Step-By-Step Development of Vertically Aligned Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. RF Magnetron Sputtering of TiN and Ni Layers
2.2. PECVD Growth of VA-CNTs
2.3. Characterization
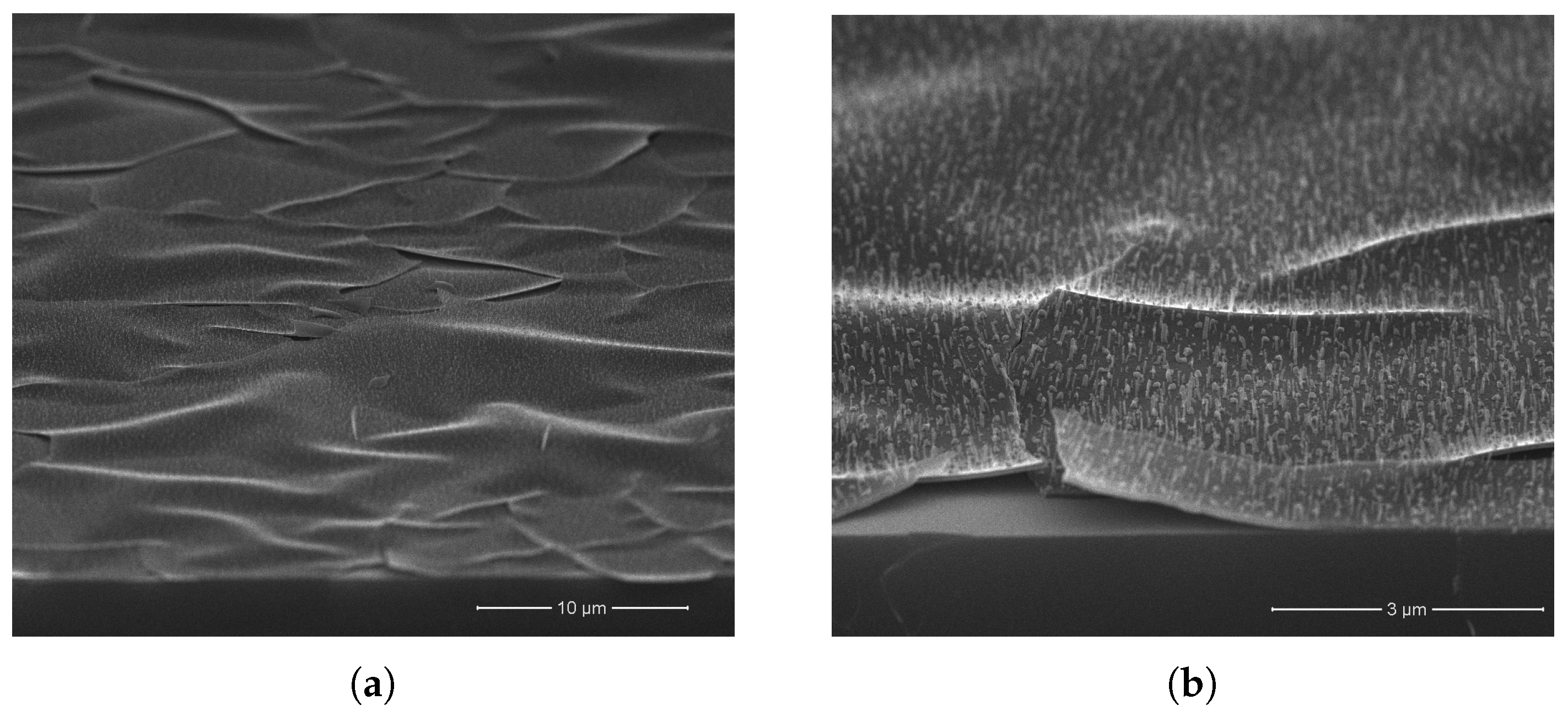
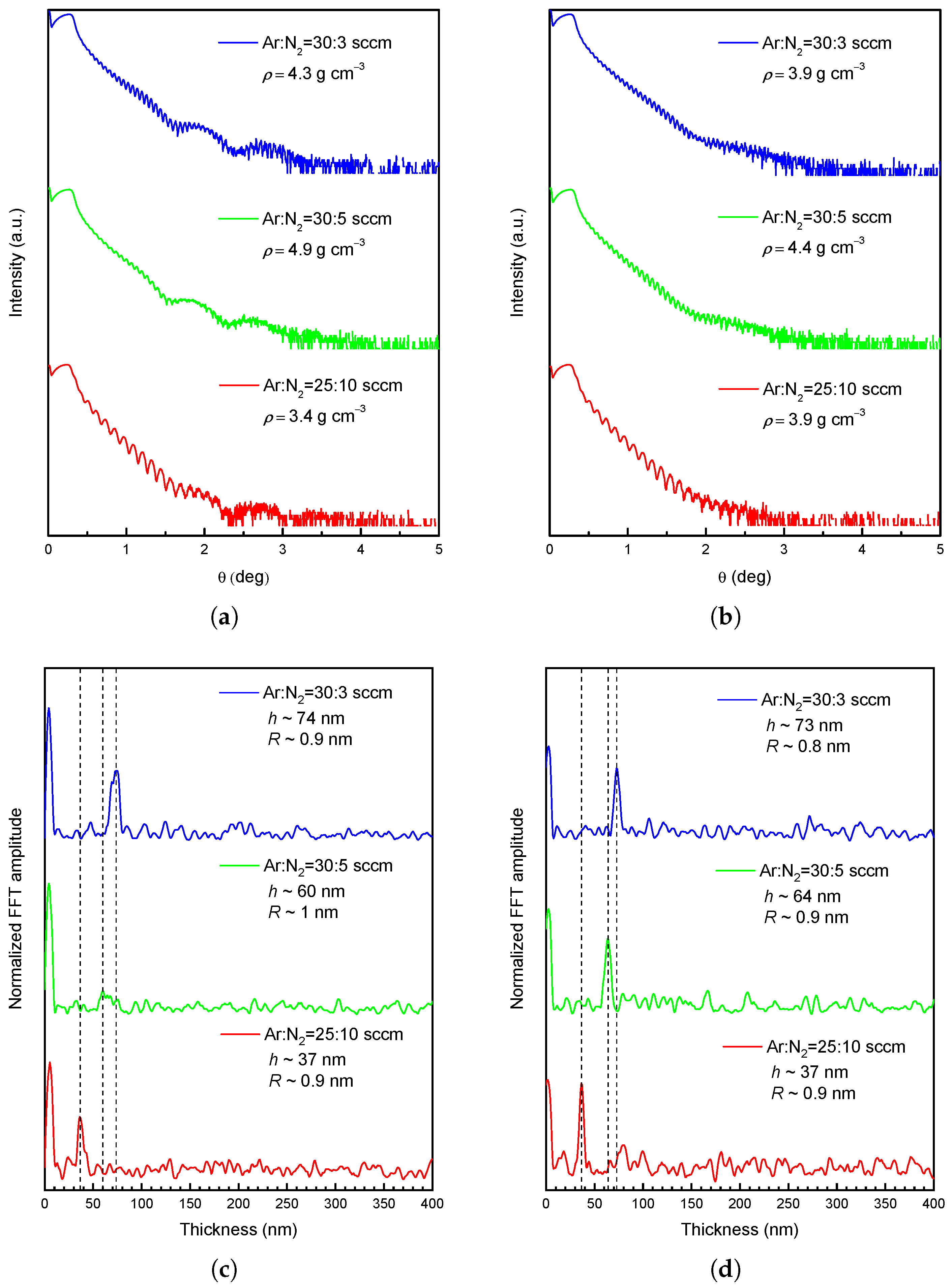
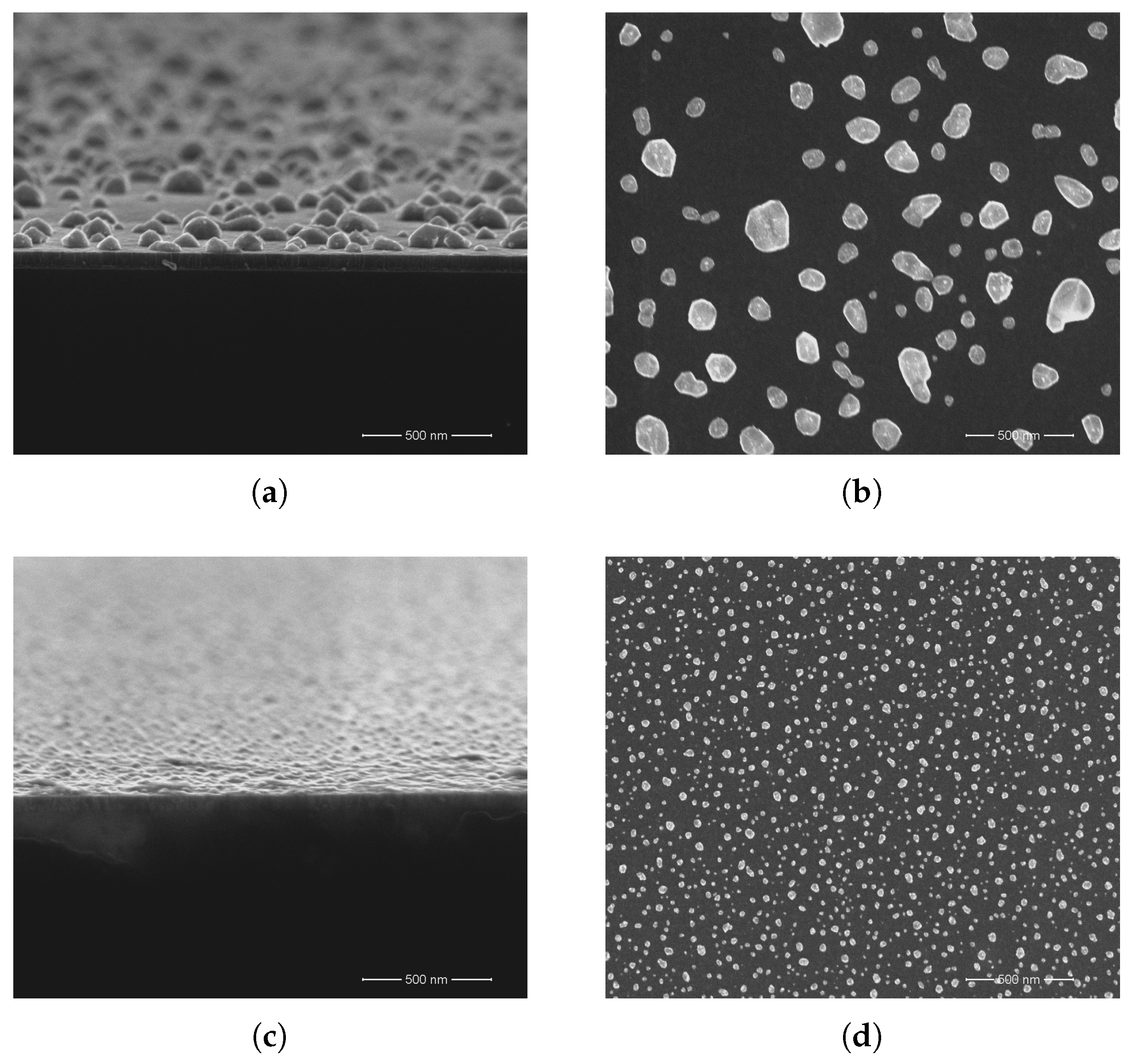
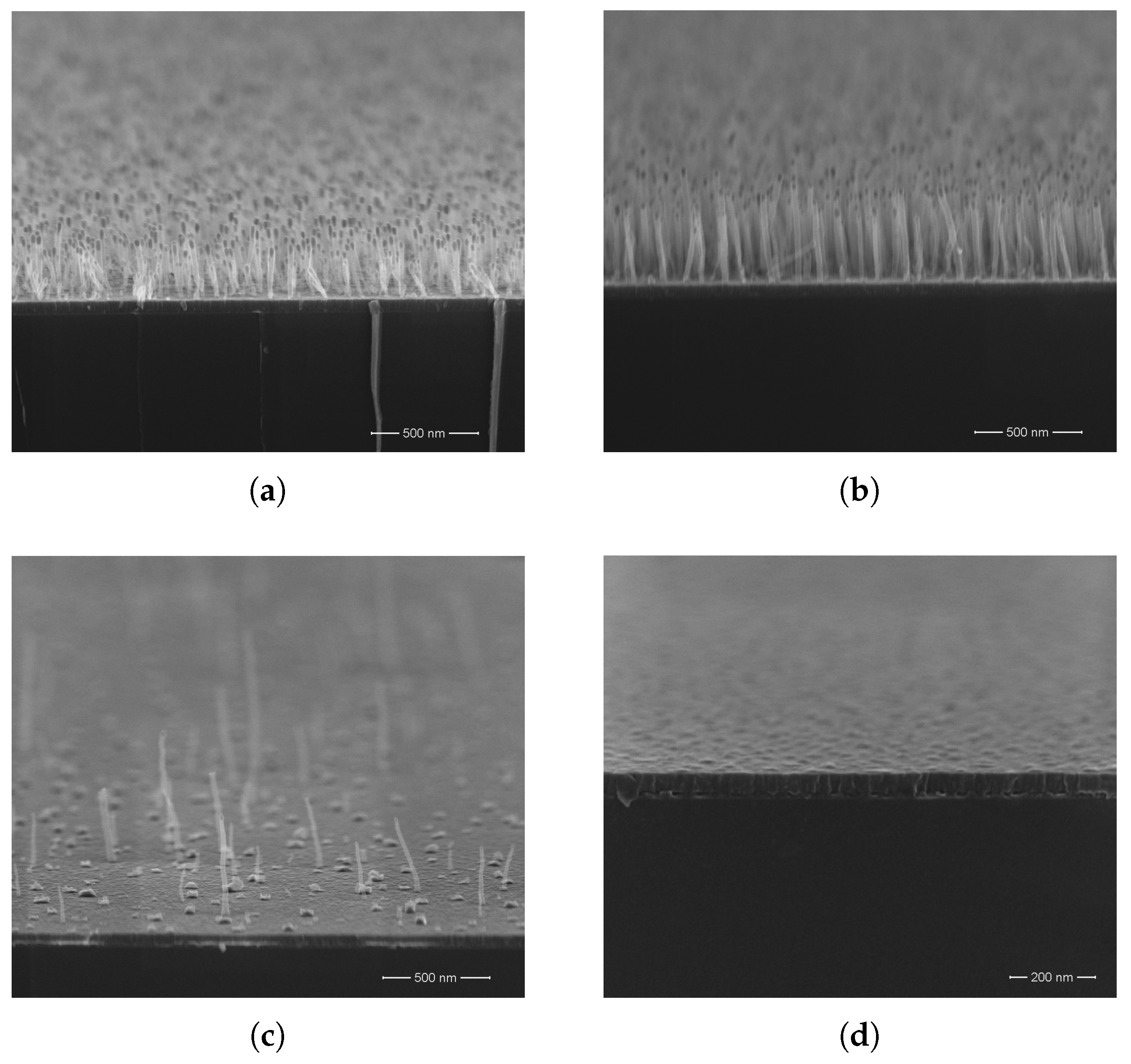
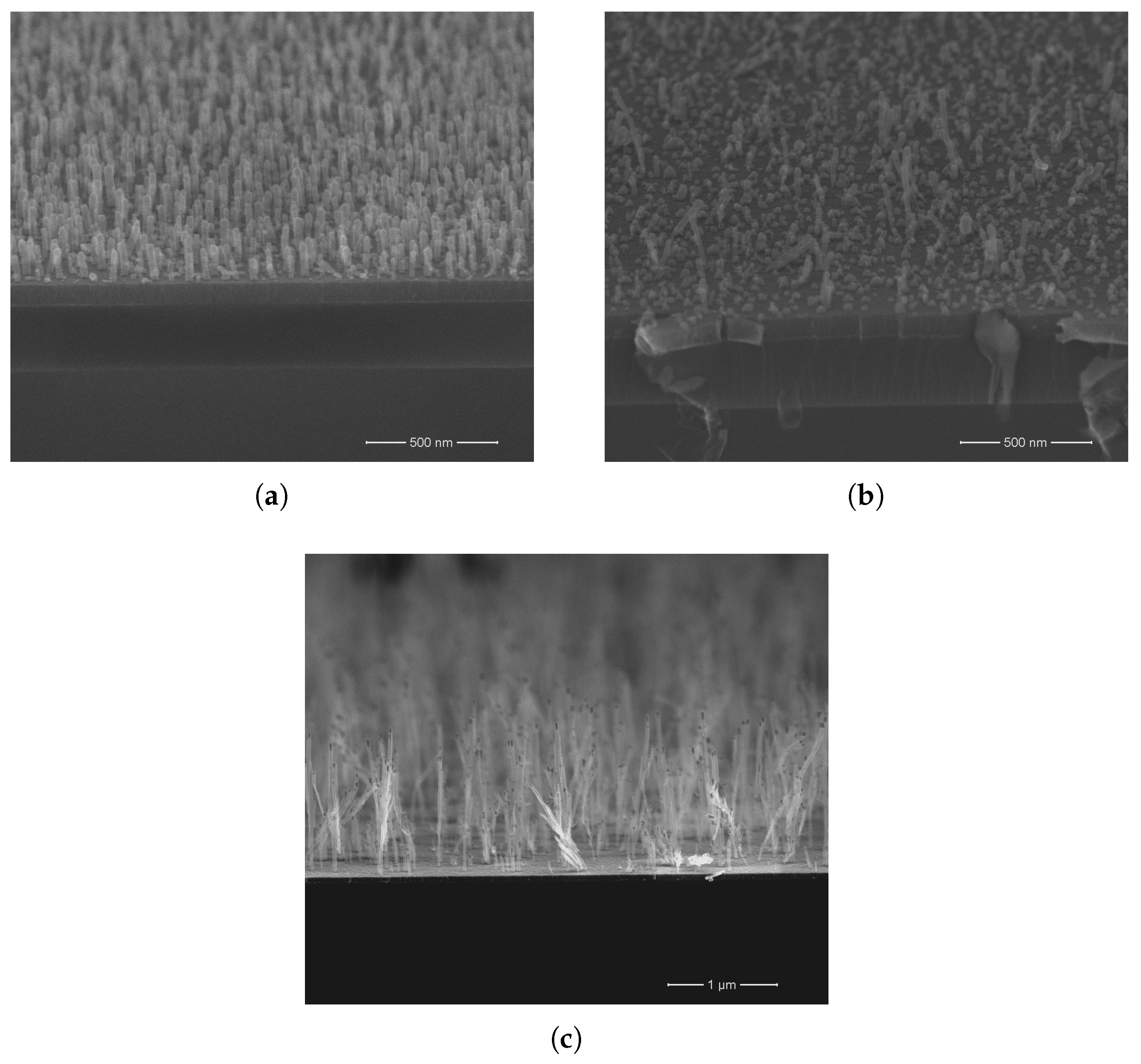
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VA-CNTs | Vertically aligned carbon nanotubes |
| DC | Direct current |
| MW | Microwave |
| NEMS | Nano-electromechanical systems |
| MEMS | Micro-electromechanical systems |
| CVD | Chemical vapor deposition |
| TCVD | Thermal chemical vapor deposition |
| PECVD | Plasma-enhanced chemical vapor deposition |
| RF | Radio frequency |
| MS | Magnetron sputtering |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| GIXRD | Grazing incidence X-ray diffraction |
| XRR | X-ray reflectivity |
| FFT | Fast Fourier transform |
| CCs | Catalytic centers |
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Terrones, M. Science and Technology of the Twenty-First Century: Synthesis, Properties, and Applications of Carbon Nanotubes. Ann. Rev. Mater. Res. 2003, 33, 419–501. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Salvetat, J.P.; Bonard, J.M.; Thomson, N.H.; Kulik, A.J.; Forró, L.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Appl. Phys. A-Mater. Sci. Process. 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, J.; Çagin, T.; Goddard, W.A. Thermal conductivity of carbon nanotubes. Nanotechnology 2000, 11, 65–69. [Google Scholar] [CrossRef]
- Hone, J.; Llaguno, M.C.; Nemes, N.M.; Johnson, A.T.; Fischer, J.E.; Walters, D.A.; Casavant, M.J.; Schmidt, J.; Smalley, R.E. Electrical and thermal transport properties of magnetically aligned single wall carbon nanotube films. Appl. Phys. Lett. 2000, 77, 666–668. [Google Scholar] [CrossRef]
- Fujii, M.; Zhang, X.; Xie, H.; Ago, H.; Takahashi, K.; Ikuta, T.; Abe, H.; Shimizu, T. Measuring the Thermal Conductivity of a Single Carbon Nanotube. Phys. Rev. Lett. 2005, 95, 065502. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.; Wang, X.; Fan, S. Measuring the thermal conductivity of individual carbon nanotubes by the Raman shift method. Nanotechnology 2009, 20, 145702. [Google Scholar] [CrossRef]
- Fischer, J.E.; Johnson, A.T. Electronic properties of carbon nanotubes. Curr. Opin. Solid State Mater. Sci. 1999, 4, 28–33. [Google Scholar] [CrossRef]
- McEuen, P.; Fuhrer, M.; Park, H. Single-walled carbon nanotube electronics. IEEE Trans. Nanotechnol. 2002, 1, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef] [Green Version]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.; Zhang, C.; Huang, B.; Li, Y.; Lai, W.; Wang, X.; Liu, X. Low temperature preparation of highly fluorinated multiwalled carbon nanotubes activated by Fe3O4 to enhance microwave absorbing property. Nanotechnology 2018, 29, 365703. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef]
- Sun, X.; Sun, J.; Li, T.; Zheng, S.; Wang, C.; Tan, W.; Zhang, J.; Liu, C.; Ma, T.; Qi, Z.; et al. Flexible Tactile Electronic Skin Sensor with 3D Force Detection Based on Porous CNTs/PDMS Nanocomposites. Nano-Micro Lett. 2019, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Kordás, K.; Tóth, G.; Moilanen, P.; Kumpumäki, M.; Vähäkangas, J.; Uusimäki, A.; Vajtai, R.; Ajayan, P.M. Chip cooling with integrated carbon nanotube microfin architectures. Appl. Phys. Lett. 2007, 90, 123105. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Nabiollahi, N.; Wang, T.; Wang, S.; Hu, Z.; Carlberg, B.; Zhang, Y.; Wang, X.; Liu, J. A complete carbon-nanotube-based on-chip cooling solution with very high heat dissipation capacity. Nanotechnology 2012, 23, 045304. [Google Scholar] [CrossRef]
- Dahmardeh, M.; Vahdani Moghaddam, M.; Hian Tee, M.; Nojeh, A.; Takahata, K. The effects of three-dimensional shaping of vertically aligned carbon-nanotube contacts for micro-electro-mechanical switches. Appl. Phys. Lett. 2013, 103, 231606. [Google Scholar] [CrossRef]
- Keidar, M.; Levchenko, I.; Arbel, T.; Alexander, M.; Waas, A.M.; Ostrikov, K.K. Increasing the length of single-wall carbon nanotubes in a magnetically enhanced arc discharge. Appl. Phys. Lett. 2008, 92, 043129. [Google Scholar] [CrossRef]
- Kingston, C.T.; Simard, B. Recent Advances in Laser Synthesis of Single-Walled Carbon Nanotubes. J. Nanosci. Nanotechnol. 2006, 6, 1225–1232. [Google Scholar] [CrossRef]
- Endo, M.; Takeuchi, K.; Igarashi, S.; Kobori, K.; Shiraishi, M.; Kroto, H.W. The production and structure of pyrolytic carbon nanotubes (PCNTs). J. Phys. Chem. Solids 1993, 54, 1841–1848. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical Vapor Deposition of Carbon Nanotubes: A Review on Growth Mechanism and Mass Production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [Green Version]
- See, C.H.; Harris, A.T. A Review of Carbon Nanotube Synthesis via Fluidized-Bed Chemical Vapor Deposition. Ind. Eng. Chem. Res. 2007, 46, 997–1012. [Google Scholar] [CrossRef]
- Ahmad, M.; Silva, S.R.P. Low temperature growth of carbon nanotubes—A review. Carbon 2020, 158, 24–44. [Google Scholar] [CrossRef]
- Merkulov, V.I.; Melechko, A.V.; Guillorn, M.A.; Simpson, M.L.; Lowndes, D.H.; Whealton, J.H.; Raridon, R.J. Controlled alignment of carbon nanofibers in a large-scale synthesis process. Appl. Phys. Lett. 2002, 80, 4816–4818. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Hatakeyama, R. Growth of Single-Walled Carbon Nanotubes by Plasma CVD. J. Nanotechnol. 2010, 2010, 256906. [Google Scholar] [CrossRef] [Green Version]
- Meyyappan, M.; Delzeit, L.; Cassell, A.; Hash, D. Carbon nanotube growth by PECVD: A review. Plasma Sources Sci. Technol. 2003, 12, 205–216. [Google Scholar] [CrossRef]
- Carbonari, M.; Martinelli, J. Effects of hot isostatic pressure on titanium nitride films deposited by physical vapor deposition. Mater. Res. 2001, 4, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Nogan, J.; Yang, Z.P.; Kort-Kamp, W.J.M.; Ross, W.; Luk, T.S.; Dalvit, D.A.R.; Azad, A.K.; Chen, H.T. Highly Plasmonic Titanium Nitride by Room-Temperature Sputtering. Sci. Rep. 2019, 9, 15287. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L. The Diffraction of X-Rays by Small Crystalline Particles. Phys. Rev. 1939, 56, 972–977. [Google Scholar] [CrossRef]
- Bowen, D.; Tanner, B. High Resolution X-ray Diffractometry And Topography; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 1998; p. 252. [Google Scholar] [CrossRef]
- Als-Nielsen, J.; McMorrow, D. Elements of Modern X-ray Physics; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 434. [Google Scholar]
- Birkholz, M. Thin Film Analysis by X-ray Scattering; Wiley-VCH: Hoboken, NJ, USA, 2005; p. 378. [Google Scholar]
- Kiessig, H. Untersuchungen zur Totalreflexion von Röntgenstrahlen. Ann. Phys. 1931, 402, 715–768. [Google Scholar] [CrossRef]
- Kiessig, H. Interferenz von Röntgenstrahlen an dünnen Schichten. Ann. Phys. 1931, 402, 769–788. [Google Scholar] [CrossRef]
- Xiao, Y.; Ahmed, Z.; Ma, Z.; Zhou, C.; Zhang, L.; Chan, M. Low Temperature Synthesis of High-Density Carbon Nanotubes on Insulating Substrate. Nanomaterials 2019, 9, 473. [Google Scholar] [CrossRef] [Green Version]
- Il’in, O.I.; Il’ina, M.V.; Rudyk, N.N.; Fedotov, A.A.; Ageev, O.A. Vertically Aligned Carbon Nanotubes Production by PECVD. In Perspective of Carbon Nanotubes; Saleh, H.E.D., El-Sheikh, S.M.M., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 5. [Google Scholar] [CrossRef] [Green Version]
- Na, N.; Kim, D.Y.; So, Y.G.; Ikuhara, Y.; Noda, S. Simple and engineered process yielding carbon nanotube arrays with 1.2 × 1013 cm−2 wall density on conductive underlayer at 400 °C. Carbon 2015, 81, 773–781. [Google Scholar] [CrossRef]



| Catalyst Treatment | Step | Heating Rate | Temperature | Time | Pressure | RF Power | Gas Flow Rate [sccm] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (°C min−1) | (°C) | (min) | (mTorr) | (W) | Ar | H2 | NH3 | CH4 | ||
| NH3 | Heat-up | 15 | − | 1000 | − | 200 | − | 50 | − | |
| Treatment | − | 700–900 | 2–5 | 1000 | − | − | − | 100 | − | |
| H2 | Heat-up | 15 | − | 1000 | − | − | 200 | − | − | |
| Treatment | − | 700–900 | 2–5 | 1000 | 200 | − | 200 | − | − | |
| Growth | − | 700–900 | 30–300 | 1000 | 200 | − | − | 30–60 | 70 & 80 | |
| Cool-down | 9 | − | − | − | − | 200 | − | − | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simionescu, O.-G.; Brîncoveanu, O.; Romaniţan, C.; Vulpe, S.; Avram, A. Step-By-Step Development of Vertically Aligned Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition. Coatings 2022, 12, 943. https://doi.org/10.3390/coatings12070943
Simionescu O-G, Brîncoveanu O, Romaniţan C, Vulpe S, Avram A. Step-By-Step Development of Vertically Aligned Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition. Coatings. 2022; 12(7):943. https://doi.org/10.3390/coatings12070943
Chicago/Turabian StyleSimionescu, Octavian-Gabriel, Oana Brîncoveanu, Cosmin Romaniţan, Silviu Vulpe, and Andrei Avram. 2022. "Step-By-Step Development of Vertically Aligned Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition" Coatings 12, no. 7: 943. https://doi.org/10.3390/coatings12070943
APA StyleSimionescu, O.-G., Brîncoveanu, O., Romaniţan, C., Vulpe, S., & Avram, A. (2022). Step-By-Step Development of Vertically Aligned Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition. Coatings, 12(7), 943. https://doi.org/10.3390/coatings12070943









