Micropatterned Polypyrrole/Hydroxyapatite Composite Coatings Promoting Osteoinductive Activity by Electrical Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Micropatterned PPy Coatings
2.3. Fabrication of Micropatterned PPy/HA Composite Coatings
2.4. Characterization
2.5. Bioactivity Assessment
2.6. Cell Culture
2.7. Effect of Micropatterned PPy/HA Composite Coatings with ES on MC3T3-E1 Cell Adhesion, Proliferation and Differentiation
2.7.1. Cell Morphology
2.7.2. MTT Assay
2.7.3. Determination of the BSA Protein Content, Alkaline Phosphatase Activity (ALP) and Inorganic Calcium Content
3. Results and Discussion
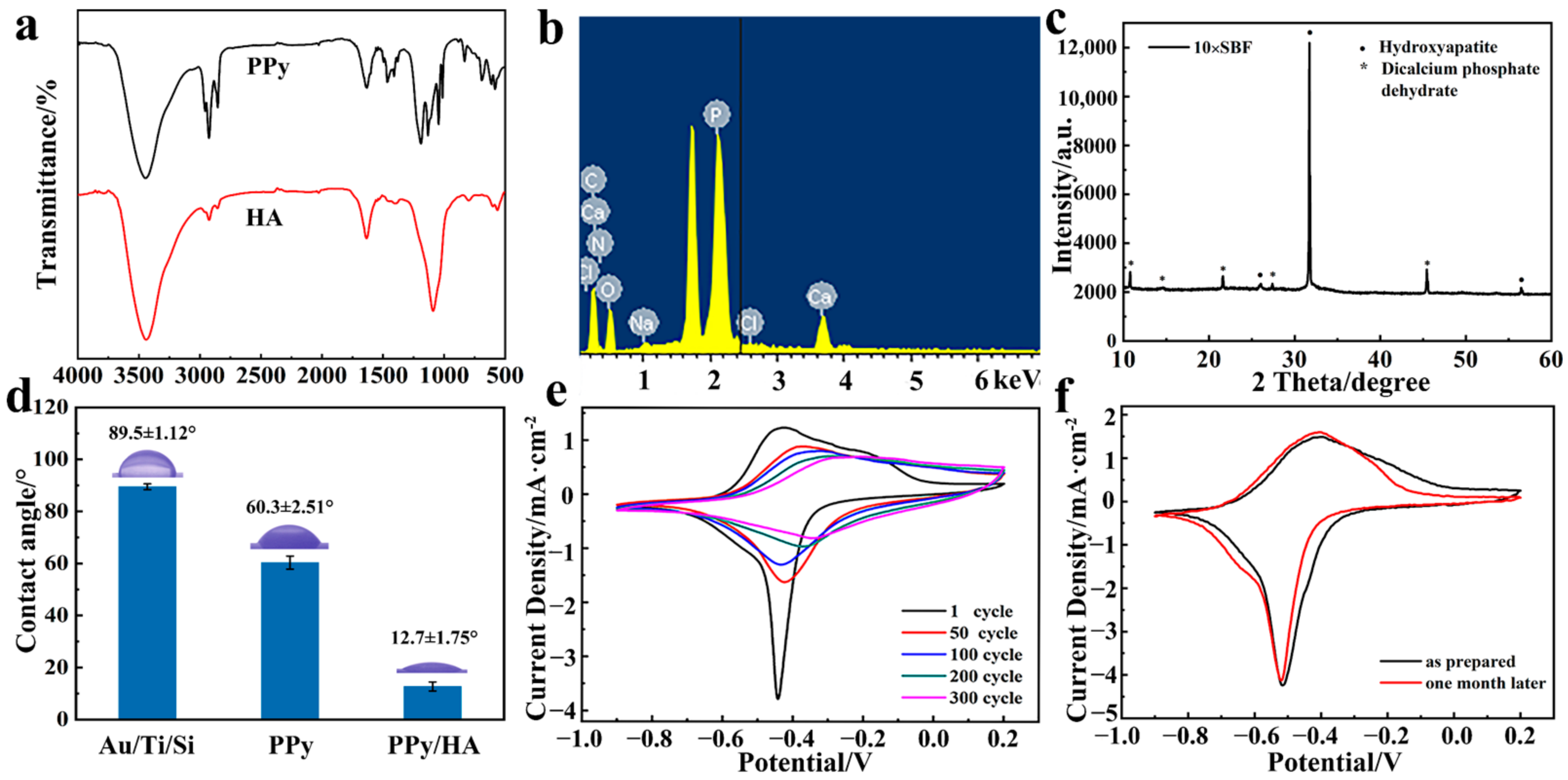
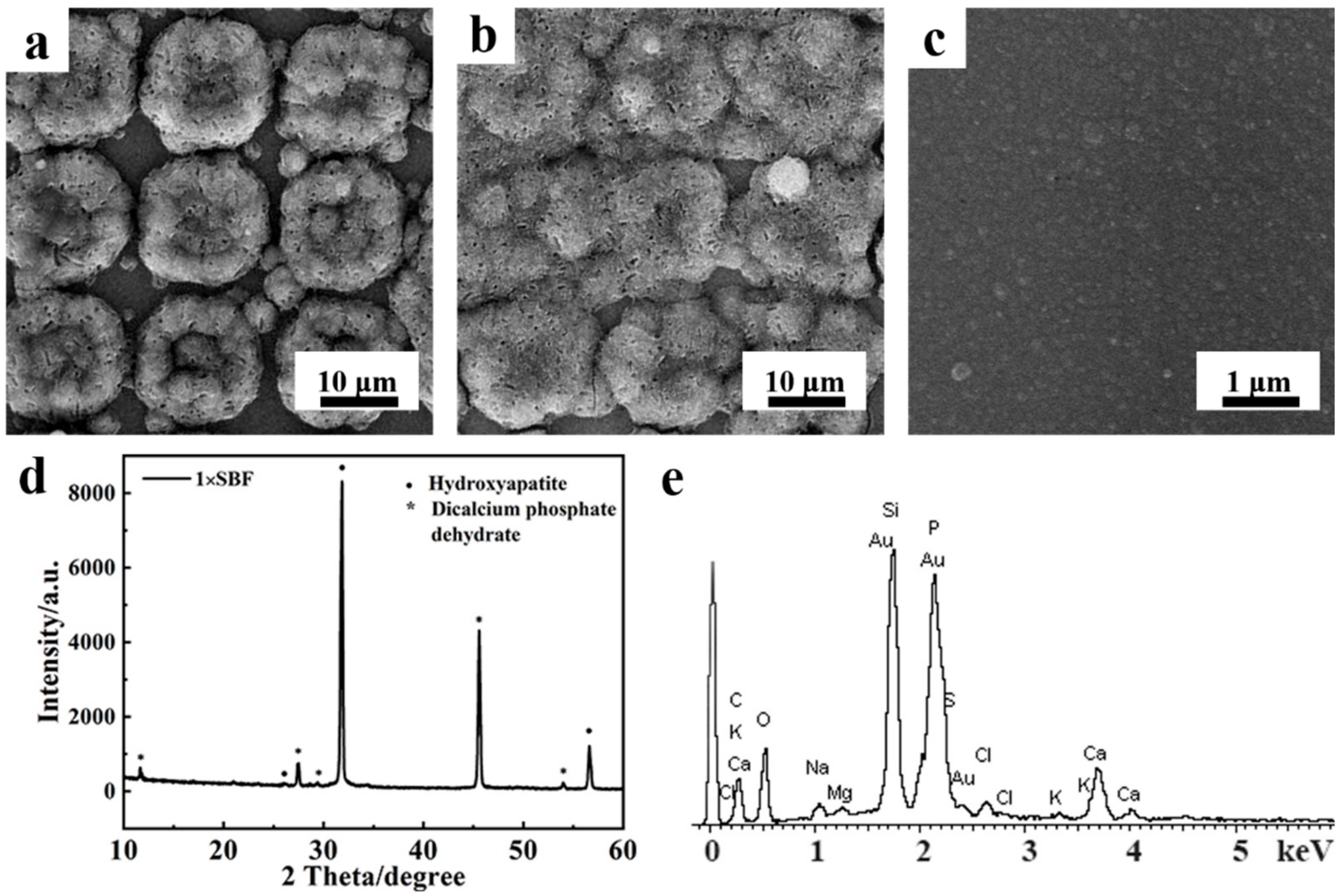
3.1. Preparation and Characterization of Micropatterned PPy/HA Composite Coatings
3.2. Bioactivity Assessment of Micropatterned PPy/HA Composite Coatings
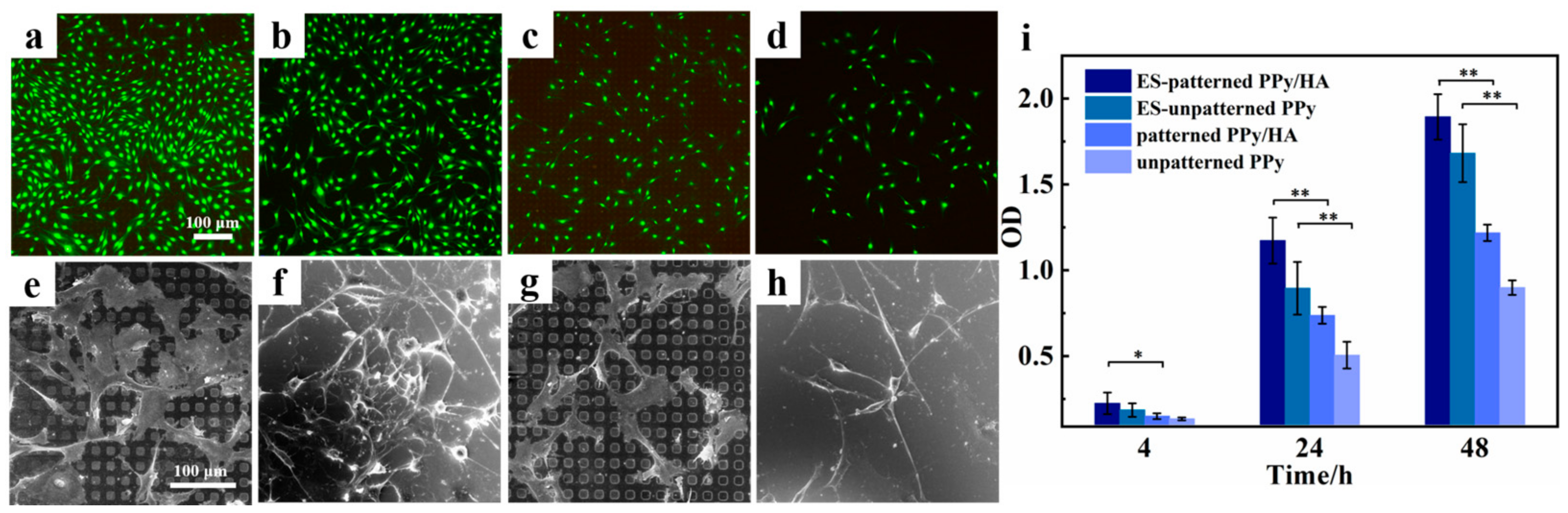
3.3. Effect of Micropatterned PPy/HA Composite Coatings with ES on Cell Activity
3.4. Effect of Micropatterned PPy/HA Composite Coatings with ES on Cell Functional Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isaacson, B.M.; Bloebaum, R.D. Bone bioelectricity: What have we learned in the past 160 years? J. Biomed. Mater. Res. Part A 2010, 95, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jing, W.; Li, Y.; Cai, Q.; Yang, X. Composites made of polyorganophosphazene and carbon nanotube up-regulating osteogenic activity of BMSCs under electrical stimulation. Colloids Surf. B 2021, 204, 111785. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Ziebart, J.; Jonitz-Heincke, A.; Hansmann, D.; Dauben, T.; Bader, R. Alternating electric fields modify the function of human osteoblasts growing on and in the surroundings of titanium electrodes. Int. J. Mol. Sci. 2020, 21, 6944. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Huang, Y.; Zhang, X.; Cai, Q.; Deng, X.; Yang, X. Mimicking the electrophysiological microenvironment of bone tissue using electroactive materials to promote its regeneration. J. Mater. Chem. B 2020, 8, 10221–10256. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Oliveira, K.M.; Bhavsar, M.B.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Eischen-Loges, M.; Oliveira, K.M.; Bhaysar, M.B.; Barker, J.H.; Leppik, L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 2018, 6, 4959. [Google Scholar] [CrossRef]
- Bhavsar, M.B.; Han, Z.; DeCoster, T.; Leppik, L.; Oliveira, K.M.; Barker, J.H. Electrical stimulation-based bone fracture treatment, if it works so well why do not more surgeons use it? Eur. J. Trauma Emerg. Surg. 2020, 46, 245–264. [Google Scholar] [CrossRef]
- Samadian, H.; Mobasheri, H.; Hasanpour, S.; Ai, J.; Azamie, M.; Faridi-Majidi, R. Electro-conductive carbon nanofibers as the promising interfacial biomaterials for bone tissue engineering. J. Mol. Liq. 2020, 298, 112021–112028. [Google Scholar] [CrossRef]
- Li, P.; Zhang, S.; Li, K.; Wang, J.; Liu, M.; Gu, X.; Fan, Y. The promoting effect on pre-osteoblast growth under electrical and magnetic double stimulation based on PEDOT/Fe3O4/PLGA magnetic-conductive bi-functional scaffolds. J. Mater. Chem. B 2018, 6, 4952–4962. [Google Scholar] [CrossRef]
- Das, R.; Curry, E.J.; Le, T.T.; Awale, G.; Liu, Y.; Li, S.; Contreras, J.; Bednarz, C.; Millender, J.; Xin, X.; et al. Biodegradable nanofiber bone-tissue scaffold as remotely-controlled and self-powering electrical stimulator. Nano Energy 2020, 76, 105028–105039. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Lee, P.S.; Hess, R.; Haenchen, V.; Basu, B.; Schamweber, D. Interplay of substrate conductivity, cellular microenvironment, and pulsatile electrical stimulation toward osteogenesis of human mesenchymal stem cells in vitro. ACS Appl. Mater. Interfaces 2015, 7, 23015–23028. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wan, Z.; Wei, P.; Lu, X.; Mao, J.; Cai, Q.; Zhang, X.; Yang, X. Dual-controlled release of icariin/Mg2+ from biodegradable microspheres and their synergistic upregulation effect on bone regeneration. Adv. Healthc. Mater. 2020, 9, 2000211–2000224. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qi, Z.; Zheng, C.; Xue, P.; Fu, C.; Pan, S.; Yang, X. Enhanced Cell Proliferation and Osteogenesis Differentiation through a Combined Treatment of Poly-L-Lysine-Coated PLGA/Graphene Oxide Hybrid Fiber Matrices and Electrical Stimulation. J. Nanomater. 2020, 2020, 5892506. [Google Scholar] [CrossRef]
- Golvari, P.; Kuebler, S.M. Fabrication of functional microdevices in SU-8 by multi-photon lithography. Micromachines 2021, 12, 472. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.A.; Maiti, T.K.; et al. Electroconductive multi-functional polypyrrole composites for biomedical applications. Appl. Mater. Today 2021, 24, 101117–101152. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Liang, Y.; Goh, J. Polypyrrole-incorporated conducting constructs for tissue engineering applications: A review. Bioelectricity 2020, 2, 101–119. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, F.; Zhang, Y.; Zhang, H.; Zhao, Y.; Xu, X.; Qin, M.; Ding, C.; Li, J. Electrically facilitated mineralization of osteoblasts and polypyrrole micro-bowl coatings for promotion of the osteogenic activity. Colloid Interface Sci. Commun. 2021, 43, 100450–100459. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Mu, J.; Dai, L.; Zhang, Z.; Sun, Y.; Shi, W.; Ge, D. Synthesis of polypyrrole nanowires with positive effect on MC3T3-E1 cell functions through electrical stimulation. Mater. Sci. Eng. C 2017, 71, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Hsu, Y.T.; Cheng, Y.C.; Li, C.; Ruaan, R.C.; Chien, C.C.; Chung, C.A.; Tsao, C.W. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C-Mater. 2014, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yu, P.; Zhou, L.; Tu, L.; Fan, L.; Zhang, F.; Dai, C.; Liu, Y.; Ning, C.; Du, J.; et al. Polypyrrole nanocones and dynamic piezoelectric stimulation-induced stem cell osteogenic differentiation. ACS Biomater. Sci. Eng. 2019, 5, 4386–4392. [Google Scholar] [CrossRef]
- He, Y.; Dai, L.; Zhang, X.; Sun, Y.; Shi, W.; Ge, D. The Bioactive Polypyrrole/polydopamine nanowire coating with enhanced osteogenic differentiation ability with electrical stimulation. Coatings 2020, 10, 1189. [Google Scholar] [CrossRef]
- Liao, J.; Chen, W.; Yang, M.; Zhou, J.; Wang, Z.; Zhou, Y.; Ning, C.; Yuan, H. Conducting photopolymers on orthopeadic implants having a switch of priority between promoting osteogenic and antibacterial activity. Mater. Horiz. 2018, 5, 545–552. [Google Scholar] [CrossRef]
- Zhou, T.; Yan, L.; Xie, C.; Li, P.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Wang, K.; Wang, Y.; et al. A mussel-inspired persistent ROS-scavenging, electroactive, and osteoinductive scaffold based on electrochemical-driven in situ nanoassembly. Small 2019, 15, 1805440–1805453. [Google Scholar] [CrossRef]
- Wu, H.; Gao, Y.; Xiao, L.; Wei, Q.; Zhang, N.; Su, Z.; Ma, C.; Ye, T.; Wang, Y. Polypyrrole doping-regulated construction of dexamethasone/hydroxyapatite composite coating on titanium surface for sustained osteoinduction. Mater. Des. 2021, 202, 109571–109582. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiao, Y.; Zhu, J.; Li, Y.; Li, C.; Lin, J.; Li, X.; Han, H.; Mao, J.; Wang, F.; et al. Electroactive and antibacterial surgical sutures based on chitosan-gelatin/tannic acid/polypyrrole composite coating. Compos. B Eng. 2021, 223, 109140–109152. [Google Scholar] [CrossRef]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.K.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications—A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic mineralization of biomaterials using simulated body fluids for bone tissue engineering and regenerative medicine. Tissue Eng. Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; No, Y.J.; Lu, Z.; Ng, P.Y.; Chen, Y.; Shi, J.; Pavlos, N.J.; Zreiqat, H. A bioceramic with enhanced osteogenic properties to regulate the function of osteoblastic and osteocalastic cells for bone tissue regeneration. Biomed. Mater. 2016, 11, 035018. [Google Scholar] [CrossRef] [PubMed]
- Sharifianjazi, F.; Esmaeilkhanian, A.; Moradi, M.; Pakseresht, A.; Asl, M.S.; Karimi-Maleh, H.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Biocompatibility and mechanical properties of pigeon bone waste extracted natural nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. B Adv. 2021, 264, 114950. [Google Scholar] [CrossRef]
- Wu, S.C.; Hsu, H.C.; Liu, M.Y.; Ho, W.F. Characterization of nanosized hydroxyapatite prepared by an aqueous precipitation method using eggshells and mulberry leaf extract. J. Korean Ceram. Soc. 2021, 58, 116–122. [Google Scholar] [CrossRef]
- Fedotov, A.Y.; Kotyakov, A.A.; Smirnov, I.V.; Zobkov, Y.V.; Baranov, O.V.; Egorov, A.A.; Teterina, A.Y.; Rad’kova, E.A.; Tyut’kova, Y.B.; Barinov, S.M.; et al. Coatings of low-temperature calcium phosphates on hydroxyapatite ceramic. Inorg. Mater. Appl. Res. 2021, 12, 940–945. [Google Scholar] [CrossRef]
- Shi, P.; Liu, M.; Fan, F.; Yu, C.; Lu, W.; Du, M. Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts. Mater. Sci. Eng. C 2018, 90, 706–712. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Nezafati, N.; Farokhi, M.; Heydari, M.; Hesaraki, S.; Nasab, N.A. In vitro bioactivity and cytocompatablity of an injectable calcium phosphate cement/silanated gelatin microsphere composite bone cement. Compos. B Eng. 2019, 175, 107146. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc silicate/nano-hydroxyapatite/collagen scaffolds promote angiogenesis and bone regeneration via the p38 MAPK pathway in activated monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Kostyuchenko, A.V.; Kochlar, G.S.; Putlyaev, V.I. Structure and nanohardness of compact hydroxyapatite-based ceramics. Inorg. Mater. 2019, 55, 1054–1060. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, T.; Jiao, C.; Liang, H.; Chen, R.; Tian, Z.; Zou, A.; Yang, Y.; Wei, Z.; Wang, C.; et al. Fabrication and properties of zirconia/hydroxyapatite composite scaffold based on digital light processing. Ceram. Int. 2020, 46, 2300–2308. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Chueva, T.R.; Gamurar, N.V.; Kalita, V.I.; Komlev, D.I.; Radyuk, A.A.; Komlev, V.S.; Teterina, A.Y.; Shamray, V.F.; Mikhailova, A.B. Influence of titanium substrate temperature on phase structure of a plasma hydroxyapatite coating. Inorg. Mater. Appl. Res. 2022, 13, 386–392. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Gross, K.A.; Berndt, C.C. Thermal processing of hydroxyapatite for coating production. J. Biomed. Mater. Res. 1998, 39, 580–587. [Google Scholar] [CrossRef]
- Wei, M.; Ruys, A.J.; Milthorpe, B.K.; Sorrell, C.C.; Evans, J.H. Electrophoretic deposition of hydroxyapatite coatings on metal substrates: A nanoparticulate dual-coating approach. J. Sol-Gel Sci. Technol. 2001, 21, 39–48. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Manna, J.S.; Saha, P.; Dhara, S. Synthesis, characterization and cytocompatibility assessment of hydroxyapatite-polypyrrole composite coating synthesized through pulsed reverse electrochemical deposition. Mater. Sci. Eng. C 2019, 94, 597–607. [Google Scholar] [CrossRef]
- Song, F.; Jie, W.; Zhang, T.; Li, W.; Jiang, Y.; Wan, L.; Liu, W.; Li, X.; Liu, B. Room-temperature fabrication of a three-dimensional reduced-graphene oxide/polypyrrole/hydroxyapatite composite scaffold for bone tissue engineering. RSC Adv. 2016, 6, 92804–92812. [Google Scholar] [CrossRef]
- Wang, H.B.; Lee, J.K.; Moursi, A.; Lannutti, J.J. Ca/P ratio effects on the degradation of hydroxyapatite in vitro. J. Biomed. Mater. Res. Part A 2003, 67, 599–608. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Cavin, R.; Ong, J.L. Protein adsorption on titanium surfaces and their effect on osteoblast attachment. J. Biomed. Mater. Res. Part A 2003, 67, 344–349. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; He, Y.; Sun, Y.; Zhang, X.; Yi, Y.; Shi, W.; Ge, D. Micropatterned Polypyrrole/Hydroxyapatite Composite Coatings Promoting Osteoinductive Activity by Electrical Stimulation. Coatings 2022, 12, 849. https://doi.org/10.3390/coatings12060849
Xu J, He Y, Sun Y, Zhang X, Yi Y, Shi W, Ge D. Micropatterned Polypyrrole/Hydroxyapatite Composite Coatings Promoting Osteoinductive Activity by Electrical Stimulation. Coatings. 2022; 12(6):849. https://doi.org/10.3390/coatings12060849
Chicago/Turabian StyleXu, Ji, Yuan He, Yanan Sun, Xiuming Zhang, Yunfeng Yi, Wei Shi, and Dongtao Ge. 2022. "Micropatterned Polypyrrole/Hydroxyapatite Composite Coatings Promoting Osteoinductive Activity by Electrical Stimulation" Coatings 12, no. 6: 849. https://doi.org/10.3390/coatings12060849
APA StyleXu, J., He, Y., Sun, Y., Zhang, X., Yi, Y., Shi, W., & Ge, D. (2022). Micropatterned Polypyrrole/Hydroxyapatite Composite Coatings Promoting Osteoinductive Activity by Electrical Stimulation. Coatings, 12(6), 849. https://doi.org/10.3390/coatings12060849




