Application of SiON Coatings in Sandstone Artifacts Conservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Protection Performance Tests
2.3.1. Porosity Test
2.3.2. Waterproofing Test
2.3.3. Test of Water Vapor Permeability
2.3.4. Test of Weather Resistance
3. Results and Discussion
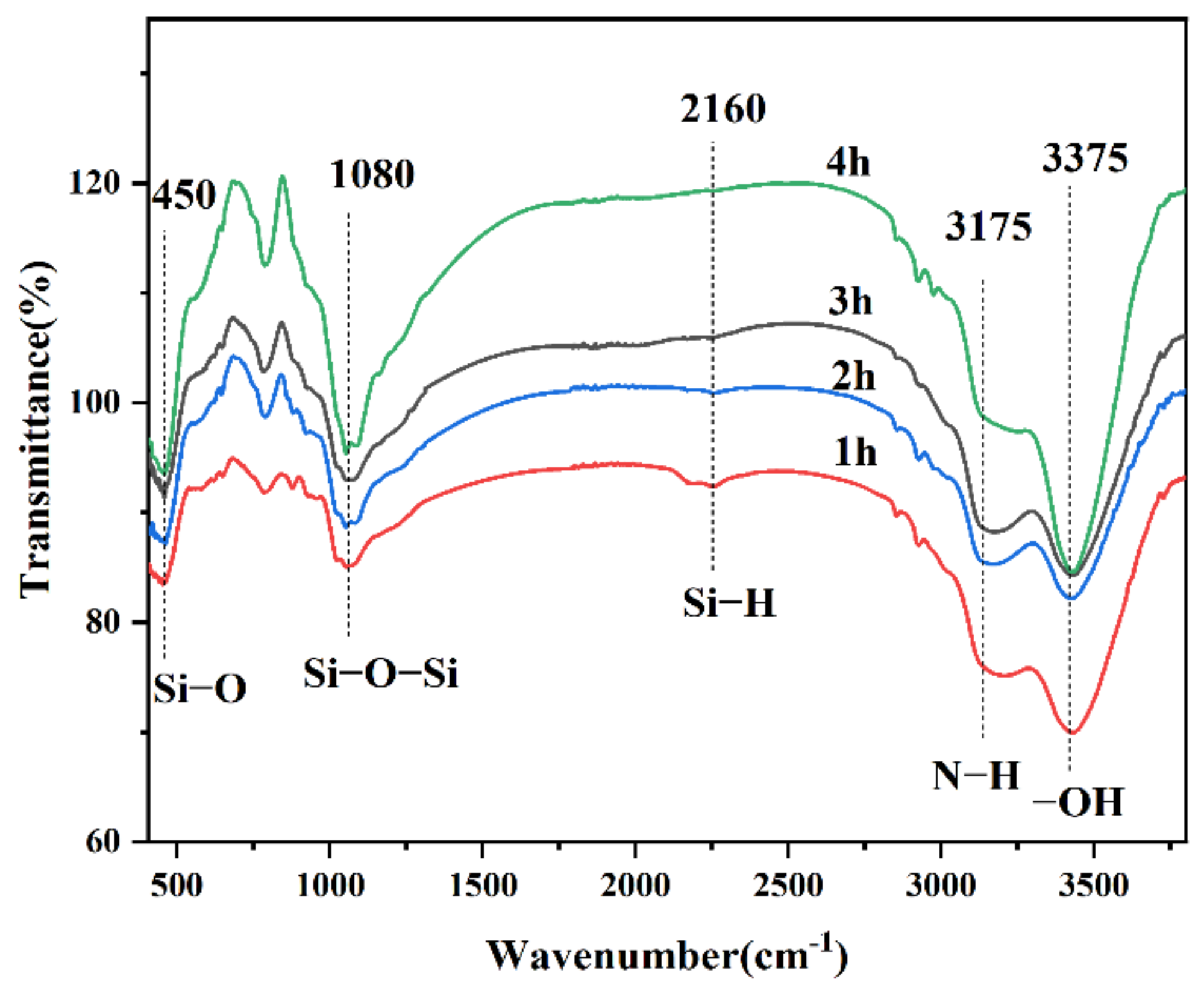
3.1. Fourier Transform Infrared Absorption Spectrum Analysis
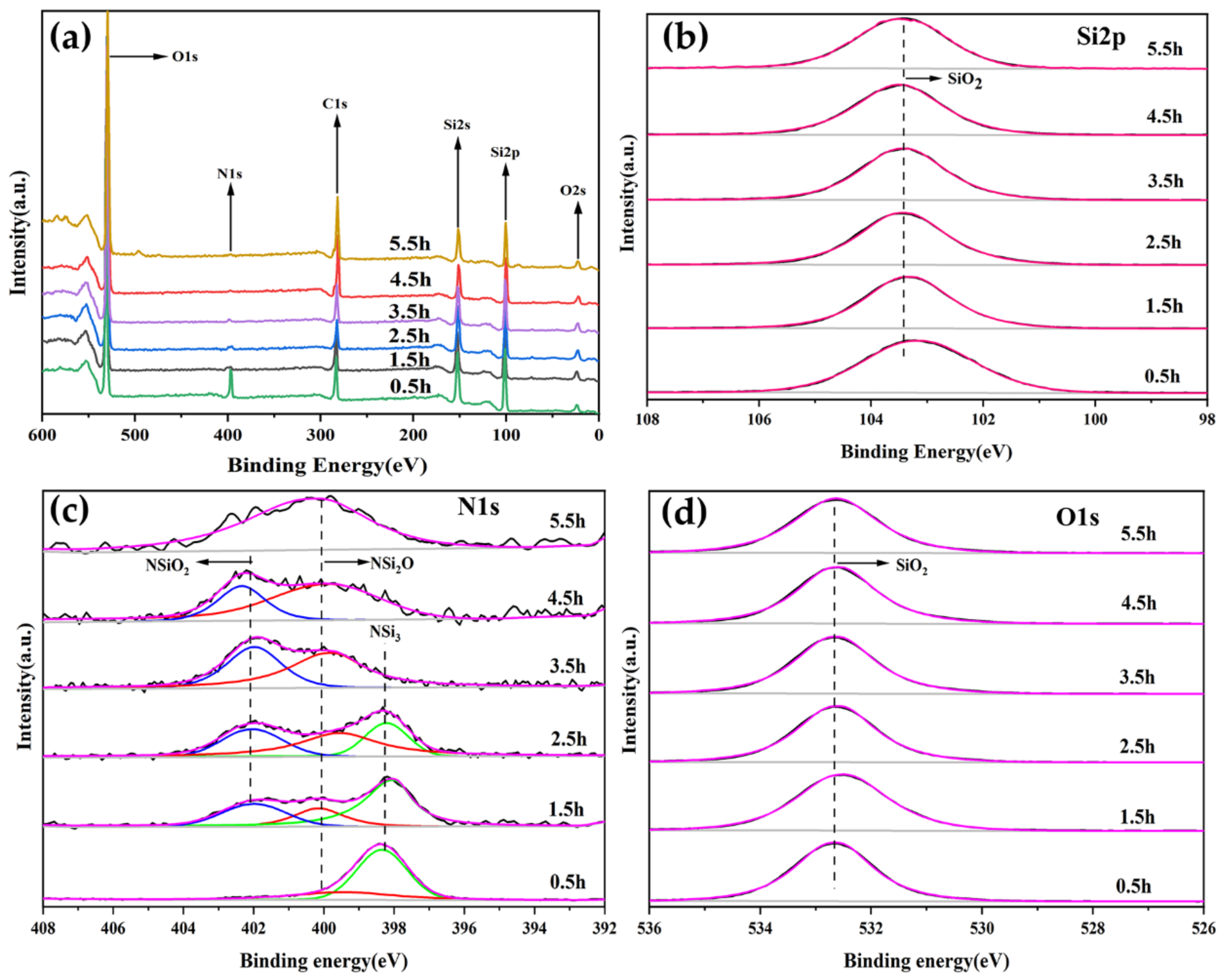
3.2. XPS Survey and Core Level Studies
3.3. Color Changes
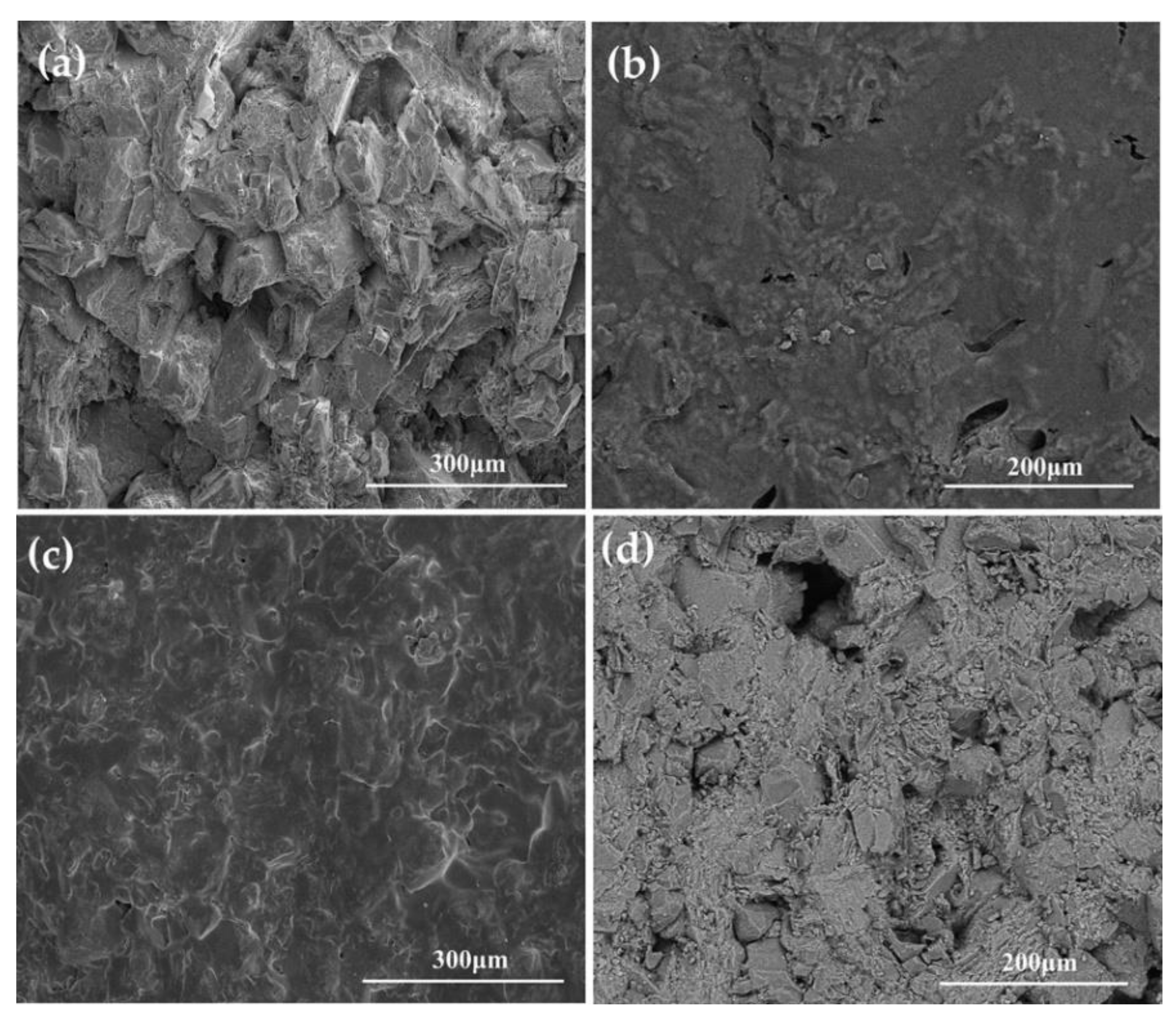
3.4. Micromorphological Analysis
3.5. Water Vapor Permeability Results
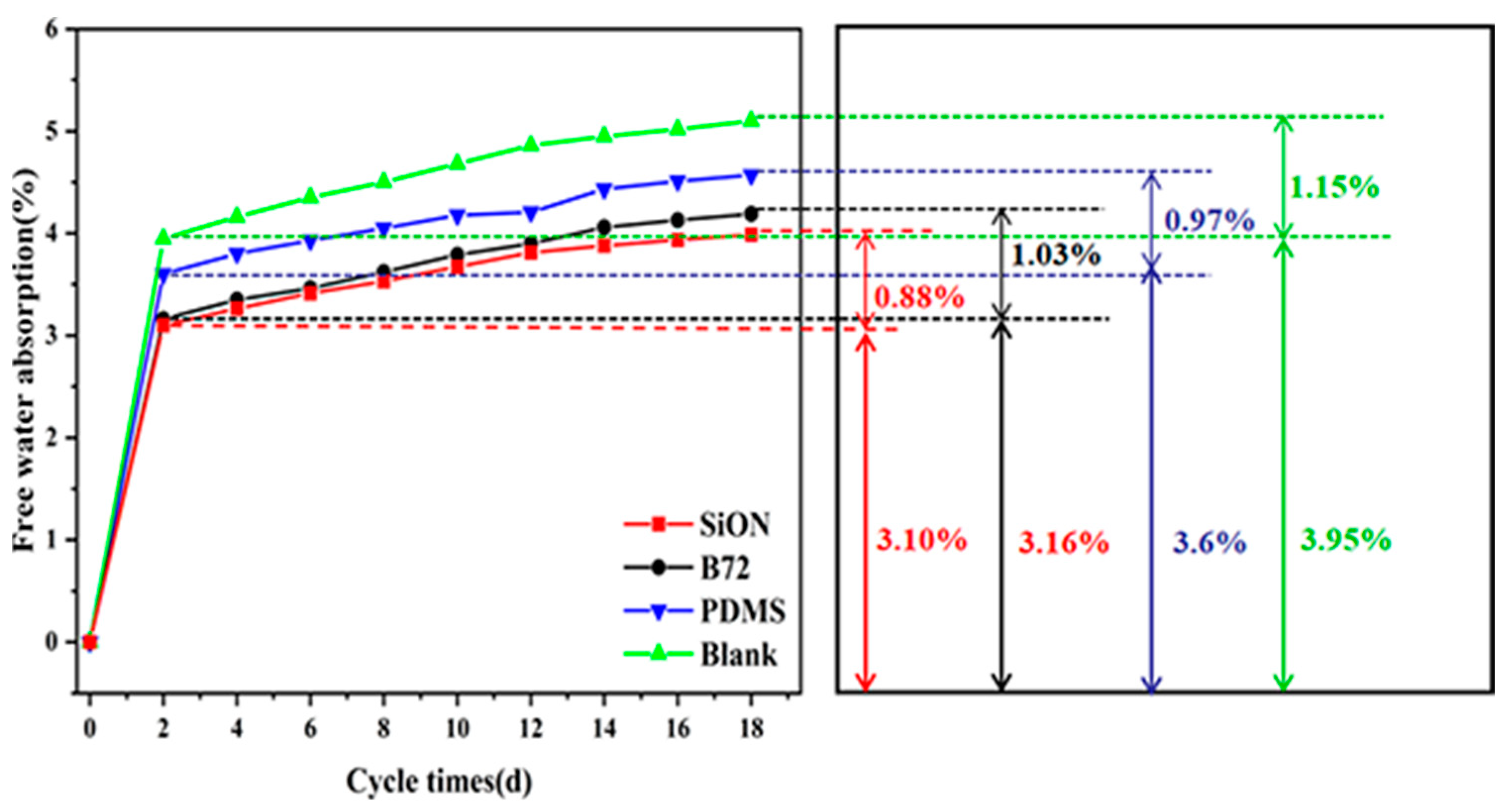
3.6. Water Repellency Analysis
3.7. Evaluation of Anti−Salt Damage for Sandstone
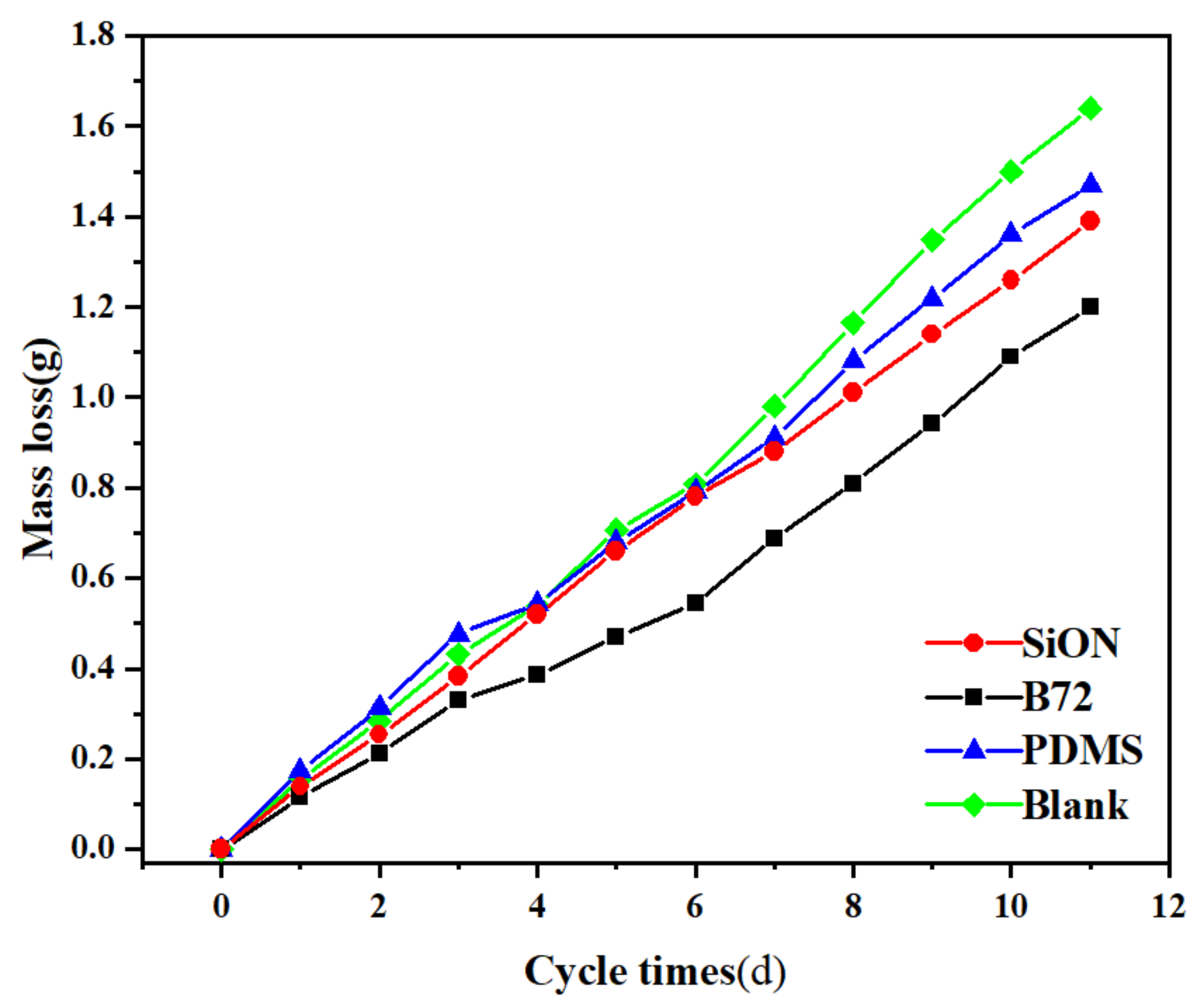
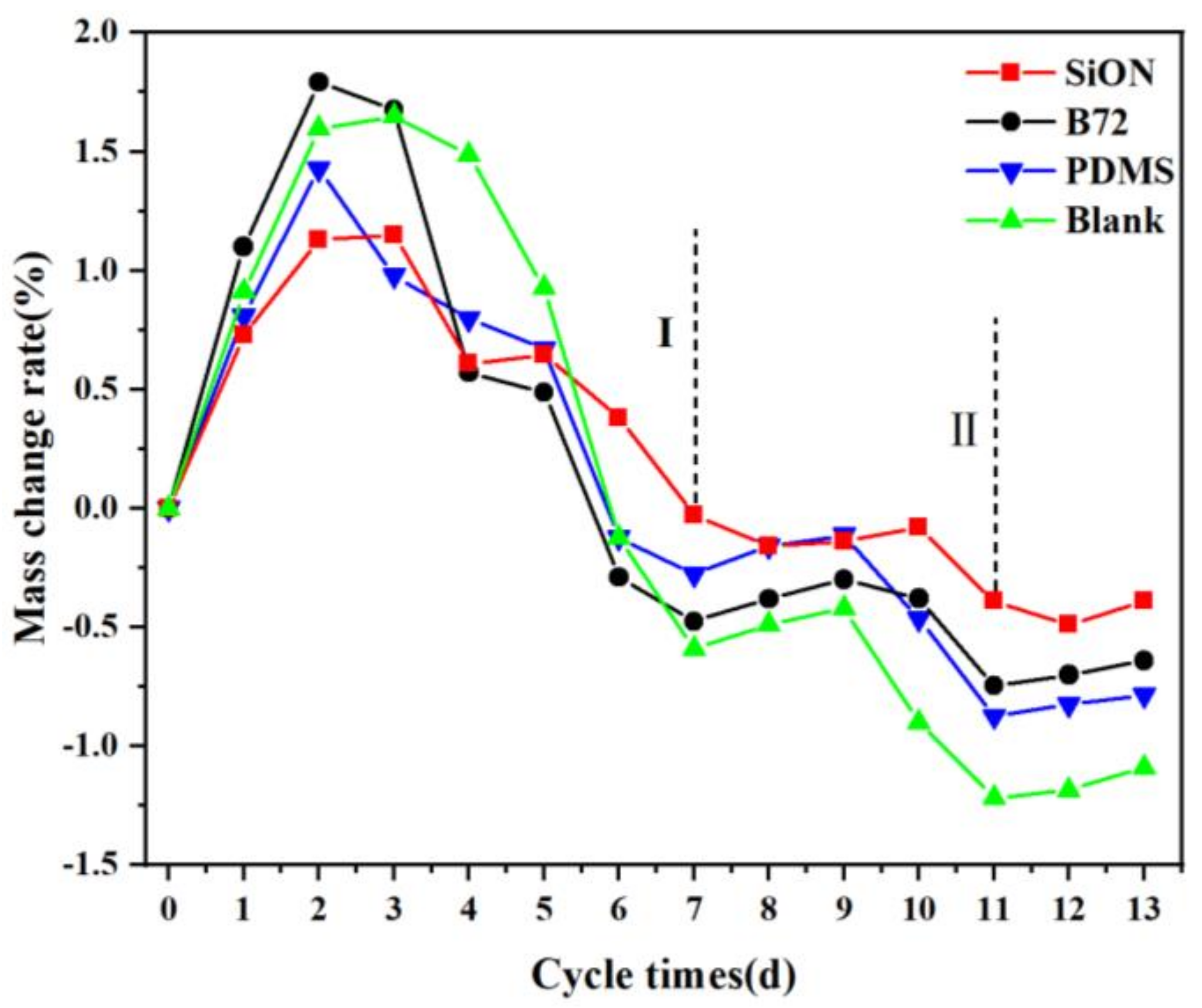
3.7.1. Mass Change in Salt Weathering Test
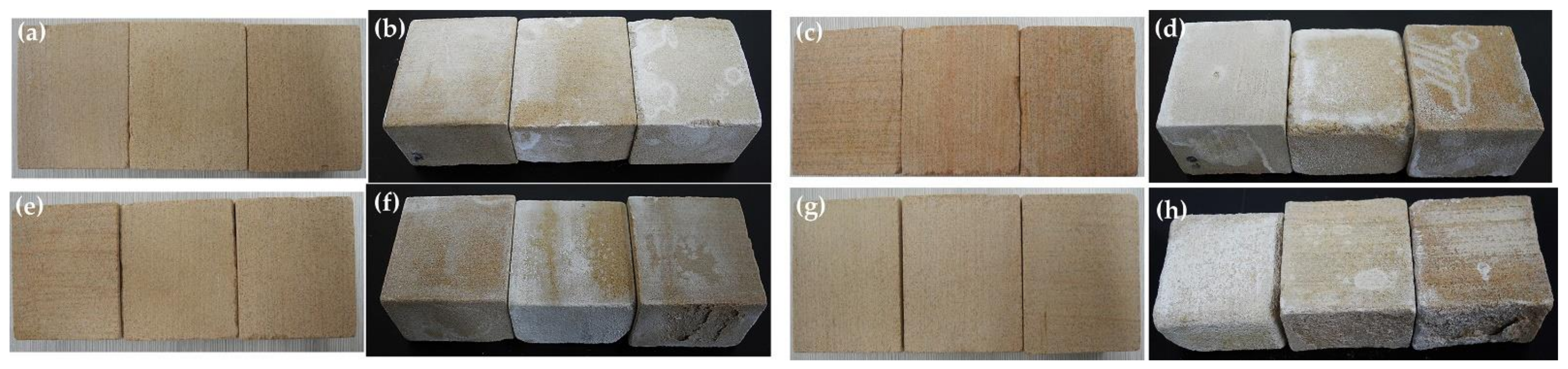
3.7.2. Appearance of Sandstone
3.7.3. SEM Analysis of Sandstone after Salt Weathering
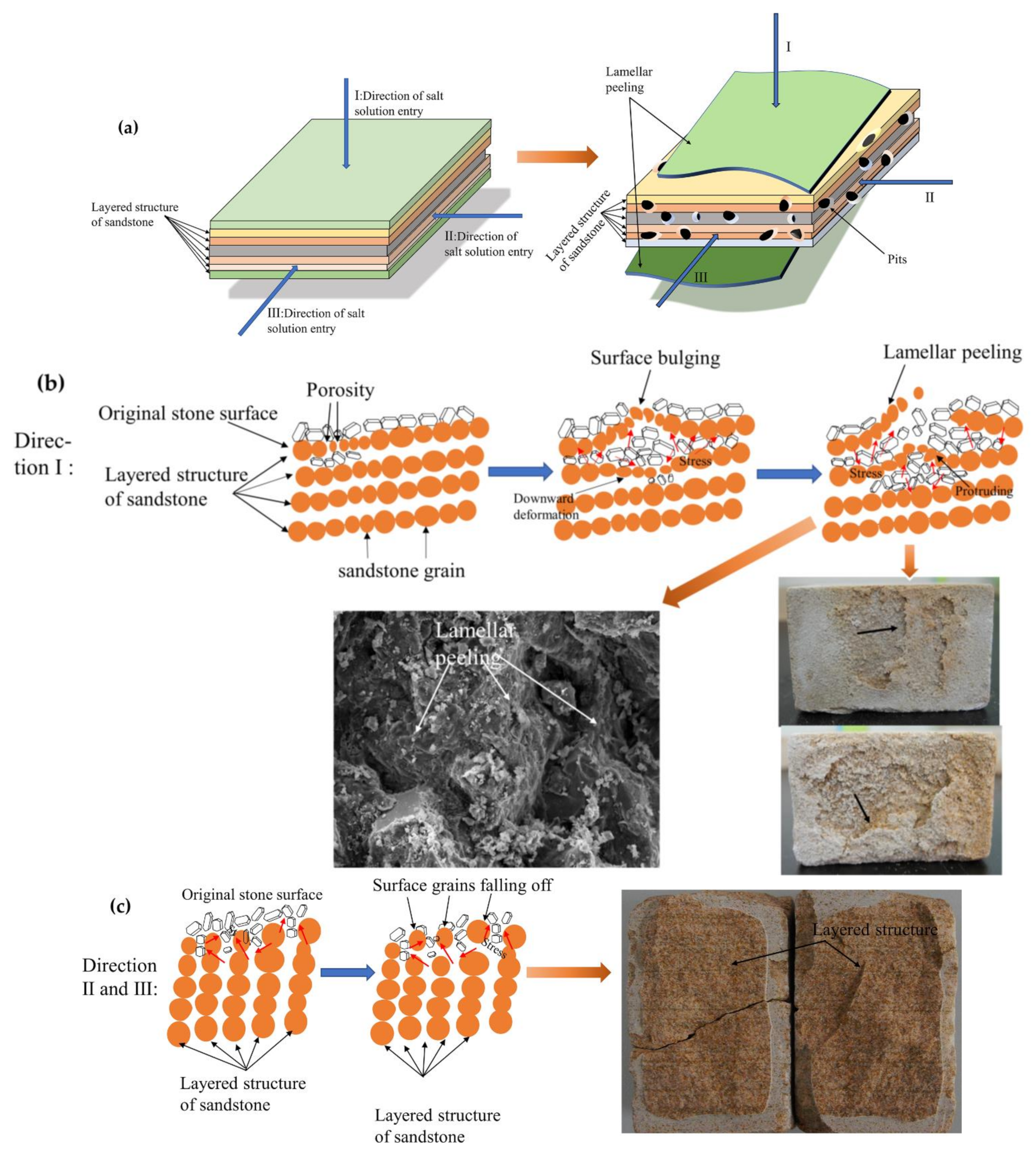
3.7.4. Analysis of Sandstone Laminar Exfoliation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Zhang, B.J. A New Method on Laboratory Evaluation of the Effectiveness of Consolidant Treatments for the Conservation of Historic Stone Buildings and Sculptures. Adv. Mater. Res. 2010, 108–111, 184–189. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Morelli, A.; Pipoli, M.; Frigione, M. Oleo/hydrophobic coatings containing nano-particles for the protection of stone materials having different porosity. Coatings 2018, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and Future Perspectives for Biocides and Antifouling Products for Stone−Built Cultural Heritage: Ionic Liquids as a Challenging Alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Turkington, A.V. Stone decay in the architectural environment. Geol. Soc. Am. 2005, 390, 39–49. [Google Scholar] [CrossRef]
- Thomas, D.S.G. Arid Zone Geomorphology: Process, form and Change in Drylands; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Smith, B.J.; Turkington, A. Stone Decay: Its Causes and Controls; Routledge: Abingdon, UK, 2016. [Google Scholar]
- Wang, Y.H.; Viles, H.; Desarnaud, J.; Yang, S.L.; Guo, Q.L. Laboratory simulation of salt weathering under moderate ageing conditions: Implications for the deterioration of sandstone heritage in temperate climates. Earth Surf. Processes Landf. 2021, 46, 1055–1066. [Google Scholar] [CrossRef]
- Jia, M.; Liang, J.; He, L.; Zhao, X.; Simon, S. Hydrophobic and hydrophilic SiO2−based hybrids in the protection of sandstone for anti−salt damage. J. Cult. Herit. 2019, 40, 80–91. [Google Scholar] [CrossRef]
- Çelik, M.Y.; Aygün, A. The effect of salt crystallization on degradation of volcanic building stones by sodium sulfates and sodium chlorides. Bull. Eng. Geol. Environ. 2018, 78, 3509–3529. [Google Scholar] [CrossRef]
- Manohar, S.; Bala, K.; Santhanam, M.; Menon, A. Characteristics and deterioration mechanisms in coral stones used in a historical monument in a saline environment. Constr. Build. Mater. 2020, 241, 118102. [Google Scholar] [CrossRef]
- Derluyn, H.; Vontobel, P.; Mannes, D.; Derome, D.; Lehmann, E.; Carmeliet, J. Saline Water Evaporation and Crystallization−Induced Deformations in Building Stone: Insights from High−Resolution Neutron Radiography. Transp. Porous Med. 2018, 128, 895–913. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M. Novel attribute of organic–inorganic hybrid coatings for protection and preservation of materials (stone and wood) belonging to cultural heritage. Coatings 2018, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, K.; Subashini, B.C.; Venkatakrishnan, B.; Ramesh, A.S. Comparison of dimensional stability of die stone and die silicone: An In Vitro comparative study. J. Interdiscip. Dent. 2018, 8, 92–95. [Google Scholar] [CrossRef]
- Tesser, E.; Antonelli, F. Evaluation of silicone based products used in the past as today for the consolidation of venetian monumental stone surfaces. Mediterr. Archaeol. Archaeom. 2018, 18, 159–170. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Frigione, M. Influence of stone particles on the rheological behavior of a novel photopolymerizable siloxane−modified acrylic resin. J. Appl. Polym. Sci. 2011, 122, 942–947. [Google Scholar] [CrossRef]
- Negri, A.; Nervo, M.; Di Marcello, S.; Castelli, D. Consolidation and Adhesion of Pictorial Layers on a Stone Substrate. The Study Case of the Virgin with the Child from Palazzo Madama, in Turin. Coatings 2021, 11, 624. [Google Scholar] [CrossRef]
- Ling, H.; Maiqian, N.; Guozheng, L. Preparation and feasibility analysis of fluoropolymer to the sandstone protection. Prog. Org. Coat. 2008, 62, 206–213. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; La Russa, M.F. Nanostructured Coatings for Stone Protection: An Overview. Front. Mater. 2019, 6, 147. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Liu, T.; Zhang, B. The preservation damage of hydrophobic polymer coating materials in conservation of stone relics. Prog. Org. Coat. 2013, 76, 1127–1134. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, B.J. Assessment of damage from organic protective coating treatments to historic stone buildings and sculptures. Appl. Mech. Mater. 2011, 44, 610–613. [Google Scholar] [CrossRef]
- Ershad−Langroudi, A.; Fadaei, H.; Ahmadi, K. Application of polymer coatings and nanoparticles in consolidation and hydrophobic treatment of stone monuments. Iran. Polym. J. 2018, 28, 1–19. [Google Scholar] [CrossRef]
- Terlikowski, W.; Gregoriou, M.; Kedzierski, M.; Sobczynska, E.; Wasilewski, K. Polymeric materials used in the preservation of historical stone buildings. Polimery 2020, 65, 289–296. [Google Scholar] [CrossRef]
- Xu, F.; Zeng, W.; Li, D. Recent advance in alkoxysilane−based consolidants for stone. Prog. Org. Coat. 2019, 127, 45–54. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Evaluation of preventive performance of kaolin and calcium hydroxide nanocomposites in strengthening the outdoor carved limestone. Archaeol. Anthrop. Sci. 2018, 11, 3389–3405. [Google Scholar] [CrossRef] [Green Version]
- Della Volpe, C.; Penati, A.; Peruzzi, R.; Siboni, S.; Toniolo, L.; Colombo, C. The combined effect of roughness and heterogeneity on contact angles: The case of polymer coating for stone protection. J. Adhes. Sci. Technol. 2000, 14, 273–299. [Google Scholar] [CrossRef]
- Skoda, D.; Pucalikova, R.; Kuritka, I.; Kroftova, K. Paraloid B72 Nanodispersion Preparation Technology and Its Possibilities for Use in the Monument Care. Civ. Eng. J.—Staveb. Obz. 2018, 27, 31. [Google Scholar] [CrossRef]
- Baalousha, M.; Yang, Y.; Vance, M.E.; Colman, B.P.; McNeal, S.; Xu, J.; Blaszczak, J.; Steele, M.; Bernhardt, E.; Hochella, M.F., Jr. Outdoor urban nanomaterials: The emergence of a new, integrated, and critical field of study. Sci. Total Environ. 2016, 557–558, 740–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrini, D.; Bartolozzi, G.; Bracci, S.; Carlesi, S.; Cucci, C.; Picollo, M. Evaluation of the efficacy and durability of the “barium hydroxide method” after 40 years. Multi−analytical survey on the Crocifissione by Beato Angelico. J. Cult. Herit. 2020, 45, 362–369. [Google Scholar] [CrossRef]
- Arizzi, A.; Gomez−Villalba, L.S.; Lopez−Arce, P.; Cultrone, G.; Fort, R. Lime mortar consolidation with nanostructured calcium hydroxide dispersions: The efficacy of different consolidating products for heritage conservation. Eur. J. Mineral. 2015, 27, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez−Navarro, C.; Vettori, I.; Ruiz−Agudo, E. Kinetics and Mechanism of Calcium Hydroxide Conversion into Calcium Alkoxides: Implications in Heritage Conservation Using Nanolimes. Langmuir 2016, 32, 5183–5194. [Google Scholar] [CrossRef]
- Funayama, O.; Tashiro, Y.; Kamo, A.; Okumura, M.; Isoda, T. Conversion mechanism of perhydropolysilazane into silicon nitride−based ceramics. J. Mater. Sci. 2004, 29, 4883–4888. [Google Scholar] [CrossRef]
- Back, H.S.; Kim, M.J.; Baek, J.J.; Kim, D.H.; Shin, G.; Choi, K.H.; Cho, J.H. Intense−pulsed−UV−converted perhydropolysilazane gate dielectrics for organic field-effect transistors and logic gates. RSC Adv. 2019, 9, 3169–3175. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ahn, H.-Y.; Han, J.S.; Ju, B.-K.; Lee, S.Y.; Jang, H.S.; Byun, J.Y.; Cho, S.-H. Luminescent silica films prepared using perhydropolysilazane and Mn-doped ZnS nanophosphors. Appl. Surf. Sci. 2020, 511, 145441. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.H.; Kim, J.H.; Park, K.; Jang, J.S.; Park, J.; Park, N. Inorganic Encapsulation Method Using Solution−Processible Polysilazane for Flexible Solar Cells. ACS Appl. Energy Mater. 2020, 3, 9257–9263. [Google Scholar] [CrossRef]
- Wang, G.; Raji, A.-R.O.; Lee, J.-H.; Tour, J.M. Conducting-Interlayer SiOx Memory Devices on Rigid and Flexible Substrates. ACS Nano 2014, 8, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.P.; Qing, H.L.; Shu, H.; Liu, Q. Evaluating the Protective Effects of Calcium Carbonate Coating on Sandstone Cultural Heritage. Coatings 2021, 11, 1534. [Google Scholar] [CrossRef]
- Morlier, A.; Cros, S.; Garandet, J.-P.; Alberola, N. Thin gas−barrier silica layers from perhydropolysilazane obtained through low temperature curings: A comparative study. Thin Solid Film. 2012, 524, 62–66. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, Z.; Luo, Y.; An, P.; Zhang, M.; Xu, C. Hydrophobic, transparent and hard silicon oxynitride coating from perhydropolysilazane. Polym. Int. 2015, 64, 971–978. [Google Scholar] [CrossRef]
- Smokovych, I.; Krüger, M.; Scheffler, M. Polymer derived ceramic materials from Si, B and MoSiB filler-loaded perhydropolysilazane precursor for oxidation protection. J. Eur. Ceram. Soc. 2019, 39, 3634–3642. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Guo, Y.; Jiang, L.; Zhang, Z.; Xu, C. Resistance Switching Behavior of a Perhydropolysilazane−Derived SiOx−Based Memristor. J. Phys. Chem. Lett. 2021, 12, 10728–10734. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.W.; Zuo, G.F.; Zhang, R.X.; Wei, G.F.; Ma, Q. Protection of the surface weathering stone artworks by a chemical conversion method. Constr. Build. Mater. 2018, 182, 210–214. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Frigione, M. Durability to simulated bird guano of nano−filled oleo/hydrophobic coatings for the protection of stone materials. Prog. Org. Coat. 2020, 148, 105900. [Google Scholar] [CrossRef]
- Bajo, J.M.; Delrieux, C.; Patow, G. Physically inspired technique for modeling wet absorbent materials. Vis. Comput. 2020, 37, 2053–2068. [Google Scholar] [CrossRef]
- Yu, J.; Yao, W.; Duan, K.; Liu, X.; Zhu, Y. Experimental study and discrete element method modeling of compression and permeability behaviors of weakly anisotropic sandstones. Int. J. Rock Mech. Min. Sci. 2020, 134, 104437. [Google Scholar] [CrossRef]
- Verri, I.; Della Torre, A.; Montenegro, G.; Onorati, A.; Duca, S.; Mora, C.A.; Radaelli, F.; Trombin, G. Development of a Digital Rock Physics workflow for the analysis of sandstones and tight rocks. J. Petrol. Sci. Eng. 2017, 156, 790–800. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Doehne, E.; Sebastian, E. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cem. Concr. Res. 2000, 30, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Navarro, C.; Doehne, E. Salt weathering: Influence of evaporation rate, supersaturation and crystallization pattern. Earth Surf. Processes Landf. 1999, 24, 191–209. [Google Scholar] [CrossRef]
- Linnow, K.; Zeunert, A.; Steiger, M. Investigation of sodium sulfate phase transitions in a porous material using humidity−and temperature−controlled X-ray diffraction. Anal. Chem. 2006, 78, 4683–4689. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Li, J.; Zhao, G.; Wang, C.; Wang, Y.; Zhou, P. Experimental investigations on the durability and degradation mechanism of cast-in-situ recycled aggregate concrete under chemical sulfate attack. Constr. Build. Mater. 2021, 297, 123771. [Google Scholar] [CrossRef]
- Cooke, R.U. Laboratory simulation of salt weathering processes in arid environments. Earth Surf. Processes 1979, 4, 347–359. [Google Scholar] [CrossRef]



| SiON’s Pyrolysis Time (h) | Atomic Concentration (%) | Concentration of Different Chemical States of Binding Energy N (1s) (%) | |||
|---|---|---|---|---|---|
| N (1S) | O (1S) | NSi3 (400 ev) | NSi2O (400 ev) | NSiO2 (402 ev) | |
| 0.5 | 22.71 | 59.74 | 16.77 | 5.94 | 0 |
| 1.5 | 8.26 | 67.85 | 2.28 | 3.62 | 2.36 |
| 2.5 | 6.57 | 68.05 | 3.58 | 1.18 | 1.81 |
| 3.5 | 5.65 | 67.89 | 0 | 3.34 | 2.31 |
| 4.5 | 4.16 | 70.93 | 0 | 3.02 | 1.14 |
| 5.5 | 3.38 | 69.1 | 0 | 3.38 | 0 |
| Color Data | Samples | Treatment | |
|---|---|---|---|
| Without Coating | With Coating | ||
| L | SiON | 65.04 ± 0.42 | 63.64 ± 0.35 |
| B72 | 61.81 ± 0.43 | 57.67 ± 0.51 | |
| PDMS | 64.03 ± 0.63 | 62.90 ± 0.48 | |
| a | SiON | 7.45 ± 0.13 | 8.29 ± 0.08 |
| B72 | 8.53 ± 0.12 | 9.61 ± 0.15 | |
| PDMS | 7.54 ± 0.17 | 7.85 ± 0.17 | |
| b | SiON | 13.38 ± 0.56 | 13.26 ± 0.51 |
| B72 | 13.87 ± 0.82 | 16.34 ± 0.93 | |
| PDMS | 12.9 ± 0.57 | 12.68 ± 0.46 | |
| ΔEab | SiON | —— | 1.63 ± 0.40 |
| B72 | —— | 4.93 ± 0.35 | |
| PDMS | —— | 1.19 ± 0.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.; Wen, Y.; Liu, Q. Application of SiON Coatings in Sandstone Artifacts Conservation. Coatings 2022, 12, 748. https://doi.org/10.3390/coatings12060748
Qin H, Wen Y, Liu Q. Application of SiON Coatings in Sandstone Artifacts Conservation. Coatings. 2022; 12(6):748. https://doi.org/10.3390/coatings12060748
Chicago/Turabian StyleQin, Huoliang, Yaping Wen, and Qiang Liu. 2022. "Application of SiON Coatings in Sandstone Artifacts Conservation" Coatings 12, no. 6: 748. https://doi.org/10.3390/coatings12060748
APA StyleQin, H., Wen, Y., & Liu, Q. (2022). Application of SiON Coatings in Sandstone Artifacts Conservation. Coatings, 12(6), 748. https://doi.org/10.3390/coatings12060748






