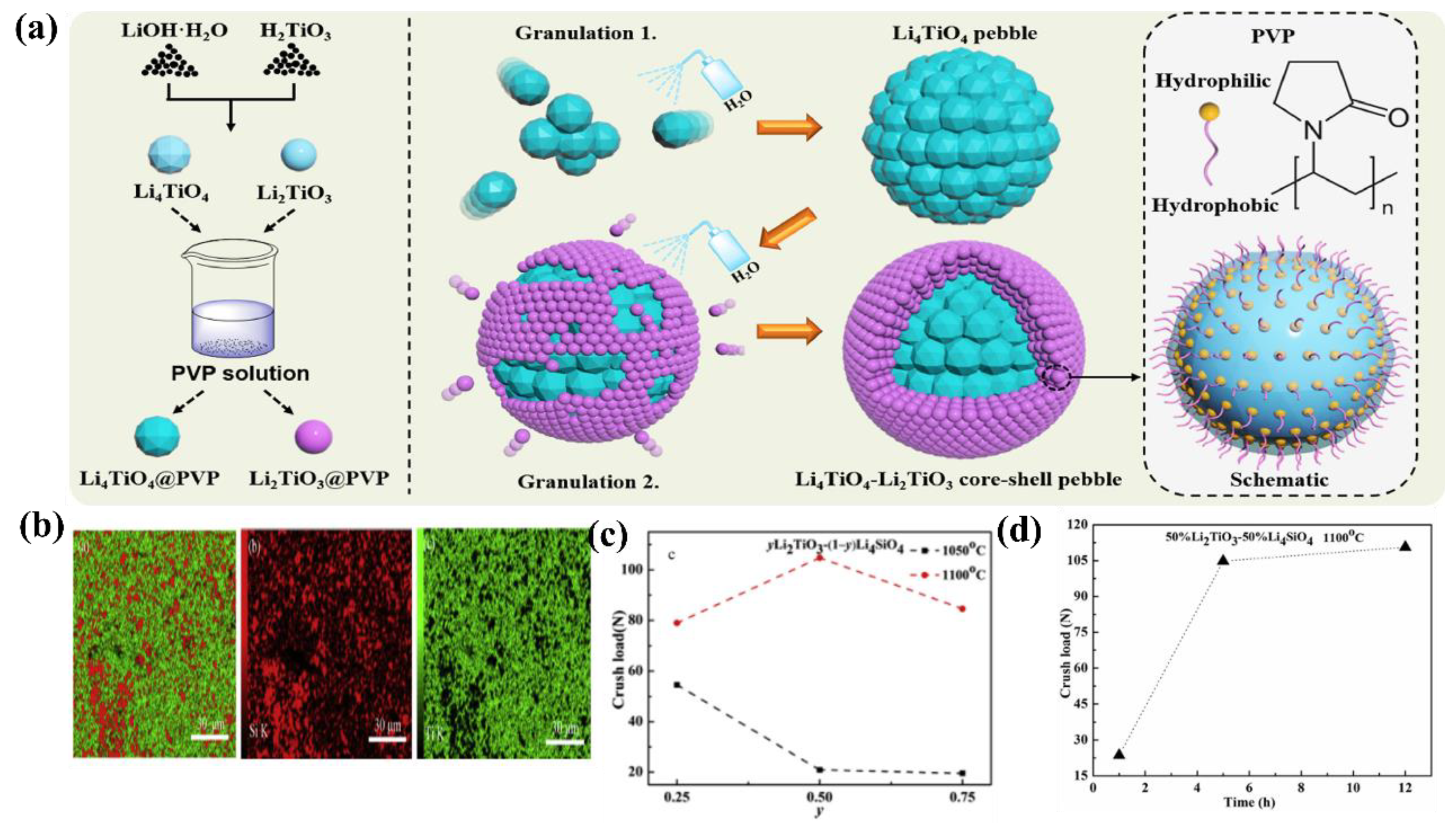
- Chen, R.; Liao, Z.; Shi, Y.; Wang, H.; Guo, H.; Zeng, Y.; Qi, J.; Shi, Q.; Lu, T. Preparation of Li4TiO4-Li2TiO3 Core-Shell Ceramic Pebbles with Thick Shells and High Strength through an Improved Granulation Method. J. Nucl. Mater. 2021, 543, 152580. [Google Scholar] [CrossRef]
- Xiang, M.; Zhang, Y.; Zhang, Y.; Liu, S.; Liu, H.; Wang, C.; Gu, C. Preparation of Li2TiO3-Li4SiO4 Core-Shell Ceramic Pebbles with Enhanced Crush Load by Graphite Bed Process. J. Nucl. Mater. 2015, 466, 477–483. [Google Scholar] [CrossRef]
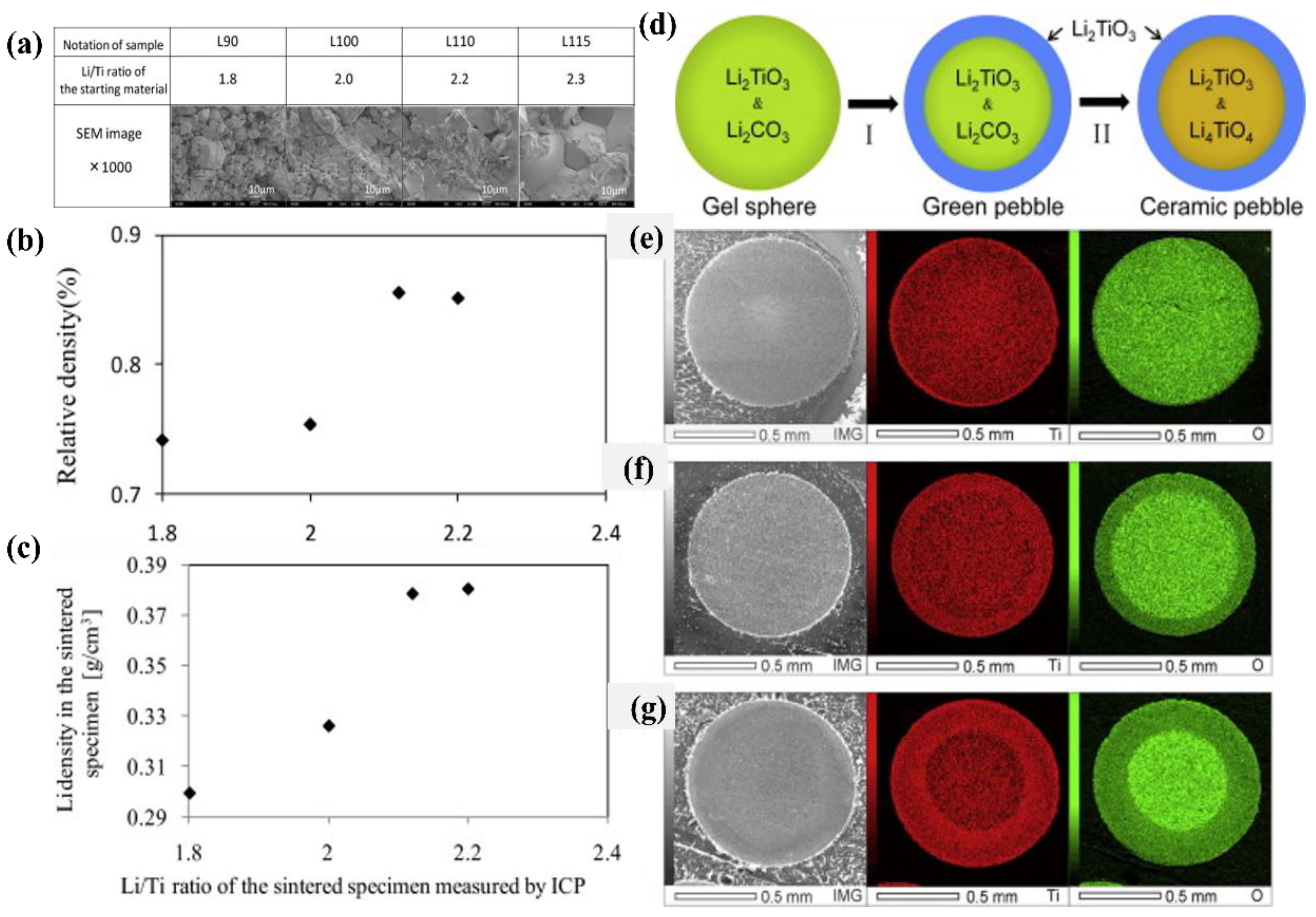




| Organization | Pebble Size/mm | Density (% T.D.) | Grain Size | Crush Load (N) | Ref. |
|---|---|---|---|---|---|
| NFRI, Korea | 1 mm | ~ | 150 nm | 7.5 N | [36] |
| SCU, China | ~ | 85.1% | 19.6 nm | 42.8 N | [37] |
| PMD, India | ~ | 98% | 2–3 μm | ~ | [38] |
| France | 0.8–1.2 mm | 90% | 1–2 μm | 38–66 N | [39] |
| WUT, China | ~ | 96.5% | 2–3 μm | 20–30 N | [40] |
| Organization | Pebble Size/mm | Density (% T.D.) | Grain Size | Crush Load (N) | Ref. |
|---|---|---|---|---|---|
| SIC, China | 1.4 mm | 68% | 10 μm | ~ | [44] |
| SIC, China | 1.18–1.3 mm | 85% | 5 μm | ~ | [45] |
| FCD, India | 0.6–0.7 mm | 90% | 100 μm | ~ | [46] |
| INCT, Poland | ~ | 88% | 250 nm | ~ | [47] |
| USTB, China | 1.3–1.5 mm | 84.9% | 2.85 μm | 67 N | [49] |
| Organization | Pebble Size/mm | Density (% T.D.) | Grain Size | Crush Load (N) | Ref. |
|---|---|---|---|---|---|
| HUST, China | 1.5 mm | 81% | 0.82 μm | 34 N | [52] |
| HUST, China | 1.0–1.2 mm | 81% | 100 nm | 35 N | [53] |
| USTB, China | 1.2 mm | 85.2% | 1.4 μm | 53 N | [55] |
| SCU, China | 1.7 mm | 89.71% | 90 nm | 99.93 N | [56] |
| SCU, China | 1.5 mm | 89% | 40 nm | 90 N | [57] |
| Organization | Pebble Size/mm | Density (% T.D.) | Grain Size | Crush Load (N) | Ref. |
|---|---|---|---|---|---|
| HUST, China | ~ | 85.8% | 5 nm | 37.2 | [59] |
| HUST, China | ~ | 92.6% | 1 μm | ~ | [60] |
| NMTDD, Korea | 2 mm | 80%–87% | ~ | ~ | [61] |
| HUST, China | ~ | 90% | 800 nm | ~ | [62] |
| KAERI, China | ~ | ~ | 0.3–0.5 μm | 30 N | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Qi, C.; Wang, B. Recent Progress in Research of Solid Tritium Breeder Materials Li2TiO3: A Review. Coatings 2022, 12, 1053. https://doi.org/10.3390/coatings12081053
Xu K, Qi C, Wang B. Recent Progress in Research of Solid Tritium Breeder Materials Li2TiO3: A Review. Coatings. 2022; 12(8):1053. https://doi.org/10.3390/coatings12081053
Chicago/Turabian StyleXu, Kun, Chao Qi, and Bo Wang. 2022. "Recent Progress in Research of Solid Tritium Breeder Materials Li2TiO3: A Review" Coatings 12, no. 8: 1053. https://doi.org/10.3390/coatings12081053
APA StyleXu, K., Qi, C., & Wang, B. (2022). Recent Progress in Research of Solid Tritium Breeder Materials Li2TiO3: A Review. Coatings, 12(8), 1053. https://doi.org/10.3390/coatings12081053






