Colorants Produced by Penicillium murcianum Are a Natural Moldicide against Trichoderma and Other Penicillium Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Species
2.2. Incorporating Fungal Colorants to Culture Media
2.3. Culturing and Monitoring
2.4. Data Analysis
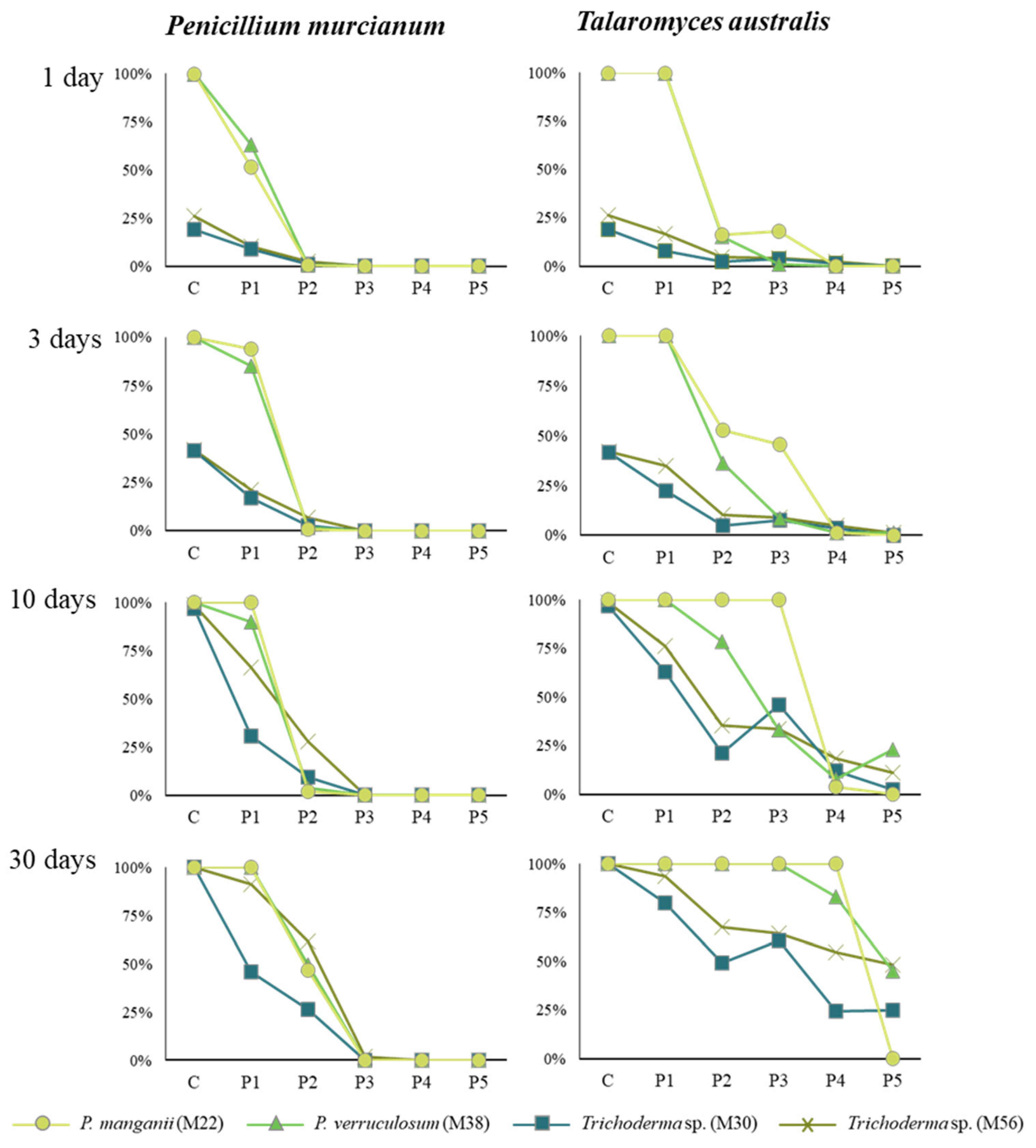
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdoğrul, Ö.; Azirak, S. A review on the red yeast rice (Monascus purpureus). Turk. Electron. J. Biotechnol. 2004, 2, 37–49. [Google Scholar]
- Blanchette, R.A.; Wilmering, A.M.; Baumeister, M. The use of green-stained wood caused by the fungus Chlorociboria in intarsia masterpieces from the 15th century. Holzforsch.-Int. J. Biol. Chem. Phys. Technol. Wood 1992, 46, 225–232. [Google Scholar]
- Michaelsen, H.; Unger, A.; Fischer, C.-H. Blaugrüne Färbung an Intarsienhölzern des 16. bis 18. Jahrhunderts. 1992. Available online: http://www.openbibart.fr/item/display/10068/935304 (accessed on 9 April 2019).
- Robinson, S.C.; Michaelsen, H.; Robinson, J.C. Spalted Wood: The History, Science, and Art of a Unique Material; Schiffer Publishing: Atglen, PA, USA, 2016; 288p, Available online: http://www.schifferbooks.com/spalted-wood-the-history-science-and-art-of-a-unique-material-5920.html (accessed on 30 March 2017).
- Tudor, D.; Robinson, S.C.; Cooper, P. The influence of moisture content variation on fungal pigment formation in spalted wood. AMB Express 2012, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Waksman, S.A.; Horning, E.S.; Spencer, E.L. Two antagonistic fungi, Aspergillus fumigatus and Aspergillus clavatus, and their antibiotic substances. J. Bacteriol. 1943, 45, 233–248. [Google Scholar] [CrossRef]
- Waksman, S.A.; Geiger, W.B. The nature of the antibiotic substances produced by Aspergillus fumigatus. J. Bacteriol. 1944, 47, 391–397. [Google Scholar] [CrossRef]
- Packter, N.M.; Glover, J. Ubiquinone (50) and Ubichromenol in Aspergillus fumigatus, Fresenius. Nature 1960, 187, 413–414. [Google Scholar] [CrossRef]
- Packter, N.M. Studies on the biosynthesis of quinones in fungi. Incorporation of 6-methylsalicylic acid into fumigatin and related compounds in Aspergillus fumigatus IMI 89353. Biochem. J. 1965, 97, 321–332. [Google Scholar] [CrossRef][Green Version]
- Gould, B.S.; Raistrick, H. Studies in the biochemistry of micro-organisms: The crystalline pigments of species in the Aspergillus glaucus series. Biochem. J. 1934, 28, 1640. [Google Scholar] [CrossRef]
- Howard, B.H.; Raistrick, H. Studies in the biochemistry of micro-organisms. 81. The colouring matters of Penicillium islandicum Sopp. Part 2. Chrysophanic acid, 4: 5-dihydroxy-2-methylanthraquinone. Biochem. J. 1950, 46, 49. [Google Scholar] [CrossRef]
- Howard, B.H.; Raistrick, H. Studies in the biochemistry of micro-organisms. 92. The colouring matters of Penicillium islandicum Sopp Part 4. Iridoskyrin, rubroskyrin and erythroskyrine. Biochem. J. 1954, 57, 212. [Google Scholar] [CrossRef]
- Takeda, N.; Seo, S.; Ogihara, Y.; Sankawa, U.; Iitaka, I.; Kitagawa, I.; Shibata, S. Studies on fungal metabolites—XXXI: Anthraquinonoid colouring matters of Penicillium islandicum sopp and some other fungi (–) luteoskyrin, (–) rubroskyrin, (+) rugulosin and their related compounds. Tetrahedron 1973, 29, 3703–3719. [Google Scholar] [CrossRef]
- Stark, A.A.; Townsend, J.M.; Wogan, G.N.; Demain, A.L.; Manmade, A.; Ghosh, A.C. Mutagenicity and antibacterial activity of mycotoxins produced by Penicillium islandicum Sopp and Penicillium rugulosum. J. Environ. Pathol. Toxicol. 1978, 2, 313–324. [Google Scholar] [PubMed]
- Saikawa, Y.; Watanabe, T.; Hashimoto, K.; Nakata, M. Absolute configuration and tautomeric structure of xylindein, a blue–green pigment of Chlorociboria species. Phytochemistry 2000, 55, 237–240. [Google Scholar] [CrossRef]
- Vega Gutierrez, S.M.; Van Court, R.C.; Stone, D.W.; Konkler, M.J.; Groth, E.N.; Robinson, S.C. Relationship between Molarity and Color in the Crystal (‘Dramada’) Produced by Scytalidium cuboideum, in Two Solvents. Molecules 2018, 23, 2581. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Gupta, C.; Aggarwal, S.; Nagpal, N. Pigment Extraction from Fungus for Textile Dyeing; NISCAIR-CSIR: New Delhi, India, 2012. [Google Scholar]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Hernández, V.A.; Galleguillos, F.; Thibaut, R.; Müller, A. Fungal dyes for textile applications: Testing of industrial conditions for wool fabrics dyeing. J. Text. Inst. 2019, 110, 61–66. [Google Scholar] [CrossRef]
- Weber, G.; Chen, H.-L.; Hinsch, E.; Freitas, S.; Robinson, S.C. Pigments extracted from the wood-Staining fungi Chlorociboria aeruginosa, Scytalidium cuboideum, and S. ganodermophthorum show potential for use as textile dyes. Color Technol. 2014, 130, 445–452. [Google Scholar] [CrossRef]
- Hinsch, E.M. A Comparative Analysis of Extracted Fungal Pigments and Commercially Available Dyes for Colorizing Textiles. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2015. Available online: http://ir.library.oregonstate.edu/xmlui/handle/1957/55651 (accessed on 1 May 2017).
- Hinsch, E.M.; Weber, G.; Chen, H.-L.; Robinson, S.C. Colorfastness of Extracted Wood-staining Fungal Pigments on Fabrics: A new potential for textile dyes. J. Text. Appar. Technol. Manag. 2015, 9. Available online: https://ojs.cnr.ncsu.edu/index.php/JTATM/article/view/8018 (accessed on 25 September 2021).
- Palomino Agurto, M.E.; Vega Gutierrez, S.M.; Chen, H.L.; Robinson, S.C. Wood-Rotting Fungal Pigments as Colorant Coatings on Oil-Based Textile Dyes. Coatings 2017, 7, 152. [Google Scholar] [CrossRef]
- Nagia, F.A.; El-Mohamedy, R.S.R. Dyeing of wool with natural anthraquinone dyes from Fusarium oxysporum. Dye. Pigment. 2007, 75, 550–555. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Hassan, M.A.; Taha, T.H. Production and Application of Extracellular Laccase Produced by Fusarium oxysporum EMT. Int. J. Agric. Biol. 2016, 18. [Google Scholar] [CrossRef]
- Galleguillos, F.; Hernández, V.; Figueroa, F.; Palfner, G.; Hernandez, V.A.; Robinson, S.C. Potential Use of Native Fungi for Value-Added Spalting in Chile. Prod. J. 2015. Available online: http://www.forestprodjournals.org/doi/abs/10.13073/FPJ-D-14-00093 (accessed on 12 April 2021). [CrossRef]
- Galleguillos, G.F.A. Hongos Asociados a Escarabajos Lignícolas en Bosque de Nothofagus su Aplicación en Control Biológico de Ophiostoma y en Spalting. Ph.D. Thesis, Universidad de Concepción, Concepción, Chile, 2016. [Google Scholar]
- Hernández, V.A.; Machuca, Á.; Saavedra, I.; Chavez, D.; Astuya, A.; Barriga, C. Talaromyces australis and Penicillium murcianum pigment production in optimized liquid cultures and evaluation of their cytotoxicity in textile applications. World J. Microbiol. Biotechnol. 2019, 35, 160. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.M.; Todd, A.; Neilson, A. Structure of Xylindein. In Proceedings of the Chemical Society of London, Sheffield, UK, 4 April 1962; 27p. [Google Scholar]
- Edwards, R.L.; Kale, N. The structure of xylindein. Tetrahedron 1965, 21, 2095–2107. [Google Scholar] [CrossRef]
- Donner, C.D.; Cuzzupe, A.N.; Falzon, C.L.; Gill, M. Investigations towards the synthesis of xylindein, a blue-green pigment from the fungus Chlorociboria aeruginosa. Tetrahedron 2012, 68, 2799–2805. [Google Scholar] [CrossRef]
- Campbell, A.H. Zone lines in plant tissues I. The black lines formed by Xylaria polymorpha (Pers.) Grev. in hardwoods. Ann. Appl. Biol. 1933, 20, 123–145. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Robinson, S.C.; Laks, P.E. Wood species and culture age affect zone line production of Xylaria polymorpha. Open Mycol. J. 2010, 4, 18–21. [Google Scholar] [CrossRef]
- ForIntek Canada Corporation. Discolorations on Wood Products. Forintek Canada Corp. 2002. Available online: https://cwc.ca/wp-content/uploads/assessingdecay-Discolorations_on_wood.pdf (accessed on 8 March 2022).
- Hernández, V.A.; Galleguillos, F.A.; Sagredo, N.; Machuca, Á. A note on the dyeing of wool fabrics using natural dyes extracted from rotten wood-inhabiting fungi. Coatings 2018, 8, 77. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 May 2021).
- RStudio Team. In RStudio: Integrated Development Environment for R RStudio; PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 1 May 2021).
- Marx, F.; Binder, U.; Leiter, É.; Pócsi, I. The Penicillium chrysogenum antifungal protein PAF, a promising tool for the development of new antifungal therapies and fungal cell biology studies. Cell. Mol. Life Sci. 2008, 65, 445–454. [Google Scholar] [CrossRef]
- Brown, A.G.; Smale, T.C.; King, T.J.; Hasenkamp, R.; Thompson, R.H. Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J. Chem. Soc. Perkin Trans. 1976, 1, 1165–1170. [Google Scholar] [CrossRef]
- Bladt, T.T.; Frisvad, J.C.; Knudensen, P.B.; Larsen, T.O. Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Yilmaz, N.; Houbracken, J.; Spierenburg, H.; Seifert, K.A.; Peterson, S.W.; Varga, J.; Frisvas, J.C. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2007, 70, 159–183. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Oliveira, D.; Andersen, C.A.; Vega Gutierrez, S.M.; Kamke, F.A.; Robinson, S.C. Method of stabilizing heavily spalted big leaf maple as a decorative coating veneer layer for engineered wood flooring. Coatings 2019, 9, 132. [Google Scholar] [CrossRef]
- Hinsch, E.; Robinson, S.C. Comparing colorfastness of light of wood-staining fungal pigments and commercial dyes: An alternative method for colorfastness to light testing. Coatings 2018, 8, 189. [Google Scholar] [CrossRef]
- He, Y.; Cao, Y.; Hwang, H.-J.; Debarajb, H.; Vega Gutierrez, S.M.; Chen, H.-L.; Robinson, S.C.; Malhotra, R.; Chang, C.-H. Inkjet printing and in-situ crystallization of biopigments for eco-friendly and energy-efficient fabric coloration. Int. J. Precis. Eng. Manuf. Green Technol. 2021, 9, 941–953. [Google Scholar] [CrossRef]
- Räisänen, R.; Nousiainen, P.; Hynninen, P.H. Emodin and dermocybin natural anthraquinones as high-temperature disperse dyes for polyester and polyamide. Text. Res. J. 2001, 71, 922–927. [Google Scholar] [CrossRef]
- De Santis, D.; Moresi, M.; Gallo, A.M.; Petruccioli, M. Assessment of the dyeing properties of pigments from Monascus purpureus. J. Chem. Technol. Biotechnol. 2005, 80, 1072–1079. [Google Scholar] [CrossRef]
- Perumal, K.; Stalin, V.; Chandrasekarenthiran, S.; Sumathi, E.; Saravanakumar, A. Extraction and characterization of pigment from Sclerotinia sp. and its use in dyeing cotton. Text. Res. J. 2009, 79, 1178–1187. [Google Scholar] [CrossRef]
- Van Court, R.C.; Robinson, S.C. Stimulating production of pigment-type secondary metabolites from soft rotting wood decay fungi (“spalting” fungi). Adv. Biochem. Eng. Biotechnol. 2019, 169, 109–124. [Google Scholar]

| Code | Concentration | Mold Species Tested at Each Concentration |
|---|---|---|
| Control | 2% MEA | Penicillium magnanii (M22) Penicillium verruculosum (M38) Trichoderma sp. (M30) Trichoderma sp. (M56) |
| P1 | 1 mg/mL colorant + 2% MEA | |
| P2 | 2 mg/mL colorant + 2% MEA | |
| P3 | 3 mg/mL colorant + 2% MEA | |
| P4 | 4 mg/mL colorant + 2% MEA | |
| P5 | 5 mg/mL colorant + 2% MEA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega Gutierrez, P.; Hernández, V.A.; Sagredo, N.; Robinson, S.C. Colorants Produced by Penicillium murcianum Are a Natural Moldicide against Trichoderma and Other Penicillium Species. Coatings 2022, 12, 821. https://doi.org/10.3390/coatings12060821
Vega Gutierrez P, Hernández VA, Sagredo N, Robinson SC. Colorants Produced by Penicillium murcianum Are a Natural Moldicide against Trichoderma and Other Penicillium Species. Coatings. 2022; 12(6):821. https://doi.org/10.3390/coatings12060821
Chicago/Turabian StyleVega Gutierrez, Patricia, Vicente A. Hernández, Nicole Sagredo, and Seri C. Robinson. 2022. "Colorants Produced by Penicillium murcianum Are a Natural Moldicide against Trichoderma and Other Penicillium Species" Coatings 12, no. 6: 821. https://doi.org/10.3390/coatings12060821
APA StyleVega Gutierrez, P., Hernández, V. A., Sagredo, N., & Robinson, S. C. (2022). Colorants Produced by Penicillium murcianum Are a Natural Moldicide against Trichoderma and Other Penicillium Species. Coatings, 12(6), 821. https://doi.org/10.3390/coatings12060821







