The Single-Step Fabrication of a Poly (Sodium Vinylsulfonate)-Grafted Polyetheretherketone Surface to Ameliorate Its Osteogenic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.4. Cell Culture
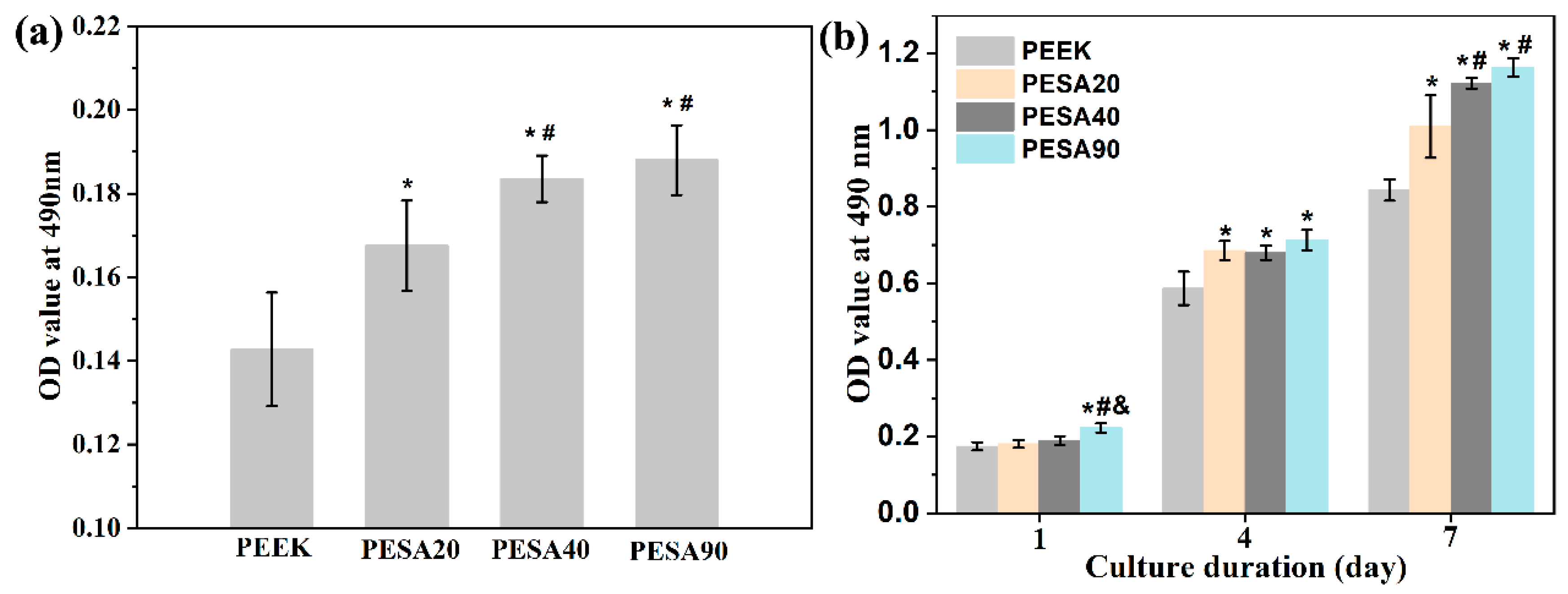
2.5. Cell Attachment and Proliferation
2.6. Cell Morphology
2.7. Osteogenic Activity Studies
2.7.1. Alkaline Phosphatase (ALP) Activity
2.7.2. Extracellular Matrix (ECM) Mineralization
2.7.3. Collagen Secretion
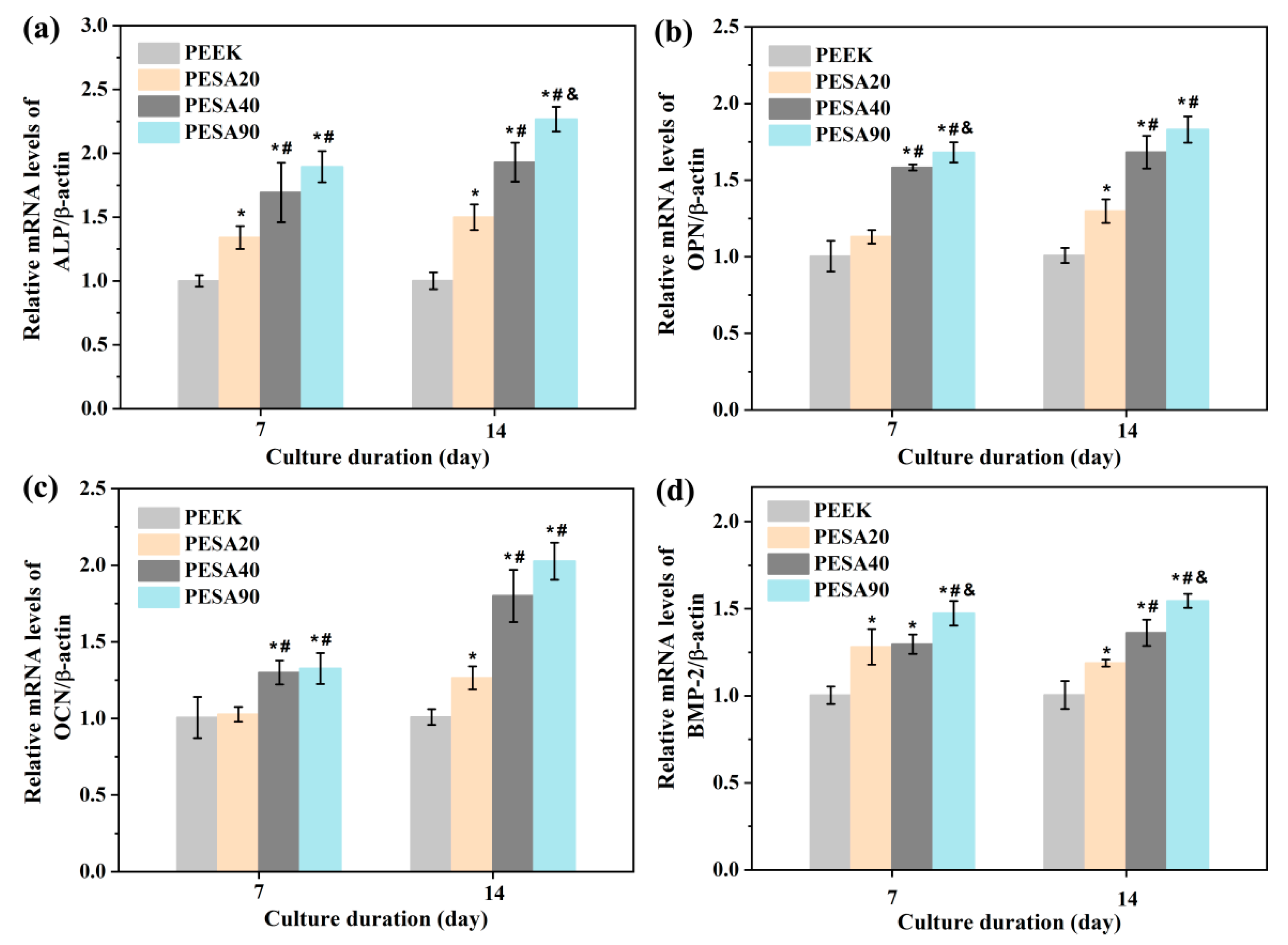
2.7.4. Osteogenesis-Related Genes Expressions
2.8. Statistical Analysis
3. Results and Discussion
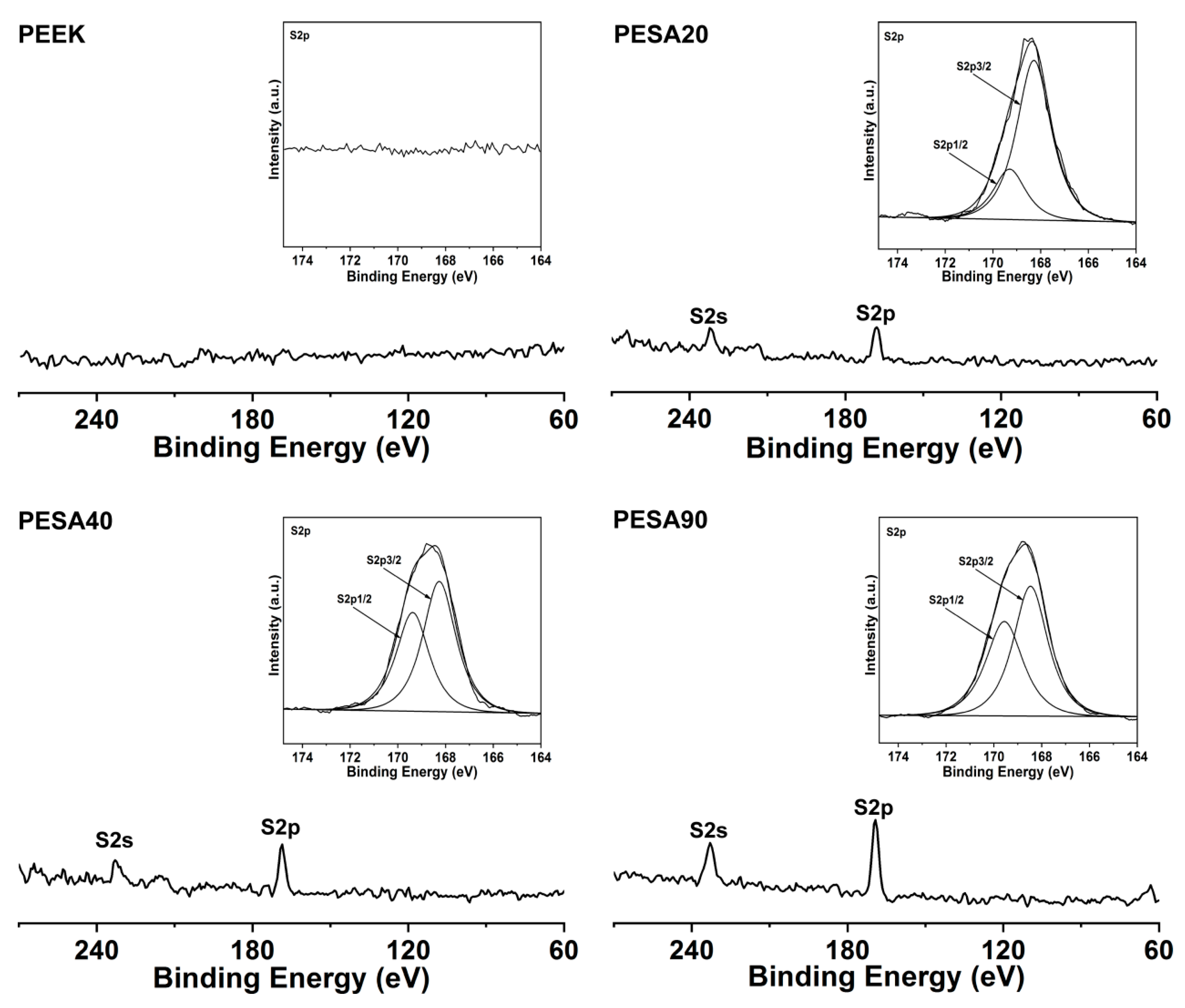
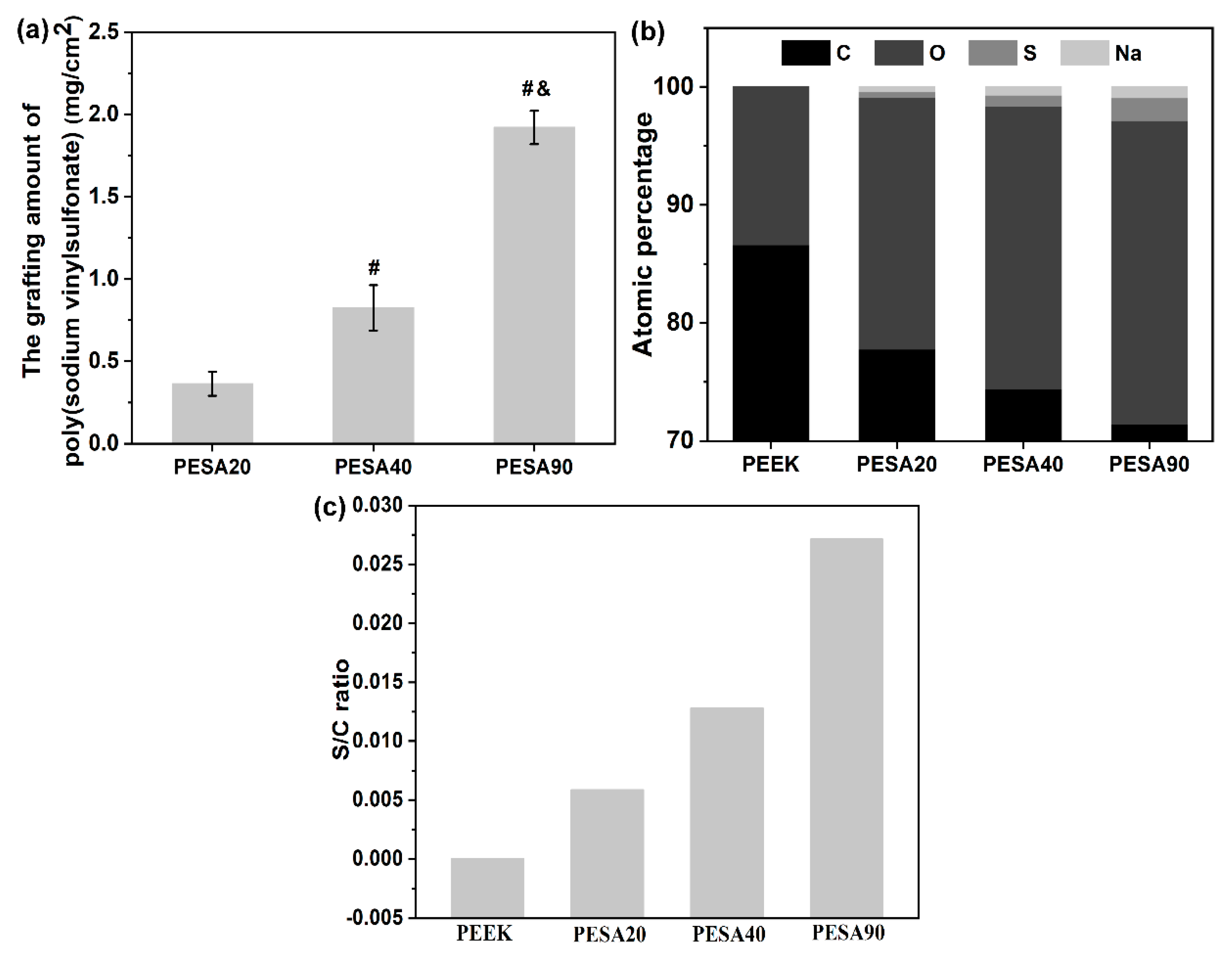
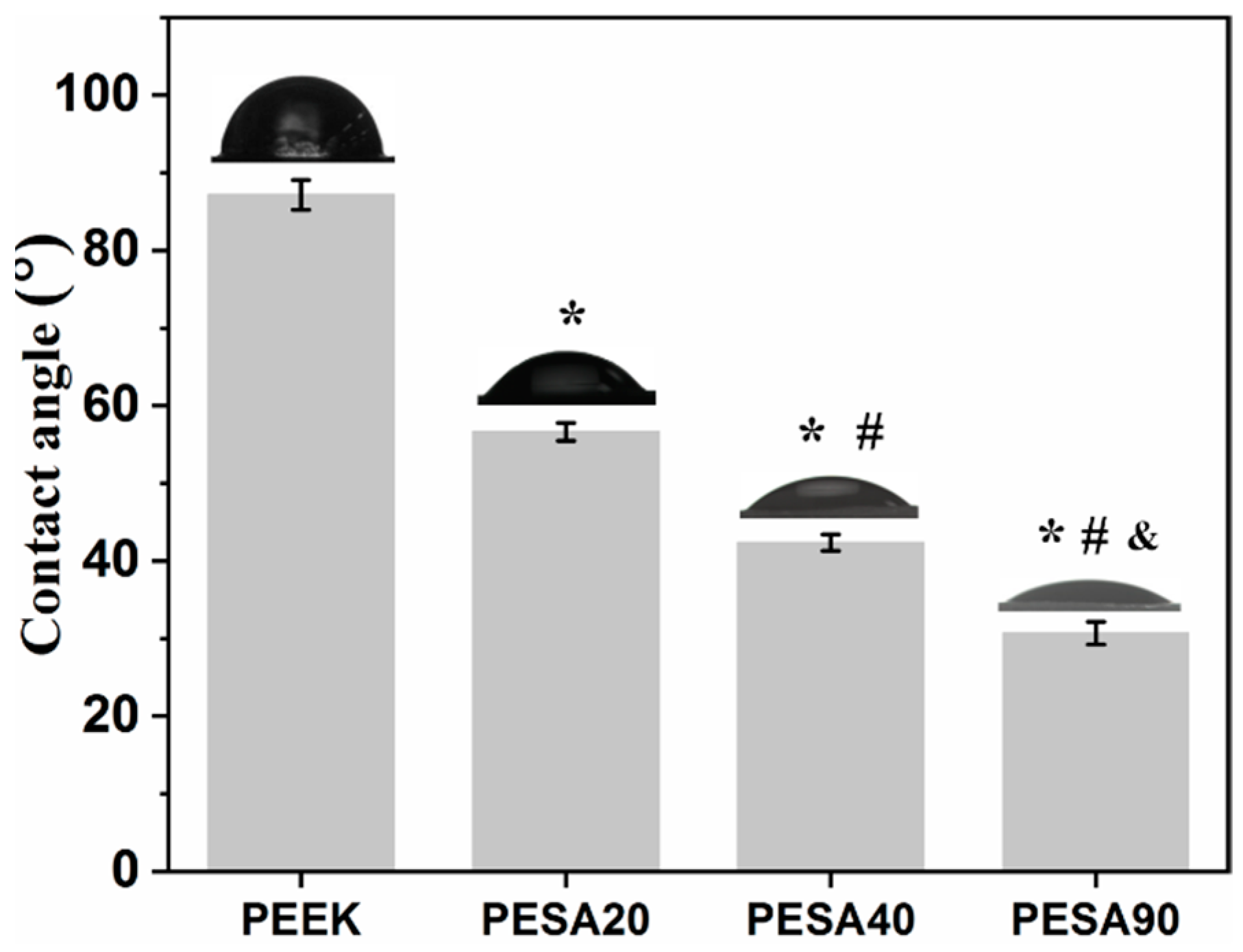
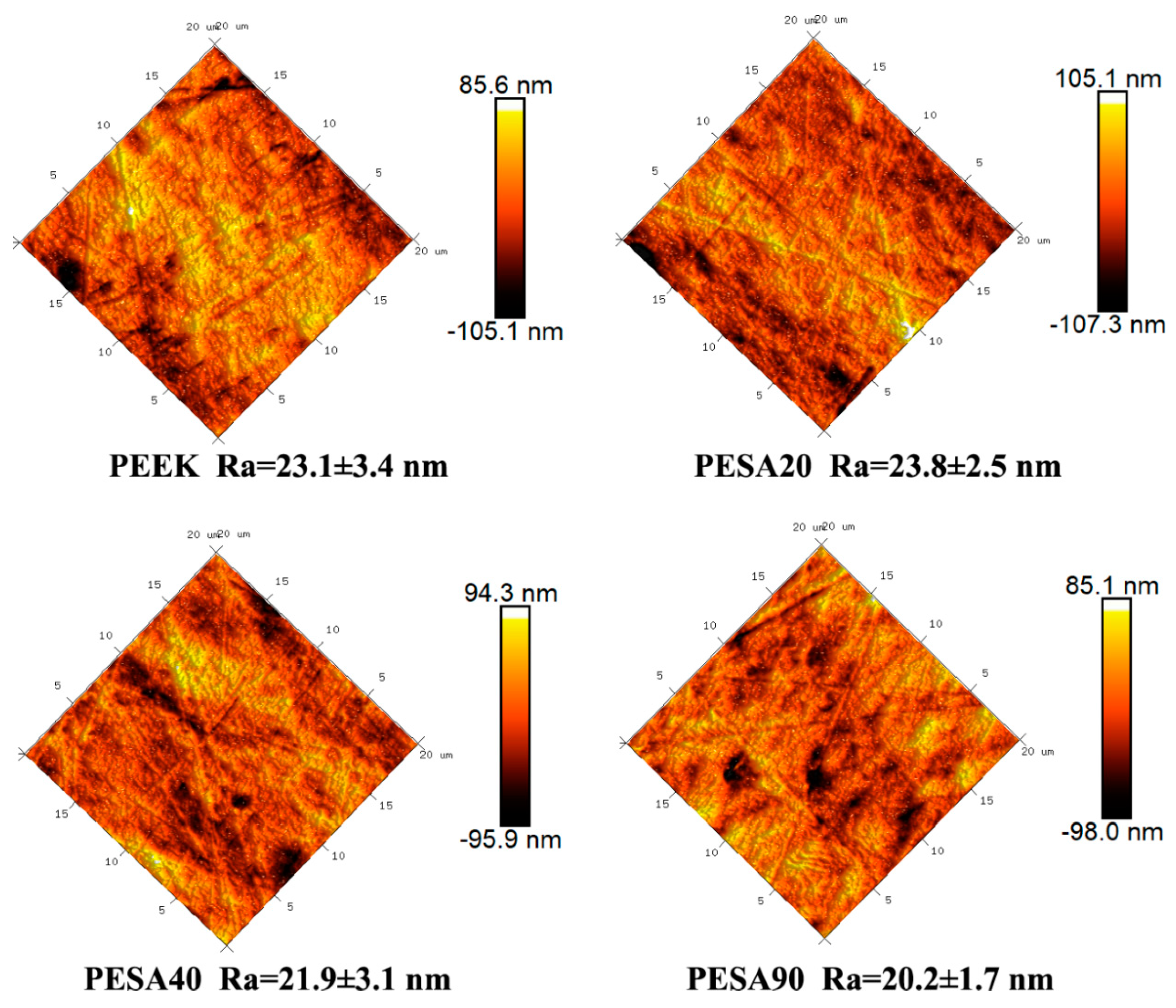
3.1. Characterization
3.2. MC3T3-E1 Osteoblast Responses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.W.; Zhong, Y.; Wang, L. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. A 2020, 108, 470–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.W.; Weir, M.D.; Wu, J.; Xu, H.H.K. Review on Development and Dental Applications of Polyetheretherketone-Based Biomaterials and Restorations. Materials 2021, 14, 408. [Google Scholar] [CrossRef]
- He, M.; Huang, Y.; Xu, H.; Feng, G.; Liu, L.; Li, Y.; Sun, D.; Zhang, L. Modification of polyetheretherketone implants: From enhancing bone integration to enabling multi-modal therapeutics. Acta Biomater. 2021, 129, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, Y.; Zhang, L.; Xiong, C. Bioactive Polyetheretherketone Implant Composites for Hard Tissue. Prog. Chem. 2017, 29, 450–458. [Google Scholar]
- Mishra, S.; Chowdhary, R. PEEK materials as an alternative to titanium in dental implants: A systematic review. Clin. Implant Dent. R 2019, 21, 208–222. [Google Scholar] [CrossRef] [Green Version]
- Buck, E.; Li, H.; Cerruti, M. Surface Modification Strategies to Improve the Osseointegration of Poly(etheretherketone) and Its Composites. Macromol. Biosci. 2020, 20, 1900271. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, N.; Kango, S.; Sharma, S. Developments of PEEK (Polyetheretherketone) as a biomedical material: A focused review. Eur. Polym. J. 2021, 147, 110295. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, W.; Yang, L.; Pan, Y.; Wang, X.; Wang, T.; Tang, S.; Yao, Y.; Hong, H.; Wei, J. Stimulation of cell responses and bone ingrowth into macro-microporous implants of nano-bioglass/polyetheretherketone composite and enhanced antibacterial activity by release of hinokitiol. Colloid Surf. B 2018, 164, 347–357. [Google Scholar] [CrossRef]
- Yu, X.; Yao, S.; Chen, C.; Wang, J.; Li, Y.; Wang, Y.; Khademhosseini, A.; Wan, J.; Wu, Q. Preparation of Poly(ether-ether-ketone)/Nanohydroxyapatite Composites with Improved Mechanical Performance and Biointerfacial Affinity. ACS Omega 2020, 5, 29398–29406. [Google Scholar] [CrossRef]
- Ma, R.; Tang, S.; Tan, H.; Qian, J.; Lin, W.; Wang, Y.; Liu, C.; Wei, J.; Tang, T. Preparation, Characterization, In Vitro Bioactivity, and Cellular Responses to a Polyetheretherketone Bioactive Composite Containing Nanocalcium Silicate for Bone Repair. ACS Appl. Mater. Interfaces 2014, 6, 12214–12225. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, A.C.; Haugen, H.J. Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Lu, T.; Wang, X.; Xu, L.; Wu, Q.; Pan, H.; Wang, D.; Liu, X.; Jiang, X. In Vitro and in Vivo Evaluation of Silicate-Coated Polyetheretherketone Fabricated by Electron Beam Evaporation. ACS Appl. Mater. Interfaces 2016, 8, 13197–13206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; He, M.; Mao, L.; Li, T.; Zhang, L.; Liu, L.; Feng, G.; Song, Y. Titanium-interlayer mediated hydroxyapatite coating on polyetheretherketone: A prospective study in patients with single-level cervical degenerative disc disease. J. Transl. Med. 2021, 19, 14. [Google Scholar] [CrossRef]
- Ren, Y.; Sikder, P.; Lin, B.; Bhaduri, S.B. Microwave assisted coating of bioactive amorphous magnesium phosphate (AMP) on polyetheretherketone (PEEK). Mater. Sci. Eng. C 2018, 85, 107–113. [Google Scholar] [CrossRef]
- Frankenberger, T.; Graw, C.L.; Engel, N.; Gerber, T.; Frerich, B.; Dau, M. Sustainable Surface Modification of Polyetheretherketone (PEEK) Implants by Hydroxyapatite/Silica Coating—An In Vivo Animal Study. Materials 2021, 14, 4589. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiong, C.; Zhang, S.; Li, X.; Zhang, L. Bone-like apatite coating on functionalized poly(etheretherketone) surface via tailored silanization layers technique. Mater. Sci. Eng. C 2015, 55, 512–523. [Google Scholar] [CrossRef]
- Velard, F.; Braux, J.; Amedee, J.; Laquerriere, P. Inflammatory cell response to calcium phosphate biomaterial particles: An overview. Acta Biomater. 2013, 9, 4956–4963. [Google Scholar] [CrossRef]
- Kassick, A.J.; Yerneni, S.S.; Gottlieb, E.; Cartieri, F.; Peng, Y.; Mao, G.; Kharlamov, A.; Miller, M.C.; Xu, C.; Oh, M.; et al. Osteoconductive Enhancement of Polyether Ether Ketone: A Mild Covalent Surface Modification Approach. ACS Appl. Bio Mater. 2018, 1, 1047–1055. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Xiong, C.; Zhang, L. Enhancement of bioactivity on modified polyetheretherketone surfaces with –COOH, –OH and –PO4H2 functional groups. Mater. Lett. 2018, 213, 84–87. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiong, C.; Li, X.; Zhang, L. Covalent attachment of cell-adhesive peptide Gly-Arg-Gly-Asp (GRGD) to poly(etheretherketone) surface by tailored silanization layers technique. Appl. Surf. Sci. 2014, 320, 93–101. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, Y.; Li, X.; Liu, X.; Yeung, K.W.K.; Wu, S.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Surface functionalization of biomaterials by radical polymerization. Prog. Mater. Sci. 2016, 83, 191–235. [Google Scholar] [CrossRef]
- Flejszar, M.; Chmielarz, P. Surface Modifications of Poly(Ether Ether Ketone) via Polymerization Methods-Current Status and Future Prospects. Materials 2020, 13, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouwatat, P.; Hirai, T.; Higaki, K.; Higaki, Y.; Sue, H.-J.; Takahara, A. Aqueous lubrication of poly(etheretherketone) via surface-initiated polymerization of electrolyte monomers. Polymer 2017, 116, 549–555. [Google Scholar] [CrossRef]
- Yousaf, A.; Farrukh, A.; Oluz, Z.; Tuncel, E.; Duran, H.; Dogan, S.Y.; Tekinay, T.; Rehman, H.U.; Yameen, B. UV-light assisted single step route to functional PEEK surfaces. React. Funct. Polym. 2014, 83, 70–75. [Google Scholar] [CrossRef]
- Kyomoto, M.; Moro, T.; Yamane, S.; Watanabe, K.; Takatori, Y.; Tanaka, S.; Ishihara, K. Smart PEEK modified by self-initiated surface graft polymerization for orthopedic bearings. Reconstr. Rev. 2014, 4, 36–45. [Google Scholar]
- Zheng, Y.; Liu, L.; Xiao, L.; Zhang, Q.; Liu, Y. Enhanced osteogenic activity of phosphorylated polyetheretherketone via surface-initiated grafting polymerization of vinylphosphonic acid. Colloid Surf. B 2019, 173, 591–598. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.; Zhang, Q.; Yu, L.; Hu, Z.; Liu, Y. Surface phosphonation treatment shows dose-dependent enhancement of the bioactivity of polyetheretherketone. RSC Adv. 2019, 9, 30076–30086. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, W.; Yuan, L.; Liu, Y.; Zheng, Y. Ameliorative antibacterial, anti-inflammatory, and osteogenic activity of sulfonate-bearing polyetheretherketone toward orthopedic and dental implants. Mater. Lett. 2021, 305, 130774. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Ma, Y.; Xiao, L.; Liu, Y. Enhanced Osteoblasts Responses to Surface-Sulfonated Polyetheretherketone via a Single-Step Ultraviolet-Initiated Graft Polymerization. Ind. Eng. Chem. Res. 2018, 57, 10403–10410. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiong, C.; Zhang, D.; Zhang, L. In vitro bioactivity evaluation of α -calcium sulphate hemihydrate and bioactive glass composites for their potential use in bone regeneration. Bull. Mater. Sci. 2018, 41, 59. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Fu, X.; Li, T.; Zhao, N.; Shi, X.; Cui, F.; Du, C.; Wang, Y. Surface chemistry from wettability and charge for the control of mesenchymal stem cell fate through self-assembled monolayers. Colloid Surf. B 2016, 148, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiong, C.; Wang, Z.; Zhang, L. Enhanced osteoblast cells adhesion, spreading, and proliferation to surface-carboxylated poly(etheretherketone). J. Bioact. Compat. Pol. 2015, 30, 302–318. [Google Scholar] [CrossRef]
- Fan, L.; Guan, P.; Xiao, C.; Wen, H.; Wang, Q.; Liu, C.; Luo, Y.; Ma, L.; Tan, G.; Yu, P.; et al. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact. Mater. 2021, 6, 2754–2766. [Google Scholar] [CrossRef]
- Wagner, A.-S.; Glenske, K.; Wolf, V.; Fietz, D.; Mazurek, S.; Hanke, T.; Moritz, A.; Arnhold, S.; Wenisch, S. Osteogenic differentiation capacity of human mesenchymal stromal cells in response to extracellular calcium with special regard to connexin 43. Ann. Anat. 2017, 209, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. A 2003, 66, 247–259. [Google Scholar] [CrossRef]
- Vaquette, C.; Viateau, V.; Guérard, S.; Anagnostou, F.; Manassero, M.; Castner, D.G.; Migonney, V. The effect of polystyrene sodium sulfonate grafting on polyethylene terephthalate artificial ligaments on in vitro mineralisation and in vivo bone tissue integration. Biomaterials 2013, 34, 7048–7063. [Google Scholar] [CrossRef] [Green Version]
- Alcheikh, A.; Pavon-Djavid, G.; Helary, G.; Petite, H.; Migonney, V.; Anagnostou, F. PolyNaSS grafting on titanium surfaces enhances osteoblast differentiation and inhibits Staphylococcus aureus adhesion. J. Mater. Sci. M 2013, 24, 1745–1754. [Google Scholar] [CrossRef]
- Hélary, G.; Noirclère, F.; Mayingi, J.; Migonney, V. A new approach to graft bioactive polymer on titanium implants: Improvement of MG 63 cell differentiation onto this coating. Acta Biomater. 2009, 5, 124–133. [Google Scholar] [CrossRef]
- Chaterji, S.; Gemeinhart, R.A. Enhanced osteoblast-like cell adhesion and proliferation using sulfonate-bearing polymeric scaffolds. J. Biomed. Mater. Res. A 2007, 83, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.; Yan, C.H.; Yeung, K.W.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primers |
|---|---|
| ALP | Forward: CTCCATCTTTGGTCTGGCTCC Reverse: CCTGGTAGTTGTTGTGAGCGTAAT |
| OCN | Forward: TGGCTGCGCTCTGTCTCTCT Reverse: TTCACTACCTTATTGCCCTCCTG |
| OPN | Forward: TAGGAGTTTCCAGGTTTCTGATGA Reverse: CTGCCCTTTCCGTTGTTGTC |
| BMP-2 | Forward: GACATCCGCTCCACAAACGA Reverse: CATCACTGAAGTCCACATACAAAGG |
| β-actin | Forward: AGATTACTGCTCTGGCTCCTAGC Reverse: ACTCATCGTACTCCTGCTTGCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Dong, J.; Zhang, W.; He, C.; Liu, Y.; Zheng, Y. The Single-Step Fabrication of a Poly (Sodium Vinylsulfonate)-Grafted Polyetheretherketone Surface to Ameliorate Its Osteogenic Activity. Coatings 2022, 12, 868. https://doi.org/10.3390/coatings12060868
Liu L, Dong J, Zhang W, He C, Liu Y, Zheng Y. The Single-Step Fabrication of a Poly (Sodium Vinylsulfonate)-Grafted Polyetheretherketone Surface to Ameliorate Its Osteogenic Activity. Coatings. 2022; 12(6):868. https://doi.org/10.3390/coatings12060868
Chicago/Turabian StyleLiu, Lvhua, Jun Dong, Weifang Zhang, Chanjuan He, Ying Liu, and Yanyan Zheng. 2022. "The Single-Step Fabrication of a Poly (Sodium Vinylsulfonate)-Grafted Polyetheretherketone Surface to Ameliorate Its Osteogenic Activity" Coatings 12, no. 6: 868. https://doi.org/10.3390/coatings12060868
APA StyleLiu, L., Dong, J., Zhang, W., He, C., Liu, Y., & Zheng, Y. (2022). The Single-Step Fabrication of a Poly (Sodium Vinylsulfonate)-Grafted Polyetheretherketone Surface to Ameliorate Its Osteogenic Activity. Coatings, 12(6), 868. https://doi.org/10.3390/coatings12060868







