DFT Study on Methanol Oxidation Reaction Catalyzed by PtmPdn Alloys
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
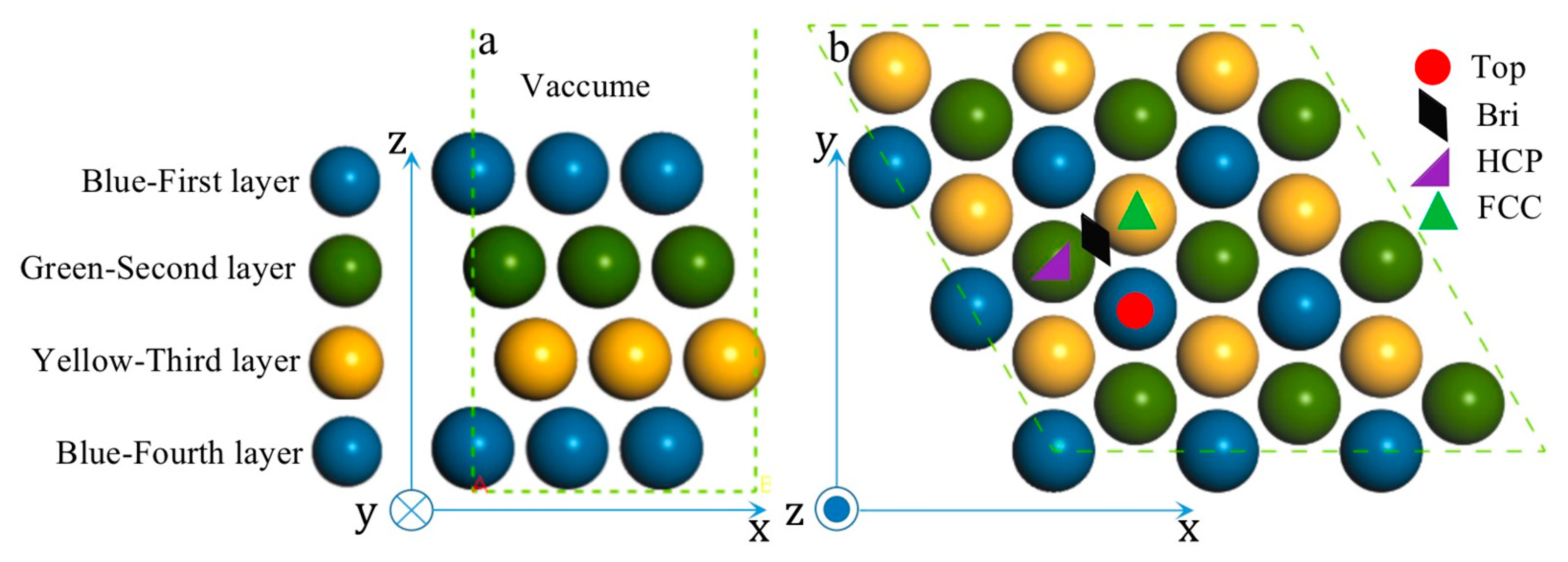
3.1. The Determination of the Most Stable Surface of the Various PtmPdn Alloys
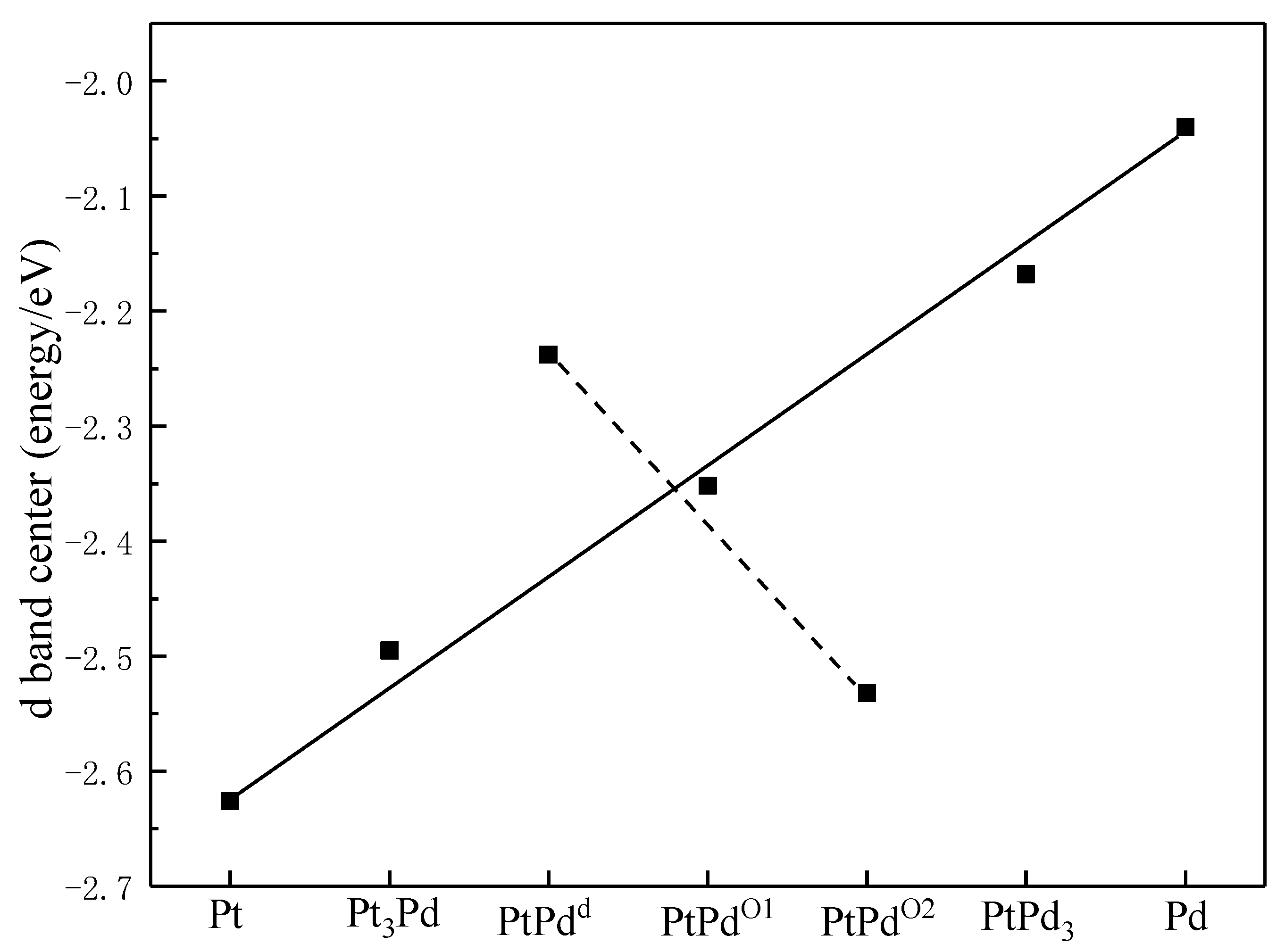
3.2. The Properties of PtmPdn Alloy
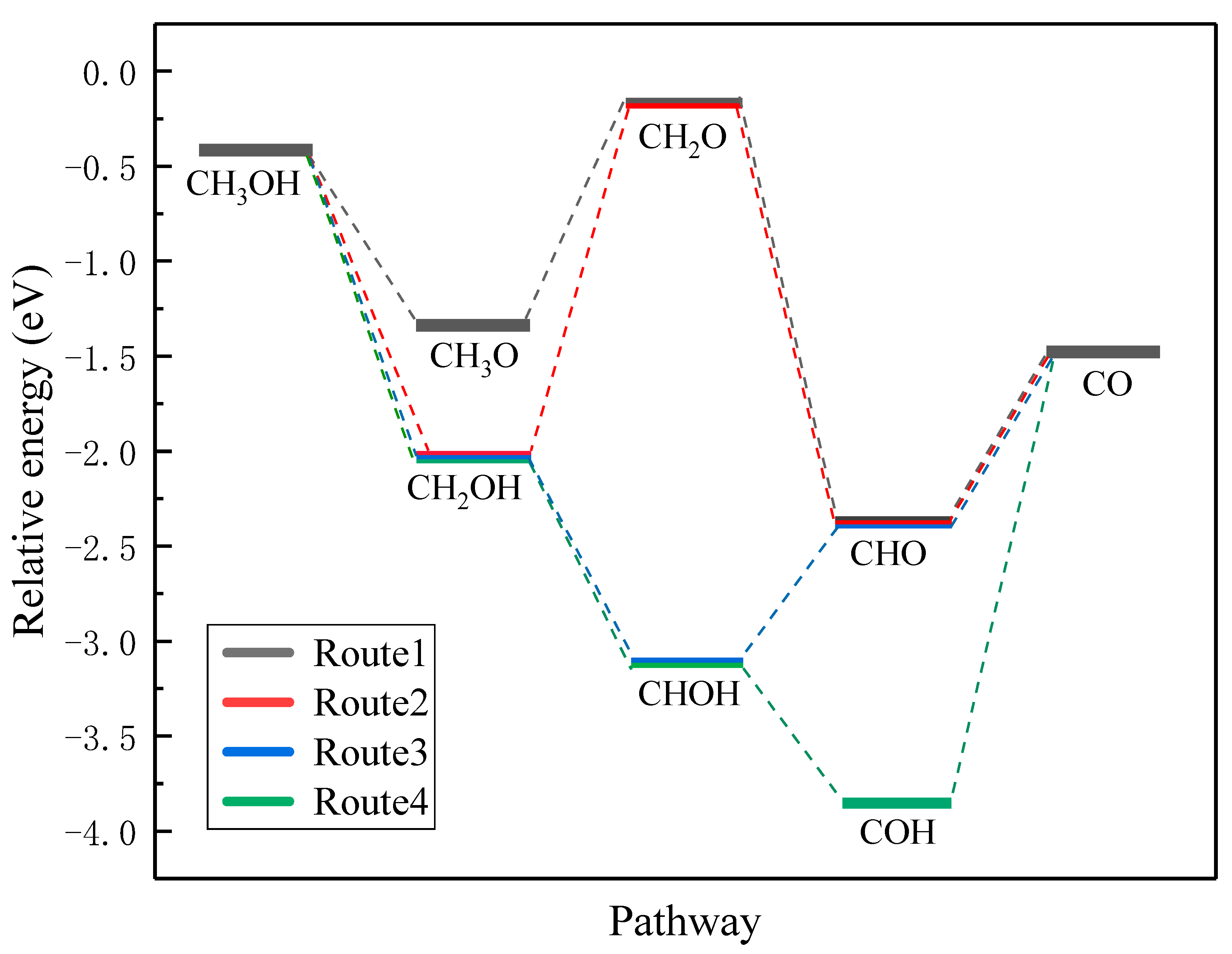
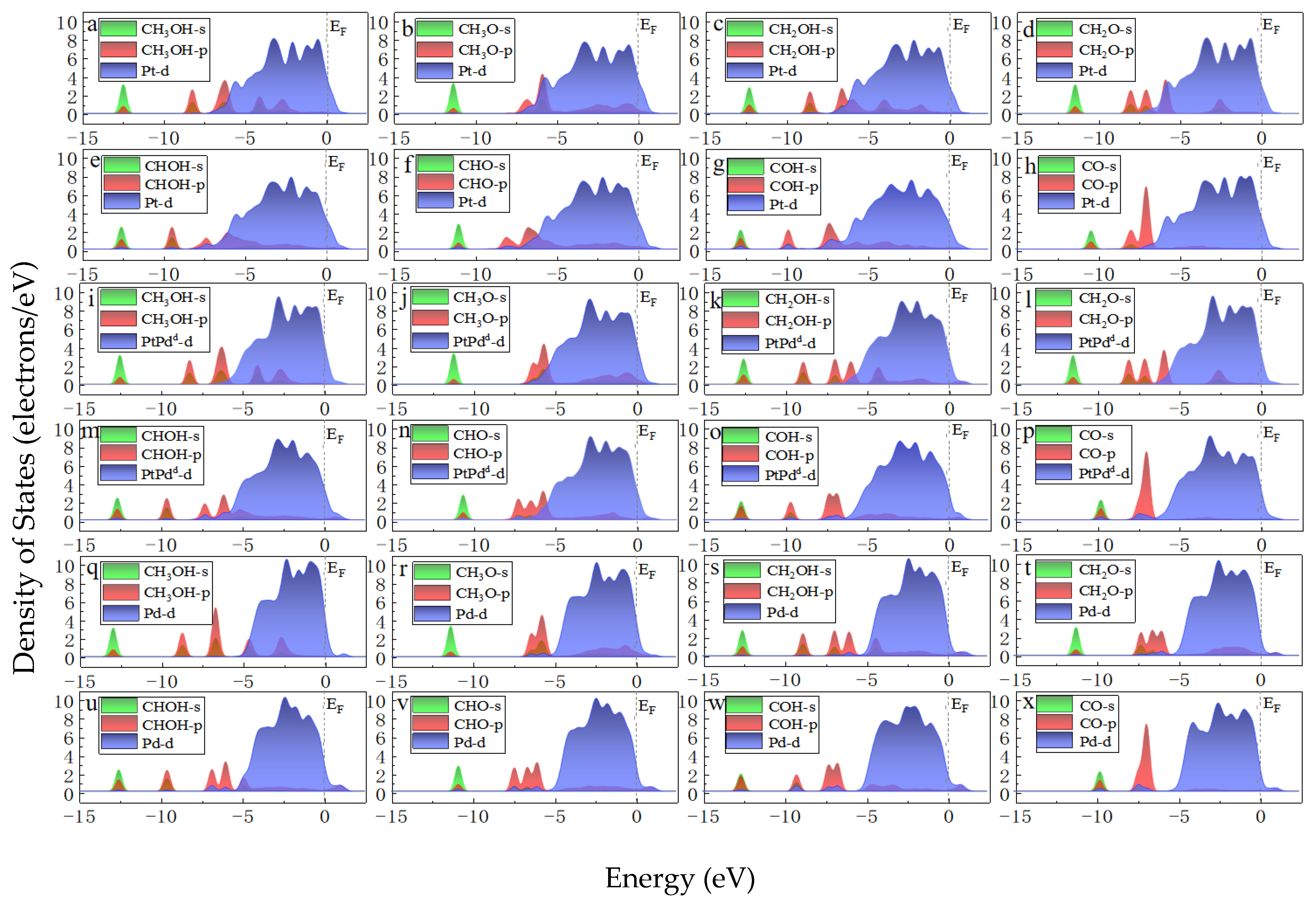
3.3. Methanol Oxidation Reaction on the Pristine Pt(111)
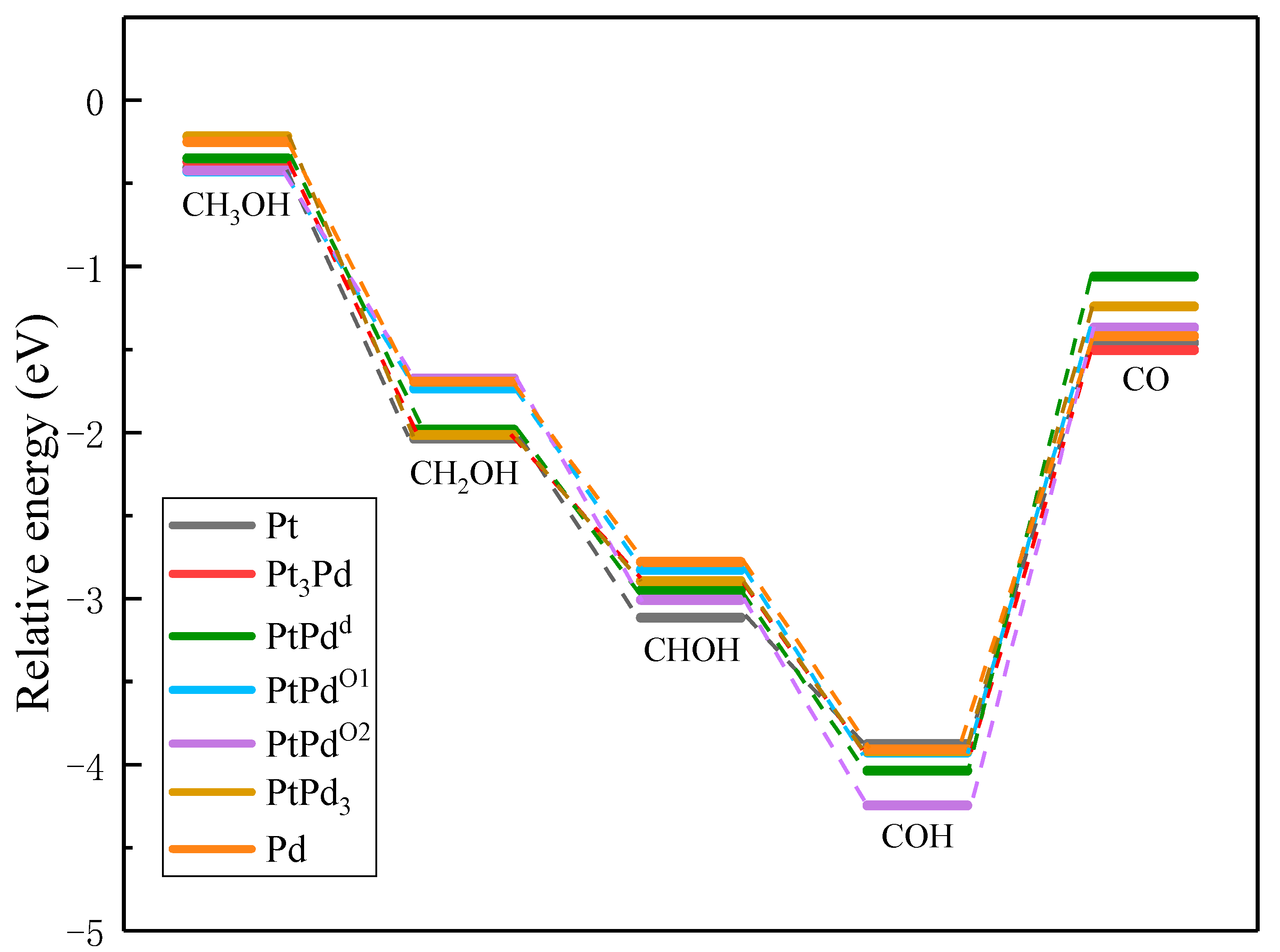
3.4. Methanol Oxidation Reaction on Various PtmPdn(111)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Si, R.; Liu, J.; Zhang, L.; Gong, X.; Gao, R.; Liu, B.; Zhang, J. Directed self-assembly pathways of three-dimensional Pt/Pd nanocrystal superlattice electrocatalysts for enhanced methanol oxidation reaction. J. Mater. Chem. A 2018, 6, 12759–12767. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B. Effect of current collector roughness on performance of passive direct methanol fuel cell. Renew. Energy 2019, 138, 272–283. [Google Scholar] [CrossRef]
- Li, H.-H.; Fu, Q.-Q.; Xu, L.; Ma, S.-Y.; Zheng, Y.-R.; Liu, X.-J.; Yu, S.-H. Highly crystalline PtCu nanotubes with three dimensional molecular accessible and restructured surface for efficient catalysis. Energy Environ. Sci. 2017, 10, 1751–1756. [Google Scholar] [CrossRef]
- Li, P.; Qi, X.; Zhao, L.; Wang, J.; Wang, M.; Shao, M.; Chen, J.S.; Wu, R.; Wei, Z. Hierarchical 3D porous carbon with facilely accessible Fe-N4 single-atom sites for Zn-air batteries. J. Mater. Chem. A 2022, 10, 5925–5929. [Google Scholar] [CrossRef]
- Nie, Y.; Qi, X.; Wu, R.; Yang, R.; Wang, H.; Deng, M.; Zhang, S.; Lu, S.; Gu, Z.; Liu, X. Structurally ordered PtFe intermetallic nanocatalysts toward efficient electrocatalysis of methanol oxidation. Appl. Surf. Sci. 2021, 569, 151004. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, C.; Peng, H.; Peng, C.; Zhang, L.; Xu, X.; Liu, W.; Wang, Z.; Zhang, N.; Wang, X. Enhanced toluene combustion performance over Pt loaded hierarchical porous MOR zeolite. Chem. Eng. J. 2018, 334, 10–18. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q.; Gu, L.; Xu, S.; Wang, P.; Liu, J.; Ding, Y.; Yao, Y.; Nan, C.; Zhao, M. Ruthenium-platinum core–shell nanocatalysts with substantially enhanced activity and durability towards methanol oxidation. Nano Energy 2016, 21, 247–257. [Google Scholar] [CrossRef]
- Saleem, F.; Ni, B.; Yong, Y.; Gu, L.; Wang, X. Ultra-small Tetrametallic Pt-Pd-Rh-Ag Nanoframes with Tunable Behavior for Direct Formic Acid/Methanol Oxidation. Small 2016, 12, 5261–5268. [Google Scholar] [CrossRef]
- Lou, Y.; Li, C.; Gao, X.; Bai, T.; Chen, C.; Huang, H.; Liang, C.; Shi, Z.; Feng, S. Porous Pt nanotubes with high methanol oxidation electrocatalytic activity based on original bamboo-shaped Te nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 16147–16153. [Google Scholar] [CrossRef]
- Peng, X.; Chen, D.; Yang, X.; Wang, D.; Li, M.; Tseng, C.-C.; Panneerselvam, R.; Wang, X.; Hu, W.; Tian, J.; et al. Microwave-assisted synthesis of highly dispersed PtCu nanoparticles on three-dimensional nitrogen-doped graphene networks with remarkably enhanced methanol electrooxidation. ACS Appl. Mater. Inter. 2016, 8, 33673–33680. [Google Scholar] [CrossRef]
- Pan, D.; Ping, W.; Chen, X.-C. Mechanism of Methanol Decomposition on the Pt3Ni(111) Surface: DFT Study. J. Phys. Chem. C 2017, 121, 9348–9360. [Google Scholar]
- Ding, Q.-Y.; Xu, W.-B.; Sang, P.-P.; Xu, J.; Zhao, L.-M.; He, X.-L.; Guo, W.-Y. Insight into the Reaction Mechanisms of Methanol on PtRu/Pt(111): A Density Functional Study. Appl. Surf. Sci. 2016, 369, 257–266. [Google Scholar] [CrossRef]
- Almeida, G.R.; López-Suárez, F.E.; Silva, L.S.; Pereira, G.F.; Bueno-López, A.; Eguiluz, K.I.; Salazar-Banda, G.R. Methanol electro-oxidation on carbon-supported PtRu nanowires. J. Nanosci. Nanotechnol. 2019, 19, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Liao, F.; Zhu, W.; Shao, C.; Shao, M. Effective PtAu nanowire network catalysts with ultralow Pt content for formic acid oxidation and methanol oxidation. Int. J. Hydrogen Energy 2020, 45, 16071–16079. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, W.; Kang, W.; Li, R.; Qu, K.; Zhang, Y.; Chen, B.; Wang, H.; Sun, Y.; Li, H. Methanol Oxidation Reaction Performance on Graphene-Supported PtAg Alloy Nanocatalyst: Contrastive Study of Electronic and Geometric Effects Induced from Ag Doping. ChemistrySelect 2018, 3, 3615–3620. [Google Scholar] [CrossRef]
- Ordoñez, L.; Roquero, P.; Ramirez, J.; Sebastian, P.J. Methanol electro-oxidation on bimetallic PtMo/C catalysts and Pt/C-Mo/C mechanical mixtures. Int. J. Electrochem. Sci. 2016, 11, 5364–5379. [Google Scholar] [CrossRef]
- Yin, S.; Wang, Z.; Li, C.; Yu, H.; Deng, K.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Mesoporous Pt@ PtM (M = Co, Ni) cage-bell nanostructures toward methanol electro-oxidation. Nanoscale Adv. 2020, 2, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Men’shchikov, V.; Guterman, V.; Belenov, S.; Spiridonova, O.; Rezvan, D. De-Alloyed PtCu/C Catalysts of Methanol Electrooxidation. Russ. J. Electrochem. 2020, 56, 850–858. [Google Scholar] [CrossRef]
- Stevanović, S.; Tripković, D.; Gavrilović-Wohlmuther, A.; Rogan, J.; Lačnjevac, U.; Jovanović, V. Carbon Supported PtSn versus PtSnO2 Catalysts in Methanol Oxidation. Int. J. Electrochem. Sci. 2021, 16, 210222. [Google Scholar] [CrossRef]
- Su, D.S.; Sun, G. Nonprecious-Metal Catalysts for Low-Cost Fuel Cells. Angew. Chem. Int. Ed. Engl. 2011, 50, 11570–11572. [Google Scholar] [CrossRef]
- Higareda, A.; Rosas, G.; Pérez, R.; Esparza, R. Characterization and Electrocatalytic Features of PtPd and PdPt Bimetallic Nanoparticles for Methanol Electro-oxidation. ChemNanoMat 2021, 7, 958–965. [Google Scholar] [CrossRef]
- Shen, L.; Ying, J.; Tian, G.; Jia, M.; Yang, X. Ultralong PtPd alloyed nanowires anchored on graphene for efficient methanol oxidation reaction. Chem. Asian J. 2021, 16, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, R.M.; Adams, B.D.; Chen, S.; Shah, B.; Chen, A. Synthesis and electrochemical study of PtPd nanodendrites. J. Electroanal. Chem. 2013, 688, 151–157. [Google Scholar] [CrossRef]
- Xiao, Y.-X.; Ying, J.; Tian, G.; Zhang, X.-Q.; Janiak, C.; Ozoemena, K.I.; Yang, X.-Y. PtPd hollow nanocubes with enhanced alloy effect and active facets for efficient methanol oxidation reaction. Chem. Commun. 2021, 57, 986–989. [Google Scholar] [CrossRef]
- Sakong, S.; Gro, A. Methanol oxidation on Pt (111) from first-principles in heterogeneous and electrocatalysis. Electrocatalysis 2017, 8, 577–586. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Yu, Y. The mechanism of methanol dehydrogenation on the PdAu (1 0 0) surface: A DFT study. Appl. Surf. Sci. 2020, 510, 145434. [Google Scholar] [CrossRef]
- Xu, J.; Guo, S.; Hou, F.; Li, J.; Zhao, L. Methanol oxidation on the PtPd (111) alloy surface: A density functional theory study. Int. J. Quantum Chem. 2018, 118, e25491. [Google Scholar] [CrossRef]
- You, G.; Jiang, J.; Li, M.; Li, L.; Tang, D.; Zhang, J.; Zeng, X.C.; He, R. PtPd (111) surface versus PtAu (111) surface: Which one is more active for methanol oxidation? ACS Catal. 2018, 8, 132–143. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Li, B. A density functional theory study of methanol dehydrogenation on the PtPd3 (111) surface. Int. J. Hydrogen Energy 2015, 40, 9656–9669. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Kang, S.; Pathak, R.; He, W.; Qi, X.; Gu, Z. Density Functional Theory Investigation of the NiO@Graphene Composite as a Urea Oxidation Catalyst in the Alkaline Electrolyte. ACS Omega 2021, 6, 14648–14654. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef] [Green Version]
- Methfessel, M.; Paxton, A. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Yang, T.; Li, P.; Wei, Z. DFT study on ORR catalyzed by bimetallic Pt-skin metals over substrates of Ir, Pd and Au. Nano Mater. Sci. 2021; in press. [Google Scholar] [CrossRef]
- Nagao, R.; Cantane, D.A.; Lima, F.H.; Varela, H. Influence of anion adsorption on the parallel reaction pathways in the oscillatory electro-oxidation of methanol. J. Phys. Chem. C 2013, 117, 15098–15105. [Google Scholar] [CrossRef]
- Bagotzky, V.; Vassiliev, Y.B.; Khazova, O. Generalized scheme of chemisorption, electrooxidation and electroreduction of simple organic compounds on platinum group metals. Chem. Interfacial Electrochem. 1977, 81, 229–238. [Google Scholar] [CrossRef]
- Parsons, R.; VanderNoot, T. The oxidation of small organic molecules: A survey of recent fuel cell related research. J. Electroanal. Chem. Interfacial Electrochem. 1988, 257, 9–45. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Ooka, H.; Huang, J.; Exner, K.S. The sabatier principle in electrocatalysis: Basics, limitations, and extensions. Front. Energy Res. 2021, 9, 155. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Li, L.; Wu, M.; Yu, Y. First principles studies of oxygen adsorption on the γ-U (1 1 0) surface and influence of Mo doping. Comput. Mater. Sci. 2020, 179, 109633. [Google Scholar] [CrossRef]


| Systems | Surface Energy (eV/Å) | ||
|---|---|---|---|
| (111) | (110) | (100) | |
| Pt | 0.075 | 0.115 | 0.106 |
| Pt3Pd | 0.078 | 0.111 | 0.106 |
| PtPdd | 0.082 | 0.114 | 0.109 |
| PtPdO1 | 0.078 | 0.113 | 0.107 |
| PtPdO2 | 0.078 | 0.113 | 0.107 |
| PtPd3 | 0.089 | 0.114 | 0.112 |
| Pd | 0.098 | 0.117 | 0.119 |
| Species | Sites | Bond Length (Å) | Eads (eV) |
|---|---|---|---|
| CH3OH | Top | d(C-O) = 1.440, d(O-Pt) = 2.470, d(C-H) = 1.099, d(O-H) = 0.970 | −0.404 |
| CH3O | Top | d(C-O) = 1.403, d(O-Pt) = 2.208, d(C-H) = 1.110 | −1.340 |
| CH2O | Top | d(C-O) = 1.216, d(C-Pt) = 2.575, d(O-Pt) = 2.261, d(C-H) = 1.119 | −0.165 |
| CHO | FCC | d(C-O) = 1.218, d(C-H) = 1.108, d(O-Pt) = 2.287 | −2.352 |
| CH2OH | Top | d(C-O) = 1.385, d(C-Pt) = 2.287, d(C-H) = 1.096, d(O-H) = 0.979 | −2.037 |
| CHOH | Bri | d(C-O) = 1.368, d(C-H) = 1.101, d(O-H) = 0.976 | −3.114 |
| COH | FCC | d(C-O) = 1.330, d(O-H) = 0.980 | −3.875 |
| CO | FCC | d(C-O) = 1.153, d(C-Pt) = 2.374 | −1.460 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Xue, Q.; Yu, X.; Qi, X.; Wu, R.; Lu, S.; Gu, Z.; Jiang, J.; Nie, Y. DFT Study on Methanol Oxidation Reaction Catalyzed by PtmPdn Alloys. Coatings 2022, 12, 918. https://doi.org/10.3390/coatings12070918
Yang T, Xue Q, Yu X, Qi X, Wu R, Lu S, Gu Z, Jiang J, Nie Y. DFT Study on Methanol Oxidation Reaction Catalyzed by PtmPdn Alloys. Coatings. 2022; 12(7):918. https://doi.org/10.3390/coatings12070918
Chicago/Turabian StyleYang, Tingting, Qian Xue, Xuewei Yu, Xueqiang Qi, Rui Wu, Shun Lu, Zhengrong Gu, Jinxia Jiang, and Yao Nie. 2022. "DFT Study on Methanol Oxidation Reaction Catalyzed by PtmPdn Alloys" Coatings 12, no. 7: 918. https://doi.org/10.3390/coatings12070918
APA StyleYang, T., Xue, Q., Yu, X., Qi, X., Wu, R., Lu, S., Gu, Z., Jiang, J., & Nie, Y. (2022). DFT Study on Methanol Oxidation Reaction Catalyzed by PtmPdn Alloys. Coatings, 12(7), 918. https://doi.org/10.3390/coatings12070918










