Evaluation of the Crystal Structure and Mechanical Properties of Cu Doped TiN Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Films
2.2. Calculation Model and Method
3. Results and Discussion
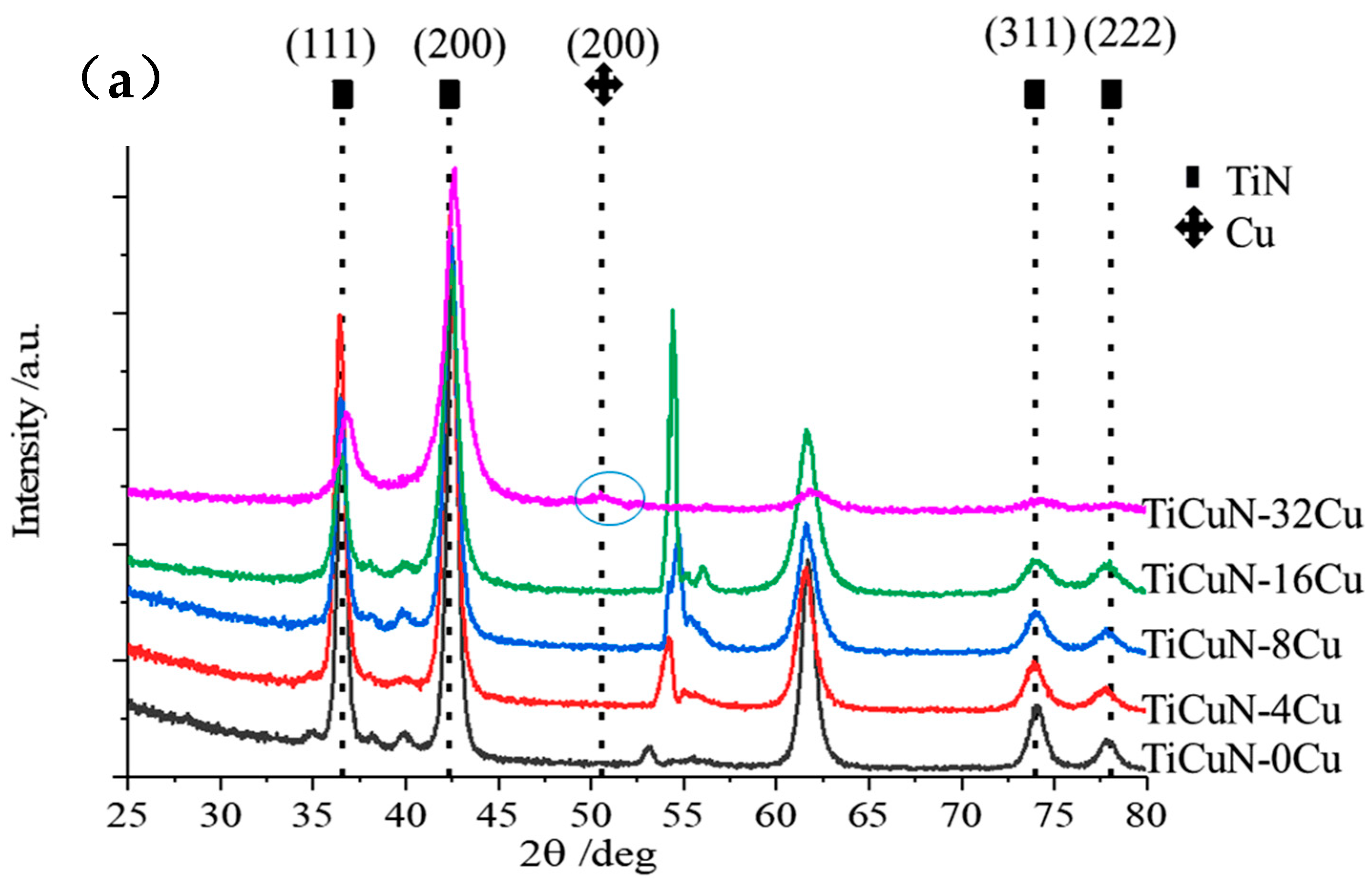
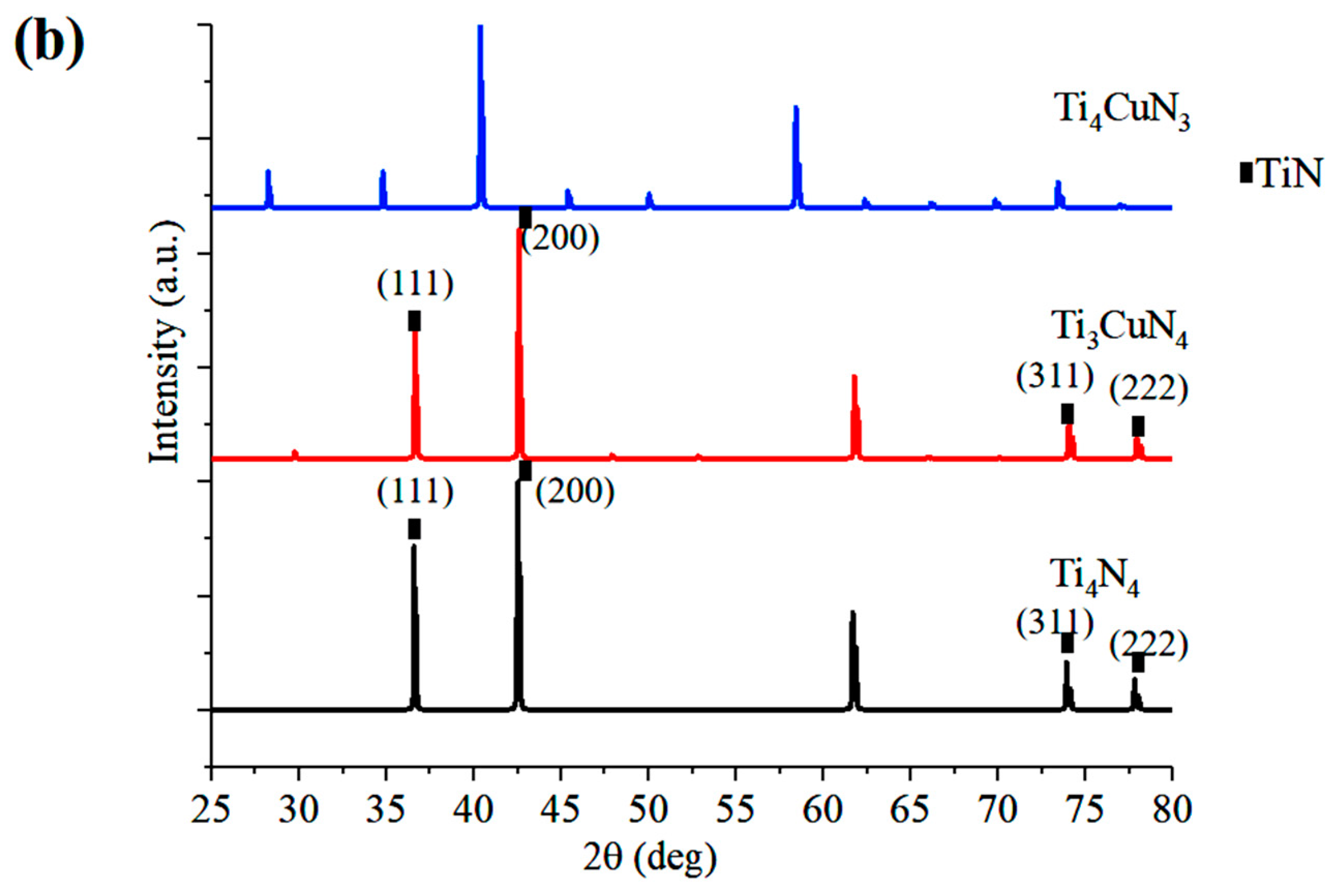
3.1. EDS and XRD
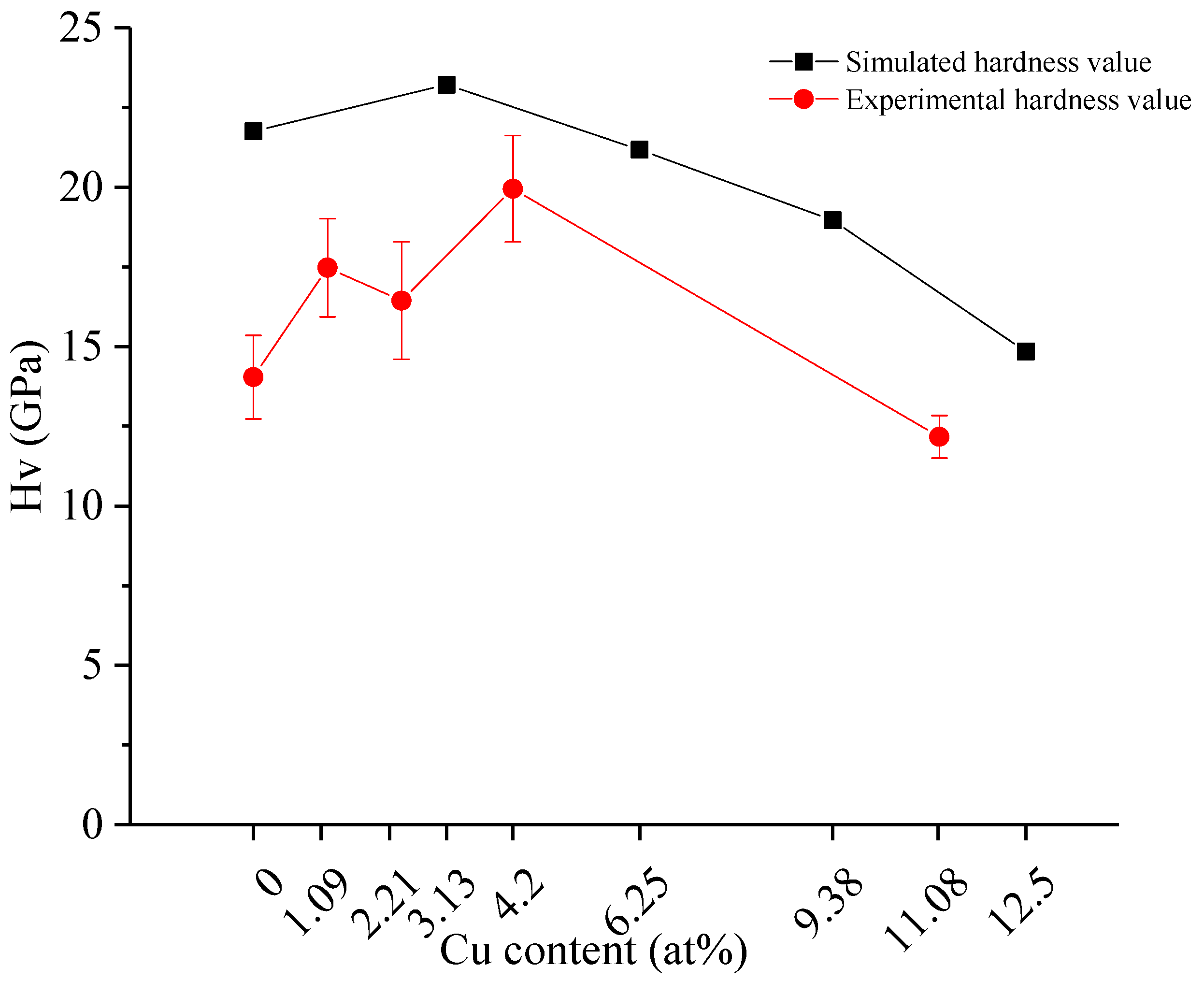
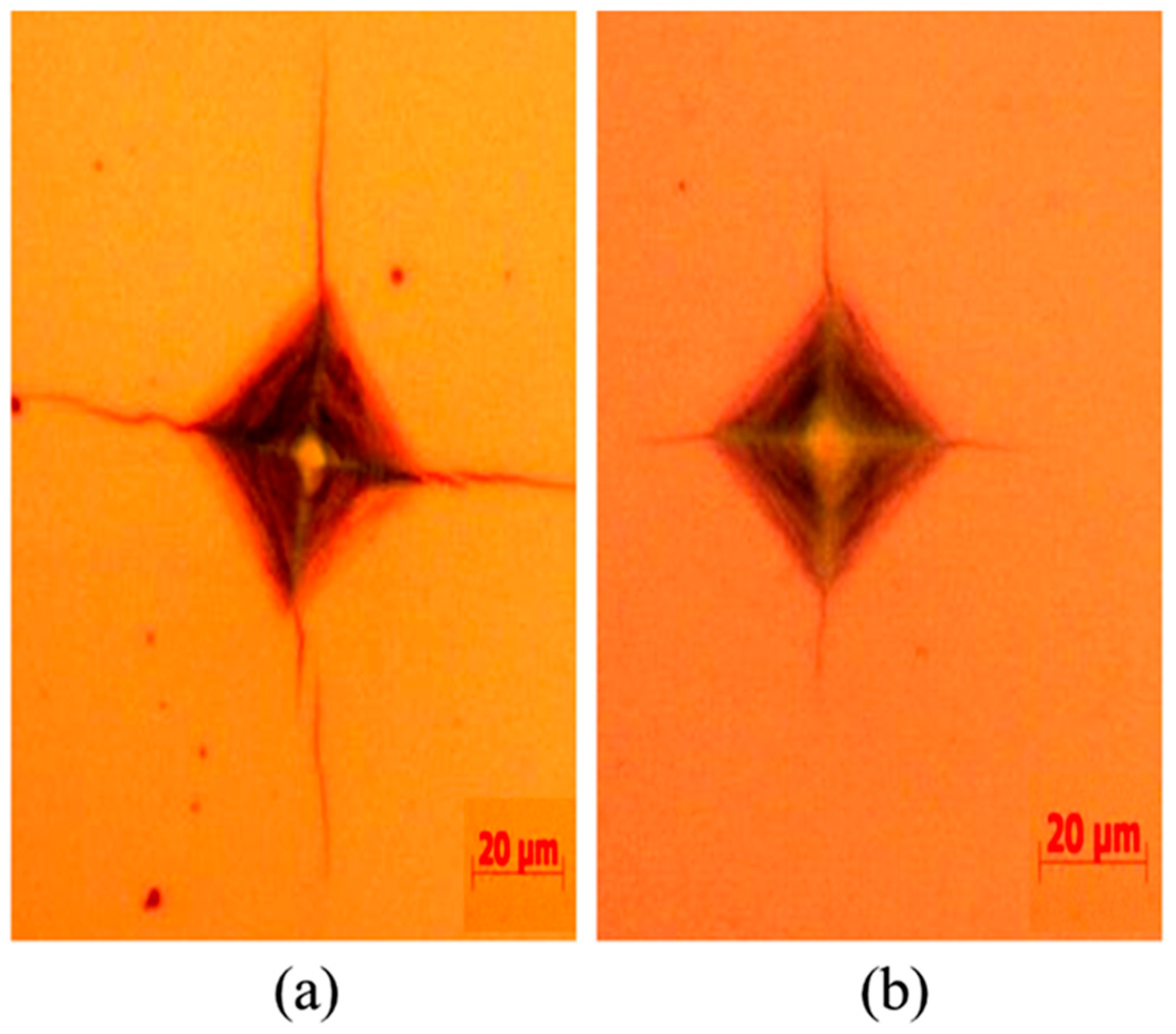
3.2. Hardness and Indentation Morphology
3.3. Calculation Results and Analysis
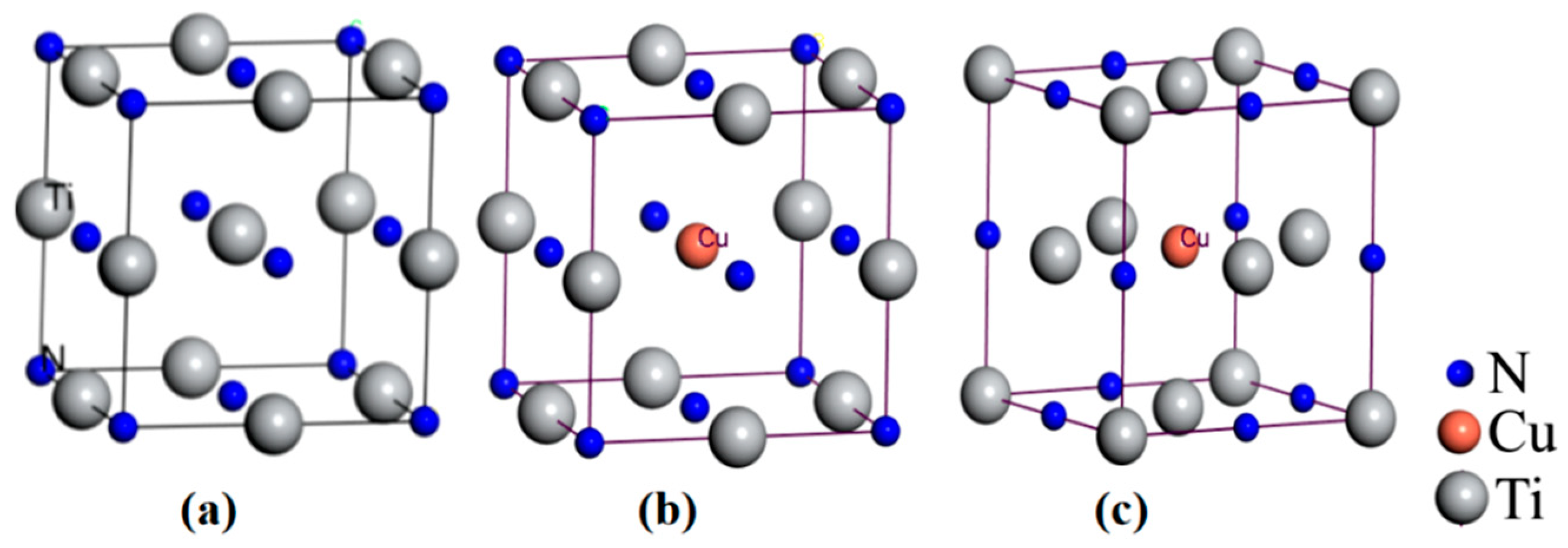
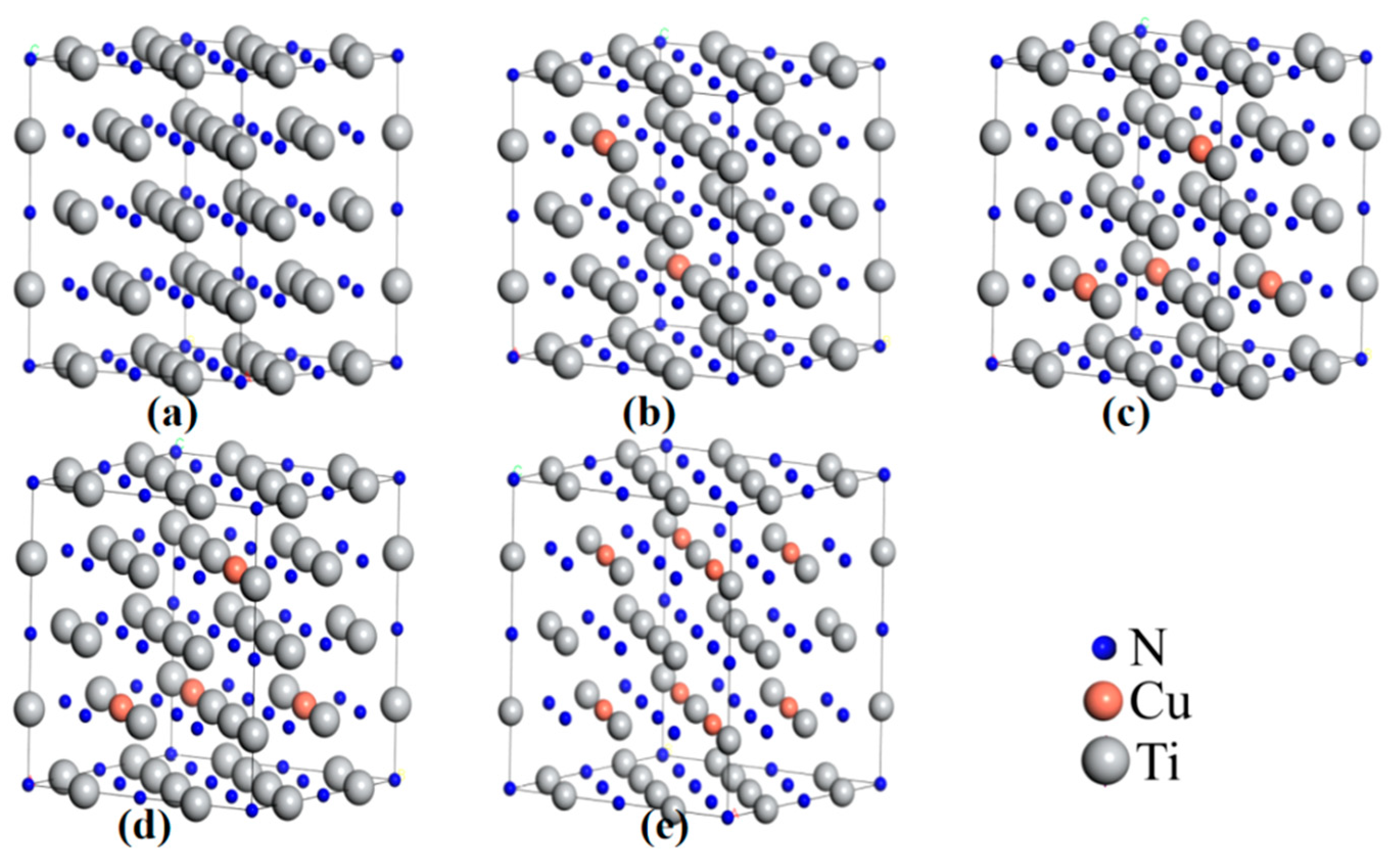
3.3.1. Cu Occupying Position in TiN Lattice
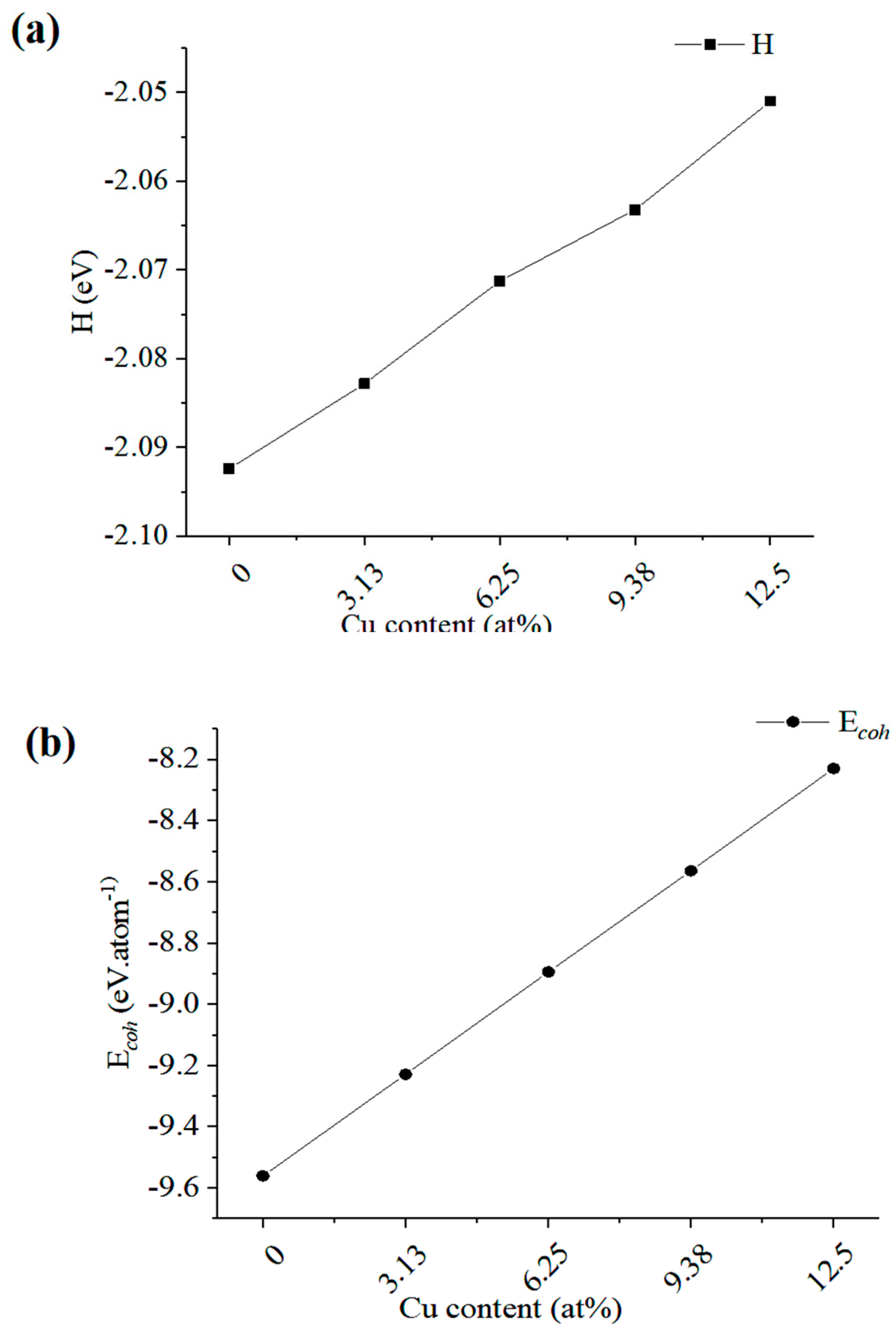
3.3.2. Forming and Binding Energies
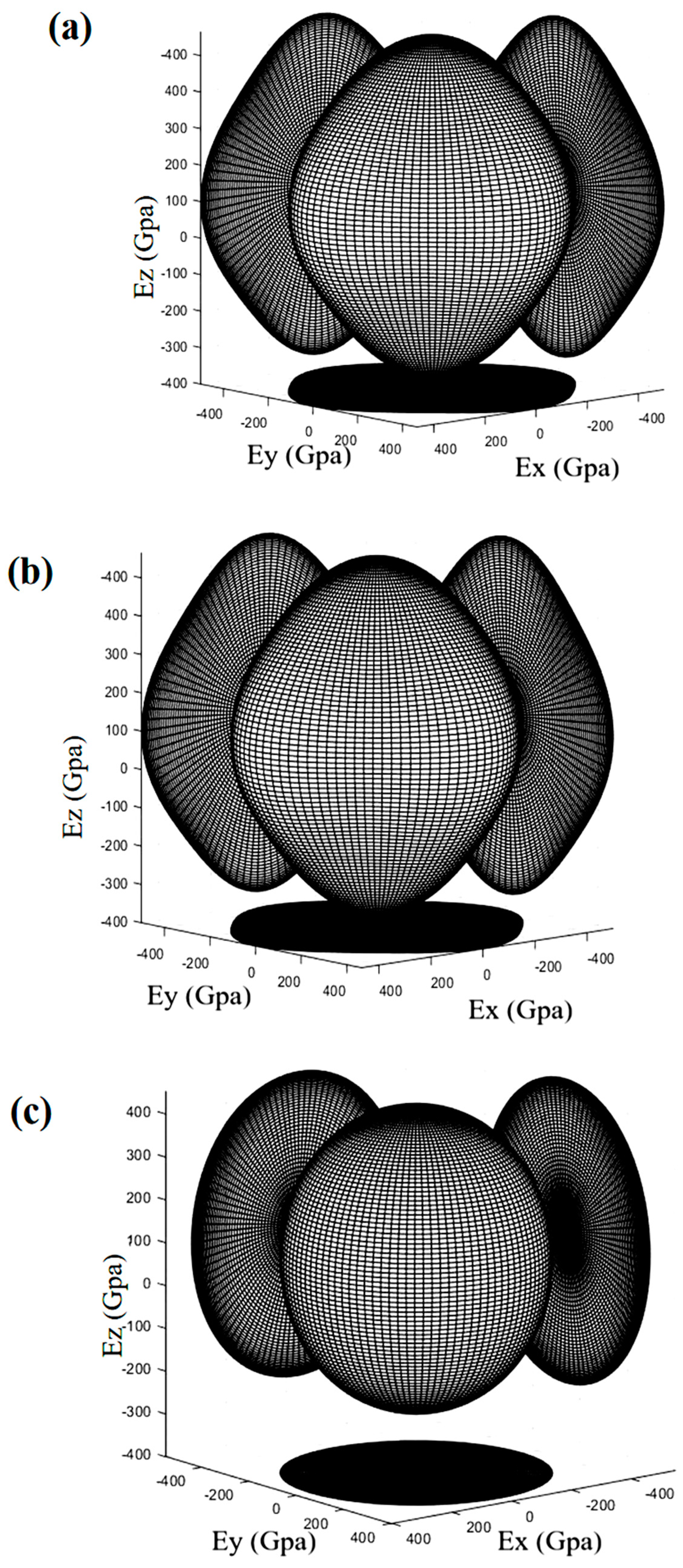
3.3.3. Elastic Coefficient and Mechanical Properties
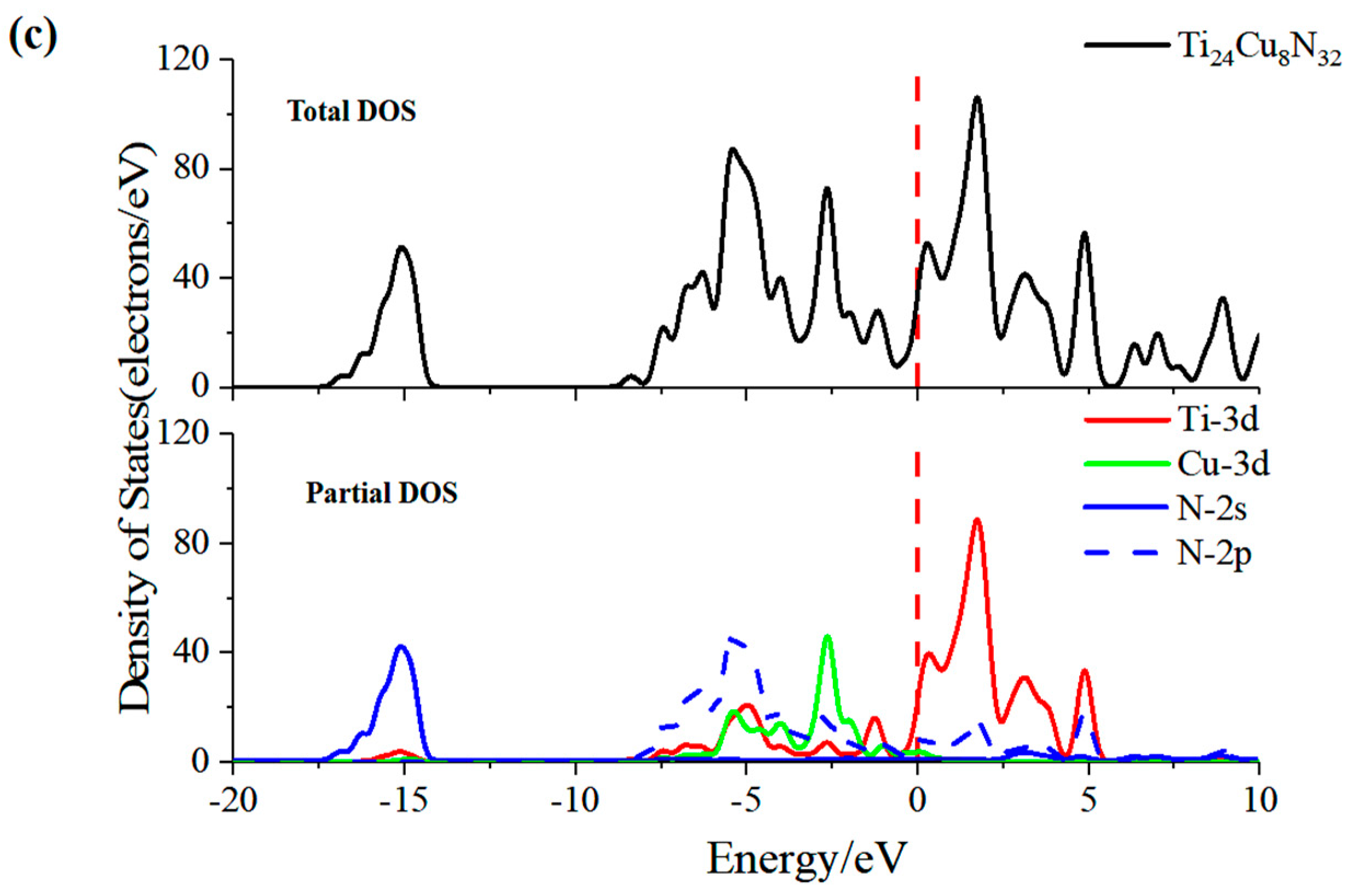
3.3.4. Density of States and Mulliken Population Analysis
4. Conclusions
- (1)
- According to the experimental and simulation spectra of XRD as well as the lattice occupation energy analysis based on DFT, Cu mainly replaced Ti atoms in TiN and formed substitution solid solution doping in the TiCuN films.
- (2)
- Both experimental and theoretical results of hardness show that with an increase in Cu content, the hardness of TiCuN increased slightly at first, and then decreased after a certain limit. The analysis of the density of states and Mulliken population reveal the effect of Cu doping on the hardness of TiCuN films. It was found that when Cu replaced Ti in TiN to form a weak Cu-N covalent bond, the bonding strength of some Ti-N bonds adjacent to Cu increased concurrently. At low Cu content, the number of Cu-N weak bonds was small, and the increase in TiCuN hardness was due to the enhanced Ti-N bond near the Cu atom. When the Cu content increased beyond a threshold, the number of weak Cu-N bonds also increased, while the increase in the number of enhanced Ti-N bonds was not significant. With the increase in the Cu-N bond beyond a certain extent, the hardness of TiCuN decreased, according to the weak bond theory.
- (3)
- The DFT results also showed that the value of B/G ratio and Poisson’s coefficient of TiCuN increased, and the isotropic elastic modulus E increased as a function of increasing Cu content. These results indicate that the toughness of the TiCuN system improved and the ability to resist cracks increased by increasing Cu composition in TiN. The analysis of the indentation morphology of both TiCuN confirmed that Cu doping improved the toughness of TiN. Therefore, the results of this study can provide theoretical and experimental guidance for improving the toughness and deformation resistance of TiN, which has potential applications in the surface modification of medical devices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tahara, H.; Ando, Y. Study of Titanium Nitride Deposition by Supersonic Plasma Spraying. Vacuum 2008, 83, 98–101. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zheng, C.B.; Fu, Q.S. Hydrothermal Oxidation Improves Corrosion and Wear Properties of Multi-Arc Ion Plated Titanium Nitride Coating for Biological Application. J. Vac. 2022, 198, 110871. [Google Scholar] [CrossRef]
- Thanka Rajan, S.; Subramanian, B.; Arockiarajan, A. A Comprehensive Review on Biocompatible Thin Films for Biomedical Application. Ceram. Int. 2022, 48, 4377–4400. [Google Scholar] [CrossRef]
- Yang, D.; Lü, X.; Hong, Y.; Xi, T.; Zhang, D. The Molecular Mechanism for Effects of TiN Coating on NiTi Alloy on Endothelial Cell Function. Biomaterials 2014, 35, 6195–6205. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yang, Y.; Guo, J.; Xiang, M.; Zhu, Q.; Ge, Y. A New Precursor for Fabricating TiN Coating on 316L Stainless Steel at Low Temperature Without Corrosive Byproducts. Ceram. Int. 2019, 45, 18265–18272. [Google Scholar] [CrossRef]
- Türkan, U.; Öztürk, O.; Eroğlu, A.E. Metal Ion Release from TiN Coated CoCrMo Orthopedic Implant Material. Surf. Coat. Technol. 2006, 200, 5020–5027. [Google Scholar] [CrossRef] [Green Version]
- Shayanfard, P.; Šandera, P.; Horníková, J.; Petruška, J.; Šittner, P.; Pokluda, J. Ni-Ti Self-Expanding Vascular Stent Configuration and Biomedical Interaction with Artery: Finite Element Analysis. Solid State Phenom. 2017, 258, 366–369. [Google Scholar] [CrossRef]
- Siddheswaran, R.; Mangalaraja, R.V.; Avila, R.E.; Manikandan, D.; Esther Jeyanthi, C.; Ananthakumar, S. Evaluation of Mechanical Hardness and Fracture Toughness of Co and Al Co-Doped ZnO. Mater. Sci. Eng. A 2012, 558, 456–461. [Google Scholar] [CrossRef]
- Din, I.U.; Salam, W.; Gillani, S.; Tahir, M.; Ikram, M.; Ali, S. Retraction notice to “Tuning the structure and optical properties of nanostructured CdS by Cu doping” [Optik 203 (2019) 164073]. Optik 2020, 203, 164707. [Google Scholar] [CrossRef]
- Zhang, S.; Bui, X.L.; Fu, Y. Magnetron-Sputtered nc-TiC/a-C (Al) Tough Nanocomposite Coatings. Thin Solid Films. 2004, 467, 261–266. [Google Scholar] [CrossRef]
- Ma, W.; Chen, F. Optical and Electronic Properties of Cu Doped Ag Clusters. J. Alloys Compd. 2012, 541, 79–83. [Google Scholar] [CrossRef]
- Khan, M.I.; Adil, F.; Majeed, S.; Farooq, W.A.; Hasan, M.S.; Jabeen, R.; Al-Mutairi, M.A.; Bukhtiar, A.; Iqbal, M. Structural, Morphological, Electrical and Optical Properties of Cu Doped DLC Thin Films. Mater. Res. Express 2019, 6, 126420. [Google Scholar] [CrossRef]
- Myung, H.S.; Lee, H.M.; Shaginyan, L.R.; Han, J.G. Microstructure and Mechanical Properties of Cu Doped TiN Superhard Nanocomposite Coatings. Surf. Coat. Technol. 2003, 163–164, 591–596. [Google Scholar] [CrossRef]
- Balashabadi, P.; Larijani, M.M.; Jafari-Khamse, E.; Seyedi, H. The Role of Cu Content on the Structural Properties and Hardness of TiN –Cu Nanocomposite Film. J. Alloys Compd. 2017, 728, 863–871. [Google Scholar] [CrossRef]
- Latushkina, S.D.; Romanov, I.M.; Zhizhchenko, A.G.; Posylkina, O.I.; Komarovskaya, V.M.; Piskunova, O.Y. Formation of Wear-Resistant Nanostructured TiN/Cu Coatings. J. Frict. Wear. 2016, 37, 27–31. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.J.; Lin, G.Q.; Han, K.C.; Ma, H. Synthesis of Cu Doped TiN Composite Films Deposited by Pulsed Bias Arc Ion Plating. Nucl. Instrum. Methods Phys. Res. B 2014, 320, 17–21. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, Y.; Jin, S.; Wang, J.; Liu, R.; Liu, H.; Shi, W.; Kolawole, S.K.; Ren, L.; Yu, B.; et al. Antibacterial TiCu/TiCuN Multilayer Films with Good Corrosion Resistance Deposited by Axial Magnetic Field-Enhanced Arc Ion Plating. ACS Appl. Mater. Interfaces 2019, 11, 125–136. [Google Scholar] [CrossRef]
- Kelly, P.J.; Li, H.; Benson, P.S.; Whitehead, K.A.; Verran, J.; Arnell, R.D.; Iordanova, I. Comparison of the Tribological and Antimicrobial Properties of CrN/Ag, ZrN/Ag, TiN/Ag, and TiN/Cu Nanocomposite Coatings. Surf. Coat. Technol. 2010, 205, 1606–1610. [Google Scholar] [CrossRef]
- Wang, H.; Shu, X.; Guo, M.; Huang, D.; Li, Z.; Li, X.; Tang, B. Structural, Tribological and Antibacterial Activities of Ti–Cu–N Hard Coatings Prepared by Plasma Surface Alloying Technique. Surf. Coat. Technol. 2013, 235, 235–240. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Xiao, J.; Yu, B.; Li, F. Ti–Cu–N Hard Nanocomposite Films Prepared by Pulse Biased Arc Ion Plating. Appl. Surf. Sci. 2011, 258, 370–376. [Google Scholar] [CrossRef]
- Pinakidou, F.; Paloura, E.C.; Matenoglou, G.M.; Patsalas, P. Nanostructural Characterization of TiN–Cu Films Using EXAFS Spectroscopy. Surf. Coat. Technol. 2010, 204, 1933–1936. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Su, D.; Wu, H. Structure and Properties of TiCuN Coatings by HCD Assisted AIP. Surf. Eng. 2016, 32, 223–228. [Google Scholar] [CrossRef]
- Luo, X.Y.; Ma, D.L.; Jing, P.P.; Gong, Y.L.; Zhang, Y.; Jing, F.J.; Leng, Y.X. In Vitro Analysis of Cell Compatibility of TiCuN Films with Different Cu Contents. Surf. Coat. Technol. 2021, 408, 126790. [Google Scholar] [CrossRef]
- Ma, Q.C.; Zhang, S.; Zhou, Q.; Shi, X.W. A First-Principles Study on Optical Properties and Dispersion Analysis of TiN Thin Films. Mater. Sci. Technol. 2019, 27, 85–89. [Google Scholar] [CrossRef]
- Kan, Q.; Yan, W.; Kang, G.; Sun, Q. Oliver–Pharr Indentation Method in Determining Elastic Moduli of Shape Memory Alloys—A Phase Transformable Material. J. Mech. Phys. Solids. 2013, 61, 2015–2033. [Google Scholar] [CrossRef]
- Lazar, P.; Redinger, J.; Podloucky, R. Density Functional Theory Applied to V N/Ti N Multilayers. Phys. Rev. B 2007, 76, 174112. [Google Scholar] [CrossRef]
- Kutschej, K.; Rashkova, B.; Shen, J.; Edwards, D.; Mitterer, C.; Dehm, G. Experimental Studies on Epitaxially Grown TiN and VN Films. Thin Solid Films 2007, 516, 369–373. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-Principles Simulation: Ideas, Illustrations and the CASTEP Code. J. Phys. Condens. Matter. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Warren, B.E. X-Ray Diffraction. Courier Corporation: North Chelmsford, MA, USA, 1990. [Google Scholar]
- Scherrer, P.; Gottingen, N.G.W. Estimation of the size and internal structure of colloidal particles by means of röntgen. Nachr. Ges. Wiss. 1918, 2, 96–100. [Google Scholar]
- Zhang, Y.J.; Qin, Y.G.; Qing, Y.A.; Deng, R.P.; Jin, H.; Li, R.Y.; Rehman, J.; Wen, M.; Zhang, K. TiCuN Solid Solution Coating: Excellent Wear-Resistant Biocompatible Material to Protect Artificial Joint. Mater. Lett. 2018, 227, 145–148. [Google Scholar] [CrossRef]
- Hu, D.C. Metal Structure and Corrosion Resistance. Aerospace Press: Beijing, China, 1987. [Google Scholar]
- Sahu, B.R. Electronic Structure and Bonding of Ultralight LiMg. Mater. Sci. Eng. B 1997, 49, 74–78. [Google Scholar] [CrossRef]
- Sevinçek, R.; Aygün, M.; Şahin, Y. Enthalpy of Formation Calculations on Five-Membered Azaborolidine Derivatives. J. Mol. Struct. 2020, 1217, 128321. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F. Necessary and Sufficient Elastic Stability Conditions in Various Crystal Systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef] [Green Version]
- Pugh, S.F. Relations Between the Elastic Moduli and the Plastic Properties of Polycrystalline Pure Metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Chen, X.; Niu, H.; Li, D.; Li, Y. Modeling Hardness of Polycrystalline Materials and Bulk Metallic Glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.S. Elastic Anisotropy of Metals; Metallurgical Industry Press: Beijing, China, 1996. [Google Scholar]
- Sofi, S.A.; Gupta, D.C. High Pressure-Temperature Study on Thermodynamics, Half-Metallicity, Transport, Elastic and Structural Properties of Co-Based Heusler Alloys: A First-Principles Study. J. Solid State Chem. 2020, 284, 121178. [Google Scholar] [CrossRef]
- Cao, Z.; Jin, N.; Ye, J.; Du, X.; Liu, Y. First-Principles Study on the Effects of N and Al Doping on the Mechanical Properties and Electronic Structures of TiC. RSC Adv. 2020, 10, 36295–36302. [Google Scholar] [CrossRef]


| Structure | Methods or Literature | a (Å) | b (Å) | c (Å) | |
|---|---|---|---|---|---|
| TiN | This study | GGA-PBE | 4.247 | 4.247 | 4.247 |
| Other studies | GGA-PBE [26] | 4.270 | 4.270 | 4.270 | |
| Experiments | [27] | 4.245 | 4.245 | 4.245 | |
| Sample | Thickness (nm) | Ti (at%) | Cu (at%) | N (at%) |
|---|---|---|---|---|
| TiCuN-0Cu | 1048 ± 31 | 51.24 | / | 48.76 |
| TiCuN-4Cu | 959 ± 10 | 48.40 | 1.09 | 50.51 |
| TiCuN-8Cu | 1011 ± 20 | 49.41 | 2.21 | 48.38 |
| TiCuN-16Cu | 946 ± 18 | 50.83 | 4.10 | 45.07 |
| TiCuN-32Cu | 947 ± 37 | 32.15 | 11.08 | 56.77 |
| Sample | (111) FWHM (°) | (200) FWHM (°) |
|---|---|---|
| TiCuN-0Cu | 0.660 ± 0.054 | 0.700 ± 0.006 |
| TiCuN-4Cu | 0.663 ± 0.004 | 0.830 ± 0.007 |
| TiCuN-8Cu | 0.668 ± 0.006 | 0.878 ± 0.007 |
| TiCuN-16Cu | 0.793 ± 0.012 | 0.987 ± 0.011 |
| TiCuN-32Cu | 0.940 ± 0.025 | 1.150 ± 0.013 |
| Structure | C11 (GPa) | C12 (GPa) | C44 (GPa) |
|---|---|---|---|
| Ti32N32 | 525 | 140 | 163 |
| Ti30Cu2N32 | 531 | 125 | 161 |
| Ti28Cu4N32 | 495 | 132 | 155 |
| Ti26Cu6N32 | 435 | 134 | 148 |
| Ti24Cu8N32 | 443 | 164 | 141 |
| Structure | B (GPa) | G (GPa) | Poisson ratio | Ratio of B/G |
|---|---|---|---|---|
| Ti32N32 | 268.18 | 174.39 | 0.23 | 1.54 |
| Ti30Cu2N32 | 260.15 | 176.60 | 0.22 | 1.47 |
| Ti28Cu4N32 | 252.68 | 165.32 | 0.23 | 1.53 |
| Ti26Cu6N32 | 234.52 | 148.94 | 0.24 | 1.57 |
| Ti24Cu8N32 | 257.20 | 140.74 | 0.27 | 1.83 |
| Structure | Cu-N | Ti-N | ||
|---|---|---|---|---|
| Number (Population = 0.06–0.11) | Number (Population = 0.27–0.29) | Number (Population = 0.29) | Number (Population = 0.29–0.4) | |
| Ti32N32 | 0 | 0 | 192 | 0 |
| Ti30Cu2N32 | 12 | 64 | 32 | 84 |
| Ti28Cu4N32 | 24 | 64 | 12 | 90 |
| Ti26Cu6N32 | 35 | 48 | 12 | 97 |
| Ti24Cu8N32 | 48 | 48 | 0 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Xie, D.; Ma, D.; Jing, F.; Matthews, D.T.A.; Ganesan, R.; Leng, Y. Evaluation of the Crystal Structure and Mechanical Properties of Cu Doped TiN Films. Coatings 2022, 12, 652. https://doi.org/10.3390/coatings12050652
Fan Y, Xie D, Ma D, Jing F, Matthews DTA, Ganesan R, Leng Y. Evaluation of the Crystal Structure and Mechanical Properties of Cu Doped TiN Films. Coatings. 2022; 12(5):652. https://doi.org/10.3390/coatings12050652
Chicago/Turabian StyleFan, Yuyuan, Dong Xie, Donglin Ma, Fengjuan Jing, D. T. A. Matthews, R. Ganesan, and Yongxiang Leng. 2022. "Evaluation of the Crystal Structure and Mechanical Properties of Cu Doped TiN Films" Coatings 12, no. 5: 652. https://doi.org/10.3390/coatings12050652
APA StyleFan, Y., Xie, D., Ma, D., Jing, F., Matthews, D. T. A., Ganesan, R., & Leng, Y. (2022). Evaluation of the Crystal Structure and Mechanical Properties of Cu Doped TiN Films. Coatings, 12(5), 652. https://doi.org/10.3390/coatings12050652







