Chemical Bonding of Biomolecules to the Surface of Nano-Hydroxyapatite to Enhance Its Bioactivity
Abstract
:1. Introduction
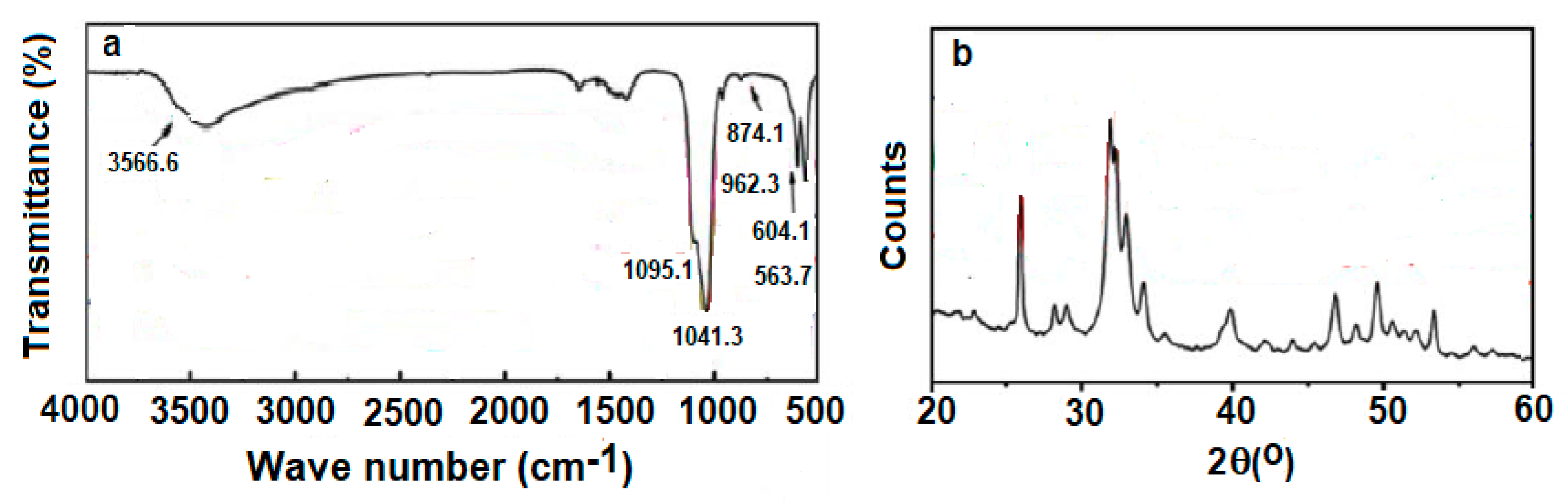
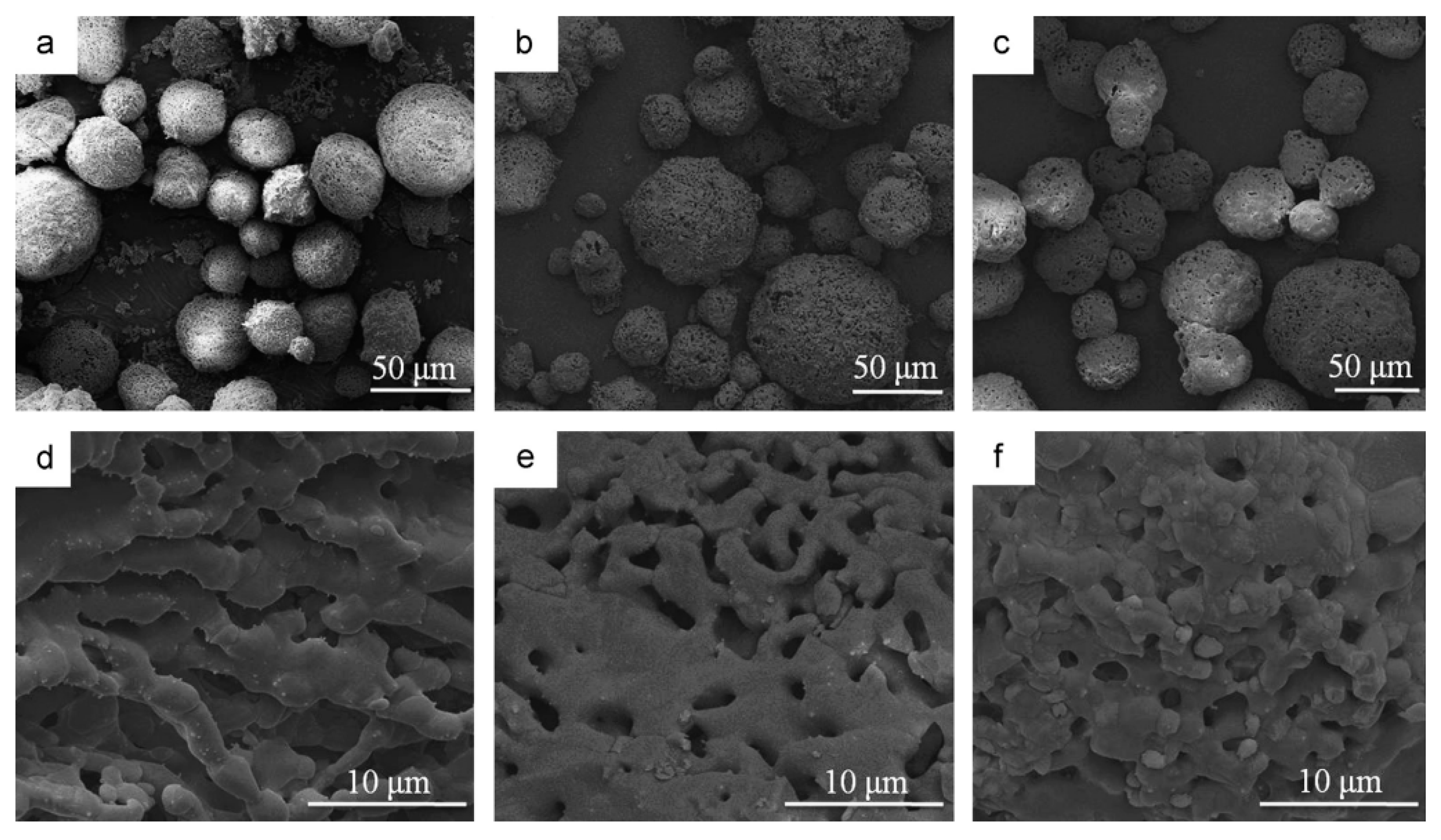
- (a)
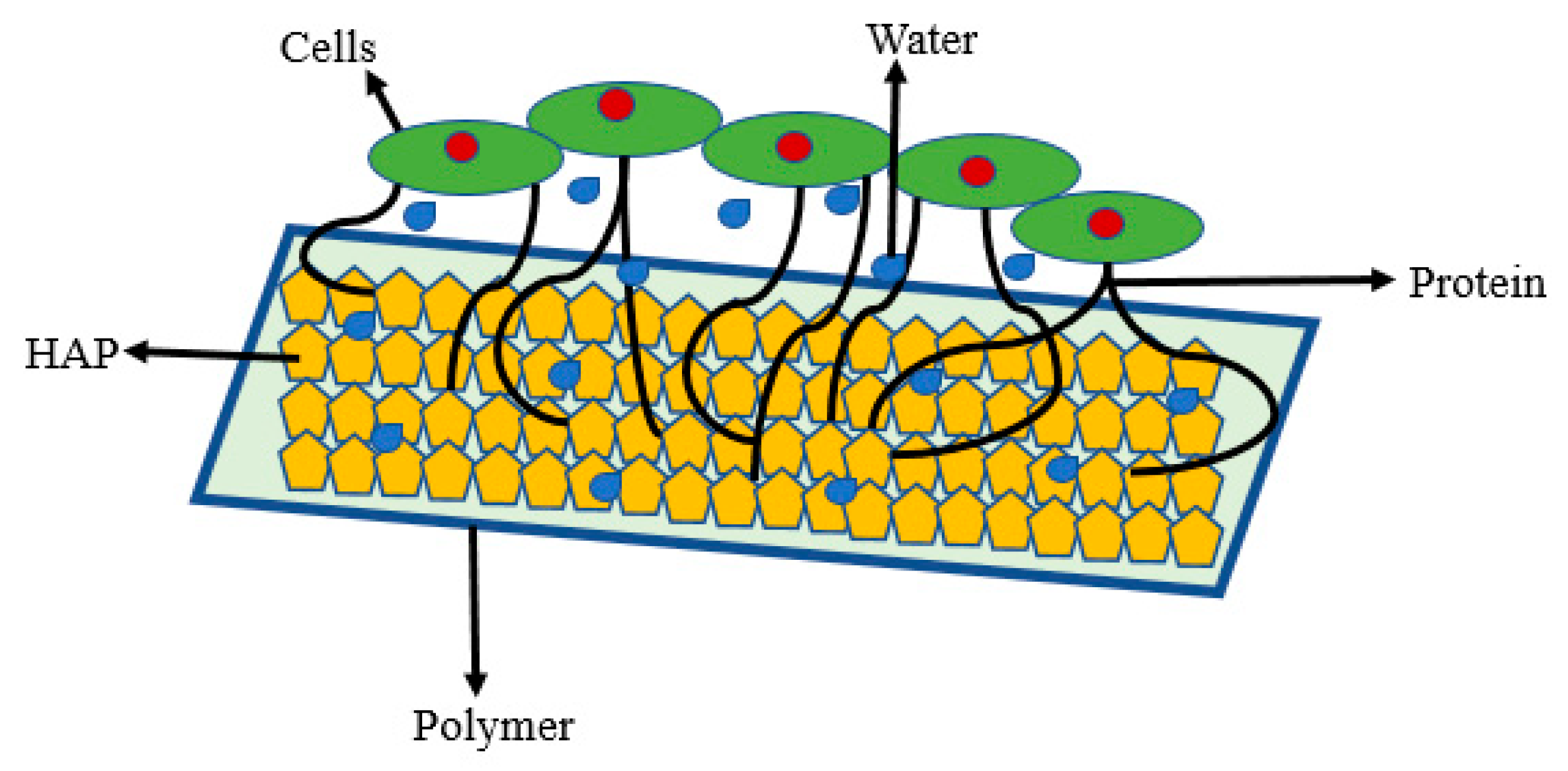
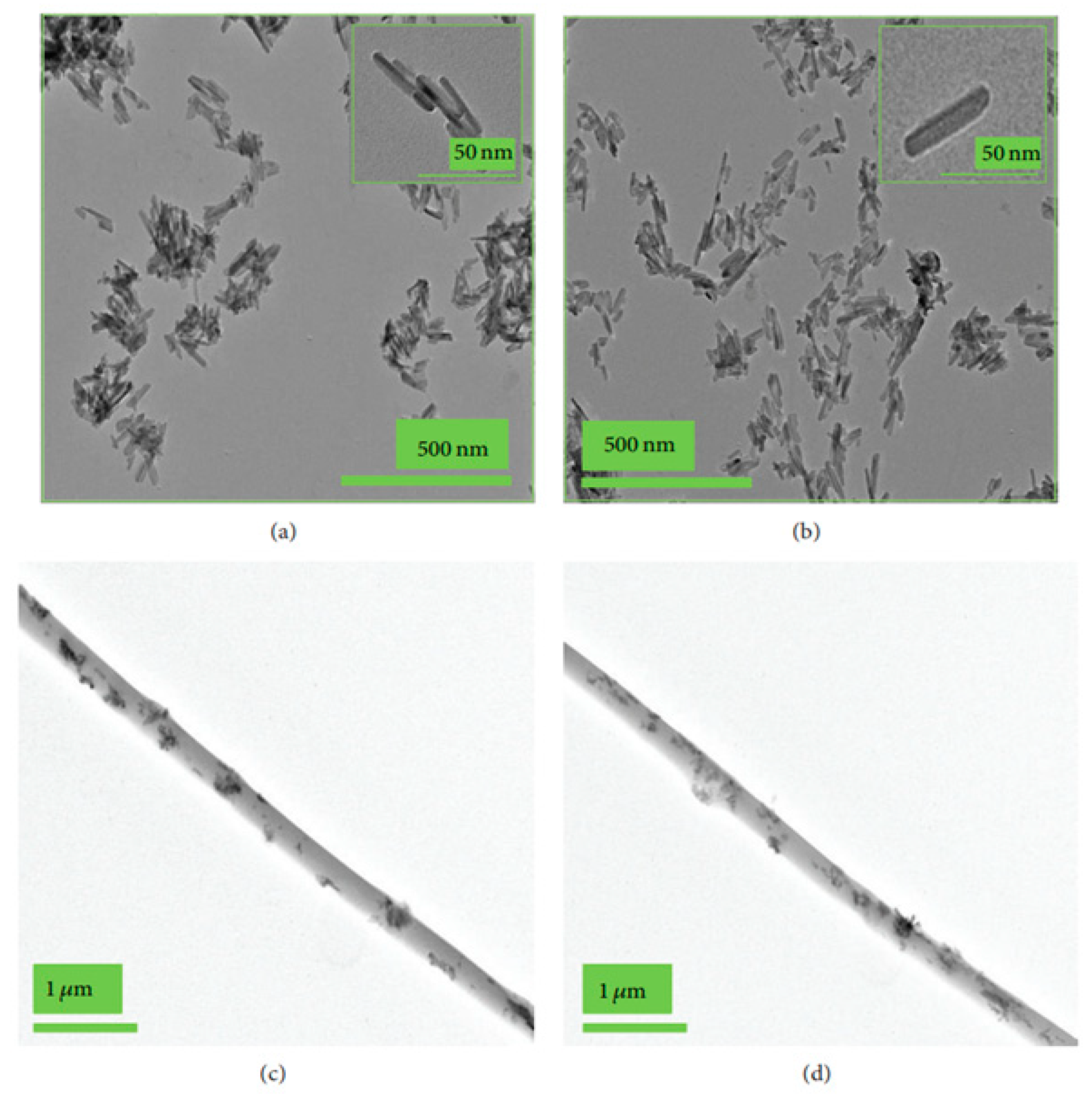
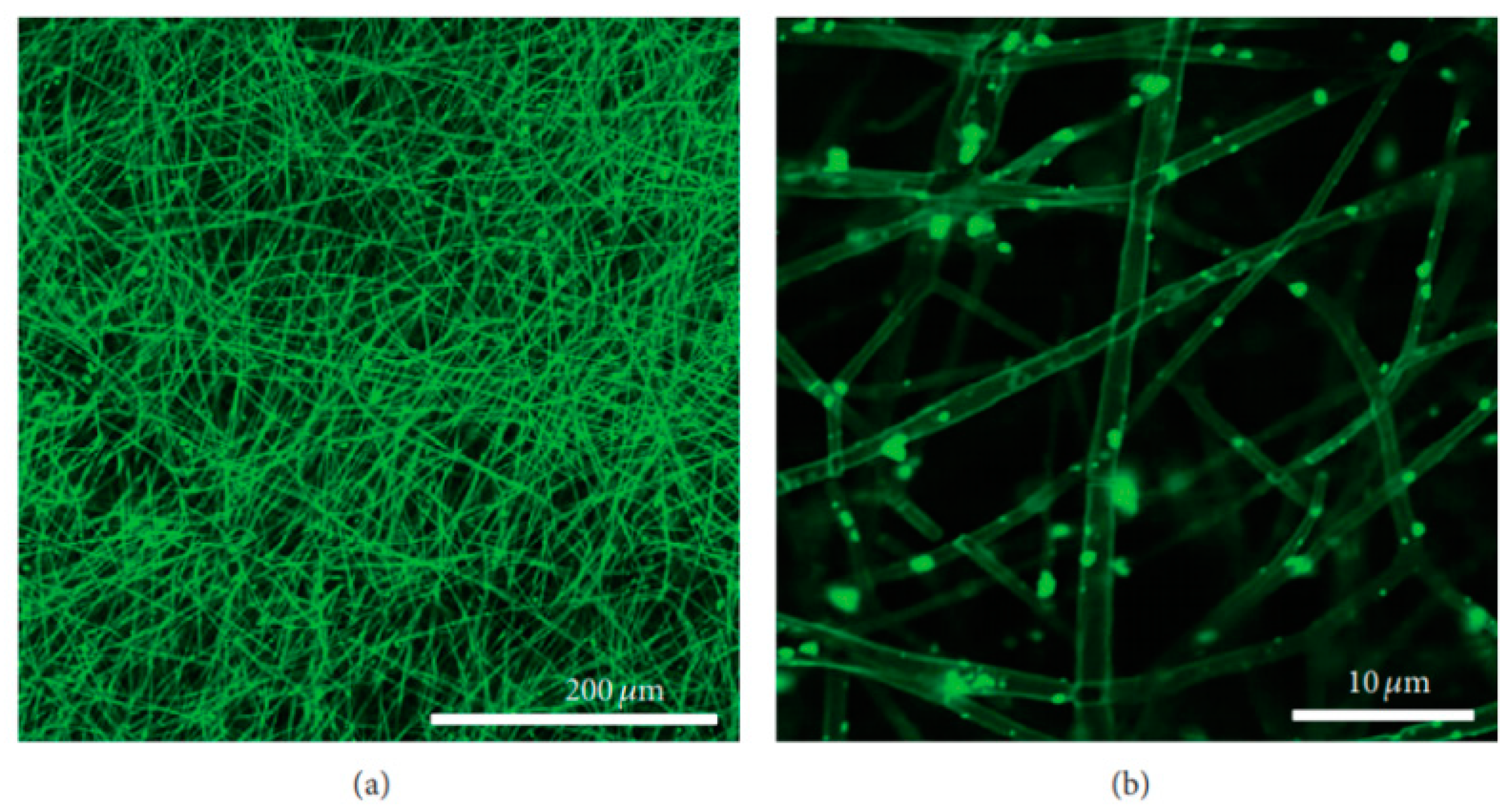
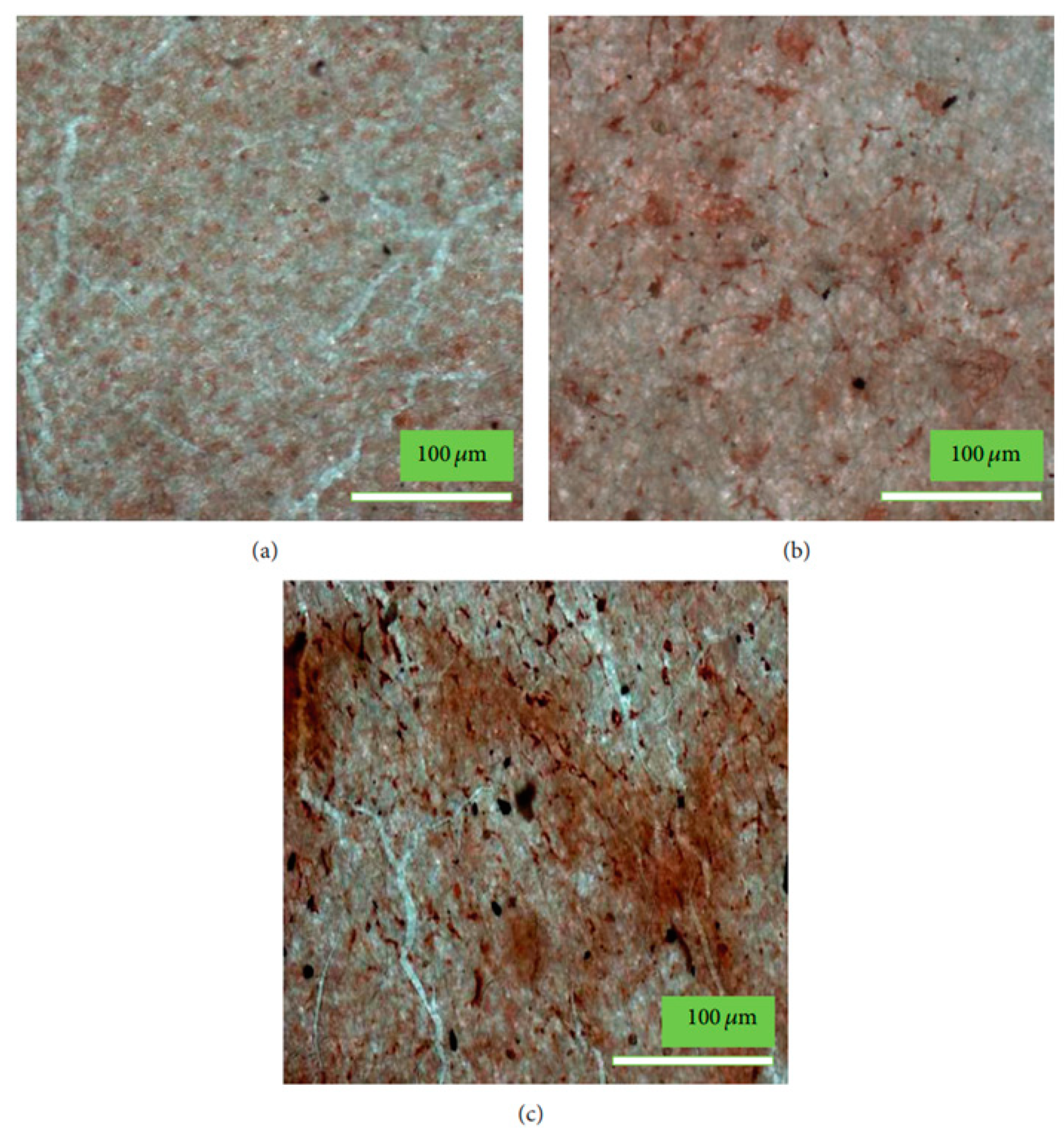
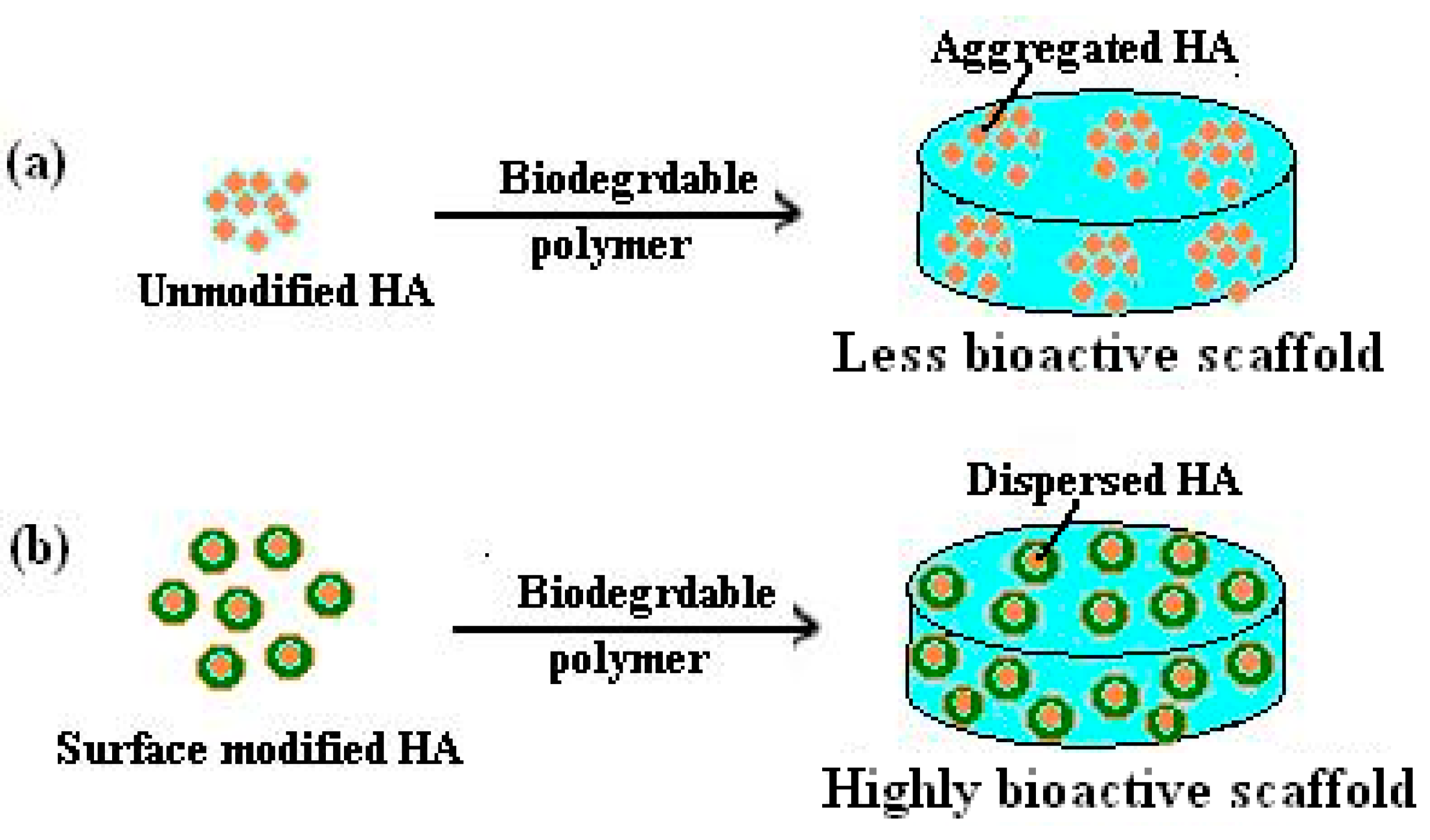
- The coating may produce morphological changes at the surfaces nHA (Figure 2a) that will influence surface roughness, which improves the mechanical properties of bone and help in stimulating the activity of cells;
- (b)
- (c)
2. Bioactivity of Scaffolds Having Nanohydroxyapatite Particles Modified with Natural Molecules or Drugs
2.1. Bioactivity of nHA Particles Modified Chemically with Natural Molecules
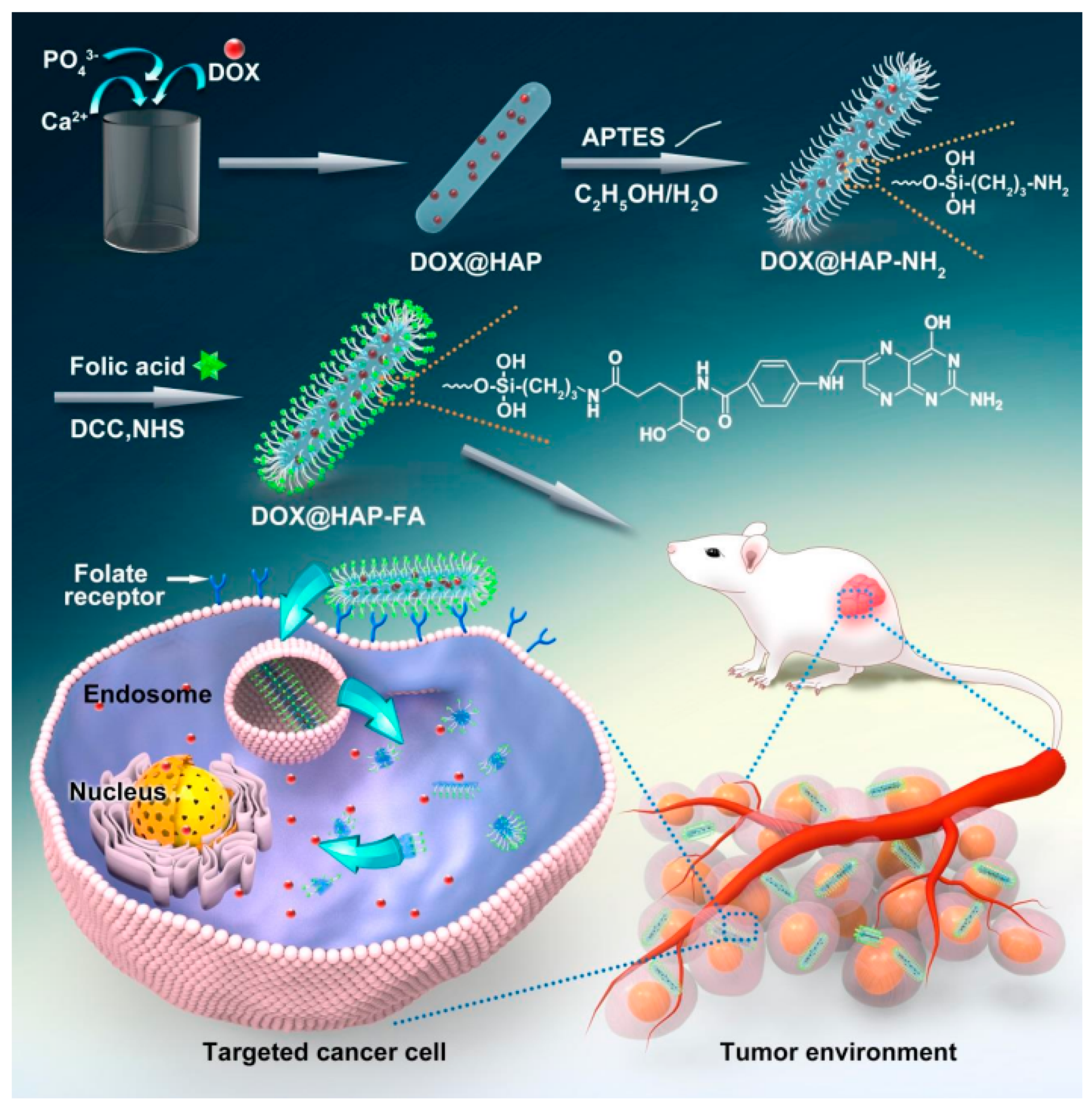
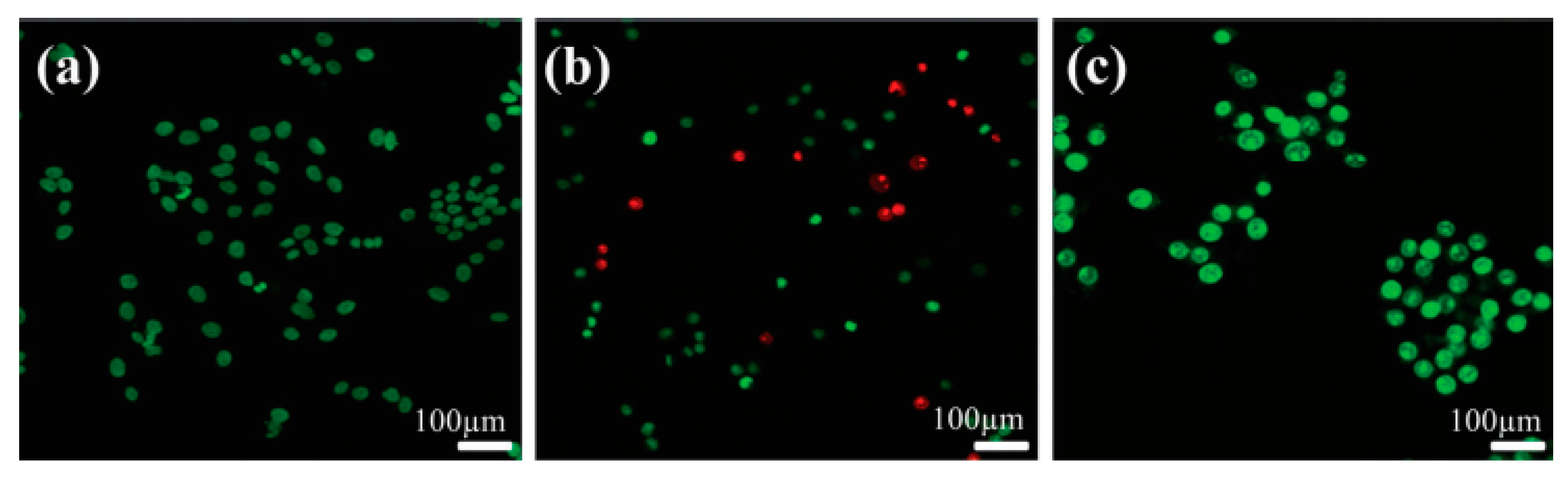
2.2. Drug-Releasing Orthopedic Implants Having Chemically Bonded Drugs at the Surface of nHA
3. Bioactivity of Scaffolds Having Small Molecules/Proteins-Modified nHA
3.1. Biological Activity of Scaffolds Having Collagen Modified nHA
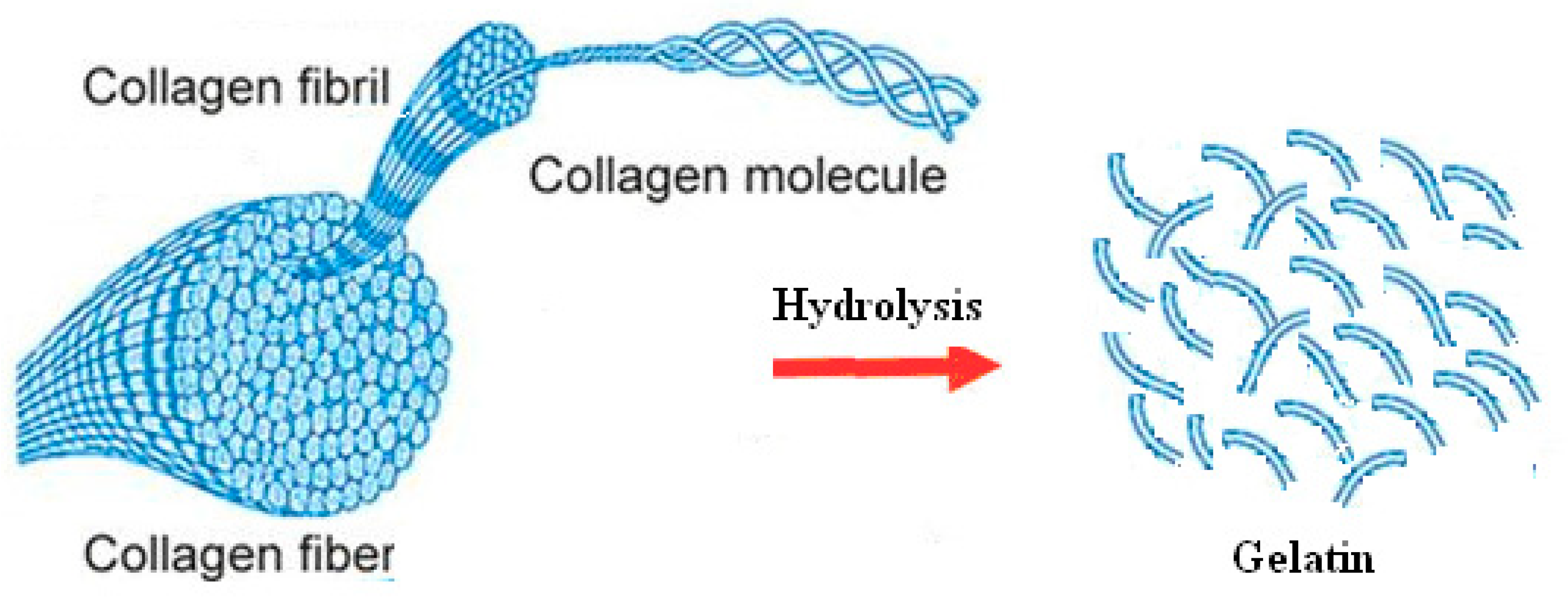
3.2. Biological Activity of Scaffolds Having Gelatin-Modified nHA
3.3. Bioactivity of Scaffolds Having Insulin-Modified nHA
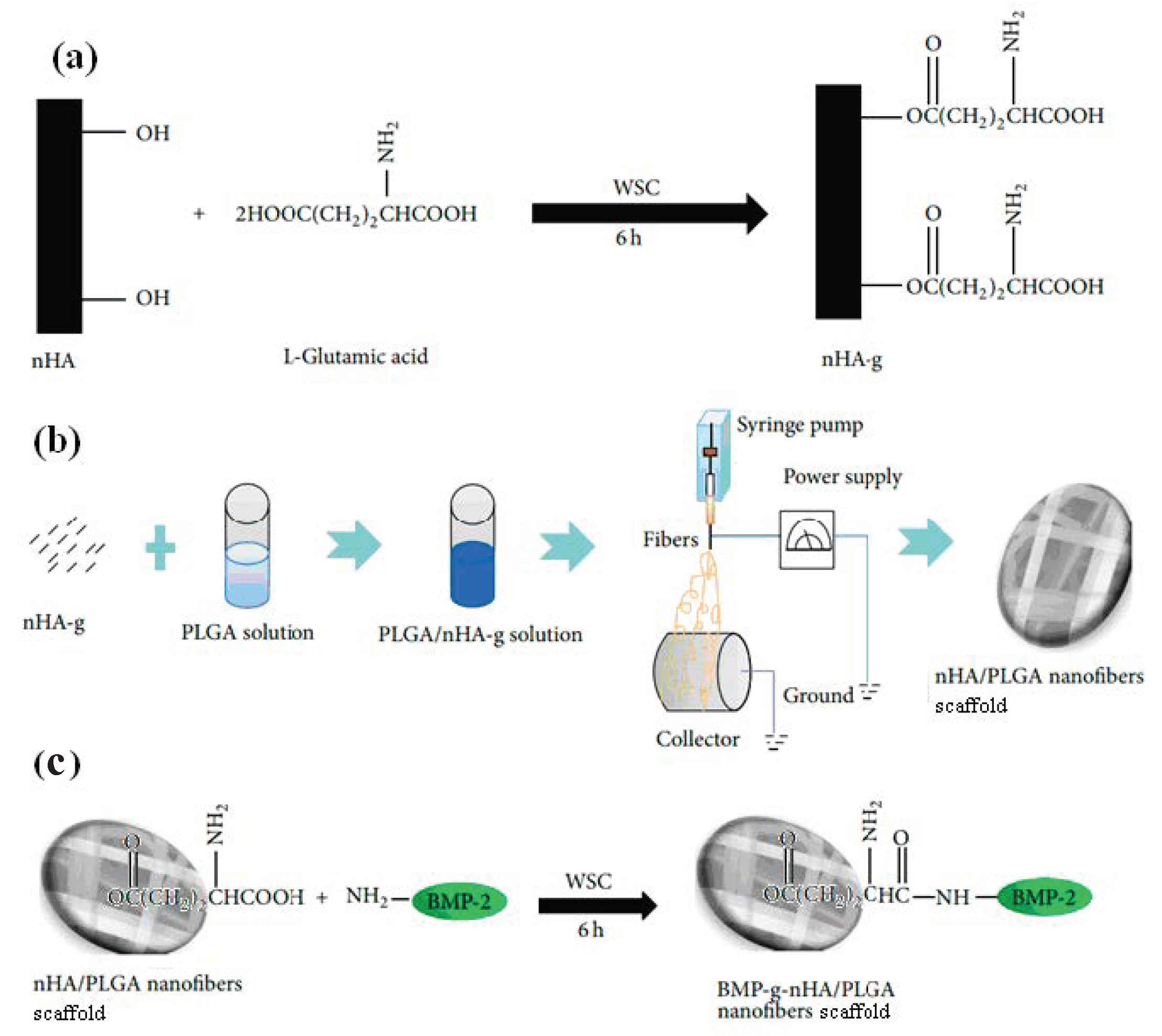
3.4. Biological Activity of Scaffolds Having BMPs-Modified nHA
3.5. Bioactivity of Scaffolds Having nHA Modified with Miscellaneous Molecules
3.6. Effect of Irradiation on Antibacterials and Bioactivity of Hydroxyapatite Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, H.Y.; Safwat, N.; Shehata, R.; Althubaiti, E.H.; Kareem, S.; Atef, A.; Qari, S.H.; Aljahani, A.H.; Al-Meshal, A.S.; Youssef, M.; et al. Synthesis of natural nanohydroxyapatite from snail shells and its biological activity: Antimicrobial, antibiofilm, and biocompatibility. Membranes 2022, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. 3D-printed hydroxyapatite and tricalcium phosphates-based scaffolds for alveolar bone regeneration in animal models: A scoping review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Zeynep, B.; Takashi, K.; Feza, K.; Hideki, Y. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Jingyi, L.; Huijun, Y.; Chuanzhong, C. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar]
- Szczes, A.; Holysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Inter. Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Islam, M.S.; Todo, M. Effects of sintering temperature on the compressive mechanical properties of collagen/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Lett. 2016, 173, 231–234. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regi, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Paul, R.; Lizhi, O.; Ching, W.Y. Electronic structure and bonding in calcium apatite crystals: Hydroxyapatite, fluorapatite, chlorapatite, and bromapatite. Phys. Rev. B 2004, 70, 155104. [Google Scholar]
- Masahiro, O.; Takuya, M. Synthesis and modification of apatite nanoparticles for use in dental and medical applications. Jpn. Dent. Sci. Rev. 2015, 51, 85–95. [Google Scholar]
- Carfi, P.F.; Conoscenti, G.; Greco, S.; La Carrubba, V.; Ghersi, G.; Brucato, V. Preparation, characterization and in vitro test of composites poly-lactic acid/hydroxyapatite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2018, 119, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Durucan, C.; Brown, P.W. Low temperature formation of calcium-deficient hydroxyapatite-PLA/PLGA composites. J. Biomed. Mater. Res. 2000, 51, 717–725. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, H.J.; Choi, J.B.; Park, J.C.; Kim, J.K. The effect of poly (lactic-co-glycolic acid)/hydroxyapatite composite scaffold on chondrocyte cyto-compatibility (II). Biomater. Res. 2008, 12, 77–81. [Google Scholar]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly (lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.G.; Escorba-Barrios, V.A.; Pozes-Guillen, A. RGD Functionalization of PLA/starch scaffolds obtained by electrospinning and evaluated in vitro for potential bone regeneration. Mater. Sci. Eng. C 2019, 96, 798–806. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 5953–5957. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, R.; Stephen, C.M.; George, J.D. Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar]
- Król-Morkisz, K.; Karaś, E.; Majka, T.M.; Pielichowski, K.; Pielichowska, K. Thermal stabilization of polyoxymethylene by PEG-functionalized hydroxyapatite: Examining the effects of reduced formaldehyde release and enhanced bioactivity. Adv. Polym. Technol. 2019, 2019, 9728637. [Google Scholar] [CrossRef]
- Slimani, N.; Poggetto, G.D.; Laurienzo, P.; Ksouri, D.; Khireddine, H. Chemical bonding of polycaprolactone on the surface of hydroxyapatite via silanization and ring opening polymerization. Nova Biotechnol. Chim. 2020, 19, 154–164. [Google Scholar] [CrossRef]
- Li, Y.; Weng, W. Surface modification of hydroxyapatite by stearic acid: Characterization and in vitro behaviors. J. Mater. Sci. Mater. Med. 2008, 19, 19–25. [Google Scholar] [CrossRef]
- Haider, A.; Kim, S.; Huh, M.W.; Kang, I.-K. BMP-2 grafted nHA/PLGA hybrid nanofiber scaffold stimulates osteoblastic cells growth. BioMed Res. Int. 2015, 2015, 281909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nifant’ev, I.; Gavrilov, D.; Tavtorkin, A.; Chinova, M.; Besprozvannykh, V.; Komarov, P.; Zaitsev, V.; Podoprigora, I.; Ivchenko, I. Antibacterial poly(ε-CL)/hydroxyapatite electrospun fibers reinforced by poly(ε-CL)-b-poly (ethylene phosphoric acid). Int. J. Mol. Sci. 2021, 22, 7690. [Google Scholar] [CrossRef] [PubMed]
- Tsebriienko, T.; Popov, A.I. Effect of poly (titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Baltacis, K.; Bystrov, V.; Bystrova, A.; Dekhtyar, Y.; Freivalds, T.; Raines, J.; Rozenberga, K.; Sorokins, H.; Zeidaks, M. Physical fundamentals of biomaterials surface electrical functionalization. Materials 2020, 13, 4575. [Google Scholar] [CrossRef]
- Bystrova, A.; Dekhtyar, Y.D.; Popov, A.; Coutinho, J.; Bystrov, V.S. Modified hydroxyapatite structure and properties: Modeling and synchrotron data analysis of modified hydroxyapatite structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Paramonova, E.V.; Dekhtyar, Y.; Katashev, A.; Karlov, A.; Polyaka, N.; Bystrova, A.V.; Patmalnieks, A.; Kholkin, A.L. Computational and experimental studies of size and shape related physical properties of hydroxyapatite nanoparticles. J. Phys. Condens. Matter. 2011, 23, 065302. [Google Scholar] [CrossRef] [PubMed]
- Madrid, C.C.; Paglioni, M.P.; Line, S.R.; Vasconcelos, K.G.; Brandão, T.B.; Lopes, M.A.; Santos-Silva, A.R.; Goes, M.F.D. Structural analysis of enamel in teeth from head-and-neck cancer patients who underwent radiotherapy. Caries Res. 2017, 51, 119–128. [Google Scholar] [CrossRef]
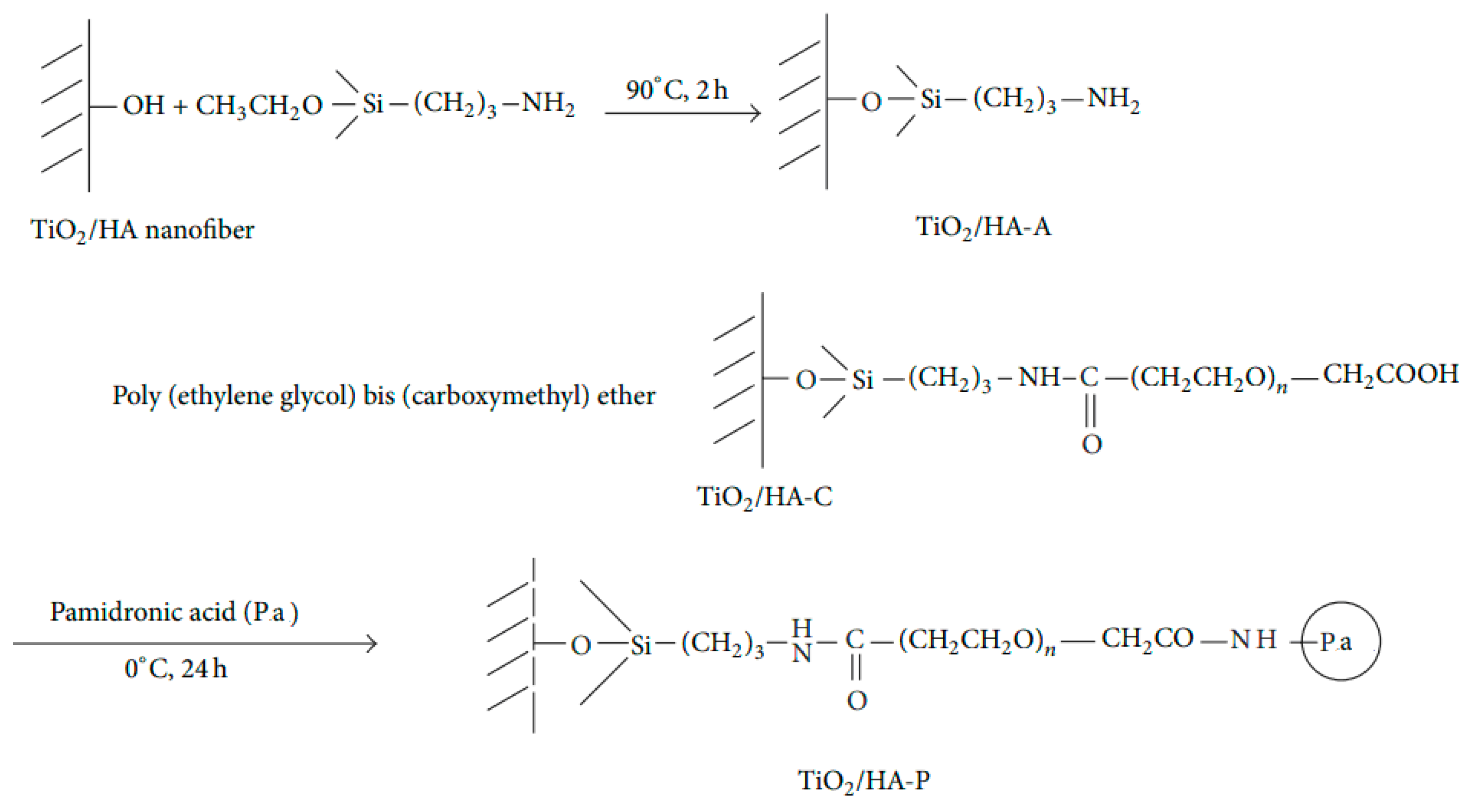
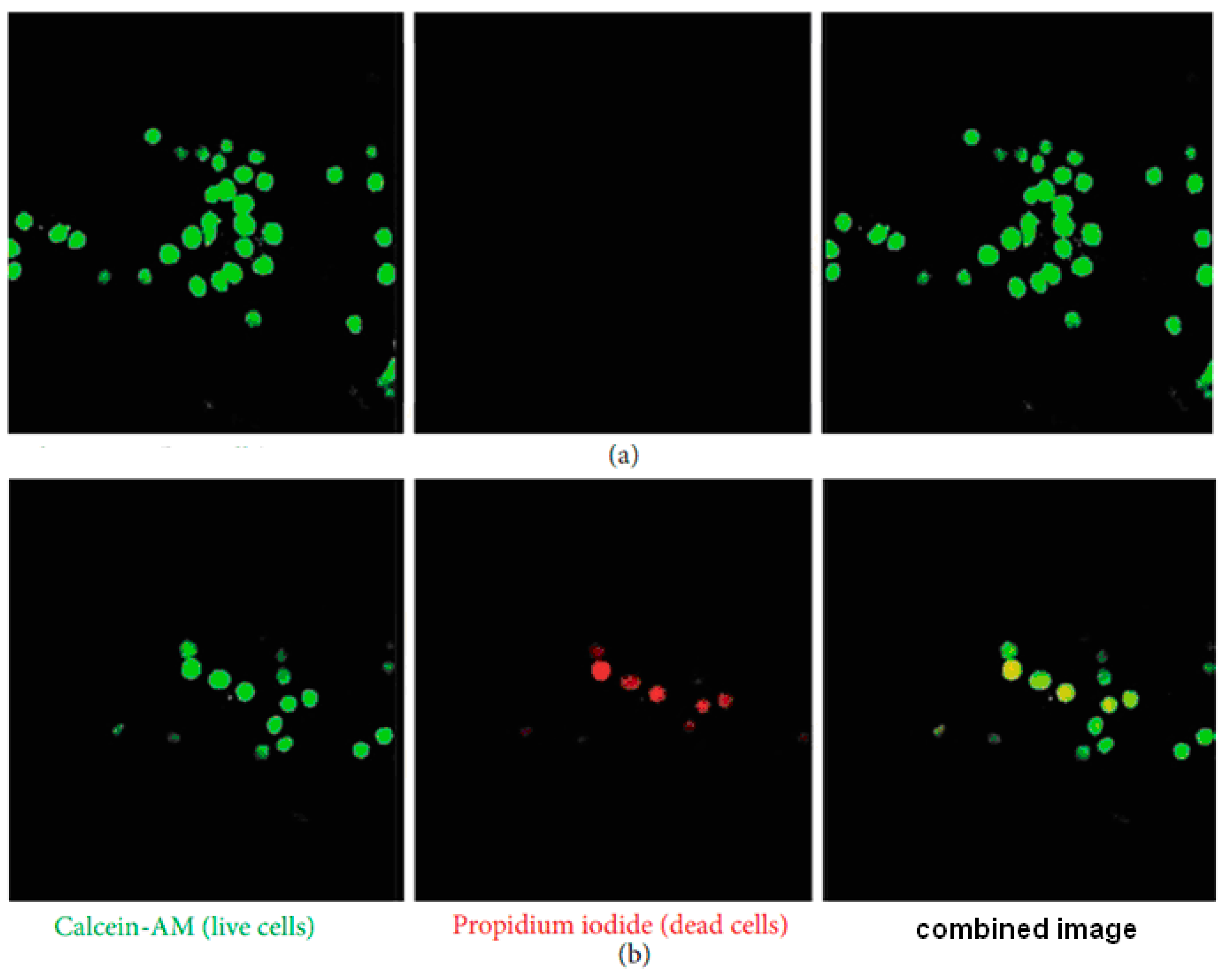
- Haider, A.; Versace, D.L.; Gupta, K.C.; Kang, I.-K. Pamidronic acid-grafted nHA/PLGA hybrid nanofiber scaffolds suppress osteoclastic cell viability and enhance osteoblastic cell activity. J. Mater. Chem. B 2016, 4, 7596–7604. [Google Scholar] [CrossRef]
- Boller, L.A.; Shiels, S.M.; Florian, D.C.; Peck, S.H.; Schoenecker, J.G.; Duvall, C.; Wenke, J.C.; Guelcher, S.A. Effects of nanocrystalline hydroxyapatite concentration and skeletal site on bone and cartilage formation in rats. Acta Biomater. 2021, 130, 485–496. [Google Scholar] [CrossRef]
- Peilong, N.; Hongyan, B.; Gang, Z.; Yingchao, H.M.; Nirmali, W.; Honglian, D.; Xinyu, W. Electrospun preparation and biological properties in vitro of polyvinylalcohol/sodium alginate/nano-hydroxyapatite composite fiber membrane. Coll. Surf. B Biointerfa. 2019, 173, 171–177. [Google Scholar]
- Zhao, L.; Cheng, S.; Liu, S.; Gao, X. Improvement of the addition amount and dispersion of hydroxyapatite in the poly(lactic acid) matrix by the compatibilizer-epoxidized soybean oil. J. Mater. Res. 2020, 35, 1523–1530. [Google Scholar] [CrossRef]
- Jiang, L.X.; Jiang, L.Y.; Xu, L.J.; Han, C.T.; Xiong, C.D. Effect of a new surface-grafting method for nano-hydroxyapatite on the dispersion and the mechanical enhancement for poly (lactide-co-glycolide). Express Polym. Lett. 2014, 8, 133. [Google Scholar] [CrossRef]
- Yu, M.; Kechao, Z.; Fuqiang, Z.; Dou, Z. Porous HA microspheres as drug delivery: Effects of porosity and pore structure on drug loading and in vitro release. Ceram. Int. 2014, 40, 12617–12621. [Google Scholar] [CrossRef]
- Sun, W.; Fan, J.; Wang, S.; Kang, Y.; Du, J.; Peng, X. Biodegradable drug-loaded hydroxyapatite nanotherapeutic agent for targeted drug release in tumors. ACS Appl. Mater. Interf. 2018, 10, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Deng, T.; Yao, P.; Song, H.; Zhou, S.; Ya, W. Development of drug loaded nanoparticles binding to hydroxyapatite based on a bisphosphonate modified nonionic surfactant. J. Nanomater. 2015, 2015, 393968. [Google Scholar] [CrossRef]
- Shin, Y.S.; Borah, J.S.; Haider, A.; Kim, S.; Huh, M.W.; Kang, I.-K. Fabrication of pamidronic acid-immobilized TiO2/hydroxyapatite composite nanofiber mats for biomedical applications. J. Nanomater. 2013, 2013, 404210. [Google Scholar] [CrossRef] [Green Version]
- Denissen, H.; van Beek, E.; van den Bos, T.; de Blieck, J.; Klein, C.; van den Hooff, A. Degradable bisphosphonate-alkaline phosphatase-complexed hydroxyapatite implants in vitro. J. Bone Miner. Res. 1997, 12, 290–297. [Google Scholar] [CrossRef]
- RoussieÁre, H.; Montavon, G.; Samia, L.B.; Janvier, P.; Alonso, B.; Fayon, F.; Petit, M.; Massiot, D.; Bouler, J.M.; Bujoli, B. Hybrid materials applied to biotechnologies; Coating of calcium phosphates for the design of implants active against bone resorption disorders. J. Mater. Chem. 2005, 15, 3869–3875. [Google Scholar] [CrossRef]
- Kurth, A.H.; Eberhardt, C.; Muller, S.; Steinacker, M.; Schwarz, M.; Bauss, F. The bisphosphonate ibandronate improves implant integration in osteopenic ovariectomized rats. Bone 2005, 37, 204–210. [Google Scholar] [CrossRef]
- Peter, B.; Gauthier, O.; Laib, S.; Bujoli, B.; Guicheux, J.; Janvier, P.; van Lenthe, G.H.; Muller, R.; Zambelli, P.Y.; Bouler, J.M.; et al. Local delivery of bisphosphonate from coated orthopedic implants increases implants mechanical stability in osteoporotic rats. J. Biomed. Mater. Res. A 2006, 76, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Peter, B.; Pioletti, D.P.; Laib, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.Y.; Bouler, J.M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Tanzer, M.; Karabasz, D.; Krygier, J.J.; Cohen, R.; Bobyn, J.D. The Otto Aufranc Award: Bone augmentation around and within porous implants by local bisphosphonate elution. Clin. Orthop. Relat. Res. 2005, 441, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echave, M.C.; Sa’nchez, P.; Pedraz, J.L.; Orive, G. Progress of gelatin-based 3D approaches for bone regeneration. J. Drug Deliv. Sci. Technol. 2017, 42, 63. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Zhao, W.; Sahai, N. A potential mechanism for amino acid-controlled crystal growth of hydroxyapatite. J. Mater. Chem. B 2015, 3, 9157–9167. [Google Scholar] [CrossRef]
- Florea, D.A.; Chircov, S.; Grumezescu, A.M. Hydroxyapatite particles-directing the cellular activity in bone regeneration processes: An up-to-date review. Appl. Sci. 2020, 10, 3483. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, Q.; Zhao, W.; Cui, Q.; Sahai, N. Essence of small molecule-mediated control of hydroxyapatite growth: Free energy calculations of amino acid side chain analogues. J. Phys. Chem. C 2018, 122, 4372–4380. [Google Scholar] [CrossRef]
- Tsuji, T.; Onuma, K.; Yamamoto, A.; Iijima, M.; Shiba, K. Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. Proc. Natl. Acad. Sci. USA. 2008, 105, 16866–16870. [Google Scholar] [CrossRef] [Green Version]
- Stevensson, B.; Edén, M. Meta dynamics simulations of the pH-dependent adsorption of phosphoserine and citrate on disordered apatite surfaces: What interactions govern the molecular binding? J. Phys. Chem. C 2021, 125, 11987–12003. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Bystrova, N.K.; Paramonova, E.V.; Dekhtyar, Y.D. Interaction of charged hydroxyapatite and living cells: I. Hydroxyapatite polarization properties. Math. Biol. Bioinform. 2009, 4, 7–11. [Google Scholar]
- Martins, M.A.; Santos, C.; Almeida, M.M.; Costa, M.E.V. Hydroxyapatite micro- and nanoparticles: Nucleation and growth mechanisms in the presence of citrate species. J. Coll. Interface Sci. 2008, 318, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Y.; Liu, X.P.; Ma, X.; Rawal, A.; Prozorov, T.; Akinc, M.; Mallapragada, S.K.; Schmidt-Rohr, K. Biomimetic self-assembling copolymer-hydroxyapatite nanocomposites with the nanocrystal size controlled by citrate. Chem. Mater. 2011, 23, 2481–2490. [Google Scholar] [CrossRef] [Green Version]
- Yeo, I.S.; Min, S.K.; Kang, H.K.; Kwon, T.K.; Jung, S.Y.; Min, B.M. Identification of a bioactive core sequence from human laminin and its applicability to tissue engineering. Biomaterials 2015, 73, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Kudo, T.; Kanetaka, H.; Miyazaki, T.; Hashimoto, M.; Kawashita, M. Adsorption of laminin on hydroxyapatite and alumina and the MC3T3-E1 cell response. ACS Biomater. Sci. Eng. 2016, 2, 1162–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandori, K.; Fudo, A.; Ishikawa, T. Study on the particle texture dependence of protein adsorption by using synthetic micrometer-sized calcium hydroxyapatite particles. Colloids Surf. B 2002, 24, 145–153. [Google Scholar] [CrossRef]
- Baht, G.S.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008, 27, 600–608. [Google Scholar] [CrossRef]
- Gordon, J.A.R.; Tye, C.E.; Sampaio, A.V.; Underhill, T.M.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 2007, 41, 462–473. [Google Scholar] [CrossRef]
- Tavafoghi Jahromi, M.; Yao, G.; Cerruti, M. The importance of amino acid interactions in the crystallization of hydroxyapatite. J. R. Soc. Interface 2013, 10, 20120906. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, Z.; Zhao, W.; Chen, W.; Miyoshi, T.; Sahai, N. Isoexergonic conformations of surface-bound citrate regulated bioinspired apatite nanocrystal growth. ACS Appl. Mater. Interfaces 2016, 8, 28116–28123. [Google Scholar] [CrossRef]
- Benaziz, L.; Barroug, A.; Legrouri, A.; Rey, C.; Lebugle, A. Adsorption of O-phospho-L-serine and L-serine onto poorly crystalline apatite. J. Coll. Interf. Sci. 2001, 238, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Skartsila, K.; Spanos, N. Adsorption monitoring of phospho-l-serine on hydroxyapatite. Colloid Polym. Sci. 2012, 290, 731–739. [Google Scholar] [CrossRef]
- Goobes, G.; Stayton, P.S.; Drobny, G.P. Solid state NMR studies of molecular recognition at protein-mineral interfaces. Prog. Nucl. Magn. Reson. Spectrosc 2007, 50, 71–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.-H.; Tseng, Y.-H.; Mou, Y.; Tsai, Y.-L.; Guo, S.-M.; Huang, S.-J.; Yu, S.S.-F.; Chan, J.C.C. Adsorption of a statherin peptide fragment on the surface of nanocrystallites of hydroxyapatite. J. Am. Chem. Soc. 2008, 130, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Palmer, L.G.; Newcomb, C.J.; Kaltz, S.R.; Spoeke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [Green Version]
- Panda, N.N.; Jonnalagadda, S.; Pramanik, K. Development and evaluation of cross-linked collagen-hydroxyapatite scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 2031–2044. [Google Scholar] [CrossRef]
- Zhang, X.; Gou, W.-G.; Cui, H.; Liu, H.-Y.; Zhang, Y.; Muller, W.E.G.; Cui, F.-Z. In vitro and in vivo enhancement of osteogenic capacity in a synthetic BMP-2 derived peptide-coated mineralized collagen composite. J. Tissue Eng. Regen. Med. 2013, 10, 99–107. [Google Scholar] [CrossRef]
- Chan, E.C.; Kuo, S.M.; Kong, A.M.; Morrison, W.A.; Dusting, G.J.; Mitchell, G.M.; Lim, S.Y.; Liu, G.S. Three dimensional collagen scaffold promotes intrinsic vascularization for tissue engineering applications. PLoS ONE 2016, 11, e0149799. [Google Scholar] [CrossRef] [Green Version]
- Haider, A.; Gupta, K.C.; Kang, I.-K. PLGA/nHA hybrid nanofiber scaffold as a nanocargo carrier of insulin for accelerating bone tissue regeneration. Nanoscale Res. Lett. 2014, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Baumann, M.J.; McCabe, L.R. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J. Biomed. Mater. Res. A 2004, 71, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Hahn, B.-D.; Kang, T.Y.; Lee, M.-J.; Choi, J.Y.; Kim, M.-K.; Kim, S.-G. Hydroxyapatite and collagen combination-coated dental implants display better bone formation in the peri-implant area than the same combination plus bone morphogenetic protein-2-coated implants, hydroxyapatite only coated implants, and uncoated implants. J. Oral Maxillofac. Surg. 2014, 72, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Altınsoy, I.; Çelebi Efe, G.; Ipek, M.; Özacar, M.; Bindal, C. 3D porous collagen/functionalize multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2018, 92, 757–768. [Google Scholar]
- Pederson, A.W.; Ruberti, J.W.; Messersmith, P.B. Thermal assembly of a biomimetic mineral/collagen composite. Biomaterials 2003, 24, 4881–4890. [Google Scholar] [CrossRef]
- Pompe, W.; Worch, H.; Epple, M.; Friess, W.; Gelinsky, M.; Greil, P.; Hempel, U.; Scharnweber, D.; Schulte, K. Functionally graded materials for biomedical applications. Mater. Sci. Eng. A 2003, 362, 40–60. [Google Scholar] [CrossRef]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cells Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef]
- Sachlos, E.; Czernuszka, J.T. Making tissue engineering scaffolds work: Review. the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell Mater. 2003, 5, 29–40. [Google Scholar] [CrossRef]
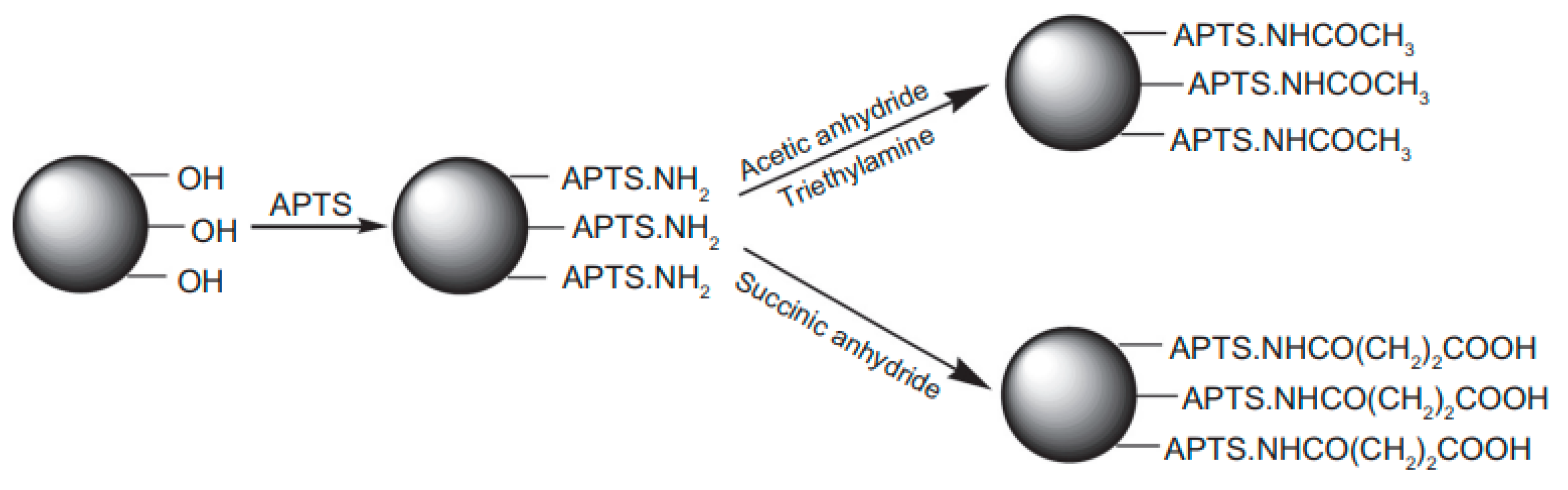
- Monkawa, A.; Ikoma, T.; Kumagai, Y.; Yunoki, S.; Tanaka, J. Immobilization of proteins on the surface of silanized hydroxyapatite. MRS Proc. 2005, 873, 918. [Google Scholar] [CrossRef]
- Xing, Z.C.; Chang, K.W.; Chun, S.; Kim, S.; Kang, I.-K. Immobilization of collagen on hydroxyapatite discs by covalent bonding and physical adsorption and their interaction with MC3T3-E1 osteoblasts. Tissue Eng. Regen. Med. 2014, 11, 99–105. [Google Scholar] [CrossRef]
- Safety Data Sheet, Version 4.13. Available online: https://www.vanderbilt.edu/vinse/facilities/safety_data_sheets/3-Aminopropyl_triethoxysilane.pdf.pdf (accessed on 6 July 2022).
- Wang, S.; Wen, S.; Shen, M.; Guo, R.; Cao, X.; Wang, J.; Shi, X. Aminopropyltriethoxysilane-mediated surface functionalization of hydroxyapatite nanoparticles: Synthesis, characterization, and in vitro toxicity assay. Int. J. Nanomed. 2011, 6, 3449–3459. [Google Scholar]
- Feng, X. Chemical and biochemical basis of cell-bone matrix interaction in health and disease. Curr. Chem. Biol. 2009, 3, 189. [Google Scholar] [PubMed] [Green Version]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, R.A.; Del, V.S.; Altankov, G.; Ginebra, M.P. Porous hydroxyapatite and gelatin/hydroxyapatite microspheres obtained by calcium phosphate cement emulsion. J. Biomed. Mater. Res. B 2011, 97, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Farbod, K.; Nejadnik, M.R.; Jansen, J.A.; Leeuwenburgh, S.C.G. Interactions between inorganic and organic phases in bone tissue as a source of inspiration for design of novel nanocomposites. Tissue Eng. B 2014, 20, 173–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, S.C.; Wang, M.-J.; Pai, N.-S.; Yen, S.-K. Preparation and characterization of gelatin–hydroxyapatite composite microspheres for hard tissue repair. Mater. Sci. Eng. C 2015, 57, 113. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Woo, E.K.; Lee, K.Y. Ionically cross-linkable hyaluronate-based hydrogels for injectable cell delivery. J. Control. Rel. 2014, 196, 146–153. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Xing, D.; Li, R.; Lv, C.; Li, Y.; Yan, X.; Ke, Y.; Xu, Y.; Du, Y.; et al. Injectable nanohydroxyapatite-chitosan-gelatin micro-scaffolds induce regeneration of knee subchondral bone lesions. Sci. Rep. 2017, 7, 16709. [Google Scholar] [CrossRef] [Green Version]
- Mazaki, T.; Shiozaki, Y.; Yamane, K.; Yoshida, A.; Nakamura, M.; Yoshida, Y.; Zhou, D.; Kitajima, T.; Tanaka, M.; Ito, Y.; et al. A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci. Rep. 2014, 4, 4457. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.; Maji, K.; Nandi, S.K. Investigating the mechanical, physiochemical and osteogenic properties in gelatin-chitosan-bioactive nanoceramic composite scaffolds for bone tissue regeneration: In vitro and in vivo. Mater. Sci. Eng. C 2019, 94, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Guo, J.; Guan, Y.; Zhang, H.; Li, X.; Chen, X.; Zhou, D.; Huang, Y. Development of organic/inorganic compatible and sustainably bioactive composites for effective bone regeneration. Biomacromolecules 2018, 19, 3637–3648. [Google Scholar] [CrossRef] [PubMed]
- Delbeck, S.; Heise, H.M. Systematic stability testing of insulins as representative biopharmaceuticals using ATR FTIR-spectroscopy with focus on quality assurance. J. Biomed. Opt. 2021, 26, 043007. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Juang, J.-H.; Hsieh, I.-C.; Wen, M.S.; Liu, S.J. Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102123. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Qi, F.; Cheng, Y.; Lu, X.; Wang, L.; Zhao, J.; Zhao, B. Enhanced bone regeneration using an insulin loaded nano-hydroxyapatite/collagen/PLGA composite scaffold. Int. J. Nanomed. 2018, 13, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, Y.; Fan, Y.; Li, X. The use of bioactive peptides to modify materials for bone tissue repair. Regen. Biomater. 2017, 4, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Goonoo, N.; Bhaw-Luximon, A. Mimicking growth factors: Role of small molecule scaffold additives in promoting tissue regeneration and repair. RSC Adv. 2019, 9, 18124–18146. [Google Scholar] [CrossRef] [Green Version]
- Tiffany, N.V.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar]
- Cui, Y.; Liu, Y.; Cui, Y.; Jing, X.; Zhang, P.; Chen, X. The nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with L-lactic acid oligomer for bone repair. Acta Biomater. 2009, 5, 2680–2692. [Google Scholar] [CrossRef]
- Zhang, H.; Migneco, F.; Lin, C.Y.; Hollister, S.J. Chemically-conjugated bone morphogenetic protein-2 on three-dimensional polycaprolactone scaffolds stimulates osteogenic activity in bone marrow stromal cells. Tissue Eng. A. 2010, 16, 3441–3448. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.J.; Kim, K.H.; Lee, J.Y.; Ku, Y.; Lee, S.J.; Min, B.M.; Chung, C.P. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotech. Appl. Biochem. 2006, 43, 17–24. [Google Scholar]
- Kempen, D.H.R.; Lu, L.; Hefferan, T.E.; Creemers, L.B.; Heijink, A.; Maran, A.; Yaszemski, M.J. Enhanced bone morphogenetic protein-2-induced ectopic and orthotopic bone formation by intermittent parathyroid hormone (1–34) administration. Tissue Eng. A 2010, 16, 3769–3777. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.J.; Milner, R.D.G. Human conditions of insulin-like growth factor I (IGF-I) deficiency. J. Trans. Med. 2012, 10, 224. [Google Scholar]
- Urbanek, O.; Pierini, F.; Choinska, E.; Sajkiewicz, P.; Bil, M.; Swie Rszkowski, W. Effect of hydroxyapatite nanoparticles addition on structure properties of poly(L-lactide-co-glycolide). After gamma sterilization. Polym. Compos. 2016, 39, 1023–1031. [Google Scholar] [CrossRef]
- Jensen, A.C.S.; Ibsen, C.J.S.; Sutherland, D.; Birkedal, H. Transparent aggregates of nanocrystalline hydroxyapatite. Cryst. Growth Des. 2014, 14, 6343–6349. [Google Scholar] [CrossRef]
- Zhang, P.; Hong, Z.; Yu, T.; Chen, X.; Jing, X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactideco-glycolide) and hydroxyapatite surface-grafted with poly(L-lactide). Biomaterials 2009, 30, 58–70. [Google Scholar] [CrossRef]
- Song, X.; Ling, F.; Ma, L.; Yang, C.; Chen, X. Electrospun hydroxyapatite grafted poly(L-lactide)/poly (lactic-co-glycolic acid) nanofibers for guided bone regeneration membrane. Compos. Sci. Technol. 2013, 79, 8–14. [Google Scholar] [CrossRef]
- Hong, Z.; Zhang, P.; He, C.; Qiu, X.; Liu, A.; Chen, L.; Chen, X.; Jing, X. Nano-composite of poly(L-lactide) and surface grafted hydroxyapatite: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 6296–6304. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, H.W.; Kim, K.J.; Lee, S.C. Modification of hydroxyapatite nanosurfaces for enhanced colloidal stability and improved interfacial adhesion in nanocomposites. Chem. Mater. 2006, 18, 5111–5118. [Google Scholar] [CrossRef]
- Hong, Z.; Qiu, X.; Sun, J.; Deng, M.; Chen, X.; Jing, X. Grafting polymerization of L-lactide on the surface of hydroxyapatite nano-crystals. Polymer 2004, 45, 6699–6706. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, L.; Hu, J.; Sun, J.; Hong, Z.; Liu, A.; Chen, X.; Jing, X. Surface-modified hydroxyapatite linked by L-lactic acid oligomer in the absence of catalyst. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5177–5185. [Google Scholar] [CrossRef]
- Liao, L.; Yang, S.; Miron, R.J.; Wei, J.; Zhang, Y.; Zhang, M. Osteogenic properties of PBLG-g-HA/PLLA nanocomposites. PLoS ONE 2014, 9, e105876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, Y.; Sun, T.; Wang, B.; Zhang, H. Bioinspired surface functionalization for improving osteogenesis of electrospun polycaprolactone nanofibers. Langmuir 2018, 34, 15544–15550. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, B.; Gong, Y.; Zhou, P.; Li, H. Mechanical properties of nanodiamond-reinforced hydroxyapatite composite coatings deposited by suspension plasma spraying. Appl. Surf. Sci. 2018, 439, 60–65. [Google Scholar] [CrossRef]
- Vu, A.A.; Robertson, S.F.; Ke, D.; Bandyopadhyay, A.; Bose, S. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [Google Scholar] [CrossRef]
- Vassal, M.F.; Nunes-Pereira, J.; Miguel, S.P.; Correia, I.J.; Silva, A.P. Microstructural, mechanical and biological properties of hydroxyapatite—CaZrO3 biocomposites. Ceramics Int. 2019, 45, 8195–8203. [Google Scholar] [CrossRef]
- Khazeni, D.; Saremi, M.; Soltani, R. Development of HA-CNTs composite coating on AZ31 magnesium alloy by cathodic electrodeposition. Part 1: Microstructural and mechanical characterization. Ceram. Int. 2019, 45, 11174–11185. [Google Scholar] [CrossRef]
- Morakul, S.; Otsuka, Y.; Ohnuma, K.; Tagaya, M.; Motozuka, S.; Miyashita, Y.; Mutoh, Y. Enhancement effect on antibacterial property of gray titania coating by plasma-sprayed hydroxyapatite-amino acid complexes during irradiation with visible light. Heliyon 2019, 5, e02207. [Google Scholar] [CrossRef] [Green Version]
- Matsuya, T.; Morakul, S.; Otsuka, Y.; Ohnuma, K.; Tagaya, M.; Motozuka, S.; Miyashita, Y.; Mutoh, Y. Visible light-induced antibacterial effects of the luminescent complex of hydroxyapatite and 8-hydroxyquinoline with gray titania coating. Appl. Surf. Sci. 2018, 448, 529–538. [Google Scholar] [CrossRef]
- Zheng, B.; Luo, Y.; Liao, H.; Zhang, C. Investigation of the crystallinity of suspension plasma sprayed hydroxyapatite coatings. J. Eur. Ceram. Soc. 2017, 37, 5017–5021. [Google Scholar] [CrossRef]
- Mohd, M.M.A.; Yuichi, O.; Kiyoshi, O.; Yukio, M. Effects of composition of hydroxyapatite/gray titania coating fabricated by suspension plasma spraying on mechanical and antibacterial properties. J. Mech. Behav. Biomed. Mater. 2022, 125, 104888. [Google Scholar]
- Matsuya, T.; Otsuka, Y.; Tagaya, M.; Motozuka, S.; Ohnuma, K.; Mutoh, Y. Formation of stacked luminescent complex of 8-hydroxyquinoline molecules on hydroxyapatite coating by using cold isostatic pressing. Mater. Sci. Eng. C 2016, 58, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Dekhtyar, Y.; Polyaka, N.; Sammons, R. Electrically charged hydroxyapatite enhances immobilization and proliferation of osteoblasts. In Proceedings of the 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics, Riga, Latvia, 16–20 June 2008; Springer: Berlin/Heidelberg, Germany, 2008; Volume 20, pp. 23–25. [Google Scholar]
- Dekhtyar, Y.; Bystrov, V.; Bystrova, A.; Dindune, A.; Katashev, A.; Khlusov, I.; Palcevskis, E.; Paramonova, E.; Polyaka, N.N.; Romanova, M.; et al. Engineering of the hydroxyapatite cell adhesion capacity. In IFMBE Proceedings, Proceedings of the International Symposium on Biomedical Engineering and Medical Physics, Riga, Latvia, 10–12 October 2012; Dekhtyar, Y., Katashev, A., Lancere, L., Eds.; Springer: Berlin/Heidelberg, Germany; Volume 38. [CrossRef] [Green Version]
- Juliadmi, D.; Fauzi, V.R.; Gunawarman, H.N.; Idris, M.H. Hydroxyapatite coating on titanium alloy Ti-6Al-4V with electrophoretic deposition (EPD) for dental root application. Inter. J. Adv. Sci. Eng. Inform. Technol. 2017, 7, 2152–2158. [Google Scholar] [CrossRef] [Green Version]
- Sisti, K.E.; de Rossi, R.; Antoniolli, A.M.B.; Aydos, R.D.; Guastaldi, A.C.; Queiroz, T.P.; Tavares, H.S. Surface and biomechanical study of titanium implants modified by laser with and without hydroxyapatite coating, in rabbits. J. Oral Implantol. 2012, 38, 231–237. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, B.S.; Gupta, K.C.; Lee, D.Y.; Kang, I.-K. Hydroxyapatite nanorod-modified sand blasted titanium disk for endosseous dental implant applications. Tissue Eng. Reg. Med. 2018, 15, 601–614. [Google Scholar] [CrossRef]
- Marco, E.; Alexandra, M.; Mansur, A.P.; Mansur, H.S. Chemical functionalization of surfaces for building three-dimensional engineered biosensors. Appl. Surf. Sci. 2013, 275, 347–360. [Google Scholar]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [Green Version]










| Bisphosphonates | Carrier | Applications | References |
|---|---|---|---|
| Zoledronate | HA | Osteoporotic bone around implants | Peter et al. [40] |
| -do- | -do- | Bone resorption around osteoporotic implants | Tanzer et al. [41] |
| -do- | -do- | -do- | Peter et al. [41] |
| -do- | -do- | -do- | Roussie et al. [38] |
| Ibandronate | -do- | -do- | Kurth et al. [39] |
| (3-Dimethylamino-1-hydroxypropylidene)-1,1-P-C-P | -do- | Alveolar bone destruction in periodontal diseases | Denisen et al. [37] |
| Pamidronate | -do- | Osteoporosis | Haider et al. [28], Shin et al. [26] |
| [Ions] at Plane | Solution pH | ||||
|---|---|---|---|---|---|
| 4.5 | 7.4 | 9.0 | 12.3 | 14.0 | |
| Ca2+ nm−2 (100) | 2.16 | 2.58 | 2.88 | 3.25 | 3.61 |
| Ca2+ nm−2 (001) | 0.37 | 1.79 | 2.48 | 3.41 | 4.35 |
| PO43− nm−2 (100) | 1.44 | 1.44 | 1.44 | 1.44 | 1.44 |
| PO43− nm−2 (001) | 3.45 | 3.73 | 3.73 | 3.73 | 3.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Haider, A.; Gupta, K.C.; Kim, H.; Kang, I. Chemical Bonding of Biomolecules to the Surface of Nano-Hydroxyapatite to Enhance Its Bioactivity. Coatings 2022, 12, 999. https://doi.org/10.3390/coatings12070999
Kang S, Haider A, Gupta KC, Kim H, Kang I. Chemical Bonding of Biomolecules to the Surface of Nano-Hydroxyapatite to Enhance Its Bioactivity. Coatings. 2022; 12(7):999. https://doi.org/10.3390/coatings12070999
Chicago/Turabian StyleKang, Sohee, Adnan Haider, Kailash Chandra Gupta, Hun Kim, and Innkyu Kang. 2022. "Chemical Bonding of Biomolecules to the Surface of Nano-Hydroxyapatite to Enhance Its Bioactivity" Coatings 12, no. 7: 999. https://doi.org/10.3390/coatings12070999
APA StyleKang, S., Haider, A., Gupta, K. C., Kim, H., & Kang, I. (2022). Chemical Bonding of Biomolecules to the Surface of Nano-Hydroxyapatite to Enhance Its Bioactivity. Coatings, 12(7), 999. https://doi.org/10.3390/coatings12070999







