Seawater Corrosion of Copper and Its Alloy Coated with Hydrothermal Carbon
Abstract
:1. Introduction
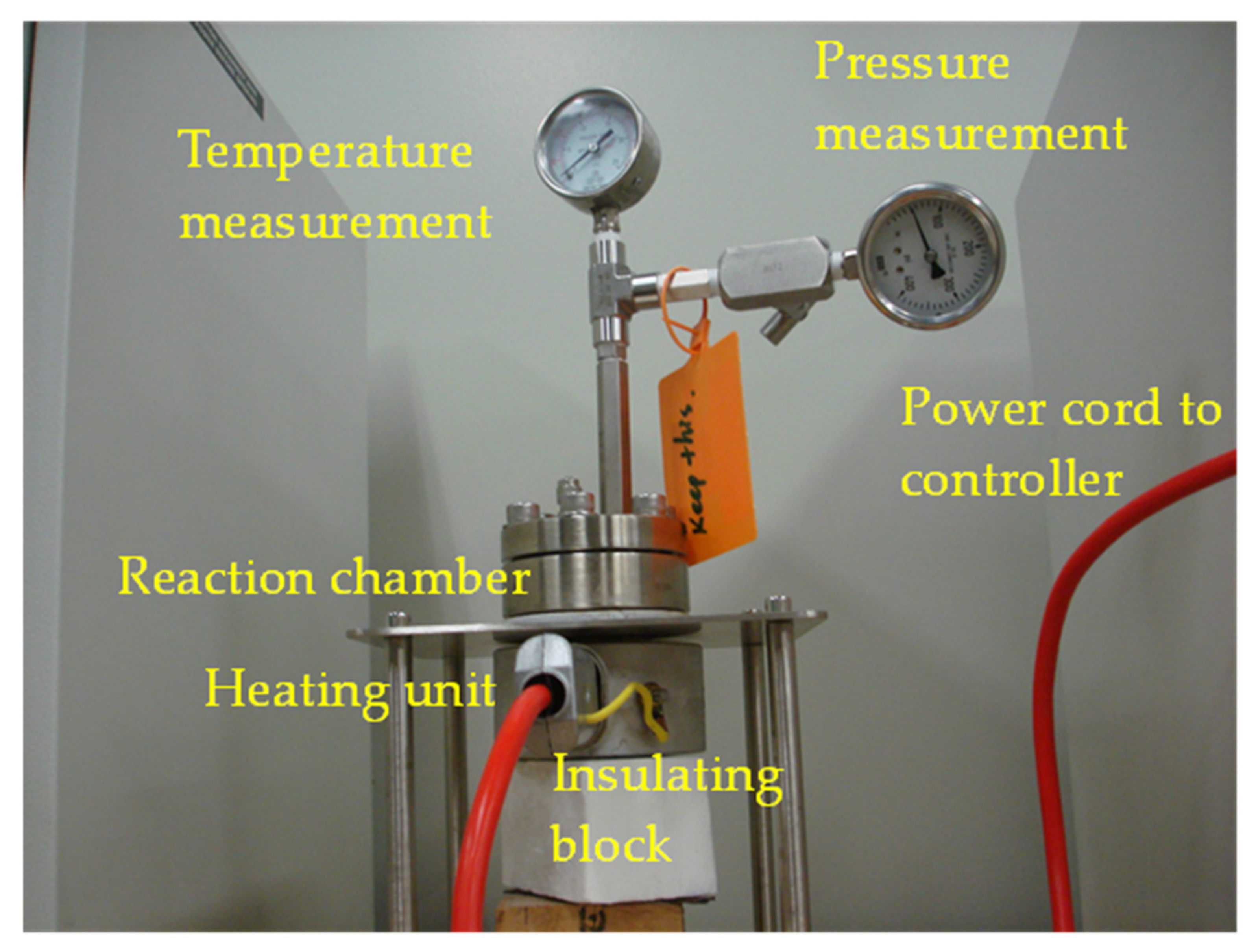
2. Materials and Methods
3. Results and Discussion
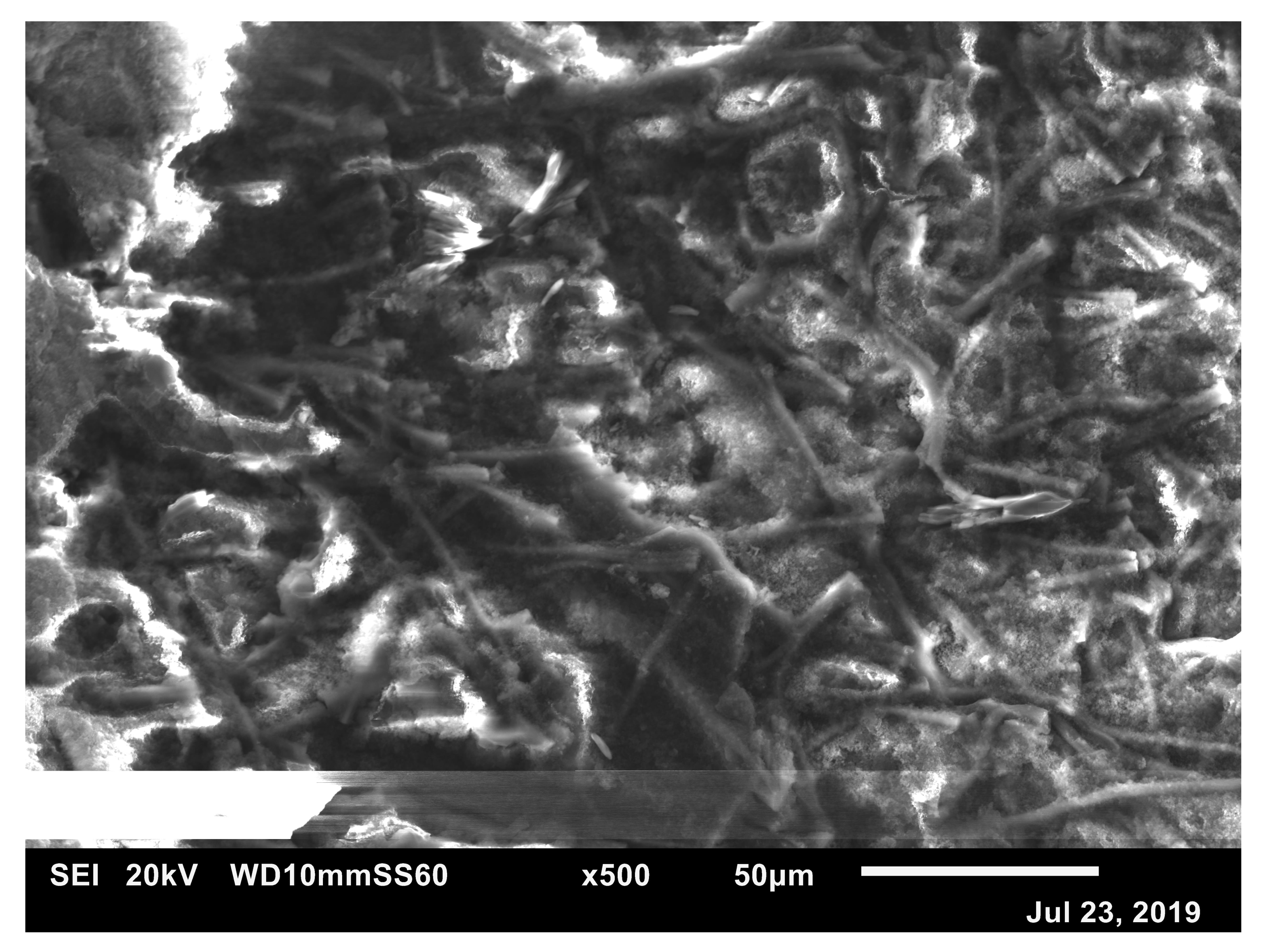
3.1. Morphology
3.2. Hardness of the Coating
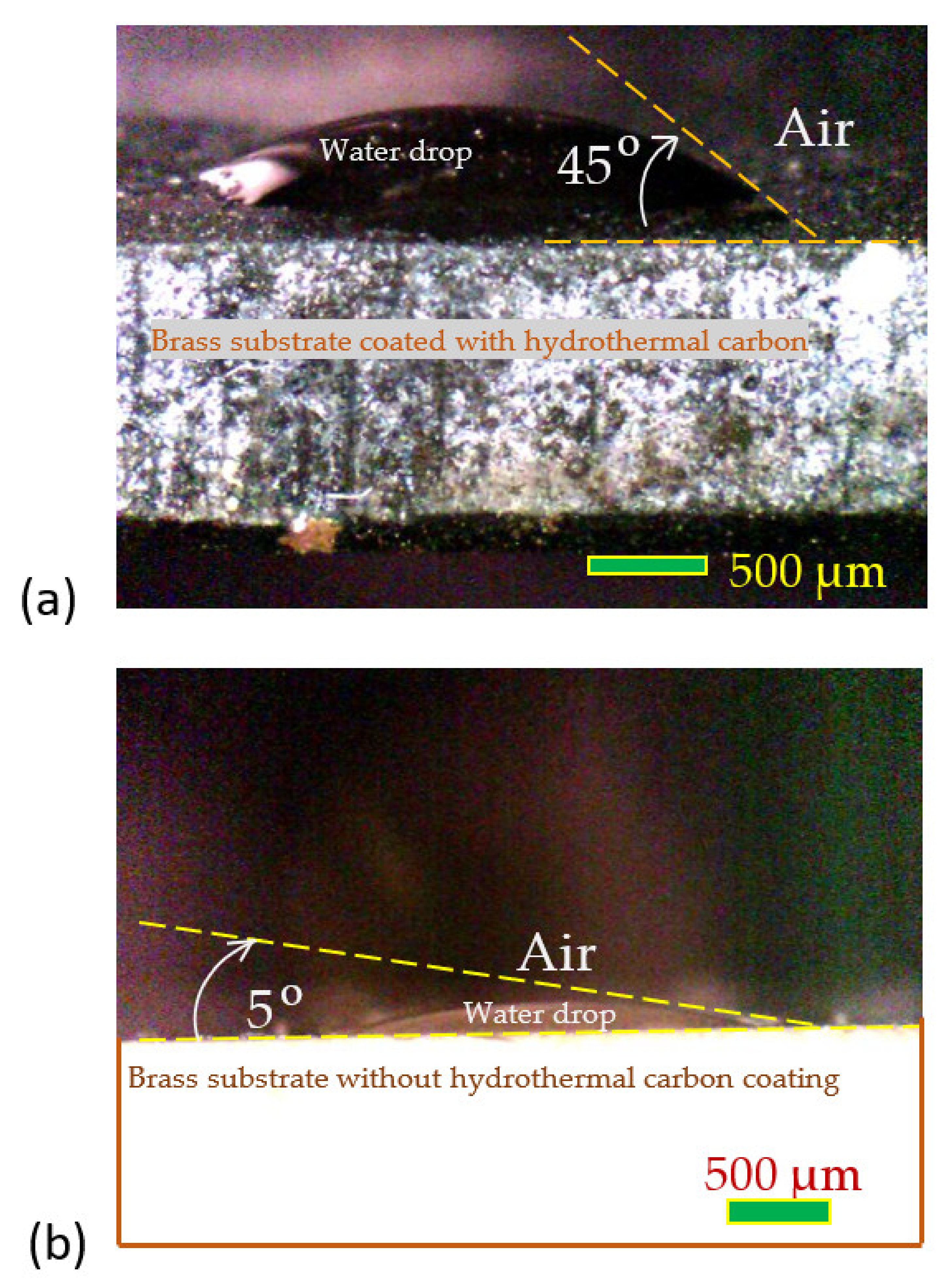
3.3. Seawater Wettability
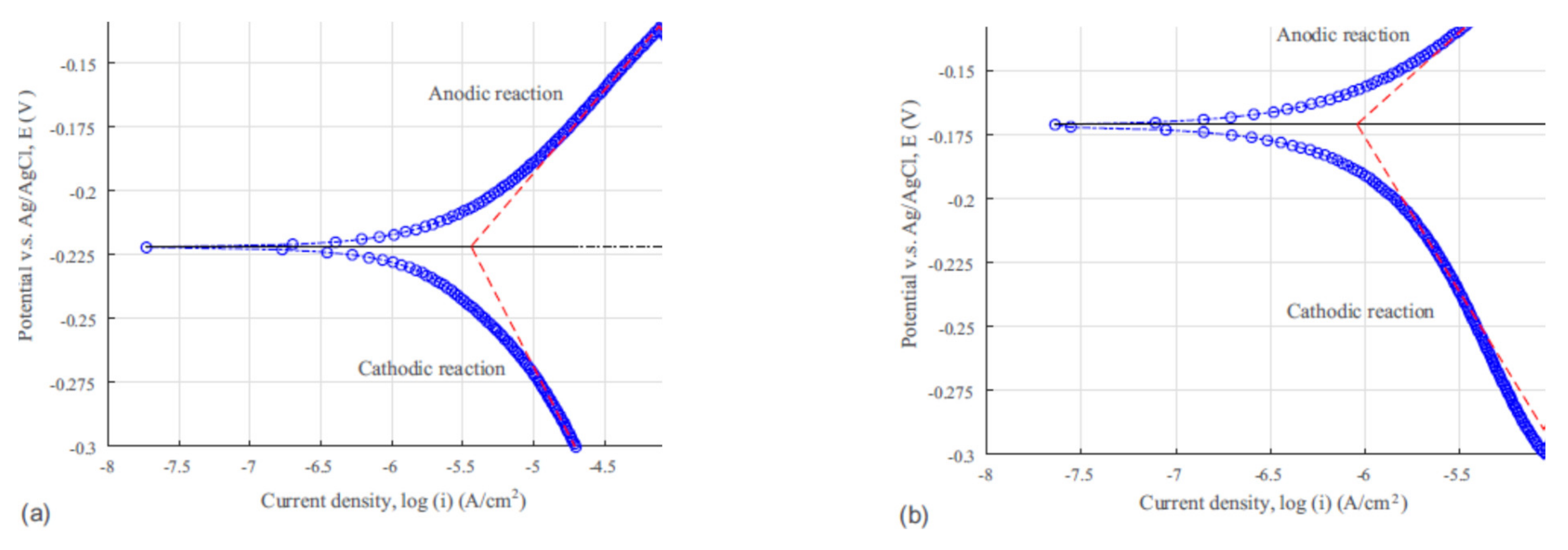
3.4. Seawater Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ling, L.; Cai, S.; Li, Q.Q.; Sun, J.Y.; Bao, X.G. Recent advances in hydrothermal modification of calcium phosphorus coating on magnesium alloy. J. Magnesium Alloys 2022, 10, 62–80. [Google Scholar] [CrossRef]
- Lin, Y.; Cai, S.; Jiang, S.; Xie, D.; Ling, R.; Sun, J.; Wei, J.; Shen, K.; Xu, G. Enhanced corrosion resistance and bonding strength of Mg substituted β-tricalcium phosphate/Mg(OH)2 composite coating on magnesium alloys via one-step hydrothermal method. J. Mech. Behav. Biomed. Mater. 2019, 90, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhu, L.G.; Cai, S.; Shen, S.B. Corrosion-resistant fluoridated Ca-Mg-P composite coating on magnesium alloys prepared via hydrothermal assisted sol-gel process. J. Mater. Res. 2018, 33, 3793–3800. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M.H. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef]
- Xie, J.S.; Zhang, J.H.; Liu, S.J.; Li, Z.H.; Zhang, L.; Wu, R.Z.; Hou, L.G.; Zhang, M.L. Hydrothermal synthesis of protective coating on Mg alloy for degradable implant applications. Coatings 2019, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Guo, G.H.; Jiang, J.H.; Zhang, L.W.; Ma, A.B.; Ma, X.L.; Chen, J.Q.; Cheng, Z.J. Hydrothermal synthesis and corrosion behavior of the protective coating on Mg-2Zn-Mn-Ca-Ce alloy. Prog. Nat. Sci. Mater. Int. 2016, 26, 590–599. [Google Scholar] [CrossRef]
- Song, D.; Li, C.; Zhang, L.W.; Ma, X.L.; Guo, G.H.; Zhang, F.; Jiang, J.H.; Ma, A.B. Decreasing bio-degradation rate of the hydrothermal-synthesizing coated Mg alloy via pre-solid-solution treatment. Materials 2017, 10, 858. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Kim, Y.K.; Ryu, M.H.; Bae, T.S.; Lee, M.H. Corrosion resistance and bioactivity enhancement of MAO coated Mg alloy depending on the time of hydrothermal treatment in Ca-EDTA solution. Sci. Rep. 2017, 7, 9061. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.D.; Zhang, K.Y.; Sun, W.; Wu, T.T.; He, H.R.; Liu, G.C. Hydrothermal synthesis of corrosion resistant hydrotalcite conversion coating on AZ91D alloy. Mater. Lett. 2013, 106, 111–114. [Google Scholar] [CrossRef]
- Wang, C.W.; Liu, C.; Lin, D.J.; Yeh, M.L.; Lee, T.M. Hydrothermal treatment and butylphosphonic acid derived self-assembled monolayers for improving the surface chemistry and corrosion resistance of AZ61 magnesium alloy. Sci. Rep. 2017, 7, 16910. [Google Scholar]
- Zhang, C.Y.; Ma, Y.L.; Liu, C.L. Hydroxyapatite coating on fluorine-treated magnesium alloy by hydrothermal method and its electrochemical corrosion behaviour in Hank’s solution. Prot. Met. Phys. Chem. Surf. 2019, 55, 127–135. [Google Scholar] [CrossRef]
- Li, L.Y.; Cui, L.Y.; Liu, B.; Zeng, R.C.; Chen, X.B.; Li, S.Q.; Wang, Z.L.; Han, E.H. Corrosion resistance of glucose-induced hydrothermal calcium phosphate coating on pure magnesium. Appl. Surf. Sci. 2019, 465, 1066–1077. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wu, G.M.; Zhao, Q.; Zhang, Y.H.; Xing, G.J.; Li, D.L. Anticorrosive magnesium hydroxide coating on AZ31 magnesium alloy by hydrothermal method. J. Phys. Conf. Ser. 2009, 188, 12044. [Google Scholar] [CrossRef]
- Roldan, L.; Santos, I.; Armenise, S.; Fraile, J.M.; Garcıa-Bordeje, E. The formation of a hydrothermal carbon coating on graphite microfiber felts for using as structured acid catalyst. Carbon 2012, 50, 1363–1372. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Luan, D.Y.; Madhavi, S.; Hu, Y.; Lou, X.W. Assembling carbon-coated α-Fe2O3 hollow nanohorns on the CNT backbone for superior lithium storage capability. Energy Environ. Sci. 2012, 5, 5252–5256. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Replication and coating of silica templates by hydrothermal carbonization. Adv. Funct. Mater. 2007, 17, 1010–1018. [Google Scholar] [CrossRef]
- Teng, F.; Hu, Z.H.; Ma, X.H.; Zhang, L.C.; Ding, C.X.; Yu, Y.; Chen, C.H. Hydrothermal synthesis of plate-like carbon-coated Li3V2(PO4)3 and its low temperature performance for high power lithium ion batteries. Electrochim. Acta 2013, 91, 43–49. [Google Scholar] [CrossRef]
- Pei, B.; Wang, Q.; Zhang, W.X.; Yang, Z.H.; Chen, M. Enhanced performance of LiFePO4 through hydrothermal synthesis coupled with carbon coating and cupric ion doping. Electrochim. Acta 2011, 56, 5667–5672. [Google Scholar] [CrossRef]
- Wang, Z.F.; Xiao, P.F.; He, N.Y. Synthesis and characteristics of carbon encapsulated magnetic nanoparticles produced by a hydrothermal reaction. Carbon 2006, 44, 3277–3284. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Asano, S.; Kudo, S.; Minh, D.P.; Appari, S.; Hisahiro, E.; Hayashi, J. The distinctive effects of glucose-derived carbon on the performance of Ni-based catalysts in methane dry reforming. Catalysts 2020, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.X.; Arjan, A.; Yik, J. Preparation of a photosensitive composite carbon fiber for spilled oil cleaning. J. Compos. Sci. 2022, 6, 28. [Google Scholar] [CrossRef]
- Kuzucu, V.; Aksoy, M.; Korkut, M.H. The effect of strong carbide-forming elements such as Mo, Ti, V and Nb on the microstructure of ferritic stainless steel. J. Mater. Proc. Technol. 1998, 82, 165–171. [Google Scholar] [CrossRef]
- Raghavan, V. C-Cu-Fe (Carbon-Copper-Iron). J. Phase Equilib. Diffus. 2012, 33, 224–225. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 10th ed.; Wiley: Hoboken, NJ, USA, 2018; p. 170. [Google Scholar]
- Marcus, P. Corrosion Mechanisms in Theory and Practices, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 47. [Google Scholar]
- Gan, Y.X.; Dong, J.; Gan, J.B. Carbon network/aluminum composite made by powder metallurgy and its corrosion behavior in seawater. Mater. Chem. Phys. 2017, 202, 190–196. [Google Scholar] [CrossRef]
- Eisenhauer, E.M.; Gan, Y.X. Corrosion behavior of a carbon network/aluminum matrix porous composite in salinated and acidic environments. ChemEngineering 2019, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Addonizio, M.L.; Ferrara, M.; Castaldo, A.; Antonaia, A. Air-stable low-emissive AlN-Ag based coatings for energy-efficient retrofitting of existing windows. Energy Build. 2021, 250, 111259. [Google Scholar] [CrossRef]



| Material | No. 1 (HRB) | No. 2 (HRB) | No. 3 (HRB) | No. 4 (HRB) | No. 5 (HRB) | No. 6 (HRB) | Average in HRB | Average in HB |
|---|---|---|---|---|---|---|---|---|
| C26000-Coated | 72.2 | 74.0 | 73.5 | 75.2 | 76.2 | 75.1 | 74.4 | 135.4 |
| C26000 | 76.1 | 81.3 | 83.8 | 82.0 | 76.7 | 80.1 | 80.0 | 149.8 |
| Cu-Coated | 68.5 | 74.8 | 70.0 | 68.5 | 72.3 | 77.0 | 71.9 | 129.4 |
| Cu | 66.6 | 67.7 | 68.1 | 68.3 | 67.5 | 69.9 | 68.0 | 120.7 |
| Material | βc (V/dec) | βa (V/dec) | icorr (A/cm2) | Ecorr (V) | Rp (Ω·cm2) |
|---|---|---|---|---|---|
| C26000-Coated | −0.136 | 0.062 | 3.535 × 10−5 | −0.173 | 1399.64 |
| C26000 | −0.306 | 0.089 | 1.472 × 10−4 | −0.281 | 370.21 |
| Cu-Coated | −0.121 | 0.067 | 9.013 × 10−7 | −0.171 | 72,327.44 |
| Cu | −0.107 | 0.065 | 3.581 × 10−6 | −0.222 | 20,079.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.X.; Chang, Y.; Chen, C.-C.; Li, M.; Gan, J.B.; Li, J. Seawater Corrosion of Copper and Its Alloy Coated with Hydrothermal Carbon. Coatings 2022, 12, 798. https://doi.org/10.3390/coatings12060798
Gan YX, Chang Y, Chen C-C, Li M, Gan JB, Li J. Seawater Corrosion of Copper and Its Alloy Coated with Hydrothermal Carbon. Coatings. 2022; 12(6):798. https://doi.org/10.3390/coatings12060798
Chicago/Turabian StyleGan, Yong X., Yizhe Chang, Chuan-Chiang Chen, Mingheng Li, Jeremy B. Gan, and Joseph Li. 2022. "Seawater Corrosion of Copper and Its Alloy Coated with Hydrothermal Carbon" Coatings 12, no. 6: 798. https://doi.org/10.3390/coatings12060798
APA StyleGan, Y. X., Chang, Y., Chen, C.-C., Li, M., Gan, J. B., & Li, J. (2022). Seawater Corrosion of Copper and Its Alloy Coated with Hydrothermal Carbon. Coatings, 12(6), 798. https://doi.org/10.3390/coatings12060798









