Study on Microstructure and Properties of Nickel-Based Self-Lubricating Coating by Laser Cladding
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Experimental Procedure
2.3. Performance Test
3. Results and Discussion
3.1. Macro Morphology
3.2. Coatings Microstructure
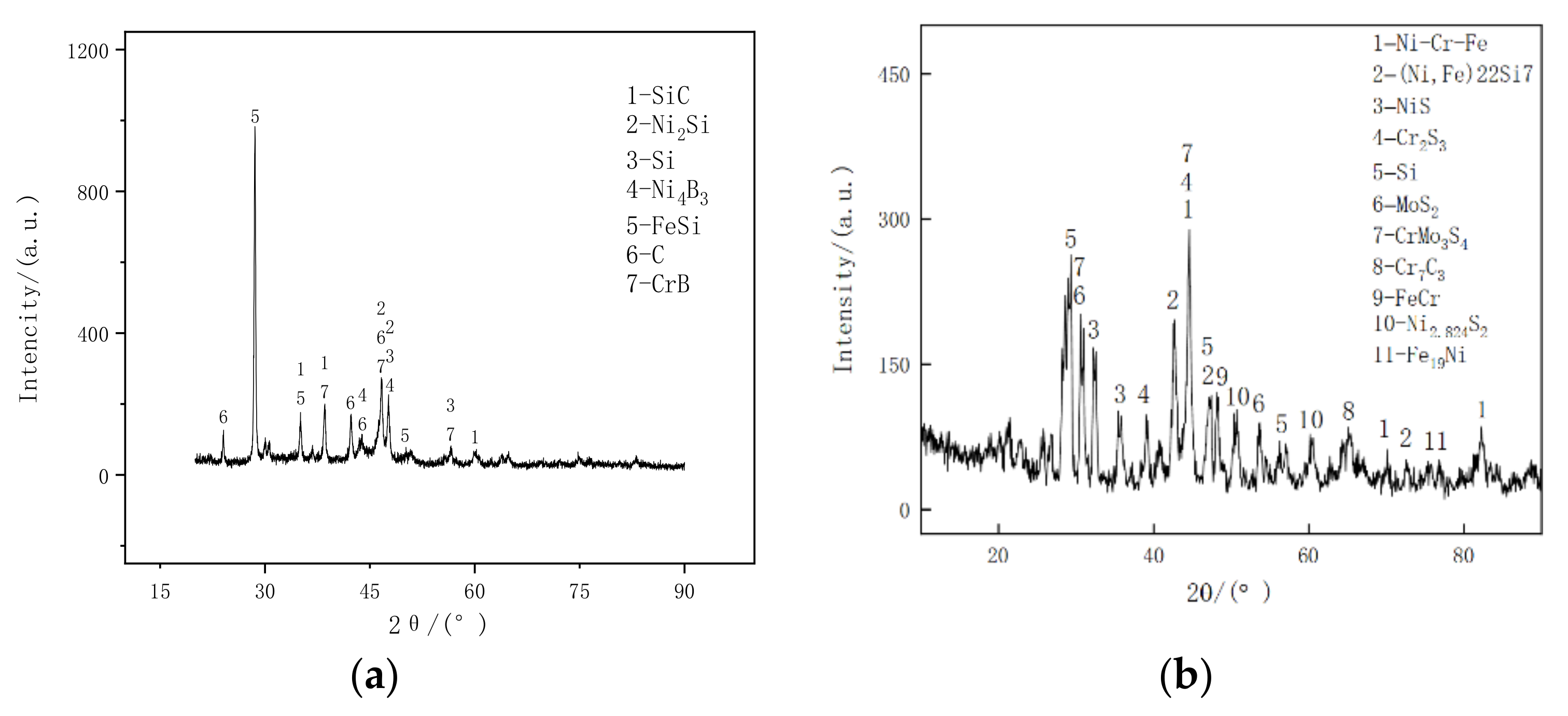
3.3. Phase Structure Analysis of Cladding Layer
3.4. Microhardness
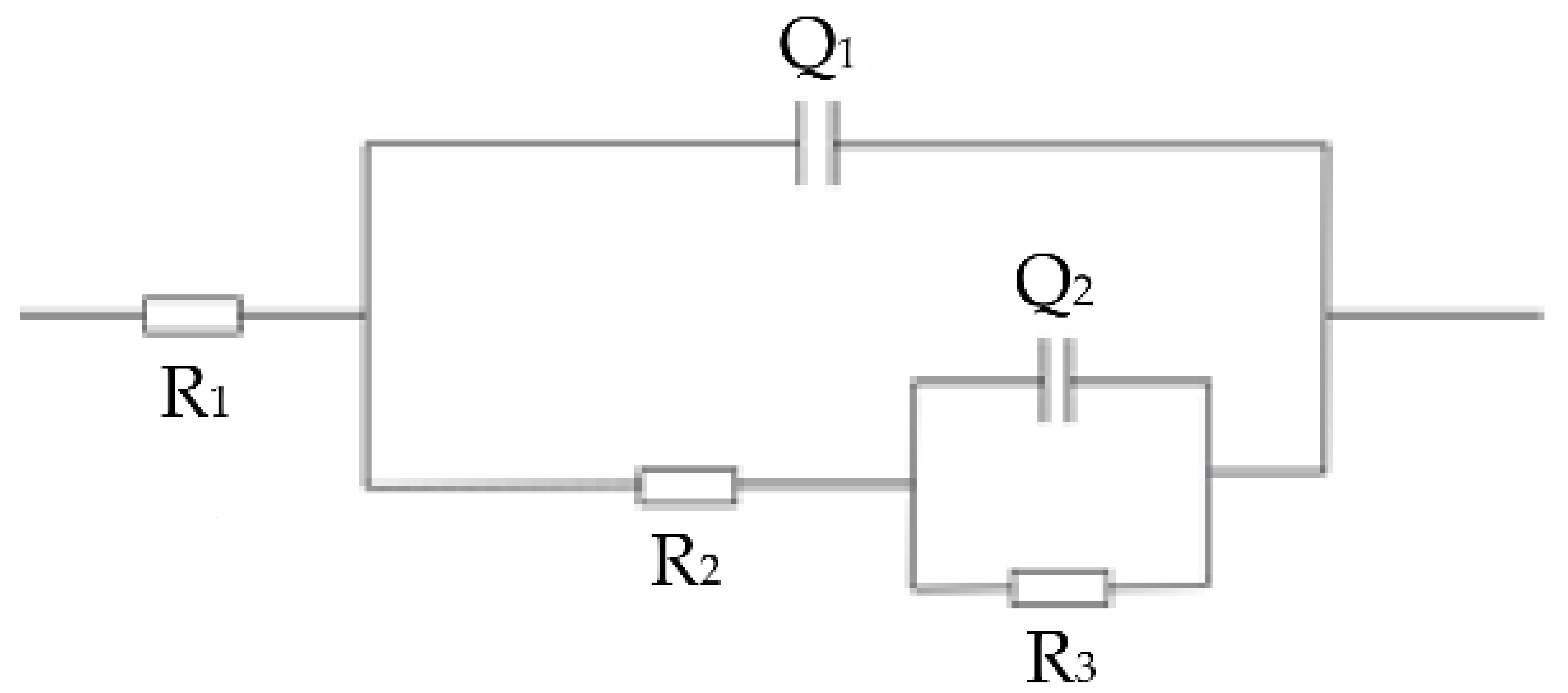
3.5. Corrosion Resistance
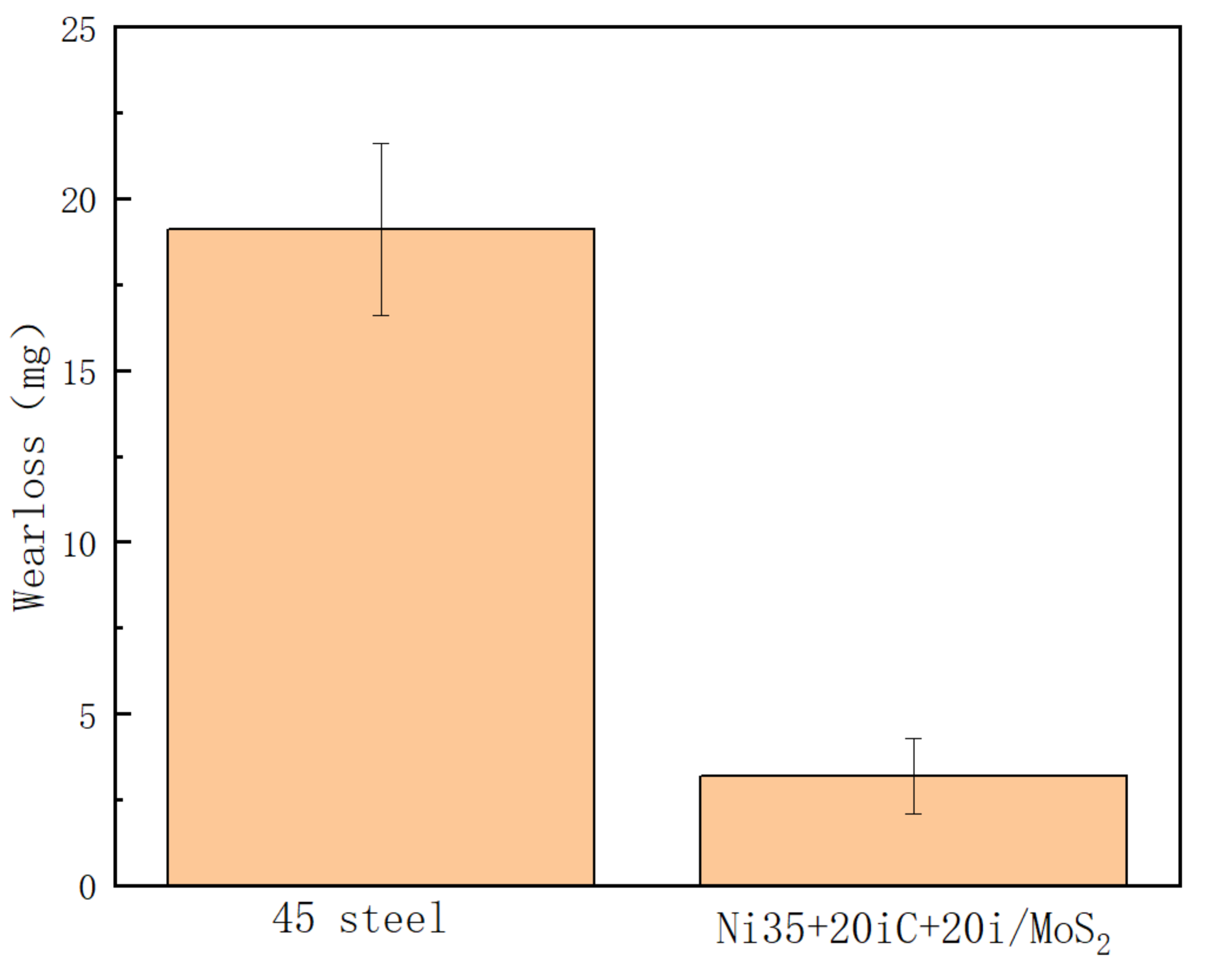
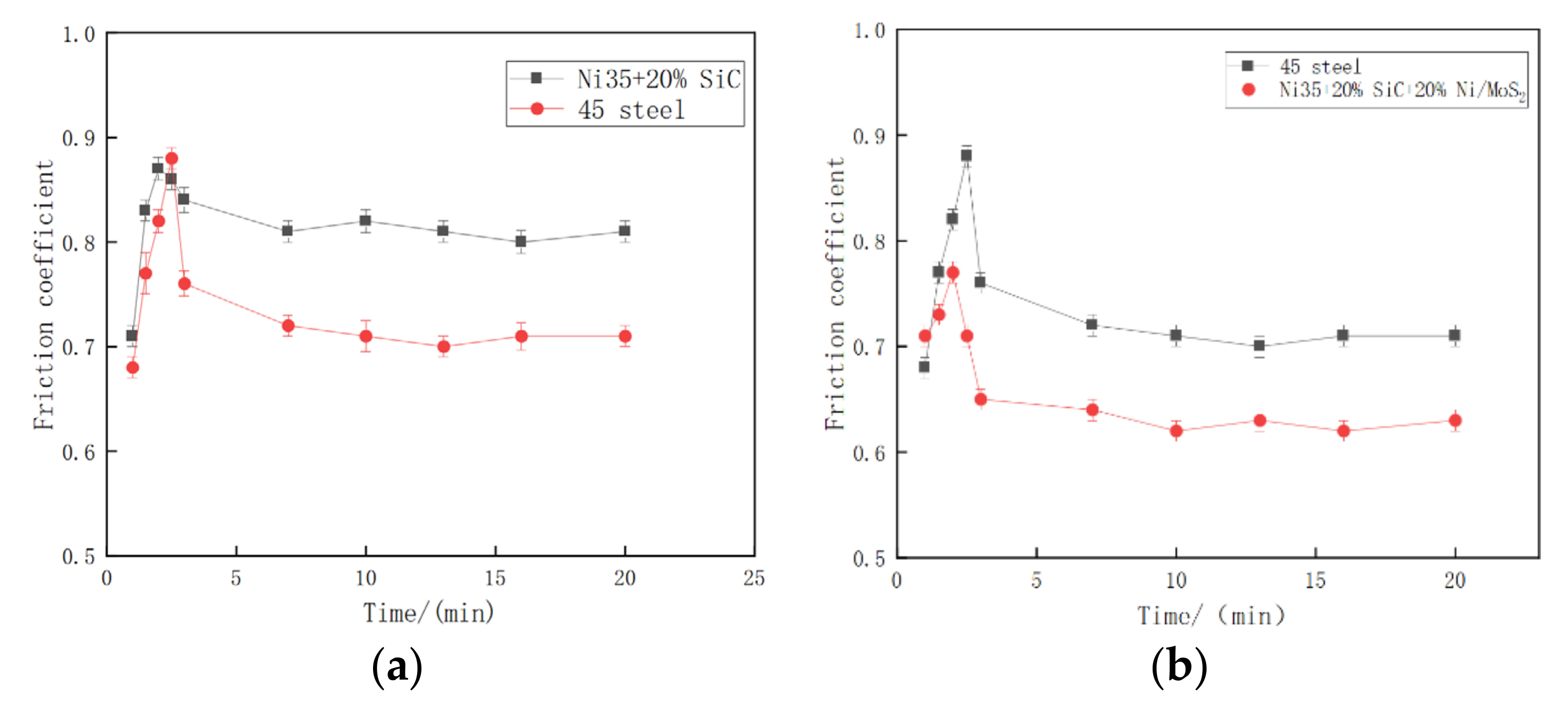
3.6. Wear Resistance
4. Conclusions
- (1)
- The microstructure of Ni35 + 20% SiC + 20% Ni/MoS2 cladding layer fabricated at 1000 W laser power and 8 mm/s scanning speed is dense and flawless, and the microstructure is mainly cellular grains and equiaxed grains.
- (2)
- During the laser cladding process, SiC are completely decomposed and Ni/MoS2 is partially dissolved. The melting layers mainly include solid-soluble Ni-Cr-Fe,(Ni, Fe)22Si7 and metal sulfide MoS2, NiS, Cr2S3, CrMo3S4. The complex phase components have a sol-id-soluble strengthening and diffuse enhancement effect, so that the coating obtains higher hardness and a lower wear rate. The microhardness of the coating is about 700 HV, the wear rate is about 0.17 times that of the grade 45 steel, and the wear resistance is significantly improved.
- (3)
- The self-corrosion potential and corrosion current density of the cladding layer in the electrochemical test were 0.052 V and 1.69 × 10−5 A∙cm−2, respectively. The comparison of the substrate shows that the cladding layer has good corrosion resistance, which indicates that the corrosion resistance of the cladding layer is obviously enhanced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, D.X. Failure analysis and repair process of Marine Chrome-plated plunger parts. Mech. Eng. 2014, 12, 226. [Google Scholar]
- Yan, X.Q.; Jie, H.; Ding, H.; Jian, C.; Wen, L.; Zheng, B.W.; Zheng, Z.B. Effects of Laser Scanning Speed on Microstructure, Microhardness, and Corrosion Behavior of Laser Cladding Ni45 Coatings. J. Chem. 2020, 2020, 1438473. [Google Scholar]
- Wang, K.M.; Chang, B.H.; Chen, J.S.; Fu, H.G.; Lin, Y.H.; Lei, Y.P. Effect of Molybdenum on the Microstructures and Properties of Stainless Steel Coatings by Laser Cladding. Appl. Sci. 2017, 7, 1065. [Google Scholar] [CrossRef]
- Ertugrul, O.; Enrici, T.M.; Paydas, H.; Saggionetto, E.; Boschini, F.; Mertens, A. Laser cladding of TiC reinforced 316L stainless steel composites: Feedstock powder preparation and microstructural evaluation. Powder Technol. 2020, 375, 384–396. [Google Scholar] [CrossRef]
- Song, B.X.; Yu, T.B.; Jiang, X.Y.; Xi, W.C.; Lin, X.L. Development mechanism and solidification morphology of molten pool generated by laser cladding. Int. J. Therm. Sci. 2021, 159, 106579. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Cui, R.; Wang, H.Z.; Han, Z.T. Effect of processing parameters of laser on microstructure and properties of cladding 42CrMo steel. Int. J. Adv. Manuf. Technol. 2018, 96, 1715–1724. [Google Scholar] [CrossRef]
- Zhang, J.C.; Shi, S.H.; Gong, Y.Q.; Yu, S.Q.; Shi, T.; Fu, G.Y. Advances in laser cladding technology. Surf. Technol. 2020, 49, 1–11. [Google Scholar]
- Morgado, T.; Valente, C. Development Status of LASER Cladding and the New Metallic Alloys. Preprints 2018, 2018050417. [Google Scholar] [CrossRef]
- Yan, X.L.; Dong, S.Y.; Xu, B.S.; Cao, Y. Progress and Challenges of Ultrasonic Testing for Stress in Remanufacturing Laser Cladding Coating. Materials 2018, 11, 293. [Google Scholar] [CrossRef]
- Chai, C.; Li, X.M.; Wang, S.C.; Wang, G. Microstructure and properties of SiC reinforced Ni35 alloy laser cladding. Mater. Mech. Eng. 2021, 45, 58–66. [Google Scholar]
- Hu, Z.; Li, W.; Zhao, Y. The Effect of Laser Power on the Properties of M3B2-Type Boride-Based Cermet Coatings Prepared by Laser Cladding Synthesis. Materials 2020, 13, 1867. [Google Scholar] [CrossRef]
- Preedawiphat, P.; Mahayotsanun, N.; Sa-Ngoen, K.; Mai, N.; Pongsak, T.; Sedthawatt, S.; Kuniaki, D. Mechanical Investigations of ASTM A36 Welded Steels with Stainless Steel Cladding. Coatings 2020, 10, 844. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.Z.; Tong, X.Y. Application of thermal spraying technology in Marine environment equipment. Sci. Technol. Inf. 2013, 02, 057. [Google Scholar]
- Liu, F.J.; Li, A.D.; Shen, Z.K.; Chen, H.Y.; Ji, Y. Microstructure and corrosion behavior of Al-Ti-TiC-CNTs/AZ31 magnesium matrix composites prepared using laser cladding and high speed friction stir processing. Opt. Laser Technol. 2022, 152, 108078. [Google Scholar] [CrossRef]
- Devoyno, O.; Drozdov, P.; Dovoretskiy, Y.; Kardapolova, M.; Lutsko, N.; Tamanis, E. Influence of Laser Cladding Parameters on the Distribution of Elements in the Beads of Nickel-Based Ni-Cr-B-Si Alloy. Latv. J. Phys. Tech. Sci. 2012, 49, 61–70. [Google Scholar] [CrossRef][Green Version]
- Xu, X.C.; Wen, H.J.; Wang, J.Y.; Miao, M. Optimization of laser cladding process parameters for remanufacturing of equipment Parts based on response surface. Chin. J. Vac. Sci. Technol. 2018, 38, 615–620. [Google Scholar]
- Oglezneva, S.; Morozov, E.; Usynin, E. Determination of the limiting values of the laser parameters to heat treatment of powder carbon steels surface. MATEC Web Conf. 2019, 298, 00058. [Google Scholar] [CrossRef]
- Zeng, M.; Yan, H.; Yu, B.B.; Hu, Z. Microstructure, hardness and corrosion resistance of Laser cladding Ni-WC Coating on AlSi5Cu1Mg Surface. Trans. Nonferrous Met. Soc. China 2021, 31, 2716–2728. [Google Scholar] [CrossRef]
- Jiang, S.S.; Liang, L.S.; Shu, F.Y. Optimization of co-based alloy coating process for laser cladding of 45 steel surface. Mater. Rep. 2020, 34, 448–451. [Google Scholar]
- Lu, H.F.; Pan, C.Y.; Tan, E.W.; Wu, S.H.; Ji, H.; Wang, B. Microstructure and properties of laser cladding WC/Ni base alloy composite coating on 45 steel. Heat Treat. Met. 2019, 44, 19–25. [Google Scholar]
- Yang, X.H.; Chen, J.F.; Wang, Z.; Chen, G.Y. Wear and corrosion resistance of Laser Cladding Ni35 powder on 45 steel surface. Hot Work. Technol. 2015, 44, 150–152. [Google Scholar]
- Sun, R.L.; Niu, W.; Li, T.; Jiang, H.F.; Jiang, L.X.; Zhen, H.Q. Tribological properties of NiCrBSi + Ni/MoS2 coatings in space. Spacecr. Environ. Eng. 2017, 34, 533–537. [Google Scholar]
- Torres, H.; Caykara, T.; Rojacz, H.; Prakash, B.; Ripoll, M.R. The tribology of Ag/MoS2 -based self-lubricating laser claddings for high temperature forming of aluminium alloys. Wear 2020, 442–443, 203110. [Google Scholar] [CrossRef]
- Torres, H.; Vuchkov, T.; Rodríguez Ripoll, M.; Prakash, B. Tribological behaviour of MoS2 -based self-lubricating laser cladding for use in high temperature applications. Tribol. Int. 2018, 126, 153–165. [Google Scholar] [CrossRef]
- Qu, C.C.; Li, J.; Juan, Y.F.; Shao, J.Z.; Song, R.; Bai, L.L.; Chen, J.L. Effects of the content of MoS2 on microstructural evolution and wear behaviors of the laser-clad coatings. Surf. Coat. Technol. 2019, 357, 811–821. [Google Scholar] [CrossRef]
- Chai, C. Microstructure and Properties of Laser Cladding Ni35 + SiC Coatings; Xinjiang University: Xinjiang, China, 2021. [Google Scholar]
- Pham, N.T.H.; Nguyen, V.T. Behaviour of TiC Particles on the Co50-Based Coatings by Laser Cladding: Morphological Characteristics and Growth Mechanism. Adv. Mater. Sci. Eng. 2020, 2020, 8462607. [Google Scholar] [CrossRef]
- Ma, S.B.; Su, B.B.; Wang, X.; Xia, Z.W.; Liu, J.; Xu, Y. Wear resistance of SiC/Ni composite coatings based on laser cladding. J. Mater. Eng. 2016, 44, 77–82. [Google Scholar]
- Wang, Z.J.; Wang, Z.Y.; Song, H.W.; Li, W. Study on solidification heat transfer of LASER cladding pool of TC4 titanium alloy. Mach. Des. Manuf. 2018, 10, 85–88. [Google Scholar]
- Li, Z.Q. Study on Process and Microstructure of Sulphuric Acid Corrosion-Resistant Alloy Cladding by Multiple Heat Sources; Lanzhou University of Technology: Lanzhou, China, 2017. [Google Scholar]
- Li, A.N.; Wei, C.L.; Liu, J.X.; Zhou, W.L.; Wang, H.J.; Kuang, S.T. Laser melting iron -based Cr3C2/MoS2 Organization and friction and wear performance. Chin. Surf. Eng. 2015, 28, 77–85. [Google Scholar]
- Yang, Y.F.; Zheng, J.; Di, C.C.; Ma, J.S.; Cui, K.B. PCrMo steel laser melting MoS2 lubrication coating rubbing learning performance Study. Tribology 2016, 2, 14. [Google Scholar]
- Fang, L.Y.; Yan, H.; Yao, Y.S.; Zhang, P.L.; Gao, Q.S.; Qin, Y. Reactive Fabrication and Effect of NbC on Microstructure and Tribological Properties of CrS Co-Based Self-Lubricating Coatings by Laser Cladding. Materials 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Liu, X.B.; He, X.M.; Wang, M.D. Research progress of solid self lubricating coatings. Mater. Rep. 2011, 25, 536–538, 542. [Google Scholar]
- Xue, L. Research on Brinell Hardness Indentation Measurement Technology Based on Machine Vision; Zhongbei University: Zhongbei, Taiyuan, 2020. [Google Scholar]
- Liu, X.G. Simulation Study on Surface Hardness Calculation Method of Micro/Nano Scale Materials; Harbin Institute of Technology: Harbin, China, 2014. [Google Scholar]
- Liu, K.W.; Yan, H.; Zhang, P.L.; Zhao, J.; Yu, Z.S.; Lu, Q.H. Wear Behaviors of TiN/WS2 + hBN/NiCrBSi Self-Lubricating Composite Coatings on TC4 Alloy by Laser Cladding. Coatings 2020, 10, 747. [Google Scholar] [CrossRef]
- He, Y.Y.; Liu, Y.J.; Yang, J.Q.; Xie, F.; Huang, W.Y.; Zhu, Z.W.; Cheng, J.H.; Shi, J.P. Effect of Interlayer Cooling on the Preparation of Ni-Based Coatings on Ductile Iron. Coatings 2020, 10, 544. [Google Scholar] [CrossRef]
- Zhang, W.P.; Liu, S.; Ji, G.H.; Ma, Y.T. Study on grain refinement behavior of laser Cladding cermet fine grain composite coating. J. Mater. Eng. 2004, 8, 12–24. [Google Scholar]
- Zhai, Z.K. In-Situ Growth of Zirconium-Based Doped Molybdenum Composite Nanomaterials and Its Application in Iron Corrosion Prevention; Zhengzhou University: Zhengzhou, China, 2019. [Google Scholar]
- Silva, D.; Lima, L.; Araújo, A.J.M.; Silva, V.D.; Gomes, R.M. The Effect of Microstructural Changes on Mechanical and Electrochemical Corrosion Properties of Duplex Stainless Steel Aged for Short Periods. Materials 2020, 13, 5511. [Google Scholar] [CrossRef]
- Li, D.D.; Wu, Z.L.; Xu, J.N.; Zhang, S.P. Corrosion behavior of QAL10-4-4, QAL9-4 and ZCuAl10Fe3 copper alloys in simulated Marine environment. Corros. Prot. 2016, 37, 7. [Google Scholar]
- Suo, Z.Y.; Wang, X.; Zhang, Z.H. Scour corrosion behavior of Ti-Zr-Be-Cu-Co bulk amorphous alloy in NaCl solution. Mater. Sci. Technol. 2015, 23, 5. [Google Scholar]
- Hao, L.S.; Zheng, F.; Chen, X.; Li, J.; Fan, Y. Erosion Corrosion Behavior of Aluminum in Flowing Deionized Water at Various Temperatures. Materials 2020, 13, 779. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Montoya-Rangel, M.; Cabral-Miramontes, J.A.; Estupian-López, F.; Almeraya-Calderón, F. Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique. Coatings 2020, 10, 521. [Google Scholar] [CrossRef]
- Hu, B. Wear Analysis of Coal Dropping Pipe in Coal Transportation System Based on EDEM Simulation Technology. Jilin Electr. Power 2019, 47, 5–8. [Google Scholar]
- Yun, R.D.; Ding, B. A new contact model considering multi-scale contact states. J. Mech. Eng. 2019, 9, 55. [Google Scholar]
- Chen, W.; Liu, B.; Chen, L.; Xu, J.P.; Zhu, Y.X. Effect of Laser Cladding Stellite 6-Cr3C2-WS2 Self-Lubricating Composite Coating on Wear Resistance and Microstructure of H13. Metals 2020, 10, 785. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, L.; Huang, Z.Y. Microstructure Characteristics and Properties of HVOF Sprayed Ni-Based Alloy Nano-h-BN Self-Lubricating Composite Coatings. Adv. Tribol. 2015, 2015, 621278-1–621278-6. [Google Scholar] [CrossRef]
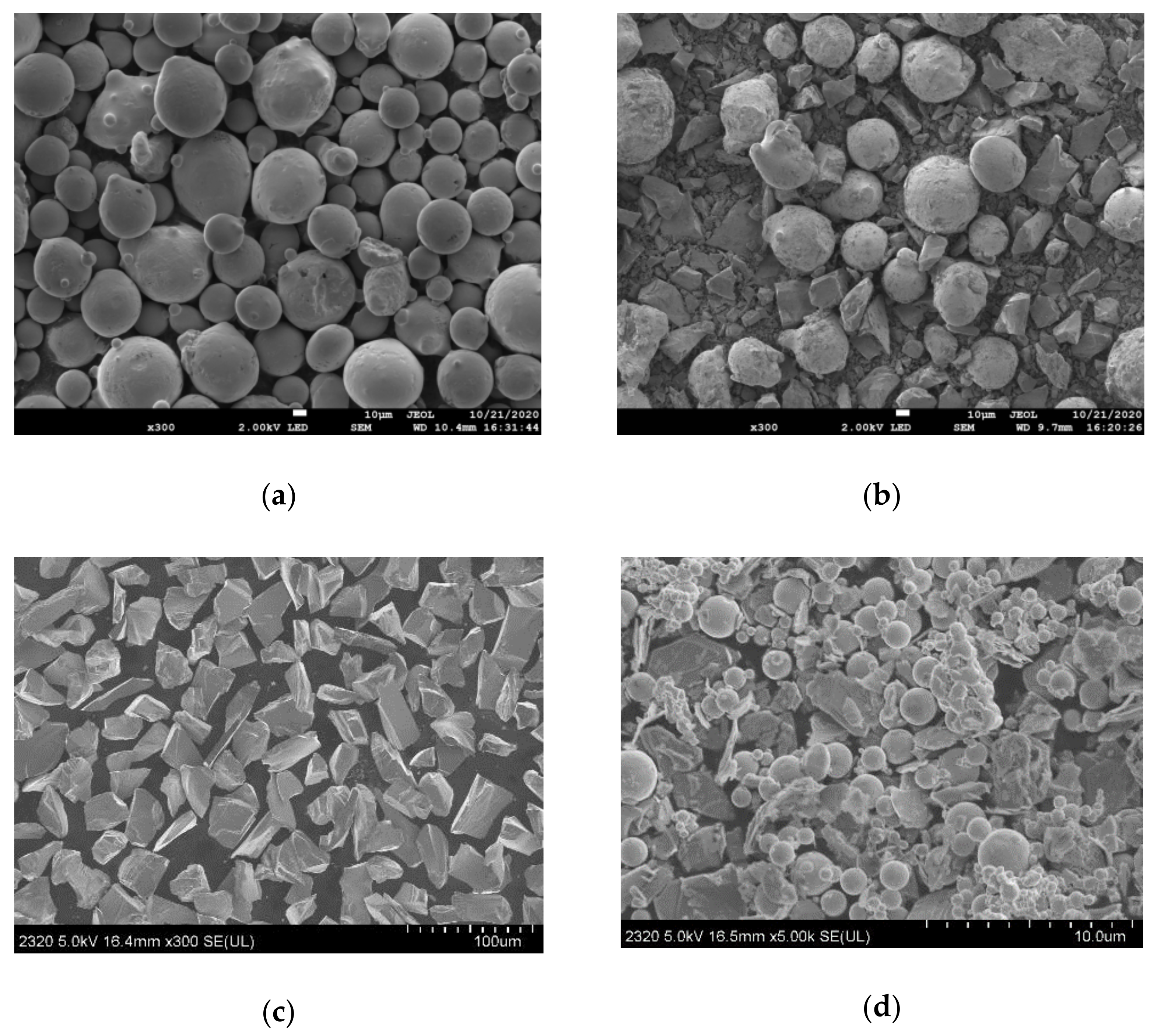
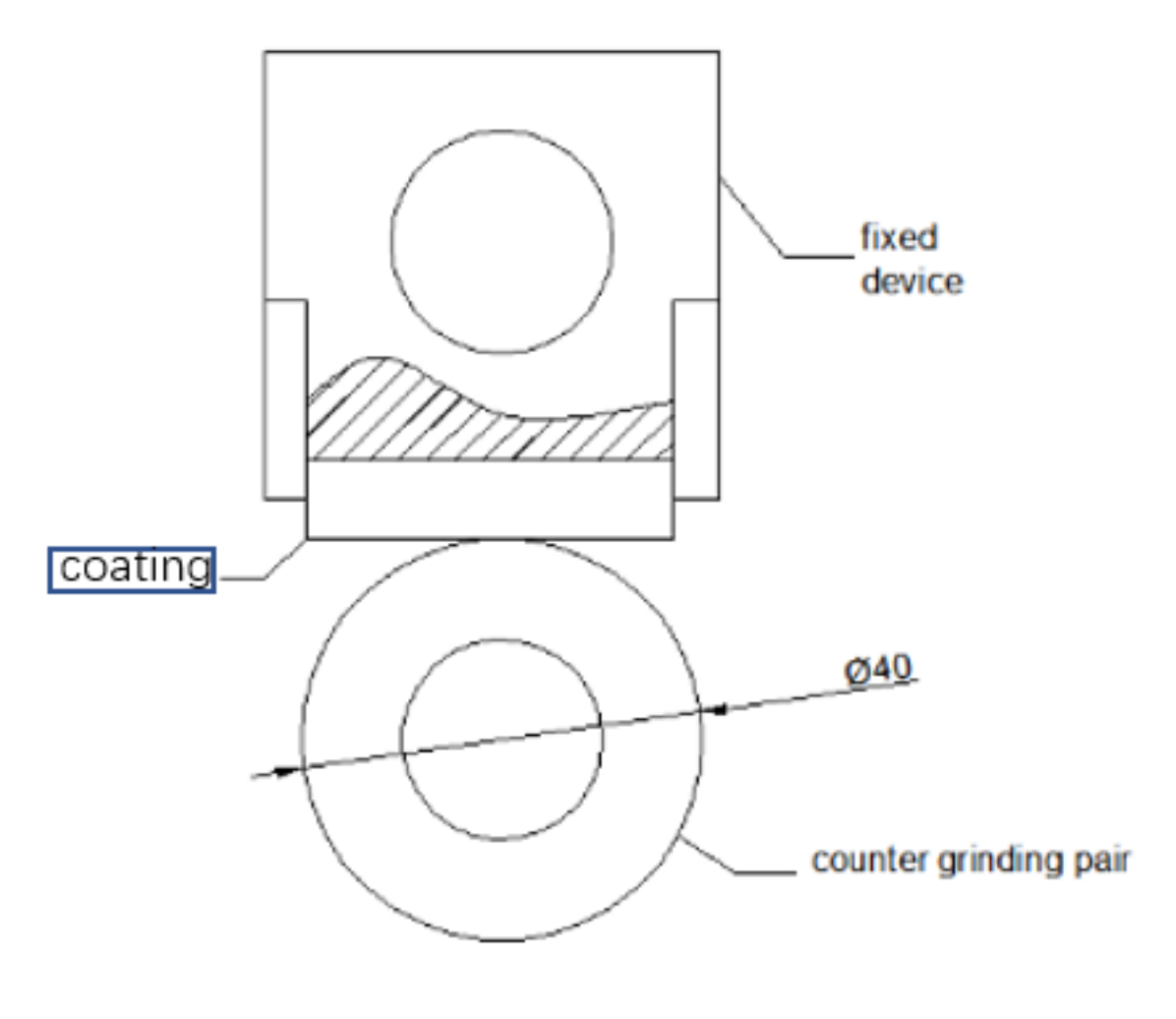
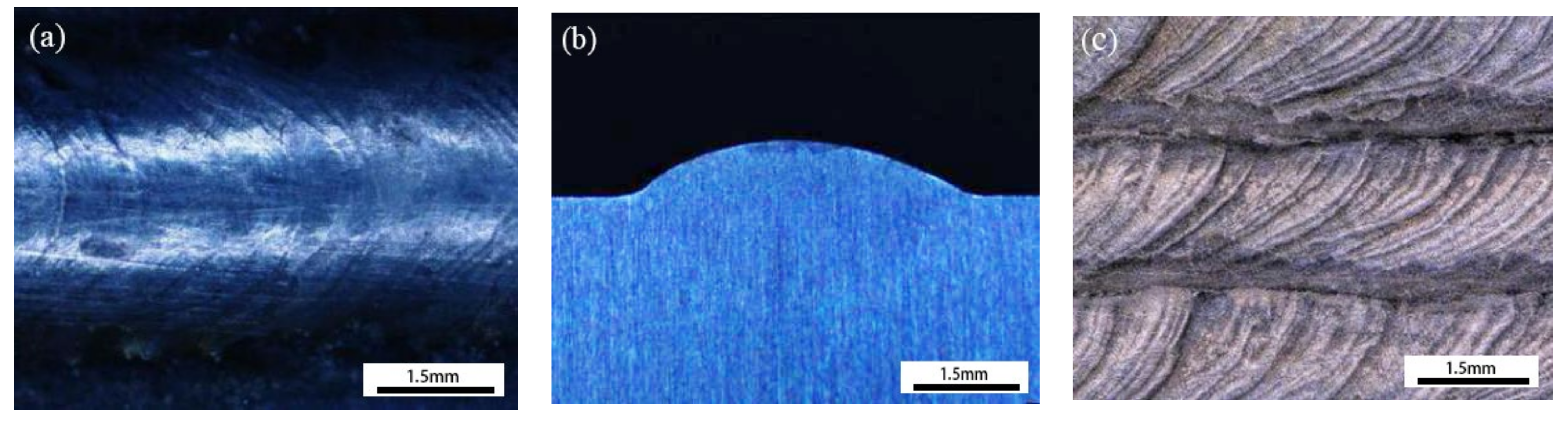
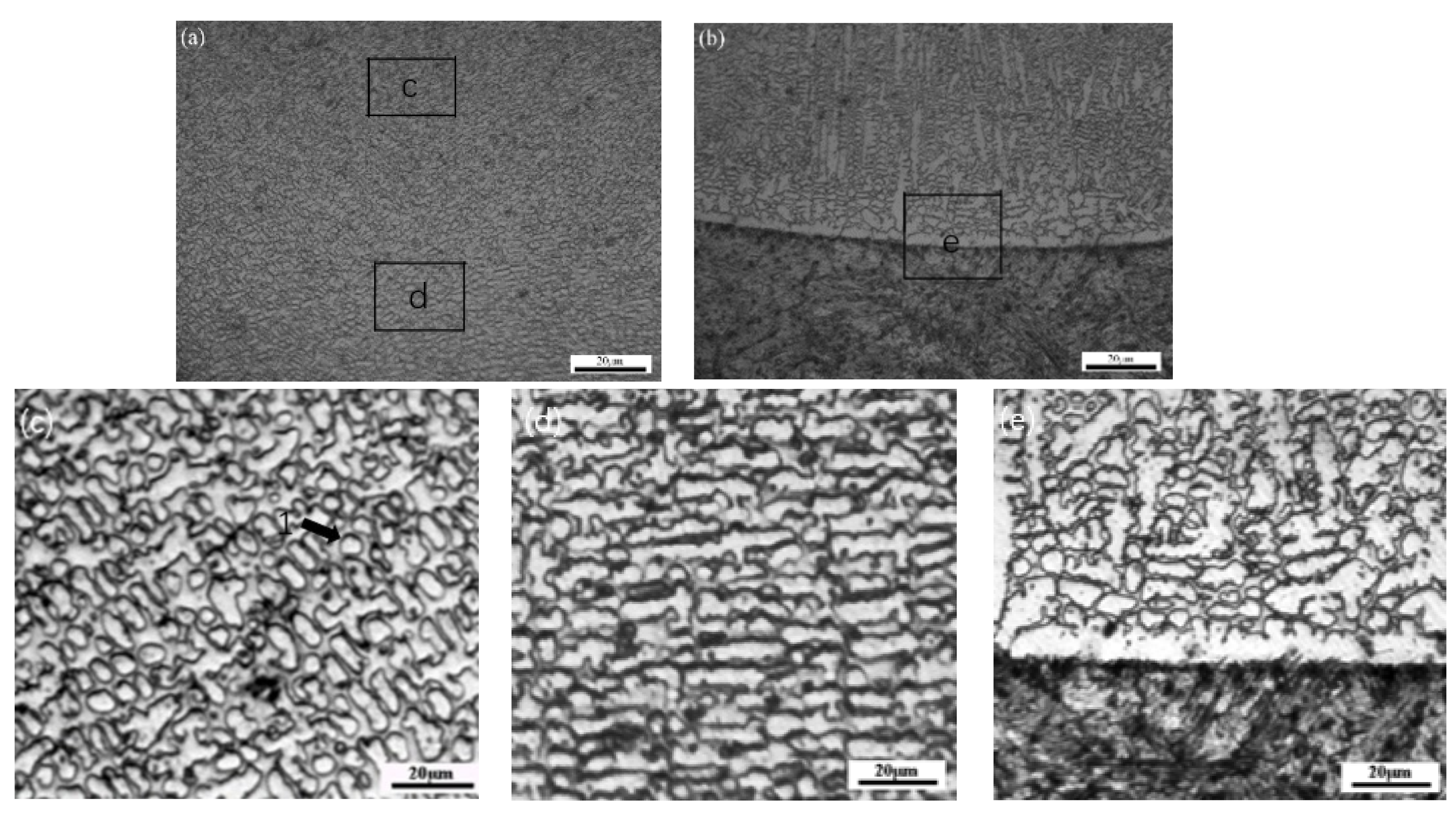



| Element | C | Si | Mn | Cr | Ni | B | Fe |
|---|---|---|---|---|---|---|---|
| grade 45 steel | 0.45 | 0.21 | 0.67 | 0.15 | 0.16 | - | Bal. |
| Ni35 | 0.5 | 3 | 1 | 12 | Bal. | 2 | 10 |
| Parameters | Values |
|---|---|
| Laser power/W | 1000 |
| Scanning speed/(mm/s) | 8 |
| Spot diameter/mm | 2 |
| Focus-deviating/mm | 10 |
| Overlap rate/% | 50% |
| Position | Cr | S | Mo | Fe | Ni |
|---|---|---|---|---|---|
| 1 | 46.67 | 40.15 | 7.02 | 3.42 | 2.52 |
| Material | R1 /Ω·cm2 | Q1 /Ω·cm2·sn | n1 | R2 /Ω·cm2 | Q2 /Ω·cm2·sn | n2 | R3 /Ω·cm2 |
|---|---|---|---|---|---|---|---|
| Grade 45 steel | 6.757 | 0.00504 | 0.5988 | 1.268 | 0.0132 | 0.6539 | 615 |
| Ni35 + 20% SiC | 4.196 | 0.00123 | 0.6362 | 26.97 | 0.00129 | 0.6189 | 221.0 |
| Ni35 + 20% SiC + 20% Ni/MoS2 | 4.61 | 0.007866 | 0.662 | 2.134 | 0.001972 | 1 | 2000.5 |
| Sample Mass Loss | First/mg | Second/mg | Third/mg | Average/mg |
|---|---|---|---|---|
| substrate | 19.15 | 19.12 | 19.03 | 19.10 |
| Ni35 + 20% SiC | 4.88 | 4.92 | 4.90 | 4.90 |
| Ni35 + 20% SiC + 20% Ni/MoS2 | 3.19 | 3.18 | 3.23 | 3.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Yang, X.; Li, X.; Chai, C.; Liu, W. Study on Microstructure and Properties of Nickel-Based Self-Lubricating Coating by Laser Cladding. Coatings 2022, 12, 753. https://doi.org/10.3390/coatings12060753
Chen W, Yang X, Li X, Chai C, Liu W. Study on Microstructure and Properties of Nickel-Based Self-Lubricating Coating by Laser Cladding. Coatings. 2022; 12(6):753. https://doi.org/10.3390/coatings12060753
Chicago/Turabian StyleChen, Wenjie, Xianchen Yang, Xinmei Li, Cheng Chai, and Weibin Liu. 2022. "Study on Microstructure and Properties of Nickel-Based Self-Lubricating Coating by Laser Cladding" Coatings 12, no. 6: 753. https://doi.org/10.3390/coatings12060753
APA StyleChen, W., Yang, X., Li, X., Chai, C., & Liu, W. (2022). Study on Microstructure and Properties of Nickel-Based Self-Lubricating Coating by Laser Cladding. Coatings, 12(6), 753. https://doi.org/10.3390/coatings12060753





