Abstract
Carrier particle spray freeze-drying is a new technology with high added value for thermosensitive powder spray freeze-drying. The technology includes the following steps: atomization, coating, freezing, and drying. Due to the action of carrier particles, the condensation of frozen droplets in the conventional spray freeze-drying process is overcome. However, there are many influencing factors involved in the process of freezing coating. The mechanism of the complex droplet collision freezing process still needs to be studied. In this paper, from the perspective of spray freezing coating after atomized droplets collide with low-temperature carrier particles, the coating process and freezing process of single droplets impacting the sphere are analyzed microscopically. The freezing coating processes of static and dynamic carrier particles are reviewed. Moreover, the surface evaluation of powder and equipment development for creating powder products is discussed.
1. Introduction
Spray freeze-drying (SFD) is a low temperature preparation method suitable for high value-added heat-sensitive powder particles such as medicine, biological products, and food. The atomized droplets are directly prepared into powders after freezing and freeze-drying. Without the need for secondary crushing, the product has high porosity and uniform particle size distribution [1].
Compared with spray drying, low-temperature processing is more suitable for the preparation of thermosensitive materials, which avoids the damage of spray drying on the effective components of products. The rapid freezing process also minimizes the phase separation of solutes so that the effective components of drugs can be uniformly distributed in the excipient materials in drug processing. Moreover, the solubility of drugs produced by spray freeze-drying can be significantly enhanced. The prepared drug particles have the characteristics of low density, loose porosity, and moderate particle size, which are beneficial to lung deposition and show better aerodynamics performance than traditional spray drying products. Therefore, drug spray freeze-drying can be applied to enhance apparent solubility, lung administration, intradermal ballistic administration, and delivery of vaccines to nasal mucosa. The process of spray freeze-drying can be matched according to the requirements of dosage form and administration route [2].
The preparation of functional powder by SFD technology is a hot research topic at present. Many scholars try to study the influence of the change of powder morphology and microstructure on the physical and chemical properties of powder particles, such as flowability, wettability, solubility, and stability [3,4,5]. The solution concentration and freezing medium have significant effects on the specific surface area of spray freeze-dried powder. The lower freezing temperature is beneficial to the formation of the porous structure. Compared with high concentration liquid, low concentration is also beneficial to increasing the specific surface area of the powder after drying [6].
Currently, the processing capacity of spray freeze-drying is generally less than several ml/min–mass production of several grams of products or less [7]. Increasing the processing capacity of SFD is a technical problem that needs to be solved for its commercialization. Research and development enterprises focusing on freeze-dryers are also trying to achieve mass processing of SFD technology. The Tofflon is China’s largest freeze-dryer manufacturer. In 2015, the spray freezing granulation device and automatic closed spray freeze-drying device were designed, but there are at this stage no reports of production [8]. In 2018, The Meridion developed a closed SFD system for freeze-drying microspheres, combining a droplet freezing tower, a granulation nozzle, and a rotary drum vacuum freeze-drying system. Using a high-precision jet, the liquid is broken into individual droplets with a diameter of 200–800 μm and frozen into round particles of uniform size in a cooling tank. Then, dynamic freeze-drying is carried out in the rotary freeze-dryer which gives good product consistency, eliminating the typical limitations of traditional freeze-drying products, such as poor fluidity and dose performance, inhomogeneity in the process of treatment, dust emission, and powder product appearance. The technology is on the verge of commercialization [2], but the size of the freeze-dried particles also limits the application of the technology. For example, the preparation of micro-nano drug powder cannot be realized, which is an urgent problem in the pharmaceutical field.
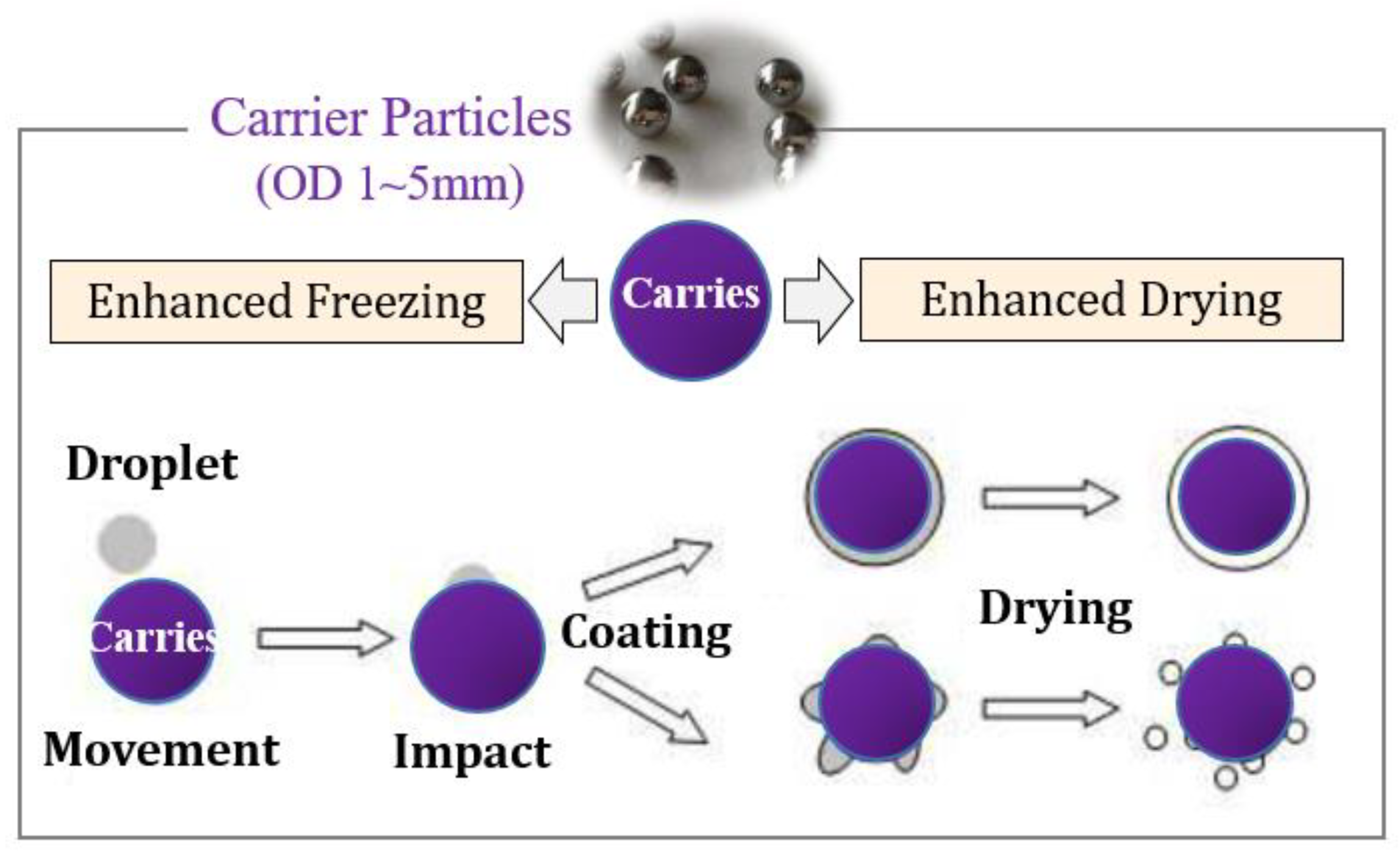
Li et al. [9] proposed a new spray freeze-drying technology of carrier particle spray freezing coating technology. As shown in Figure 1, atomized droplets are sprayed on the surface of cold carrier particles. The cold carrier particles make the droplets freeze rapidly on the surface into ice particles (or ice films). The rapid heat transfer of carrier particles and uniform coating of materials during freeze-drying can realize rapid sublimation of ice crystals to produce dry powder products.
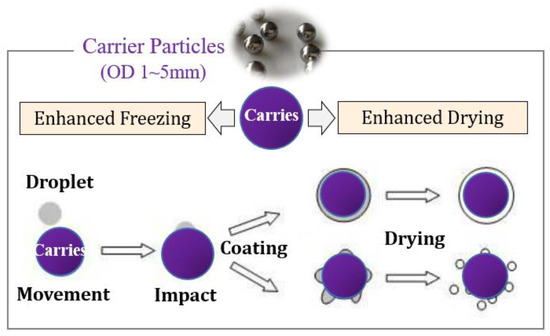
Figure 1.
Schematic diagram of spray freeze-drying on fluidization particle surface.
Due to the function of carrier particles, this technology overcomes the agglomeration of frozen droplets in the conventional spray freezing process, so it does not need an additional stirring device. Droplets are coated on the surface of cold carrier particles rather than directly sprayed into liquid nitrogen (−196 °C) as in most literature, thus overcoming the problem of freezing solution treatment and the special requirements for atomizers. Figure 2 shows the flow chart of the spray freeze-drying device for carrier particles. The low temperature in the drying chamber is maintained by circulating cold gas. The atomized droplets fall behind and freeze in the air or freeze rapidly to form a frozen film after coating the surface of the carrier particles. Then, the vacuum freeze-drying process was carried out in the same drying chamber, and the spray freeze-drying powder was obtained by standard screening.
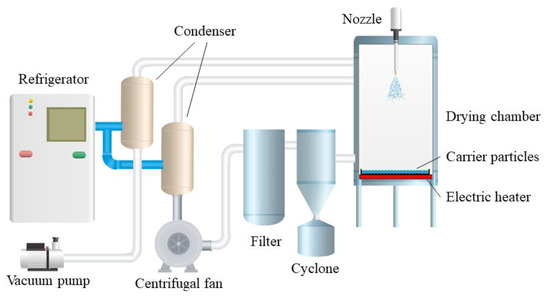
Figure 2.
Flow chart of carrier particle spray freeze-drying device [10].
At present, after the liquid spray freezes, the frozen powder is generally moved to the tray, bottle, or fixed bed for freeze-drying operation. However, because of the low voidage of the powder bed and the mass transfer resistance, the drying time is long. When fluidization technology is adopted, the smaller the particle size of the powder, the shorter the drying time, and the carrier fluidized bed (spouted bed) is thus conducive to the fluidization of the powder [11]. Since the carrier particles are in motion during the coating process, the binding force between coating and carrier is weak. The binding force can be reduced by selecting the carrier material and treating its surface. In addition, the collision between particles and the porosity of the dry surface make it easy to peel off the carrier particles. The stripping of dry surface powder and the formation of carrier particles as a “heat transfer media unit” help to promote mass transfer and heat transfer and to improve the speed of freeze-drying, thereby reducing the drying time. Compared with the traditional fluidized bed drying, this technology can not only efficiently heat and mass transfer, but also rapidly freeze the liquid on the surface of the carrier particles through the rapid freezing process. Hence, the dried powder forms a porous structure. Since the operation process is carried out at a low temperature, the fluidized bed spray freeze-drying technology can also effectively maintain the physical and chemical properties of the materials to be dried. The carrier particles can strengthen the freezing and drying process, strengthen the mass and heat transfer, and overcome the agglomeration of frozen droplets in the conventional spray freezing process. The application of this technology improves the operability and processing capacity of the spray freeze-drying process, which is conducive to the application and development of spray freeze-drying technology.
The most important factor in carrier particle spray freezing coating technology is the impact and freezing of atomized droplets and metal carrier particles, named as the freezing coating. This process also has a great influence on the structure and characteristics of the final product. According to the static and movement of metal carrier particles, the process of freezing coating is also different. From the perspective of droplet collision and freezing on a solid surface, the collision and freezing dynamic behaviors between droplet and substrate with different temperature, shape, and material in carrier particle spray freezing coating technology are summarized and analyzed. The research on drying product quality is summarized and the development trend and direction of equipment development and improvement and new technology application are presented. Carrier particles spray freeze-drying is a new spray freeze-drying technology; this review explores the strengthening effect of carrier particles on spray freeze-drying–especially the important influence of the spray freezing coating stage on spray freeze-drying–to effectively improve the batch volume of spray freeze-drying and promote the development of spray freeze-drying technology.
2. Method
To fulfill the aim, this review adopts the following steps: literature investigation, screening, analysis, and summary. The Web of Science and CNKI were used as search engines for the literature survey. After selecting the database, the literature was searched for structurally. Coating, spray freeze-drying, carrier particles, and droplets were the four core themes, with the following terms being researched: “coating” or “depositing” or “impact”, and “spray freeze drying” or “spray freeze” or “SFD”, and “carrier particles” or “inert particles”, and “drop” or “drill”. The starting date of publication was not determined, and papers published before the middle of 2022 were retrieved. In order to ensure the relevance and qualification of the retrieved papers, the authors carefully examined them on a case by case basis according to the full text of each paper. Language types included English and Chinese, and literature types were limited to journal papers, master’s degree papers, conference papers, and patents. In addition, the research results and summaries of our team over the years were cited. Through the selected papers, a clear report can be completed.
3. Microscopic Analysis of Dancing Single Droplet
Droplet impact particle is a very complicated process. Interference often occurs between droplets and carrier particles, which not only depends on the nature of the droplet (such as the size of the droplets, surface tension, density, viscosity) but also relates to the nature of the surface shape (such as surface roughness, temperature, radius of curvature) [12]. Therefore, it is necessary to study the impact between a single droplet and a single particle to better understand the process of coating and freezing.
3.1. Coating Process of Single Droplet Impacting on Spherical Surface
Since 1876, when Worthington studied droplets hitting smoky glass plates [13], the impact of a droplet on a solid surface has aroused extensive research interest. The impact process is mostly studied by single droplet impact. Reitz et al. [14] first described the whole collision process with the famous adhesion, reflection, and wall-hitting model. They divided the collision into five processes, namely the movement process, spreading process, relaxation process, wetting process, and rebound process (or splashing process).
A single droplet will form a spherical coronal film on the surface of a smooth sphere after impact. Due to the action of the inertia force and surface tension, it will go through four stages: the movement stage, spreading stage, contraction stage, and oscillatory balance stage.
For an incident droplet, it is usually described by a dimensionless number, including Equations (1)–(4):
where, ρ—density of feed liquid, V—velocity at which a droplet strikes a solid surface, D—initial diameter of the droplet, —viscosity of the droplet, σ—surface tension of the droplet. The We represents the ratio of the inertial force of the droplet to the surface tension, the Re represents the ratio of inertial force to viscous force, and the Oh number is used to describe the ratio of viscous force to surface tension. To describe the spreading state of the droplet after collision, the spread rate β is introduced as Equation (5):
K = We0.5Re0.25
Wachters et al. [15] were the first to study the phenomenon of the vertical impact of water droplets on high-temperature surfaces. It is found that the occurrence of rebound and wall attachment after droplet impact is determined by the dimensionless We; the critical value is 80. Zhang et al. [16] studied the spreading, splashing, and solidification of a metallic zirconia droplet impacting on an incline, and obtained the critical conditions for deposition and splashing. When the Reynolds number is 3535 and the impact angle is less than 44° or the Sommerfeld number K > 58, the droplet starts to splash. Otherwise, the droplet will deposit, which opens up the study of molten metal droplets impacting the plate surface.
Xu et al. [17] studied the coating process of a droplet impacting a sphere. Xu defined the coating degree as the coating rate, as shown in Equation (6):
where, R—radius of sphere; H—crown height.
The coating process of a droplet impinging on the sphere is affected by the initial kinetic energy of the droplet, and there may be different situations that occur such as spreading oscillation, coating the sphere, breaking, and splashing. The impact velocity has a great influence on the coating effect. The greater the impact velocity, the greater the energy introduced into the system and the greater the coating rate. The coating rate decreases with the increase in spherical diameter, but there is a critical K value corresponding to the diameter. When the spherical diameter is much larger than the droplet diameter, the coating rate is close to the droplet collision with the plane [17].
Bakshi et al. [18] studied fluid flow and coating in the process of droplets impacting a sphere of different diameters. The dynamic process of droplet impingement on a spherical surface can be divided into three stages: initial droplet deformation stage, inertial force dominant stage, and viscous force dominant stage. At the low Reynolds, the dominant stage of viscous force is advanced and the film thickness increases. With the increase in spherical diameter, the residual thickness of the film increases and the thinning process slows down.
Li et al. [19] carried out a three-dimensional numerical simulation of the process of a droplet impacting a spherical surface with low impact energy, focusing on the influence of the droplet impact velocity and spherical curvature radius on the droplet deposition behavior. The droplet spreading rate and spreading area increase with the increase in impact velocity, and the droplet spreading area decreases with the increase in spherical curvature radius. The relationships between the local rupture and Weber and radius of spherical curvature are established.
For the impacted surface, there are various surface properties, including hydrophilic and hydrophobic [20]; they are also imporous or porous. This mainly affects the wettability of the droplet, and the wettability often determines the size of the contact angle. It is usually described by contact angle and wettability, and the equation for evaluating wettability is mainly the Young Equation (7):
where θ is the contact angle of the droplet, , , are the interfacial tension of liquid–gas, solid–liquid, and solid–gas, respectively. Dupre defined the bond work between solid and liquid as Equation (8):
substitute this equation into the Young equation to obtain the Young–Dupre Equation (9):
for the given value of , the larger the bond work between solid and liquid, the smaller the contact angle and the better wettability [21]. The dynamic contact angle, contact linear velocity, and dimensionless spread factor are all functions of time [22].
Lunkad et al. [23] simulated the spread of droplets on a porous spherical surface due to the influence of pore structure in order to solve the problem of spreading and adsorption of droplets on the spherical filler in the packed column. The spreading behavior of different droplets on a porous spherical surface is obtained. It is found that the greater the number of pores, the easier the droplet spreads, but the effect of pores on droplet spread decreases with the increase in surface wettability (static contact angle). The spread of a droplet over a porous surface supports the idea of a dual role for the hole, with one edge of the droplet moving away from the hole and the other accelerating towards it. Mantle et al. [24] introduced magnetic resonance imaging (MRI) to study the shape and spreading imaging of single droplet on a porous surface. Gunjal et al. [25] also studied the collision between a droplet and spherical surface catalyst in the spray bed reactor to better explore the wetting characteristics of the catalyst.
Bang et al. [26] studied the velocity evaluation of the gas and liquid phase after the impact induced by the impact droplet. They numerically simulated how the gas pressure affected the impact of the droplet and calculated the internal and external pressures of the impact droplet. The fluid velocity was calculated using the BEF method to reflect the change in the momentum of the falling droplet and surrounding gas.
3.2. Freezing Process of Single Droplet Impacting on Spherical Surface
Mass and heat transfer can change the droplet properties after impact and the heat-inducing effect can be inferred experimentally. Precise experiments can be used to calculate the heat transfer during an impact by measuring liquid–solid contact (e.g., deposition of plate mass, contact time, mixing rate, gas release rate, etc.). The mass and heat transfer in the collision is mainly determined by the temperature difference between the droplet and the plate surface transferred from the high temperature object to the low temperature object. The droplet surface temperature distribution can be used to judge the droplet evolution behavior after impact. This temperature distribution can be as an evaluation index of the impact process to have an important influence on the fluid dynamics characteristics of the droplet during impact. Heterogeneous nucleation occurs at the liquid-solid interface when the droplet collides with the supercooled surface. Cossali et al. [27] studied the morphology and heat transfer mechanism of droplet impact, and introduced image analysis technology (IAT) into the experimental post-processing to analyze the change in the diameter of a secondary atomized droplet over time. Bhardwaj et al. [28] used a high-resolution laser surface temperature meter to measure the heat transfer in the process of microdroplet spreading. This non-contact temperature measurement method greatly simplified the operation complexity. In the diffusion stage and cooling stage after droplet impact, two condensation stages occur due to the interaction between the droplet impact and vapor mass diffusion, and three different condensation halos form [29].
When a droplet collides with a cryogenic surface, heat is transferred from the liquid phase to the solid phase, during which the droplet rapidly spreads out and freezes in the cryogenic sphere. As the droplet diffusion time is much shorter than the solidification time, different shapes of frozen droplets are formed in the process of spreading and shrinking. A single droplet impacting on a cold surface was investigated by analyzing changes in droplet shape and dimensions recorded with a high-speed camera by Xu et al. [30]. At low surface temperatures (−5 and −10 °C), a droplet spreads in a very short period upon impact and then retracts. Higher impact velocity expands the spreading diameter, but at lower surface temperatures (i.e., −20 °C) the droplet can be classified into two categories: instantaneous freezing and non-instantaneous freezing on different material surfaces. The results of this work show a general indication of the effect of the wall material on the collision problem, and thus proper selection of dryer wall material may be considered to reduce the deposition problem.
On this basis, Xu and Wang et al. [31,32] simulated the coating freezing process of a low viscosity and low density droplet impacting a flat plate by using the method of multi-physics coupling. Combining the two-phase flow level set method and Marin’s curing model, the curing model of the level set method was established by replacing the enthalpy equation with the temperature-based heat conduction equation and adding the latent heat of melting. The spread rate and spread diameter after the droplet impacts the cold plate surface are mainly affected by the viscosity of the feed liquid rather than the temperature.
The impact and freezing process of water droplets on different spherical cold surfaces are experimentally studied. Ju et al. [33] found that the diffusion factor of a low-temperature surface was larger than that of a normal temperature surface. Due to the effect of cold carrier particles, the liquid–solid interface was first formed at the bottom of the water droplets. When more liquid water became ice, the liquid–solid interface moved upward. After the whole droplet froze, a small protrusion appeared at the top of the ice bead. However, the change in temperature and spherical curvature radius did not lead to the obvious change of ice bead shape.
The study of coating and freezing between a single droplet and a sphere mainly focuses on millimeter-scale droplets, which are relatively easy to operate; research regarding micron droplets is more difficult. Wu et al. [34,35] studied the freezing behavior of a single droplet of Pullulan polysaccharide on a micron scale (240–600 μm) impacting the cryosphere with a high-speed camera. It was found that the droplet formed an ice film with the decrease in temperature during the coating process. The low temperature increases the surface stress and viscous force of the droplet, and produces resistance to the droplet’s oscillation retraction. The oscillation frequency of the droplet is lower than that of the normal temperature. This process is fast and stable. The spreading diameter of the liquid film decreases and the thickness of the coating layer becomes thinner. Changing the temperature has a greater effect on the liquid film spreading than changing the droplet diameter and carrier particle diameter.
Therefore, the high-speed camera method can be used to study the collision between droplets and low-temperature spherical carrier particles, and the coating degree can be used to describe the collision process. When studying the critical state of a droplet spreading evenly and rebound splashing on the surface of carrier particles, it should focus on the situation of the droplet film coating sphere and droplet solid adhesion sphere. Researchers can study the influence of droplet characteristics (such as size, viscosity, composition, impact velocity, etc.) on the coating degree of the carrier sphere (material, surface characteristics, etc.). The researchers used Comsol Multiphysics software to simulate the process of a droplet impacting the spherical surface and to compare with the experimental results. The mathematical model of droplet impacting on the spherical surface freezing was established to realize the freezing coating of droplet and carrier particles. The interaction relationship between droplet and carrier particles can be provided for the study of droplet spray freezing coating.
4. Spray Freeze Coating on the Stationary Carrier Particles
4.1. Freezing and Flow of Atomized Droplets in Flow Field
The atomization freezing of a droplet group is mainly defined by spray freezing dynamics, that is, heat and mass transfer, supercooling, and the freezing rate of the droplet. Therefore, the establishment of an appropriate freezing model is of great significance for the development, industrial design, and scale of SFD equipment. Sebastiao et al. [36] established a single droplet spray freezing model to study the influence of different process parameters on the droplet freezing process and the theoretical operating limit of SFD equipment. The model described the evolution process of the position, total mass, speed, temperature, and solute concentration of the single droplet during its fall in the cooling tower. They found that the droplet went through three different states, namely initial cooling, freezing (ice crystallization), and post-freezing cooling. When the supercooled droplet reaches the nucleation temperature, the crystal nucleus is first formed inside the droplet and rapidly crystallized. The time of this process is related to the droplet temperature and solute mass fraction. In the actual spray freezing process, due to the fluctuation of airflow and other impurities in the gas, the freezing process of the droplet may start from the outside of the droplet and form a shell on the surface of the droplet. For droplets at the millimeter level, the whole process can be completed within a few seconds. Therefore, in order to avoid the freezing of the droplet before the collision with the carrier particles, it is necessary to control the temperature, flow rate of cold air, and the concentration of solute in the droplet to control the supercooling of the droplet. The carrier particles should have a lower temperature so that the droplets can freeze rapidly with a high freezing rate after contacting.
Geng et al. [37] established a mathematical model for calculating the freezing process of atomized droplets and considered the heat loss and mass change caused by droplet moisture evaporation in the pre-cooling stage into the calculation of the freezing stage. A one-dimensional layered freezing model of the freezing process is established. The influence of atomization freezing conditions on the freezing time of micron droplets was studied, and the mass loss of atomized droplets during freezing was analyzed. Compared with changing airflow velocity and ambient temperature, decreasing droplet diameter can greatly shorten the complete freezing time of the droplet, and the droplet mass loss rate is about 2% during the whole freezing process.
The freezing process of atomizing droplets is affected by many factors such as droplet size, airflow velocity, and environmental temperature. However, for the time required for the complete freezing of droplets, there are significant differences in the degree of influence of these three parameters. Among them, the droplet size has the greatest impact on the freezing time. When the airflow velocity was 10 m/s and the ambient temperature was −42 °C, the complete solidification time increased from 0.11 to 199 μs as the droplet diameter increased from 2 to 141 μm [38]. Completely solidified droplets cannot form a coating on the surface of carrier particles. Therefore, in industrial production, these three factors need to be comprehensively considered to avoid incomplete coating or complete freezing of droplets in the air.
The above model can be used as a mathematical model to calculate the freezing degree and freezing time of atomized droplets. However, atomized droplet groups will randomly move and collide in the equipment, resulting in changes in droplet size distribution. The change process of the particle size is mainly related to freezing nucleation, coalescence, and crushing.
Li et al. [38] studied the flow field, temperature distribution, and particle aggregation and fragmentation law in a spray refrigerating bed based on the PBM and DPM models. They added the population equilibrium equation (PBM) to describe the balance of the droplet group–the agglomeration fragmentation behavior generated in the process of droplet atomization. The variation of droplet size, temperature field distribution, and contact times between the droplet and solid particles were systematically studied. The variation of droplet size distribution in the atomization process and the contact collision between the carrier particles and droplet in the three-phase flow were obtained. Compared with the calculation results of DPM of the discrete phase model, the population equilibrium model can obtain detailed fog droplet size distribution based on accurate velocity field distribution. Due to the low ambient temperature, droplets will quickly freeze, form ice particles, and reach the bottom wall of the fluidized bed. After that, the droplets backflow upward and collide with other droplets, and a large number of droplets coming out near the wall will appear, which will affect the atomization process.
This study can better understand the droplet group before it reaches the carrier particles and movement in the equipment.
4.2. Three Cases of Spray Freeze Coating
In the interaction between atomized droplets and stationary carrier particles in the equipment, the coating deposition on the wall of the equipment, the coating freezing on the carrier particles, and the clearance freezing of the carrier particles will occur.
Xu et al. [39,40,41] developed a visualization system for the freezing coating of micron-level droplets hitting spherical surfaces. They collected dynamic images of atomized droplets during the coating and freezing process on the surface of a cold carrier to study the dynamic phenomenon of the freezing coating of droplets. The spray droplet group was coated and frozen on the surface of carrier particles at a low temperature (−20 °C). The effects of the carrier particle diameter, atomized liquid flow rate, and spray volume on the coating process of the atomized droplet group were studied. Through a simple and effective image analysis method based on Image J for the spray freezing coating effect of carrier particles, the projection area of the particle coating was obtained and the coating amount was calculated. A multi-parameter model of droplet group coating on carrier particles was established as Equation (10):
where, δ—coating thickness, —particle diameter, m—coating amount, N—particle number, ρ—material liquid density.
The model can predict the variation trend of the coating amount and coating thickness, and provide theoretical support for controlling the coating process. According to the coating liquid volume model, the critical coating amount and coating thickness of coated particles were obtained, and the coating thickness was about 140–180 μm. The coating thickness measured in the experiment was very close to the theoretical prediction. The results show that the theoretical model is accurate, and the detection and control of liquid film coating thickness on the surface of spray-frozen spherical particles at a low temperature are well-realized. The maximum spraying amount can be estimated when a specific mass of carrier particles is used.
Zhang et al. [10] studied in detail the microstructure of powder produced in these three cases. The surface structure and morphology of dry powders under three conditions were observed by SEM. The effects of coating freezing on the cold surface of carrier particles, cold air freezing, and wall deposition on powder morphology and microstructure were analyzed. The results show that the contact freezing of droplets with carrier particles, frozen particles, the wall of the drying chamber, and random collision between droplets can lead to different powder structures. The droplet–carrier particle contact will rapidly freeze, and the powder near the contact surface will show a cellular structure. With the decrease in freezing rate, the particle structure will have a changing trend of cell–dendritic–flake. If the operation is improper, the droplet deposited on the wall of the drying chamber will affect the sphericity of the powder, and the powder will agglomerate.
Zhang et al. [42] studied the influence of carrier particles on the process of preparing whole milk powder in combination with the above phenomena. It is found that adding carrier particles can shorten the SFD time. Compared with conventional SFD, adding 5 mm stainless steel carrier particles can shorten the drying time by 37%. Under the same atomization conditions, the powder particle size distribution range is narrower and the average particle size is smaller. The powder coated on the surface of the carrier particles is rapidly frozen to form ice particles or ice with fine ice crystal structure. After freeze-drying, a cellular powder skeleton with high porosity will be formed. The connectivity of pores is excellent, and the drying time can be shortened as a channel for ice sublimation. Adding carrier particles can strengthen freezing, make the dry powder create a porous structure, and increase the specific surface area of the powder.
Ma et al. [43] carried out the SFD experiments on the surface of carrier particles with different materials (stainless steel, glass); the collected powder had different surface structures. The powder on the surface of the stainless steel ball had a porous shell, and the powder surface had a more complete shell structure with a decrease in the thermal conductivity of the carrier particles.
In the experiment, it was found that when static carrier particles were freezing coated, the spray volume received at the atomization center was large and radially diffused outward. In order to improve the uniformity of carrier particle coating, it was considered to move carrier particles so that random carrier particles could increase the uniformity of the coating.
5. Spray Freeze Coating on Moving Carrier Particles
Zhang et al. [44,45,46] studied the freezing coating of carrier particles in a spouted bed. Materials were frozen on the surface of the spouting cold carrier particles, and low-temperature, and low-humidity air was used as the spouting gas to achieve the conditions and dynamic adjustment for the stable operation of spray freezing coating in the spouted bed.
The coating freezing process can be treated in batches by using a spouted bed, and bed stability is the key. The freezing process of droplets would agglomerate the carrier particles. By analyzing the standard deviation of the bed pressure drop and the fluctuation of the bed pressure, the stable operating conditions of the spouted bed under freezing coating were studied, and a dynamic adjustment method of the inlet air velocity to maintain stable spouting was proposed. The results show that the stable operating air velocity range can be described using the standard deviation of pressure fluctuations. Before bed collapse occurs, it will experience an unstable spouting process, which causes the bed pressure to fluctuate a standard deviation of less than 15 Pa after more than 40 Pa [46].
6. Integrated Spray Freeze Coating Equipment and its Engineering Application
The carrier particle SFD technology is an effective method to protect high volatile flavor compounds formed due to the production process at a low temperature. This SFD technology has been used to produce high activity probiotics, coffee powder with volatile compounds, high-quality milk powder, and other powdered foods. Future research tends to focus on the development and simulation of new spray freeze-drying devices [35,47], the optimization of process parameters, the online measurement of temperature and humidity distribution, and the control of multi-level continuous drying in order to realize its compact, automatic, and energy-saving properties [48]. In recent years, new spray freeze-drying devices and methods have also been reported.
Xu et al. [49] developed a freeze-drying device that can judge the critical points of primary and secondary drying in the freeze-drying process. This device can accurately judge the dividing point and end point of primary and secondary drying. It can raise the heat of the material at the exact time, shorten the drying time, and further improving the product quality. When preparing SFD powder, the ice particles are transferred to the equipment after atomization and freezing, which can realize the real-time monitoring of the material freeze-drying process.
Li et al. [50] developed an integrated carrier particle spray freeze-drying device that can shorten the freeze-drying time of bioactive materials. Compared with the traditional freeze-drying device of probiotics, the drying time of the same quality material is shortened from 37 to 15 h, which effectively improves the batch production efficiency. The equipment sets up a drying end point detection device to prevent excessive drying and save energy. The activity of probiotics is almost the same, but the powder properties of the products are greatly improved.
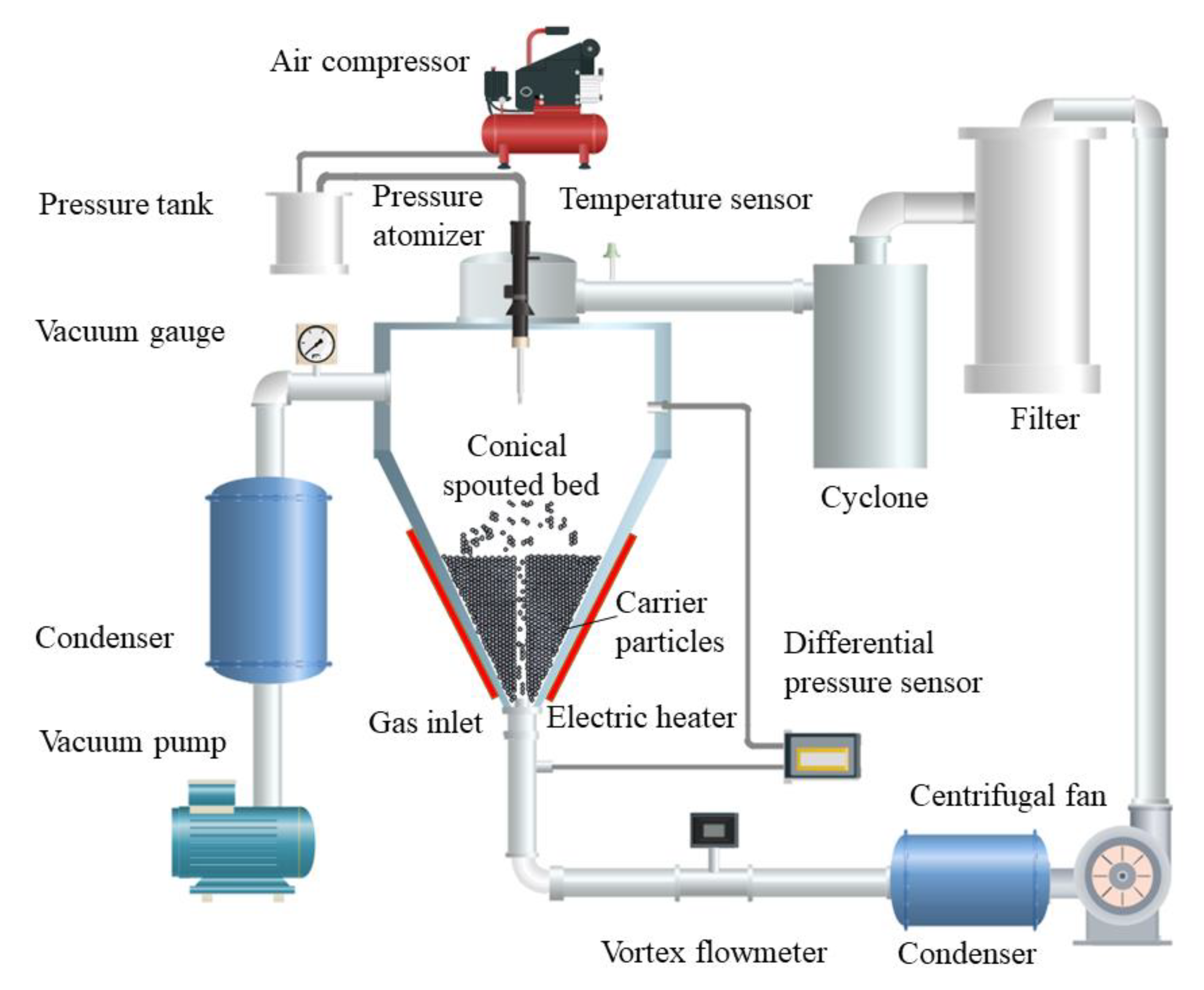
Based on the carrier particle fluidized bed [9], Li et al. invented an integrated carrier particle spouted bed spray freeze-drying device [46], as shown in Figure 3. The materials are frozen on the surface of the spouting cold particles, and low-temperature and low-humidity air is used as the spouting gas. The dry powder layer on the surface of the spouting particles presents a very high porosity. Due to the collision coating between the atomized droplets and cold carrier particles, the droplets freeze rapidly after spreading, avoiding solute segregation migration to instead form interconnected pore networks after the frozen coating dries.
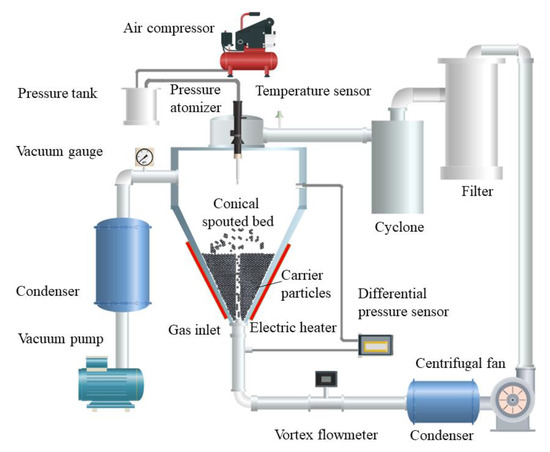
Figure 3.
Flowchart of the carrier particle spouted bed for the spray freezing process [44].
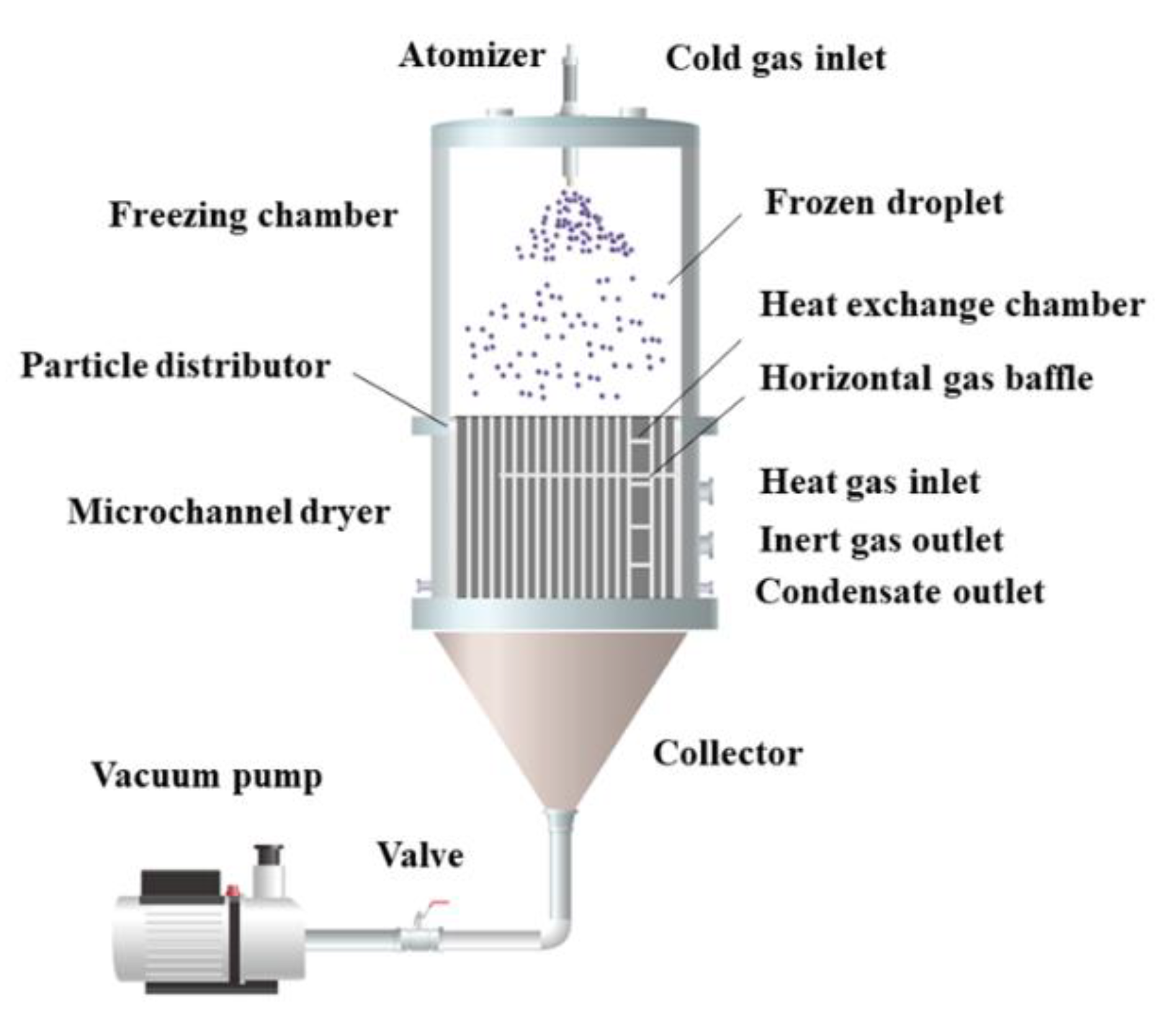
For the preparation of micro-nano powders, Li et al. [51] invented the spray freezing micro-channel drying equipment, which combined spray freezing and micro-channel drying technology, as shown in Figure 4. The atomized droplets freeze and fall in the freezing chamber, disperse through the distributor, enter the micro-channel dryer, and dry to the required moisture content in the process of movement. This device realizes the integration and automatic operation of the atomization, freezing, and drying process.
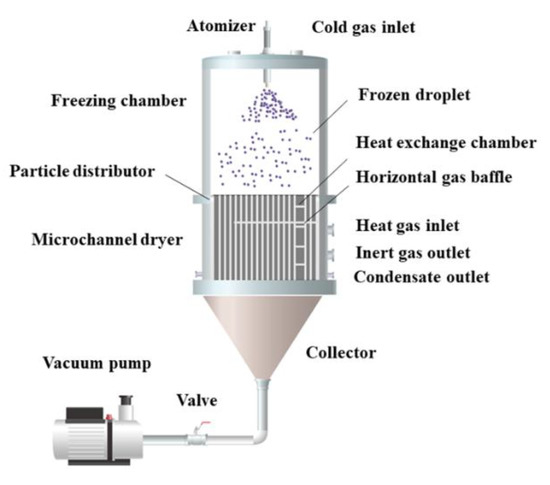
Figure 4.
Flow chart of spray freezing microchannel drying equipment.
7. Conclusions
The most important factor in the carrier particle spray freezing coating technology is the impact and freezing between the atomized droplets and carrier particles–named, cold coating. The presence of carrier particles can strengthen the freezing and drying process and strengthen mass and heat transfer to overcome the agglomeration of frozen droplets in the conventional spray freezing process. This technology can improve the operation and handling capacity of the spray freeze-drying process.
The collision between droplets and spherical surfaces is the basis for the coating technology. The freezing of a single droplet in cold air, random collision freezing of droplets, and coating freezing of droplets on the surface of carrier particles will form ice particles, and eventually lead to the change in particle size distribution and product structure.
There are many factors affecting the freezing coating of atomized droplet groups in the equipment. In the case of inappropriate gas velocity, turbulence will occur in the freezing chamber to promote the droplet–droplet and droplet–wall surface collision, and affect the particle size distribution. In order to achieve the uniformity of the coating, the static carrier particles are considered to move, and the dynamic collision and freezing process will affect the morphology of the final powder. Hence, it is of great significance to obtain a product with expected functional characteristics.
Spray freeze-drying of carrier particles is a very effective method to prepare high-quality micro-nano powder particles that are suitable for low temperature preparation of high value-added thermally sensitive powder particles, such as in drugs, biological products, and food. However, there is a poor controllability of freezing process and an agglomeration problems in the process of freezing coating. The morphology control mechanism of powder products remains to be studied to further improve product quality. The drying process mechanism and powder stripping characteristics of powder on the surface of carrier particles in a moving state need further study.
Author Contributions
Q.X., Manuscript writing, literature search, study design; R.W. (Ruixin Wang), Literature search, manuscript revision; F.Z., Manuscript writing, figures, study design; R.W. (Ruifang Wang), Study design, data collection; L.W., Manuscript revision and figures; B.L., Literature search and data collection. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support of this project by the Key-Area Research and Development Program of Guangdong Province (No. 2020B0202010004) and the National Natural Science Foundation of China (Grant No. 31571906).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Q.; Geng, X.R.; Li, Z.Y. Morphology of particle produced by spray-freeze drying. Chem. Ind. Eng. Prog. 2013, 32, 270–275. [Google Scholar] [CrossRef]
- Wanning, S.; Süverkrüp, R.; Lamprecht, A. Pharmaceutical spray freeze drying. Int. J. Pharmaceut. 2015, 488, 136–153. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Huang, L.X.; Zhou, R.J.; Mujumdar, A.S. Studies on the spray freeze drying of milk powders. Chem. Eng. Mach. 2009, 36, 219–222. [Google Scholar]
- Lei, H. Towards Tuning Microparticle Properties via Spray Drying Technique Invesitgation on Particle Formation Mechanism. Master’s Thesis, Suzhou University, Suzhou, China, 2016. [Google Scholar]
- Xu, Q.; Yao, Y.; Zhao, T.; Shi, Q.Q.; Li, Z.Y.; Tian, W. Dissolution characteristics of freeze-dried pullulan particles affected by solution concentration and freezing medium. Int. J. Food Eng. 2018, 14, 20180073. [Google Scholar] [CrossRef]
- Kamlesh, C.P.; Chen, X.D. Production of spherical and uniform-sized particles using a laboratory ink-jet spray dryer. Asia-Pac. J. Chem. Eng. 2007, 2, 415–430. [Google Scholar] [CrossRef]
- Zheng, X.D. A Fully Automatic Closed Spray Freeze-Drying Production Equipment and Method. Chinese Patent ZL201510790555.5, 10 February 2016. [Google Scholar]
- Li, Z.Y.; Xu, Q.; Wu, Z.H.; Ye, J.S.; Geng, X.R. The Invention Relates to an Inert Particle Spray Freeze Drying Device and a Method Thereof. Chinese Patent ZL201110103036.9, 26 October 2011. [Google Scholar]
- Zhang, F.; Wang, L.S.; Ma, X.Y.; Xu, Q.; Tian, W.; Li, Z.Y. Microstructure of spray freezing dried powders affected by the presence of inert particles. Int. J. Food Eng. 2020, 16, 1357–1366. [Google Scholar] [CrossRef]
- Li, Z.Y.; Pan, B.; Gao, X.Y.; Hu, Y.J. Particle mixing and segregation of binary mixtures in fluidized beds with additional pulsating air flow. Trans. Chin. Soc. Agric. Mach. 2015, 46, 247–253. [Google Scholar] [CrossRef]
- Zhu, W.Y. A Visual Experimental Study on a Droplet Impacting onto Solid Surfaces. Master’s Thesis, Dalian University of Technology, Dalian, China, 2007. [Google Scholar]
- Worthington, A.M. On the forms assumed by drops of liquids falling vertically on a horizontal plate. Proc. R. Soc. Lond. 1876, 25, 261–271. [Google Scholar] [CrossRef]
- Naber, J.D.; Reitz, R.D. Modeling engine spray wall impingement. SAE Trans. 1988, 97, 118–140. [Google Scholar]
- Wachters, L.H.J.; Westerling, N.A.J. The heat transfer from a hot wall to impinging water drops in the spheroidal state. Chem. Eng. Sci. 1966, 21, 1047–1056. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhang, H.; Zheng, L.L. Simulation of droplet spreading, splashing and solidification using smoothed particle hydrodynamics method. Int. J. Heat Mass Transf. 2008, 51, 3410–3419. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.Y.; Wang, R.F.; Zhu, S.G. Coating and the impact of single droplet on the spherical surface. J. Tianjin Univ. Sci. Technol. 2013, 28, 50–54. [Google Scholar] [CrossRef]
- Bakshi, S.; Roisman, I.V.; Tropea, C. Investigations on the impact of a drop onto a small spherical target. Phys. Fluids 2007, 19, 32102. [Google Scholar] [CrossRef]
- Li, Y.P.; Wang, H.R. Numerical study on deposition characteristics of a droplet impinging onto spherical surface with low impact energy. J. Xi’an Jaotong Univ. 2009, 43, 21–24. [Google Scholar]
- Li, X.Y.; Ma, X.H.; Lan, Z. Behavioral patterns of drop impingement onto rigid substrates with a wide range of wettability and different surface temperatures. AIChE J. 2009, 55, 1983–1992. [Google Scholar] [CrossRef]
- Mendez-Vilas, A.; Jodar-Reyes, A.B.; Gonzalez-Martin, M.L. Ultrasmall liquid droplets on solid surfaces: Production, imaging, and relevance for current wetting research. Small 2009, 5, 1366–1390. [Google Scholar] [CrossRef]
- Šikalo, Š.; Tropea, C.; Ganić, E.N. Dynamic wetting angle of a spreading droplet. Exp. Therm. Fluid Sci. 2005, 29, 795–802. [Google Scholar] [CrossRef]
- Lunkad, S.F.; Maiti, R.N.; Buwa, V.V.; Nigam, K.D.P. Numerical study of drop spreading over saturated pores. Can. J. Chem. Eng. 2010, 88, 661–670. [Google Scholar] [CrossRef]
- Mantle, M.D.; Reis, N.C.; Griffiths, R.F.; Gladden, L.F. MRI studies of the evaporation of a single liquid droplet from porous surfaces. Magn. Reson. Imaging 2003, 21, 293–297. [Google Scholar] [CrossRef]
- Gunjal, P.R.; Ranade, V.V.; Chaudhari, R.V. Experimental and computational study of liquid drop over flat and spherical surfaces. Catal. Today 2003, 79–80, 267–273. [Google Scholar] [CrossRef]
- Bang, B.H.; Yoon, S.S.; Kim, H.Y.; Heister, S.D.; Park, H.; James, S.C. Assessment of gas and liquid velocities induced by an impacting liquid drop. Int. J. Multiph. Flow 2011, 37, 55–66. [Google Scholar] [CrossRef]
- Cossali, G.E.; Marengo, M.; Santini, M. Thermally induced secondary drop atomisation by single drop impact onto heated surfaces. Int. J. Heat Fluid Flow 2008, 29, 167–177. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Longtin, J.P.; Attinger, D. A numerical investigation on the influence of liquid properties and interfacial heat transfer during microdroplet deposition onto a glass substrate. Int. J. Heat Mass Transf. 2007, 50, 2912–2923. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.G.; Zhu, F.Q.; Zhang, H.; New, T.H.; Jin, L.W.; Yang, C. Triple condensate halo from a single water droplet impacting upon a cold surface. Appl. Phys. Lett. 2019, 114, 183703. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.Y.; Wang, J.; Wang, R.F. Characteristics of single droplet impact on cold plate surfaces. Dry. Technol. 2012, 30, 1756–1762. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.Y.; XU, Q.; Wang, R.F. Numerical simulation of the impact of single droplet oncold horizontal plate surface. J. Tianjin Univ. Sci. Technol. 2014, 29, 36–41. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, J.; Li, M.M.; Li, Z.Y. Experiments and simulation of a single droplet impacting on cold surfaces. Ciesc J. 2016, 67, 4160–4168. [Google Scholar] [CrossRef]
- Ju, J.J.; Jin, Z.Y.; Zhang, H.H.; Yang, Z.G.; Zhang, J. The impact and freezing processes of a water droplet on different cold spherical surfaces. Exp. Therm. Fluid Sci. 2018, 96, 430–440. [Google Scholar] [CrossRef]
- Wu, X.S.; Xu, Q.; Wang, R.F.; Li, Z.Y. Impact of coating droplet on low temperature spherical particles. J. Tianjin Univ. Sci. Technol. 2019, 34, 43–48. [Google Scholar] [CrossRef]
- Wu, X.S. Uniformity of Liquid Film on Inert Particles by Spray Freezing Coating. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2019. [Google Scholar]
- Sebastiao, I.B.; Bhatnagar, B.; Tchessalov, S.; Ohtake, S.; Plitzko, M.; Luy, B.; Alexeenko, A. Bulk dynamic spray Freeze-Drying part 1: Modeling of droplet cooling and phase change. J. Pharm. Sci. 2019, 108, 2063–2085. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.R.; Xu, Q.; Li, Z.Y.; Song, J.T. Numerical simulation of a single droplet freezing process. Chem. Ind. Eng. Prog. 2012, 31, 981–986. [Google Scholar] [CrossRef]
- Li, M.M.; Xu, J.; Wang, X.; Xu, Q. Numerical simulation of droplets atomization in spray-freezing fluidized bed based on DPM and PBM. China Powder Sci. Technol. 2018, 24, 46–51. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, L.S.; Wu, X.S.; Zhang, F.; Li, Z.Y. Liquid film growth of spray-freezing coating on low temperature spherical particles. J. Tianjin Univ. Sci. Technol. 2021, 36, 42–47. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, X.S.; Xu, J.; Li, Z.Y. System and Method for Use in Freezing and Coating after Impact of Micron-Sized Droplets onto Sphercial Surfaces. Australian Patent 2020100794, 10 June 2020. [Google Scholar]
- Li, Z.Y.; Ma, X.Y.; Zhang, F.; Xu, Q. An Image Analysis Method of Spray Freezing Coating Effect Based on Image J. China Patent CN201910670255.1, 13 December 2019. [Google Scholar]
- Zhang, F.; Ma, X.Y.; Wu, X.S.; Xu, Q.; Tian, W.; Li, Z.Y. Inert particles as process aid in spray-freeze drying. Dry. Technol. 2020, 38, 71–79. [Google Scholar] [CrossRef]
- Ma, X.Y.; Zhang, F.; Li, Z.Y.; Xu, Q. Effect of Inert Carrier Particles on Morphology of Spray Freeze-Drying Powders. In Proceedings of the 17th National Drying Technology Exchange Conference, Nanjing, China, 26 September 2019. [Google Scholar]
- Zhang, F.; Wang, L.S.; Xu, Q.; Tian, W.; Li, Z.Y. Stability of spouted bed during spray cold coating on the surface of carrier particles. Int. J. Food Eng. 2022, 18, 119–128. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, F.; Wang, L.S.; Xu, Q. An Inert Particle Spray Freeze Drying Device and Method. China Patent CN201911249506.5, 19 June 2020. [Google Scholar]
- Li, Z.Y.; Zhang, F.; Xu, Q.; Wang, L.S.; Gao, H.P.; Lin, B. Spouting Stability Self-Regulating Spray Freezing Spouted Bed Drying Device and Drying Method. China Patent CN202011633545.8, 18 June 2021. [Google Scholar]
- Sebastião, I.B.; Bhatnagar, B.; Tchessalov, S.; Ohtake, S.; Plitzko, M.; Luy, B.; Alexeenko, A. Bulk dynamic spray Freeze-Drying part 2: Model-Based parametric study for Spray-Freezing process characterization. J. Pharm. Sci. 2019, 108, 2075–2085. [Google Scholar] [CrossRef]
- Bruttini, R.; Liapis, A.I. The drying rates of spray freeze drying systems increase through the use of stratified packed bed structures. Int. J. Heat Mass Transf. 2015, 90, 515–522. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, L.S.; Wang, H.Y.; Wang, J.J.; Dai, X.Y. Drying Device and Method for Judging the Critical Point of Primary and Secondary Drying in Freeze-Drying Process. China Patent CN202111331012.9, 15 March 2022. [Google Scholar]
- Li, Z.Y.; Zhang, F.; Ma, X.Y.; Xu, Q. Large-Scale Drying Device and Method of Probiotics Liquid Freeze Drying Time. China Patent ZL201910670268.9, 3 December 2019. [Google Scholar]
- Li, Z.Y.; Xu, Q.; Xu, J.; Li, M.M. Spray Freezing Microchannel Dryer. China Patent ZL201611020004.1, 3 May 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).