Surface Investigation of Physella Acuta Snail Shell Particle Reinforced Aluminium Matrix Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Stir Casting
2.2.2. Sample Preparation for the Analyses
3. Results and Discussion
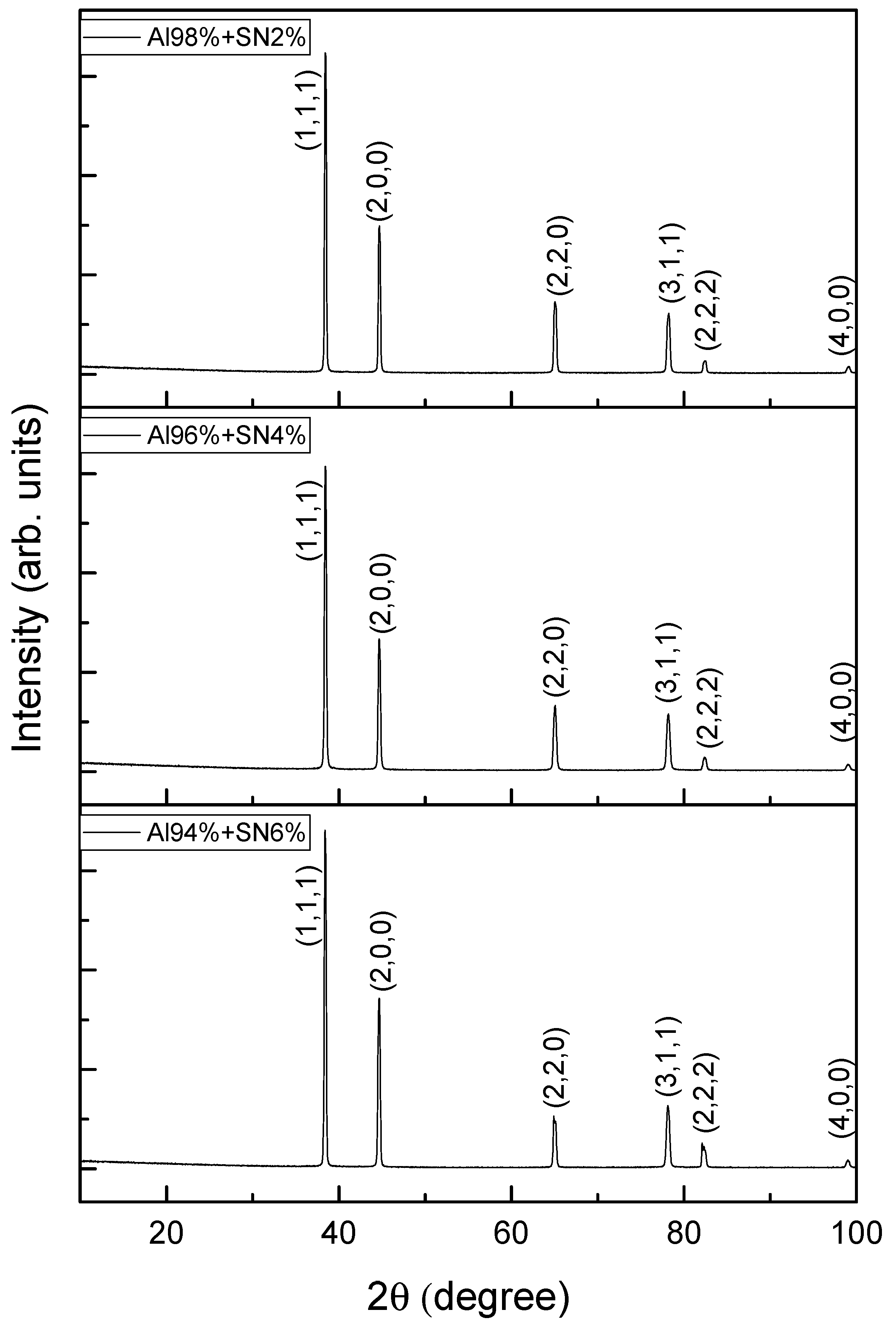
3.1. Elemental and Phase Composition
3.2. Morphology and Roughness as Received Surfaces
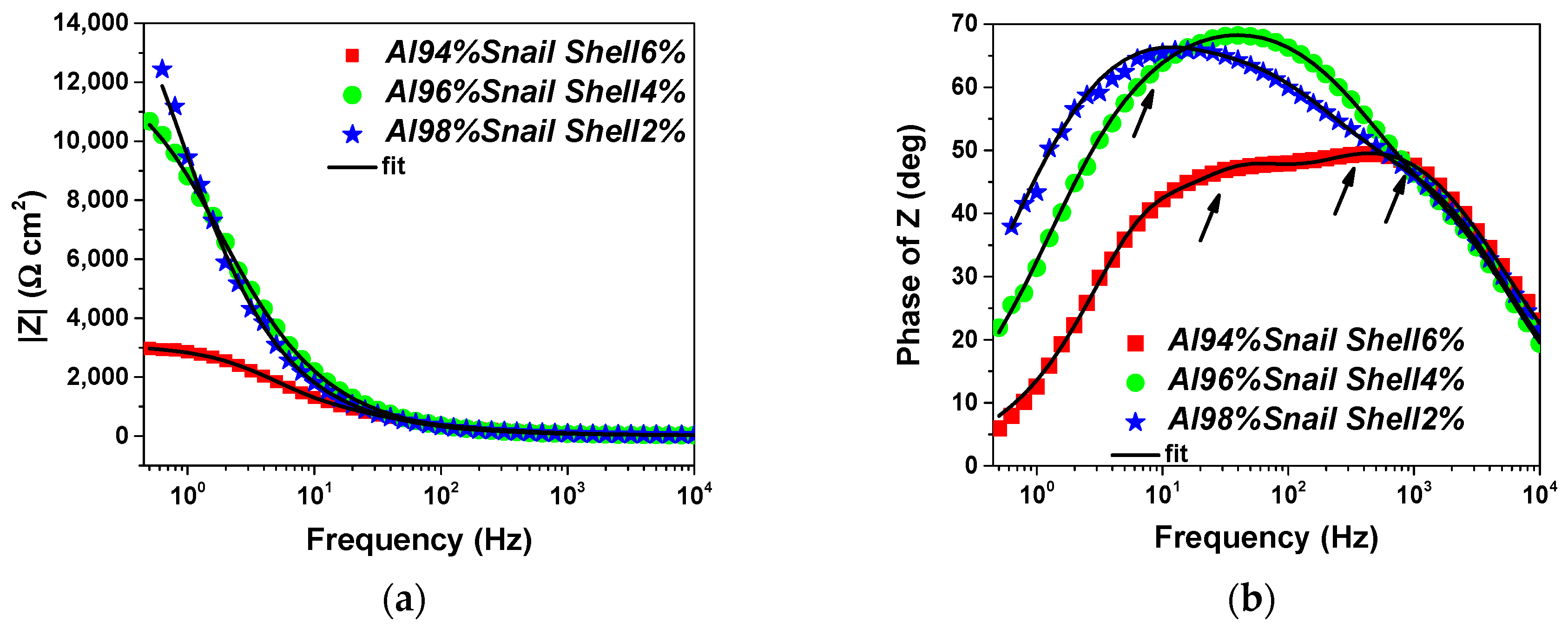
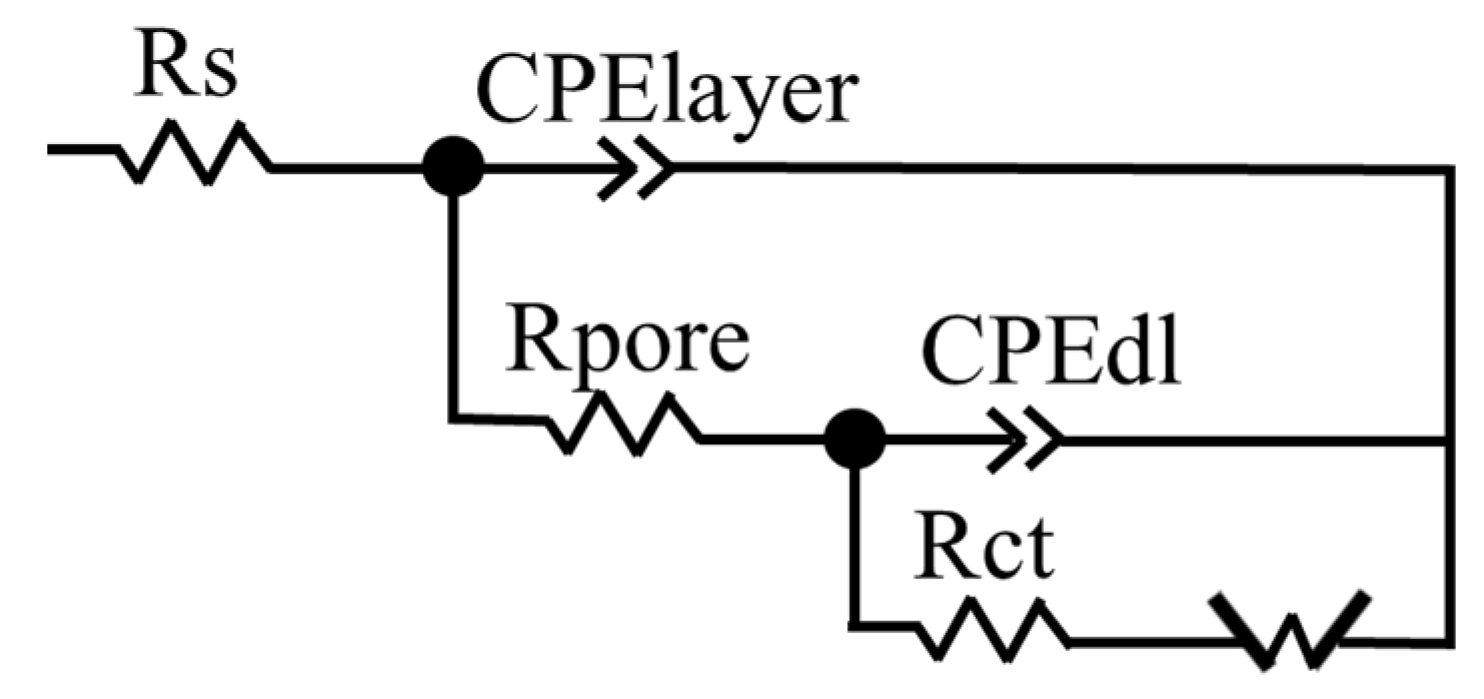
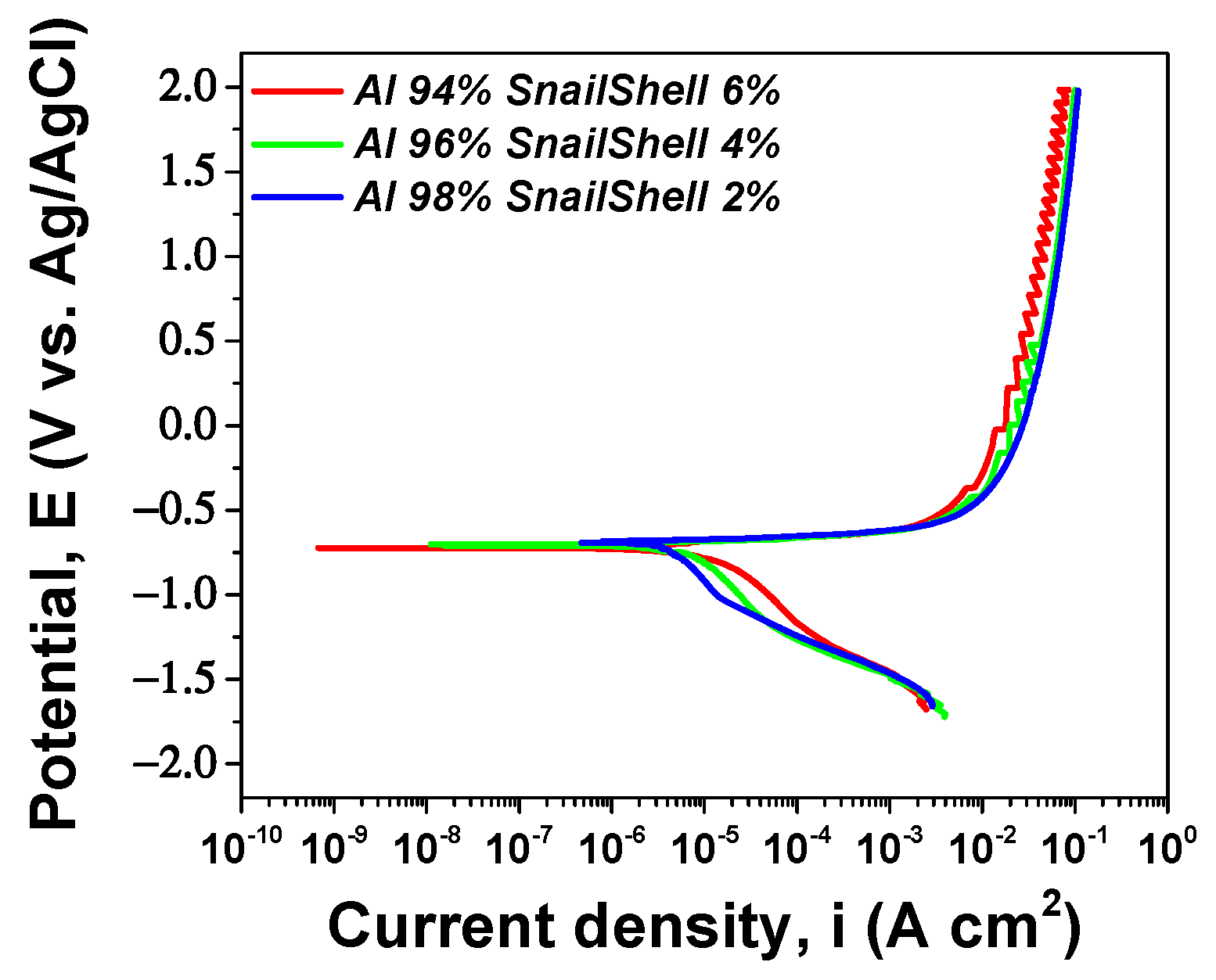
3.3. Electrochemical Performance
3.4. Elemental Composition after Corrosion
3.5. Morphology and Roughness after Corrosion
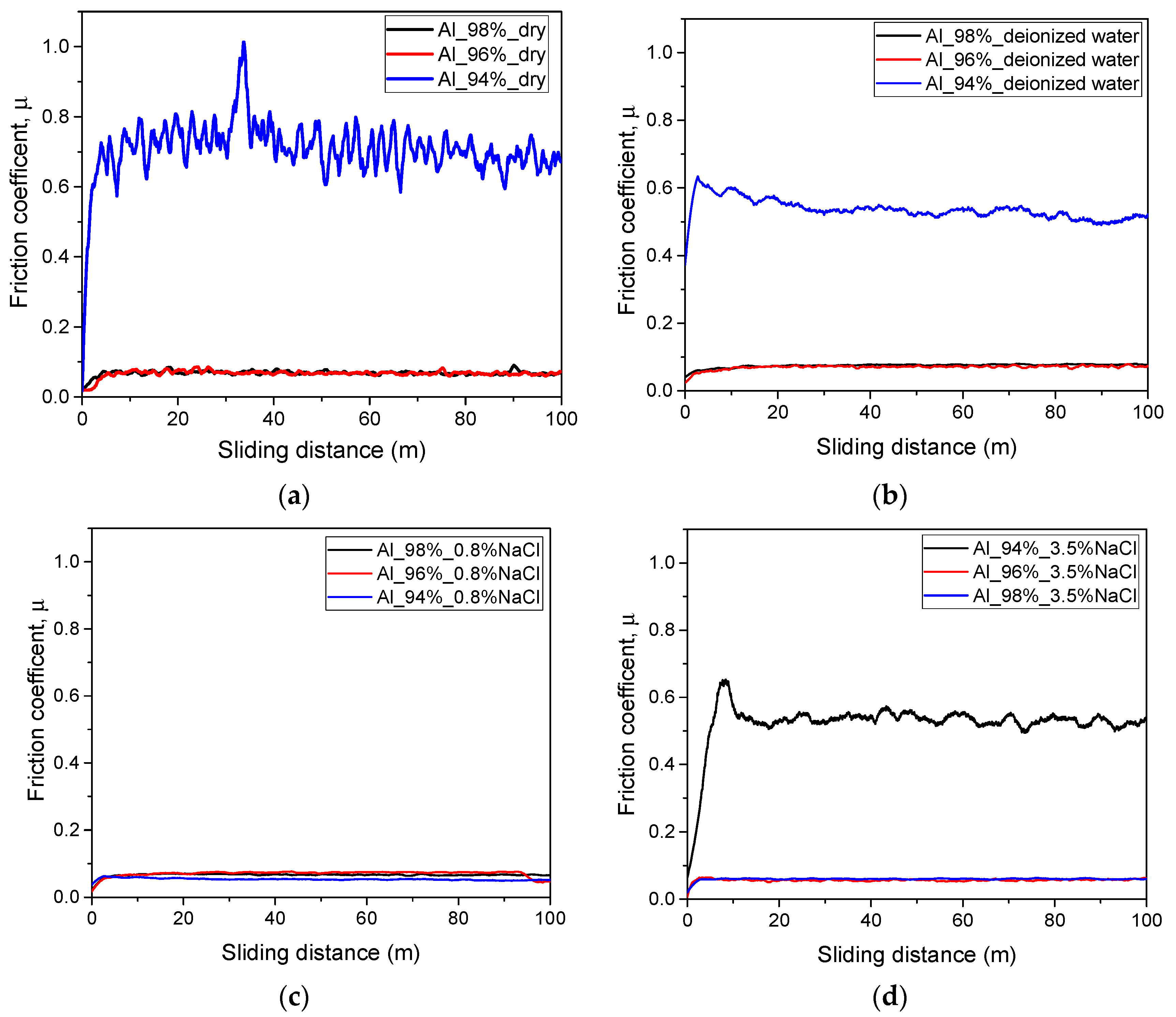
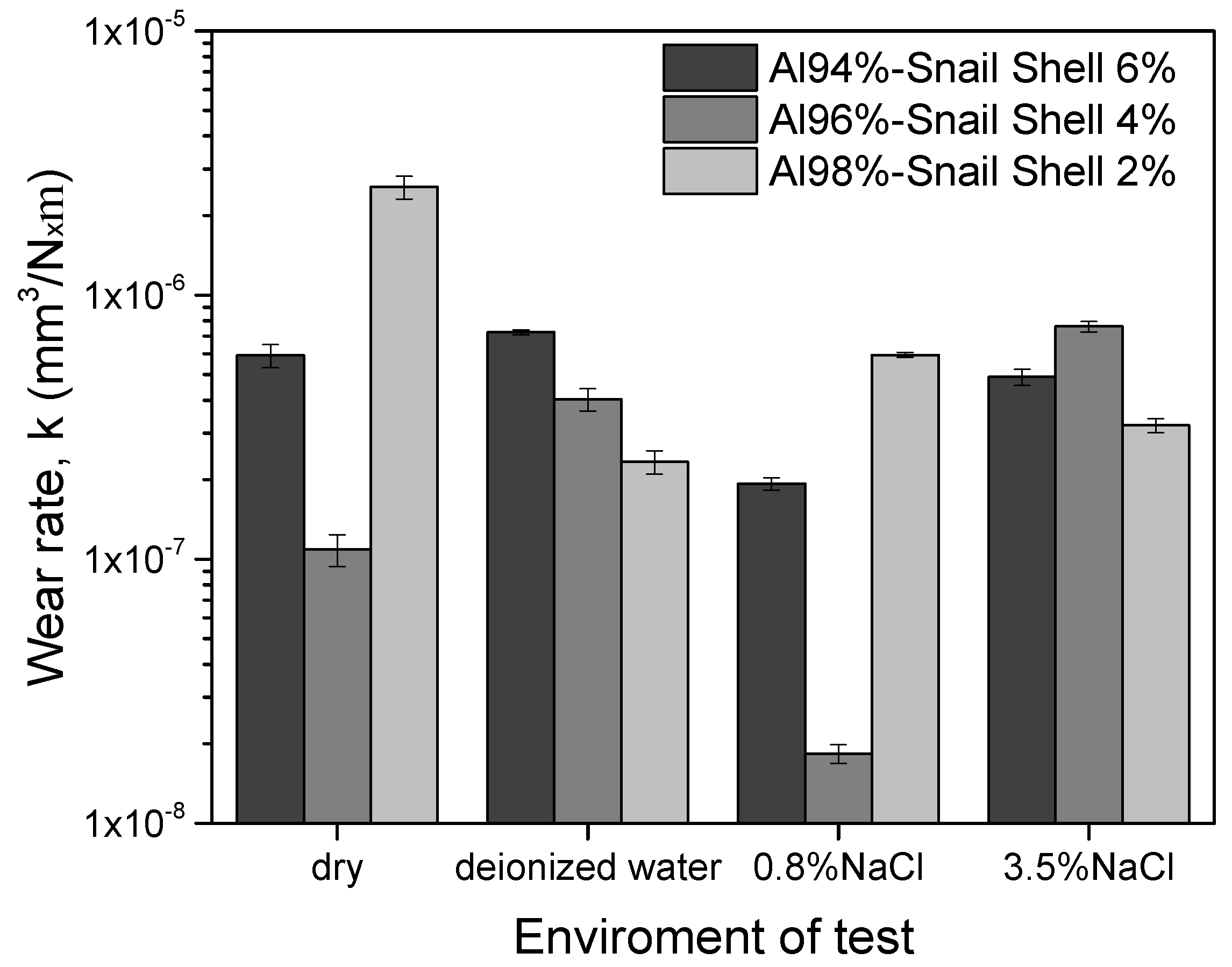
3.6. Tribological and Tribocorrosion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surappa, M.K. Aluminium matrix composites: Challenges and opportunities. Sadhana 2003, 28, 319–334. [Google Scholar] [CrossRef]
- Shaikh, M.B.N.; Arif, S.; Aziz, T.; Waseem, A.; Shaikh, M.A.N.; Ali, M. Microstructural, mechanical and tribological behaviour of powder metallurgy processed SiC and RHA reinforced Al-based composites. Surf. Interfaces 2019, 15, 166–179. [Google Scholar] [CrossRef]
- Mohapatra, J.; Nayak, S.; Mahapatra, M.M. Mechanical and tribology properties of Al-4.5%Cu-5%TiC metal matrix composites for light-weight structures. Int. J. Lightweight Mater. Manuf. 2020, 3, 120–126. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Sankaranarayanan, R.; Tharmaraj, R.; Pruncu, C.I.; Dispinar, D. A comparative study of the mechanical and tribological behaviours of different aluminium matrix-ceramic composites. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 330. [Google Scholar] [CrossRef]
- Panwar, N.; Chauhan, A. Fabrication methods of particulate reinforced aluminium metal matrix composite: A review. Mater. Today Proc. 2018, 5, 5933–5939. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Raja, S.; Tharmaraj, R.; Pruncu, C.I.; Dispinar, D. Mechanical and tribological characteristics of boron carbide reinforcement of AA6061 matrix composite. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 155. [Google Scholar] [CrossRef]
- Miracle, D. Metal matrix composites–from science to technological significance. Compos. Sci. Technol. 2005, 65, 2526–2540. [Google Scholar] [CrossRef]
- Bhoi, N.K.; Singh, H.; Pratap, S. Developments in the aluminum metal matrix composites reinforced by micro/nano particles–A review. J. Compos. Mater. 2019, 54, 813–833. [Google Scholar] [CrossRef]
- Kim, I.S.; Song, M.Y.; Kim, J.H. Effects of TieB and Si additions on microstructure and mechanical properties of Al-Cu-Mg based aluminum matrix composites. J. Alloys Compd. 2020, 832, 154827. [Google Scholar] [CrossRef]
- Selvam, J.D.R.; Dinaharan, I.; Philip, S.V.; Mashinini, P.M. Microstructure and mechanical characterization of in situ synthesized AA6061/(TiB2+Al2O3) hybrid aluminum matrix composites. J. Alloys Compd. 2018, 740, 529–535. [Google Scholar] [CrossRef]
- Gayathri, J.; Elansezhian, R. Influence of dual reinforcement (nano CuO þ reused spent alumina catalyst) on microstructure and mechanical properties of aluminium metal matrix composite. J. Alloys Compd. 2020, 829, 154538. [Google Scholar] [CrossRef]
- Gxowa-Penxa, Z.; Daswa, P.; Modiba, R.; Mathabathe, M.N.; Bolokang, A.S. Development and characterization of Al–Al3Ni–Sn metal matrix composite. Mater. Chem. Phys. 2021, 259, 124027. [Google Scholar] [CrossRef]
- Kolawole, M.Y.; Aweda, J.O.; Abdulkareem, S. Archachatina marginata bio-shells as reinforcement materials in metal matrix composites. Int. J. Automot. Mech. Eng. 2017, 14, 4068–4079. [Google Scholar] [CrossRef]
- Ferguson, H.T.; Oladiran, K.A.; Raseed, A.M.; Salawu, A.A. Snail Shell as an Inspiring Engineering Material in Science and Technology Development: A Review. Int. J. Contemp. Res. Rev. 2018, 9, 20408–20416. [Google Scholar] [CrossRef]
- Njoku, R.E.; Okon, A.E.; Ikpaki, T.C. Effects of Variation of Particle Size and Weight Fraction on the Tensile Strength and Modulus of Periwinkle Shell Reinforced Polyester Composite. Niger. J. Technol. 2011, 30, 87–93. [Google Scholar]
- Atuanya, C.U.; Aigbodion, V.S.; Obiorah, S.O.; Kchaou, M.; Elleuch, R. Emperical model for estimating the mechanical and morphological properties of recycled low density polyethylene/snail shell biocomposites. J. Assoc. Arab Univ. Basic Appl. Sci. 2016, 21, 45–52. [Google Scholar]
- Asafa, T.B.; Durowoju, M.O.; Oyewole, A.A.; Solomon, S.O.; Adegoke, R.M.; Aremu, O.J. Potentials of Snail Shell as a Reinforcement for Discarded Aluminum Based Materials. Int. J. Adv. Sci. Technol. 2015, 84, 1–8. [Google Scholar] [CrossRef]
- Idusuyi, N.; Olayinka, J.I. Dry sliding wear characteristics of aluminium metal matrix composites: A brief overview. J. Mater. Res. Technol. 2019, 8, 3338–3346. [Google Scholar] [CrossRef]
- Joseph, J.S.D.; Kumaragurubaran, B.; Sathish, S. Effect of MoS2 on the wear behavior of aluminium (AlMg0.5Si) composite. Silicon 2019, 12, 1481–1489. [Google Scholar] [CrossRef]
- Mistry, J.M.; Gohil, P.P. An overview of diversified reinforcement on aluminum metal matrix composites: Tribological aspects. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2017, 231, 399–421. [Google Scholar] [CrossRef]
- Mavhungu, S.T.; Akinlabi, E.T.; Onitiri, M.A.; Varachia, F.M. Aluminum matrix composites for industrial use: Advances and trends. Procedia Manuf. 2017, 7, 178–182. [Google Scholar] [CrossRef]
- White, M.M.; Chejlava, M.; Fried, B.; Sherma, J. The concentration of calcium carbonate in shells of freshwater snails. Am. Malacol. Bull. 2007, 22, 139–142. [Google Scholar] [CrossRef]
- Waterworld. CORROSION CONTROL: Using Caustic Soda to Control Corrosion and Calcium Carbonate Precipitation. 2022. Available online: https://www.waterworld.com/home/article/16191536/corrosion-control-using-caustic-soda-to-control-corrosion-and-calcium-carbonate-precipitation (accessed on 12 May 2022).
- Corrosionpedia. Corrosionpedia Explains Calcium Carbonate. 2022. Available online: https://www.corrosionpedia.com/definition/2032/calcium-carbonate#:~:text=Calcium%20cabonate%20is%20used%20to,white%20color%20to%20-a%20coating (accessed on 12 May 2022).
- Tianhua, C. Tribological properties of calcium carbonate powders modified with Tween 40 as lubricant additives. Funct. Mater. 2017, 24, 572–576. [Google Scholar] [CrossRef]
- Mahesh, V.P.; Praseeda, S.N.; Rajan, T.P.D.; Pai, B.C.; Hubli, R.C. Processing of surface-treated boron carbide-reinforced aluminum matrix composites by liquid–metal stir-casting technique. J. Compos. Mater. 2011, 45, 2371–2378. [Google Scholar] [CrossRef]
- Ali, M.; Mohsen, O. Investigation on mechanical properties of nano-Al2O3-reinforced aluminium matrix composites. J. Compos. Mater. 2011, 45, 2579–2586. [Google Scholar]
- Mahmoud, T.S. Tribological behaviour of A390/Grp metal–matrix composites fabricated using a combination of rheocasting and squeeze casting techniques. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2008, 222, 257–265. [Google Scholar] [CrossRef]
- Kok, M. Production and mechanical properties of Al2O3 particle-reinforced 2024 aluminium alloy composites. J. Mater. Processing Technol. 2005, 161, 381–387. [Google Scholar] [CrossRef]
- Martin, F.J.; Cheek, G.T.; Grady, W.E.; Natishan, P.M. Impedance studies of the passive film on aluminum. Corros. Sci. 2005, 47, 3187–3201. [Google Scholar] [CrossRef]
- Hagioglu, P.; Gheorghies, C.; Cantaragiu, A.M.; Moisa, S. The accelerated corrosion behaviour in saline environments of some samples made of tombac, copper and aluminium. J. Sci. Arts 2010, 10, 153–160. [Google Scholar]
- Chang, L.; Jeng, Y.-R. Effects of negative skewness of surface roughness on the contact and lubrication of nominally flat metallic surfaces. Proc. IMechE Part J Eng. Tribol. 2012, 227, 559–569. [Google Scholar] [CrossRef]



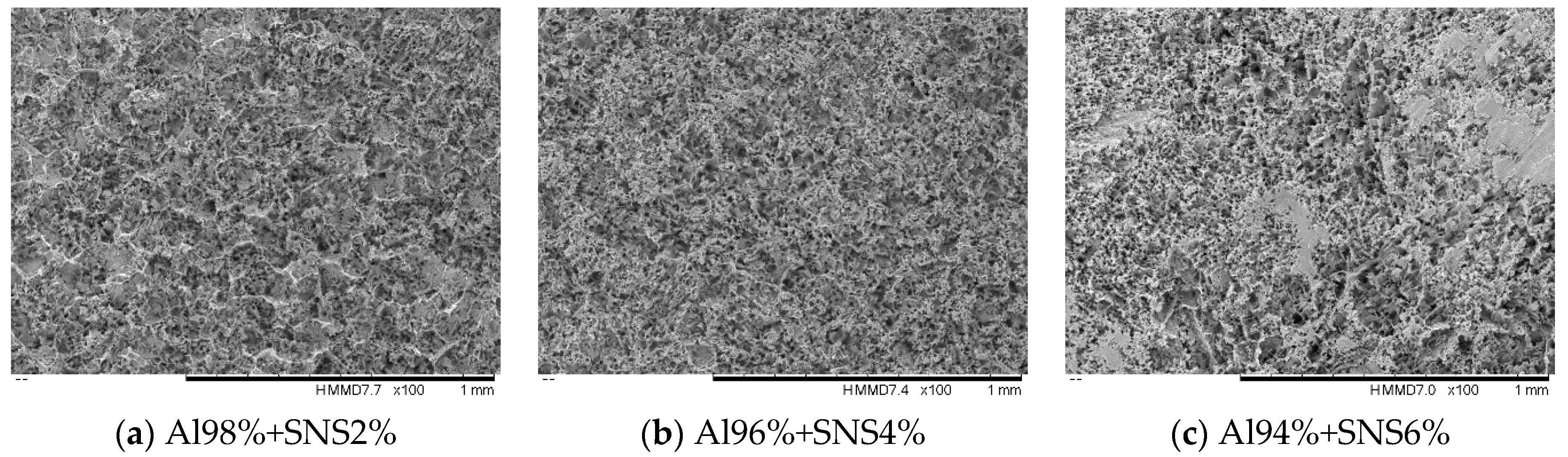
| Materials | Fe | Mn | Mg | Cu | Ni | Zn | Si | Ti | Pb | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| Presence in % | 0.40 | 0.03 | 0.03 | 0.03 | 0.03 | 0.07 | 0.30 | 0.03 | 0.03 | Balance |
| SNS Wt. % | SNS Wt. (g) | LM0 Wt. % | LM0 Wt. (g) | SNS Volume Fraction | LM0 Volume Fraction |
|---|---|---|---|---|---|
| 2 | 30 | 98 | 1470 | 4.19 | 95.81 |
| 4 | 60 | 96 | 1440 | 8.20 | 91.80 |
| 6 | 90 | 94 | 1410 | 12.03 | 87.97 |
| Element | Al98SNS2 (norm. at %) | Al96SNS4 (norm. at %) | Al94SNS6 (norm. at %) |
|---|---|---|---|
| Carbon | 2.15 ± 0.063 | 2.75 ± 0.085 | 1.94 ± 0.057 |
| Oxygen | 0.75 ± 0.017 | 0.76 ± 0.017 | 0.74 ± 0.016 |
| Silicon | 0.03 ± 0.001 | 0.07 ± 0.002 | 0.05 ± 0.002 |
| Iron | 0.04 ± 0.001 | 0.07 ± 0.002 | 0.19 ± 0.004 |
| Zinc | 0.01± 0.001 | 0.04 ± 0.001 | 0.04 ± 0.001 |
| Calcium | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 |
| Manganese | 0.01 ± 0 | 0.00 ± 0 | 0.00 ± 0 |
| Aluminum | Remaining | Remaining | Remaining |
| Sample | T(hkl) | ||||||
|---|---|---|---|---|---|---|---|
| (1,1,1) | (2,0,0) | (2,2,0) | (3,1,1,) | (2,2,2) | (4,0,0) | d (nm) | |
| Al98SNS2 | 0.388 | 0.207 | 0.132 | 0.124 | 0.027 | 0.018 | 35.17 |
| Al96SNS4 | 0.395 | 0.201 | 0.129 | 0.129 | 0.031 | 0.018 | 31.85 |
| Al94SNS6 | 0.413 | 0.252 | 0.107 | 0.142 | 0.067 | 0.018 | 30.37 |
| Scheme | Ra, nm | Rq, nm | Ssk | |||
|---|---|---|---|---|---|---|
| Average | STDEV | Average | STDEV | Average | STDEV | |
| Al98SNS2 | 5187.42 | 591.42 | 6331.80 | 519.49 | −0.15 | 0.16 |
| Al96SNS4 | 5875.75 | 408.95 | 7059.99 | 302.57 | −0.28 | 0.13 |
| Al94SNS6 | 4794.64 | 673.44 | 5805.84 | 588.04 | −0.11 | 0.14 |
| Parameters | Sample | ||
|---|---|---|---|
| Al98SNS2 | Al96SNS4 | Al94SNS6 | |
| Rs (Ω cm2) | 26.12 | 27.13 | 28.95 |
| Y0 (Ω−1 cm−2 sn) | 1.13 × 10−5 | 5.88 × 10−6 | 1.52 × 10−5 |
| n1 | 0.80 | 0.87 | 0.75 |
| Rpor (Ω cm2) | 244 | 123 | 769 |
| Y0’ (Ω−1 cm−2 sn) | 5.80 × 10−6 | 7.02 × 10−6 | 4.56 × 10−6 |
| n2 | 0.85 | 0.82 | 0.99 |
| Rct (Ω cm2) | 283.2 | 9200 | 1013 |
| W (Ω cm2 sn) | 18,627 | 3063 | 1325 |
| χ2 | 0.0005 | 0.0001 | 0.0002 |
| Parameters | Sample | ||
|---|---|---|---|
| Al98SNS2 | Al96SNS4 | Al94SNS6 | |
| Eoc (mV) | −716 | −717 | −799 |
| Rp (kΩ cm2) | 38 | 31 | 5 |
| Ecorr (mV) | −673 | −703 | −832 |
| icorr (nA cm−2) | 121 | 44 | 22.6 |
| Element | Al98SNS2 (norm. at %) | Al96SNS4 (norm. at %) | Al94SNS6 (norm. at %) |
|---|---|---|---|
| Carbon | 1.75 ± 0.052 | 1.53 ± 0.047 | 1.60 ± 0.049 |
| Oxygen | 1.70 ± 0.046 | 2.02 ± 0.060 | 2.26 ± 0.074 |
| Silicon | 0.13 ± 0.004 | 0.08 ± 0.001 | 0.12 ± 0.001 |
| Iron | 0.94 ± 0.018 | 0.49 ± 0.008 | 1.02 ± 0.042 |
| Zinc | 0.01 ± 0.001 | 0.00 | 0.00 |
| Calcium | 0.00 | 0.00 | 0.01 ± 0.001 |
| Manganese | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 |
| Aluminum | Remaining | Remaining | Remaining |
| Sample | Ra, nm | Rq, nm | Ssk | |||
|---|---|---|---|---|---|---|
| Average | STDEV | Average | STDEV | Average | STDEV | |
| Al98SNS2 | 11,747.41 | 573.35 | 14,736.48 | 406.48 | 0.51 | 0.34 |
| Al96SNS4 | 11,232.34 | 1357.63 | 14,000.86 | 1124.02 | 0.07 | 0.08 |
| Al94SNS6 | 11,414.37 | 3132.20 | 14,372.92 | 3663.33 | −0.12 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruncu, C.I.; Vladescu, A.; Hynes, N.R.J.; Sankaranarayanan, R. Surface Investigation of Physella Acuta Snail Shell Particle Reinforced Aluminium Matrix Composites. Coatings 2022, 12, 794. https://doi.org/10.3390/coatings12060794
Pruncu CI, Vladescu A, Hynes NRJ, Sankaranarayanan R. Surface Investigation of Physella Acuta Snail Shell Particle Reinforced Aluminium Matrix Composites. Coatings. 2022; 12(6):794. https://doi.org/10.3390/coatings12060794
Chicago/Turabian StylePruncu, Catalin Iulian, Alina Vladescu, N. Rajesh Jesudoss Hynes, and Ramakrishnan Sankaranarayanan. 2022. "Surface Investigation of Physella Acuta Snail Shell Particle Reinforced Aluminium Matrix Composites" Coatings 12, no. 6: 794. https://doi.org/10.3390/coatings12060794
APA StylePruncu, C. I., Vladescu, A., Hynes, N. R. J., & Sankaranarayanan, R. (2022). Surface Investigation of Physella Acuta Snail Shell Particle Reinforced Aluminium Matrix Composites. Coatings, 12(6), 794. https://doi.org/10.3390/coatings12060794





.jpg)


