Abstract
High-entropy amorphous alloys designed based on the concept of multi-principal components have the comprehensive advantages of high passivation element content and amorphous structure, and are considered as one of the promising alternative protective materials in extreme marine environments. However, based on the composition of traditional amorphous alloys, the multi-principal design significantly reduces its glass forming ability. In order to improve the glass formation ability of high-entropy amorphous alloys, this study attempts to design Fe19.6Co19.6Ni19.6Cr19.6(B13.72Si5.88)19.6Y2 alloy by microalloying on the basis of traditional FeCoNiCrBSi high-entropy amorphous alloy. The traditional Fe43.6Co6Ni17.4Cr9B17.5Si1.5Nb5 iron-based amorphous alloy was selected as the comparison material. Then, spherical alloy powders were prepared by gas atomization. The amorphous nanocrystalline composite coatings were deposited on the 304 stainless steel by laser cladding technology. The microstructure of the coatings was characterized by scanning electron microscopy and X-ray diffractometer. The corrosion behavior of laser cladding coatings in 3.5 wt.% NaCl solution were investigated in detail. The results show that the Fe43.6Co6Ni17.4Cr9B17.5Si1.5Nb5 powder is composed of FCC, Laves and boride phases. Whereas the Fe19.6Co19.6Ni19.6Cr19.6(B13.72Si5.88)19.6Y2 high-entropy amorphous alloy powder is composed of FCC and boride phases. Due to the remelting and multiple heat treatments during the preparation of the laser cladding coatings, borides were precipitated in both coatings. The microstructure of the two coatings from the bonding area with the substrate to the top layer are plane grains, dendrite, equiaxed grains and amorphous phase, respectively. Fe19.6Co19.6Ni19.6Cr19.6(B13.72Si5.88)19.6Y2 high-entropy amorphous alloy coating exhibits high corrosion potential, passivation film resistance and low corrosion current density in 3.5 wt.% NaCl solution. In addition, the passivation film formed on the coating has higher Cr content and lower defect concentration, showing more excellent corrosion resistance.
1. Introduction
Under the combined influence of corrosion, working load and environmental load, large-scale marine engineering equipment usually suffers from harsh service environments [1,2]. Due to the excellent mechanical properties, corrosion resistance and wear resistance, Fe-based amorphous alloy coatings are one of the promising candidates for the design of anti-corrosion and wear-resistant integrated coatings [3]. However, in Fe-based amorphous alloys, the content of passivation elements such as Co, Ni, and Cr is limited, which will weaken its passivation performance in corrosive environments. This is because the atomic radius of elements such as Co, Ni, Cr and Fe are very close, and high content of Co, Ni and Cr will not be conducive to increasing the atomic size difference in the alloy system. In addition, the mixing enthalpy of each of Co, Ni, and Cr elements and Fe is close to zero. Therefore, the alloy tends to form a solid solution, which is not conducive to the formation of an amorphous phase [4].
Recently, high-entropy amorphous alloys have received extensive attention due to their unique structures and excellent properties [5,6]. The design concept of high-entropy alloys breaks the limitation of choosing one or two elements as the main element in traditional alloy design. High-entropy amorphous alloys exhibit the characteristics of multi-component in composition, and also have the structural advantages of amorphous phase [5]. Up to now, the reported high-entropy amorphous alloys exhibit excellent comprehensive properties such as high hardness and excellent wear resistance, good corrosion resistance and unique thermal stability [7]. Laser cladding technology is one of the effective methods to prepare high-performance high-entropy amorphous alloy coatings [7]. It has high power density and cooling rate (104–106 K/s), which is conducive to the formation of amorphous phase in the coating [8]. A metallurgical bond is formed between the substrate and the coating, and the dilution effect of the substrate on the coating composition is controllable [9,10]. Shu et al. [7] deposited FeCrCoNiSiB high-entropy amorphous alloy coatings by laser cladding technology. The upper layer of the coating consists of amorphous phase and face-centered cubic (Fe, Ni) phase, in which the volume fraction of amorphous phase is about 49%. The higher the amorphous content in the coating, the better the corrosion resistance in HCl and NaCl solutions. Cheng et al. [11] reported that increasing the Nb content in the laser cladding (Fe0.25Co0.25Ni0.25(B0.7Si0.3)0.25)100-xNbx high-entropy amorphous alloy coating could improve its GFA and promote amorphous phase formation. Yang et al. [12] studied the effect of Fe/Co element ratio on the amorphous phase content in laser cladding FeCoCrBNiSi high-entropy amorphous coatings. The results show that when the Fe/Co ratio is about 1:1, the amorphous content of the top layer of the coating exceeds 66.7%. Sun et al. [13] investigated the effect of B/Si ratio on the formation of amorphous phase in laser clading Fe25Co25Ni25(BxSi1-x)25 (x = 0.5, 0.6, 0.7, 0.8) high-entropy amorphous alloy coatings. The results show that increase of B/Si ratio (0.7 ≤ x ≤ 0.8) promoted the precipitation of (Fe, Co, Ni)3B phase. Wu et al. [14] deposited FeCoCrNiSiB high-entropy amorphous alloy coatings on low carbon steel substrates by laser cladding. The coating exhibited layered microstructure including the columnar dendrites and the amorphous-matrix layer with β-Co phase. The content of amorphous phase in the upper surface was up to 85.1%.
Literature studies have shown that the properties of coatings are closely related to the amorphous phase content [11]. However, the amorphous phase content in the coating is closely related to the GFA of the alloy as well as the coating preparation process. Most of the high-entropy amorphous alloys reported so far are obtained by element replacement based on the composition of traditional amorphous alloys. Studies have shown that the GFA of high-entropy amorphous alloys is significantly lower than that of traditional amorphous alloys [15]. The critical dimensions of more than 40 high-entropy amorphous alloys reported so far do not exceed 2 mm. It is well known that microalloying is an effective method to improve the GFA of conventional amorphous alloys [15]. However, there are few research reports on these aspects. In addition, the preparation of high-entropy amorphous alloy powders is difficult [16], so there are few related reports on high-entropy amorphous alloy coatings.
In this paper, based on the FeCoNiCrBSi high-entropy amorphous alloy reported in the literature [7,11,12,13,14], the composition of FeCoNiCrBSiY high-entropy amorphous alloy was designed by doping with Y element. High-entropy amorphous alloy powders were prepared by argon atomization. The commercial Fe43.6Co6Ni17.4Cr9B17.5Si1.5Nb5 amorphous powder (Peshing New Metal Material Co., Ltd., Changzhou, China) was selected as the comparison material, and two kinds of coatings were deposited on the 304 stainless steel by laser cladding technology. The microstructure of the coating was characterized, and the electrochemical corrosion behaviors of these coatings in 3.5 wt.% NaCl solution were comparatively studied.
2. Experimental Procedures
2.1. Preparation of Gas Atomized Powders
High-purity Fe, Co, Ni, Cr, B, Si, and Y elements (99.99%) were selected as raw materials. High-entropy alloy ingots with uniform composition was obtained by multiple arc melting according to the chemical composition shown in Table 1. The alloy ingots were cut into the raw material size required for gas atomization powder preparation process by mechanical processing. Then, spherical powders were prepared by induction melting and argon atomization (HERMIGA 100-30 V2ICC, Peshing New Metal Material Co., Ltd., Changzhou, China). The prepared powder was processed by air classification. FeCoNiCrBSiY (45–106 μm) and Fe43.6Co6Ni17.4Cr9B17.5Si1.5Nb5 powders (53–106 μm) were selected as raw materials for the preparation of laser cladding coatings. Figure 1 presents the particle size distribution of the powders.

Table 1.
Nominal chemical composition of the FeCoNiCrBSiY and FeCoNiCrBSiNb powders (at.%).
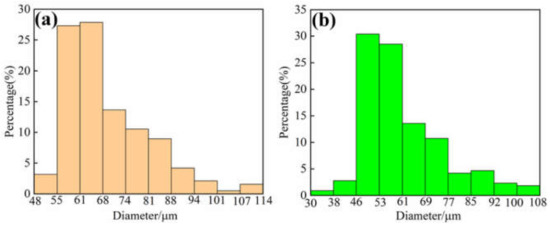
Figure 1.
Particle size distribution of raw powders: (a) FeCoNiCrBSiNb; (b) FeCoNiCrBSiY.
2.2. Preparation of Laser Cladding Coatings
SUS304 stainless steel is selected as the base material, and its chemical composition is shown in Table 2. The stainless steel was cut into samples with dimensions of 100 mm × 100 mm × 6 mm. Before preparing the coating, the surface of the sample was ground with sandpaper, cleaned with alcohol, and then dried. The coatings were deposited on a 100 mm × 100 mm surface of the stainless steel substrate using a JM-RB-3000 laser cladding system (Wuhan Precision Laser Technology Co., Ltd., Wuhan, China) with coaxial powder feeding. High-purity argon was used as the protective gas in the coating preparation process. The laser cladding process parameters of the two coatings are shown in Table 3. The overlap ratio of adjacent cladding layers is selected as 35 %, and the coating is obtained by single-layer multi-pass cladding.

Table 2.
Chemical composition of 304 stainless steel (wt.%).

Table 3.
Laser cladding process parameters.
2.3. Microstructural Characterization of Powders and Coatings
An AL-2700B X-ray diffractometer was used to analyze the phase structures of the powders and coatings (Dandong Aolong Radiative Instrument Group Co., Ltd., Dandong, China). The target material was Cu target, and the working voltage and current were 40 kV and 30 mA, respectively. The scanning range (2θ) was 10~90°, and the scanning speed was 5 min/◦.
After mixing the prepared alloy powder with phenolic resin, metallographic sam-ples for microstructure characterization of the powders were prepared using an XQ-1 metallographic sample inlay machine (Shanghai Caikon Optical Instrument Co., Ltd., Shanghai, China). The samples were ground with sandpaper and polished to a mirror surface with 0.3 µm diamond paste. The FeCoNiCrBSiNb and FeCoNiCrBSiY coatings were cut into 10 mm × 10 mm × 8 mm samples. The cross-section of the two coatings was also ground with sandpapers and then polished. The cross-sectional morphology and chemical composition of the powders and coatings were characterized by Oxford scanning electron microscopy with energy dispersive spectroscopy (Oxford Instruments Technology Ltd., Oxford, UK).
2.4. Corrosion Behavior of Laser Cladding Coatings
An electrochemical station (Multi Autolab M204, Hongkong, China) with a three-electrode cell system was used to measure the potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy curves (EIS) of the coatings. Before testing, the stainless steel substrate of the coated samples was welded with Cu wire. Then, the samples were cold mounted with resin. Only the coated surface was exposed as a working surface with an area of 1 cm2. To ensure the same surface roughness for all coated samples, all coatings were ground and polished. The corrosion behavior of the coatings was investigated in 3.5 wt.% NaCl solution at room temperature. A platinum sheet and a saturated calomel electrode (SCE) were used as counter electrode and reference, respectively.
Before corrosion performance characterization, all prepared samples were soaked in 3.5 wt.% NaCl solution for 30 min to reach a stable open circuit potential (OCP). Then, the EIS measurements were performed in a frequency range from 100 kHz down to 10 mHz at OCP using a 10 mV signal amplitude. The PDP tests were started from −0.5V and ends at 1.5 V versus OCP at a potential scan rate of 1 mV/s. The impedance data was interpreted by using the Zsimpwin software and equivalent electron circuits.
2.5. Characterization of the Passivation Film
The coating samples was held potentiostatically at 1 V for 1 h in the 3.5 wt.% NaCl solution to form a stable passivation film. Then, X-ray photoelectron spectroscopy (XPS) was used to analyze the composition of the passivation film. The commercial software XPSpeak (version 4.1) was used to analyze XPS data. The semiconducting properties of the passivation films were analyzed by Mott-Schottky (M-S) plots. The M-S plots were performed within the potential range from −1.0 VSCE to 0.5 VSCE. The scanning rate was 50 mV/steps at a perturbation voltage of 10 mV and a fixed frequency of 1 kHz.
3. Results
3.1. Microstructure of the Gas Atomized Powders
Figure 2 shows the microstructure of the FeCoNiCrBSiNb and FeCoNiCrBSiY powders. The powder particles of both alloys exhibited good spherical shape with smooth surfaces. There are a few pores on the surface of the powder particles. FeCoNiCrBSiNb powder contains a small amount of circular precipitates, the average content of which is about 11% measured by grayscale method. There are a large number of strip-like precipitates in FeCoNiCrBSiY powder, with an average content of about 75%. Table 4 shows the EDS analysis results in Figure 2b,d. It can be seen from Table 4 that the content of B element in the precipitation phase of the two powders is relatively high, indicating that borides are formed inside the powders.
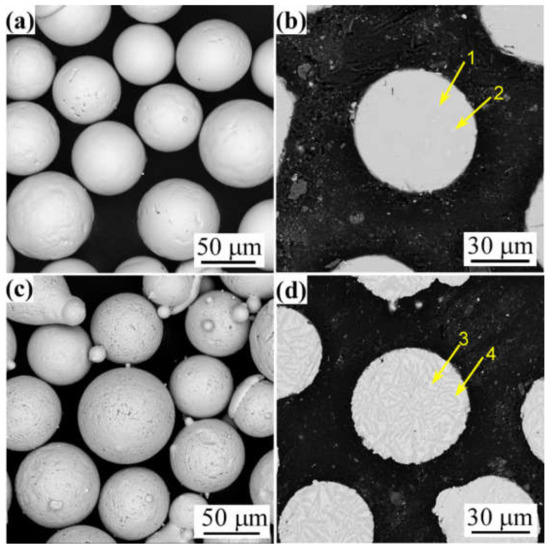
Figure 2.
Surface and cross-sectional morphologies of (a,b) FeCoNiCrBSiNb powder and (c,d) FeCoNiCrBSiY powder. (The positions 1, 2, 3 and 4 marked in the figure are the positions for energy spectrum analysis).

Table 4.
Chemical compositions of the powders obtained by EDS analysis at the marked positions in Figure 2b,d (at.%).
3.2. Phase Structure of Powders and Laser Cladding Coatings
Figure 3 illustrates the X-ray diffraction (XRD) patterns of the powder and laser cladding coatings. The results show that FeCoNiCrBSiNb powder is composed of face-centered cubic solid solution (FCC), Laves and Cr2B. According to the research results in Figure 2 and Table 4, it can be inferred that the precipitates in the powder are solid solution (FCC), Laves and Cr2B. In addition, the XRD pattern of the powder showed scattering peaks at 2θ = 51° and 75°, indicating that a small amount of amorphous phase or nanocrystals existed in the powder. After the laser cladding process, the FeCoNiCrBSiNb coating consists of face-centered cubic solid solution (FCC), Laves, Cr2B, Fe2B, Fe3B, CoB and Ni4B3 phases. This result is similar to the phase results reported by Guilherme et al. [17] in Fe63.5Cr25Ni7B4.5 alloy. The diffraction peak of the coating is significantly broadened at 2θ = 51°, indicating that the coating contains a high content of amorphous or nanocrystalline phases. This is because the powder is remelted and solidified rapidly during the laser cladding process, and the cooling rate of the coating is relatively large.
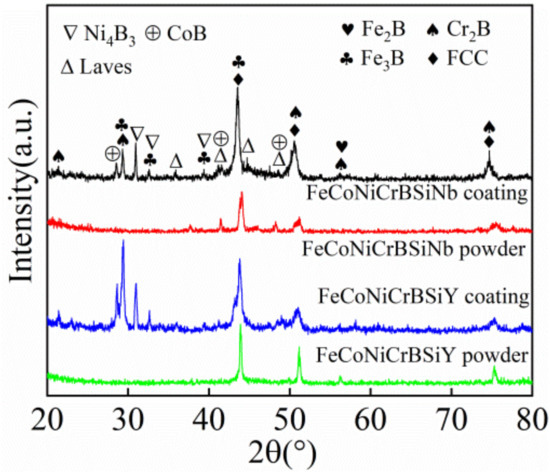
Figure 3.
X-ray diffraction (XRD) patterns of powder and laser cladding coatings.
FeCoNiCrBSiY powder is composed of face-centered cubic solid solution (FCC) and Cr2B. According to the research results in Figure 2 and Table 4, it can be inferred that the precipitates in the powder are face-centered cubic solid solution (FCC) and Cr2B. After the laser cladding process, the FeCoNiCrBSiY coating can be found to be composed of face-centered cubic solid solution (FCC), Cr2B, Fe2B, Fe3B, CoB and Ni4B3 phases. This phenomenon is similar to the findings of Cheng et al. [11] in Fe25Co25Ni25(B0.7Si0.3)25 high-entropy alloy coating. The diffraction peaks of the coating at 2θ = 43°, 51° and 75° were significantly broadened, indicating that the content of amorphous phase or nanocrystal in the coating was significantly higher than that of the powder.
3.3. Microstructure Characterization of Laser Cladding Coatings
Figure 4a,b show the cross-sectional morphologies of the laser cladding FeCoNiCrBSiNb and FeCoNiCrBSiY coatings, respectively. The results show that the microstructures of the two coatings are uniform and dense, and the coatings show metallurgical bonding with the SUS304 substrate. The average thicknesses of FeCoNiCrBSiNb and FeCoNiCrBSiY coatings are approximately 614 μm and 625 μm, respectively. It can be seen from Figure 4a that the boundaries between the multiple cladding layers in the FeCoNiCrBSiNb coating are clear. In addition, the white precipitates are mainly distributed in the bottom and middle layers of the coating. However, the difference is that no obvious boundaries between multiple cladding layers are found from the cross-sectional morphologies of the FeCoNiCrBSiY coating (Figure 4b). The white precipitates are mainly distributed in the bottom layer of the coating.
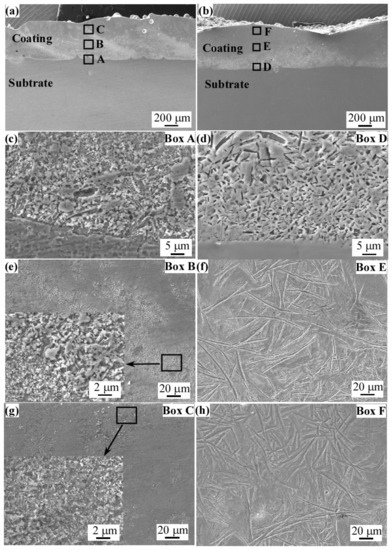
Figure 4.
Cross-sectional morphologies of (a) FeCoNiCrBSiNb and (b) FeCoNiCrBSiY coatings. (Figure (c,e,g) are the microstructures of the A, B and C regions in Figure (a). Figure (d,f,h) are the microstructures of the D, E and F regions in Figure (b).
Figure 4c,e,g show the local micro-morphologies of different regions of FeCoNiCrBSiNb coating. The results show that a large number of dendrites are precipitated in the bottom and middle layers of FeCoNiCrBSiNb coating. This is due to the fact that the liquid metal in the molten pool is in close contact with the matrix grains and completely wets them, so crystals can easily nucleate from the liquid metal on the matrix grains [18]. The solidification rate (V) is slower and the temperature gradient (G) is higher near the bonding area between the coating and the SUS304 substrate, and the larger G/V ratio leads to the formation of larger size dendrites at the bottom of the molten pool [19]. Similar findings were also reported by Cheng et al. [19] and Xiao et al. [20]. Figure 4g indicates that the precipitates in the top layer of the coating are significantly reduced and smaller in size, which is caused by the rapid cooling. The EDS surface scanning analysis results of the middle region of FeCoNiCrBSiNb coating are shown in Figure 5. It can be seen from the figure that the distribution of Fe and Co elements in the middle area of FeCoNiCrBSiNb coating is relatively uniform, while Nb and Cr elements are segregated. The white fine precipitates are rich in Nb elements, and the gray striped precipitates are rich in Cr and Nb elements. According to the XRD pattern analysis results of FeCoNiCrBSiNb coating, it can be inferred that the Nb-rich precipitation phase is Laves and the Cr-rich precipitation phase is Cr2B.
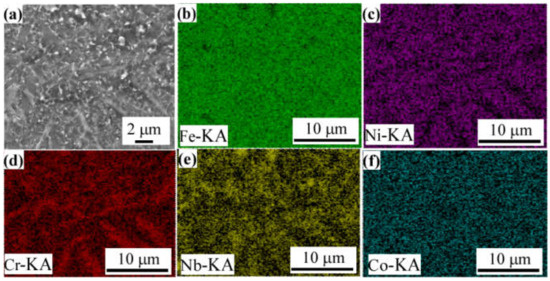
Figure 5.
Elemental distribution of the FeCoNiCrBSiNb coating. (a) cross-sectional morphology; (b) Fe; (c) Ni; (d) Cr; (e) Nb; (f) Co.
Figure 4d,f,h are the microstructures of different regions of FeCoNiCrBSiY coating. It can be seen from Figure 4d that there are a large number of dendrites in the bonding area between the FeCoNiCrBSiY coating and the substrate. The intermediate layer of the coating (Figure 4f) has a large number of band-like precipitates. Due to the faster cooling rate of the top layer of the coating, the content of the band-like precipitates decreases and the size becomes smaller (Figure 4h). Figure 6 shows the EDS analysis results of the middle region of the FeCoNiCrBSiY coating. It can be seen from Figure 6 that the strip-shaped precipitates are rich in Cr and depleted in Co and Ni elements. According to the XRD analysis results in Figure 3, the strip-like precipitates in this coating can be inferred to be Cr2B.
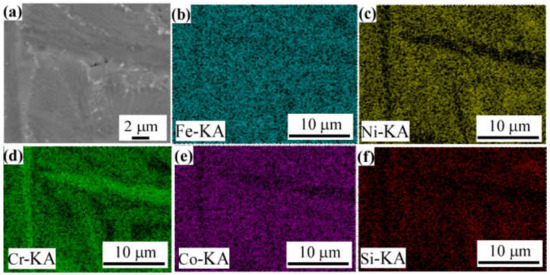
Figure 6.
Elemental distribution of the FeCoNiCrBSiY coating. (a) cross-sectional morphology; (b) Fe; (c) Ni; (d) Cr; (e) Co; (f) Si.
3.4. Corrosion Behavior of Laser Cladding Coatings
3.4.1. PDP Curves
Figure 7 shows the PDP curves of laser cladding FeCoNiCrBSiNb and FeCoNiCrBSiY coatings. Table 5 lists a series of electrochemical parameters fitted from PDP curves. Ecorr and icorr represent corrosion potential and corrosion current, respectively. The βa and βc are the slopes of the anodic and cathodic Tafel polarization curves, respectively. The polarization resistance (Rp) of the coating is calculated according to the Stern-Geary Equation (1) [21].
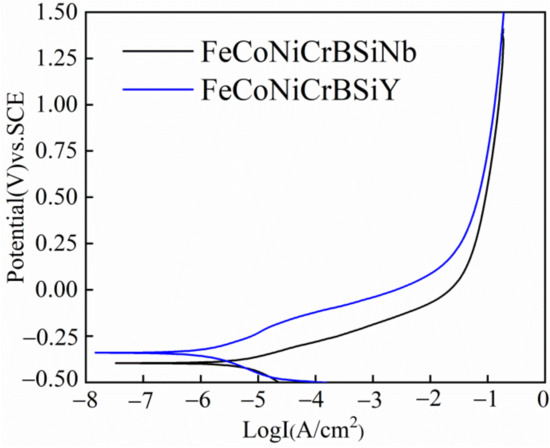
Figure 7.
Potentiodynamic polarization curves of the laser cladding FeCoNiCrBSiY coating and FeCoNiCrBSiNb coating.

Table 5.
A series of electrochemical parameters fitted from the PDP curves.
In general, high corrosion potential and low corrosion current density indicate materials with higher chemical stability and lower corrosion tendency [22]. Compared with the FeCoNiCrBSiNb coating, the FeCoNiCrBSiY coating exhibits high corrosion potential, low corrosion current density, and large polarization resistance, indicating better corrosion resistance.
Table 5 lists the electrochemical parameters of some high-entropy amorphous alloys and Fe-based amorphous alloy coatings reported in the literature under the same experimental conditions. Literature studies have shown that the corrosion resistance of amorphous coatings is closely related to the compactness, passivation elements and amorphous phase content of the coating. The amorphous phase content of the magnetron sputtering Ti21.6Al11.3Cr19.4Si23.5V22.0O2.2 high-entropy amorphous coating reported by Lin et al. [23], the atmospheric plasma sprayed Fe87.6Cr2.5Si6.7B2.5C0.7 coating reported by Bijalwan et al. [24], and the HVOF sprayed Fe48Cr15Mo14C15B6Y2 coating reported by Huang et al. [25] are as high as 100%, 93.40% and 88.95%, respectively. However, due to the high porosity or low content of passivating elements in the above coatings, their corrosion resistance is limited [26,27]. The research results of Berger et al. [28] showed that a stable passivation film can be formed on the alloy surface only when the chromium content is higher than 17%. It has been reported in the literature that the effect of the content of passivating elements in amorphous alloys on its corrosion resistance may be more important than the effect of the amorphous structure itself [29,30]. Botta et al. [31] confirmed the key role of Cr on the corrosion performance of Fe-based amorphous alloys, and Fe-based amorphous alloys without passivation elements had poor corrosion resistance.
The laser cladding high-entropy amorphous-nanocrystalline composite coatings reported in this study are highly dense. In addition, high-purity argon is used as a protective gas during the coating cladding process, which prevents the molten pool from being oxidized. There are almost no pores and oxides in the laser cladding coating, which significantly reduces the diffusion path of corrosion ions. The high content of passivation elements such as Co, Ni and Cr in the FeCoNiCrBSiY high-entropy amorphous alloy coating improves the corrosion resistance of the coating.
3.4.2. Electrochemical Impedance Spectroscopy
The EIS measurement results of laser cladding FeCoNiCrBSiNb and FeCoNiCrBSiY coatings are illustrated in Figure 8. The Nyquist plots of both coatings in 3.5 wt.% NaCl solution (Figure 8b) are incomplete semi-circles, indicating that the corrosion process of the coatings is controlled by the charge transfer process. Generally speaking, a large capacitive arc radius indicates high corrosion resistance [32]. It can be seen from Figure 8b that the capacitive arc radius of FeCoNiCrBSiY coating is significantly larger, which indicates that FeCoNiCrBSiY coating has stronger resistance to electrochemical corrosion and forms a more protective passivation film on its surface. The Bode plot (Figure 8c) shows that the slope (−0.9) of the logZ-logf curves of the two coatings is close to −1, which means that the passivation film formed on the coatings has capacitance-like properties. Furthermore, the Phase-logf plots of both coatings (Figure 8d) have only one peak, which means there is only one time constant. According to the equivalent circuit of Rs(QfilmRfilm) proposed in [33], the EIS data of this study were fitted with Zsimpwin software. Rs represents the resistance of the solution. A passivation film and an electric double layer exist at the electrode/electrolyte solution interface, and Rfilm is the total resistance of the passivation film and the electric double layer. Studies have shown that the passivation film has a high charge transfer resistance, and the charge transfer resistance value mainly depends on the passivation film [34]. The lower the Rfilm value, the easier it is for the charge to pass through the passivation film [35]. Equivalent capacitance Qfilm (constant phase angle element CPE) is used for fitting instead of pure capacitance C [36]. The equivalent circuit diagram is shown in Figure 6a, and the fitting parameters are shown in Table 6. The Chi-squared (χ2) values is related to the fitting accuracy [34]. The order of magnitude of χ2 values for both coatings is 10−4, indicating that it is reasonable to use the Rs(QfilmRfilm) equivalent circuit to fit the EIS data in this study.
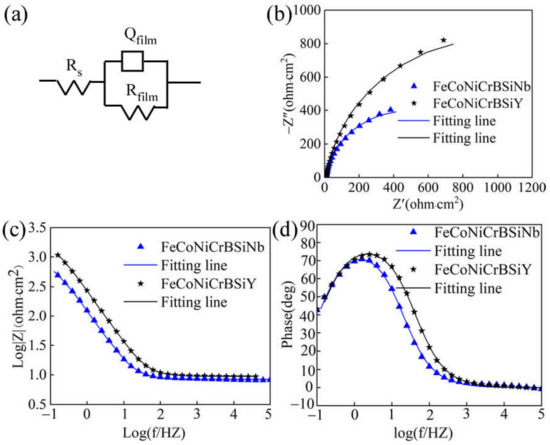
Figure 8.
(a) Equivalent circuit used to fit EIS data; (b) Nyquist plot and (c,d) Bode plot of FeCoNiCrBSiNb and FeCoNiCrBSiY coatings under OCP condition in 3.5wt% NaCl solution.

Table 6.
A series of electrochemical parameters fitted from the EIS curves.
It is reported in the literature that the capacitance of the passivation film on the coating can be represented by the equivalent capacitance Q [37]. Q includes the constant Y0(Ω−1·cm−2·s−n) and dimensionless exponent n. n is the deviation value from pure capacitive behavior. The closer the system is to an ideal capacitance, the closer the value of n is to 1 [38]. It can be found from Table 6 that the Qfilm-Y0 value (6.9 × 10−4 Ω−1·cm−2·s−n) of FeCoNiCrBSiY coating is smaller, indicating that the passivation film formed on its surface is thicker and denser [39,40]. The higher Rfilm value of FeCoNiCrBSiY coating indicates that its surface passivation film is stable and less sensitive to anions in solution.
3.5. Surface Analysis of the Passive Film
3.5.1. XPS Analysis
Figure 9 and Figure 10 show the X-ray photoelectron spectroscopy (XPS) of the passivation films formed by FeCoNiCrBSiNb and FeCoNiCrBSiY coatings in 3.5 wt.% NaCl solution, respectively. Figure 8a and Figure 9a show the spectra of Fe, Co, Cr, Ni and O, while the C peak is associated with inevitable air pollution. There is a main O peak in the measured energy spectra of FeCoNiCrBSiNb and FeCoNiCrBSiY coatings, which confirms the formation of oxide films on the coating surfaces.
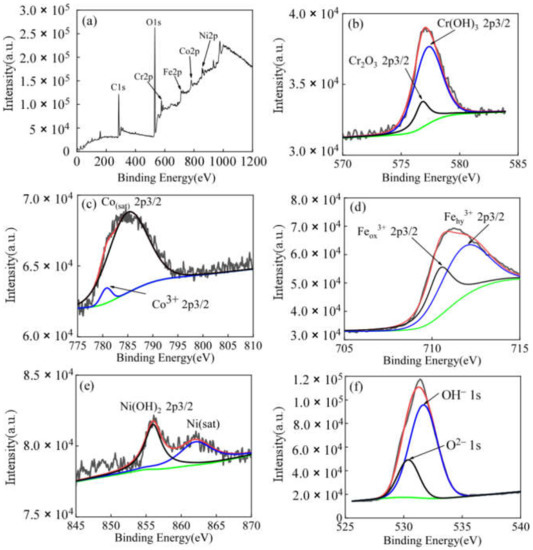
Figure 9.
High-resolution XPS pattern of the passivation film on FeCoNiCrBSiNb coating in 3.5 wt.%NaCl solution. (a) suevey spectrum; (b) Cr 2p3/2; (c) Co 2p3/2; (d) Fe 2p3/2; (e) Ni 2p3/2; (f) O 1s detail spectrums.
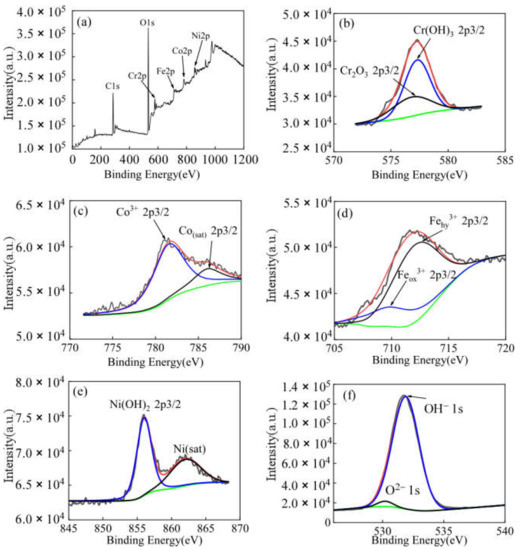
Figure 10.
High-resolution XPS pattern of the passivation film on FeCoNiCrBSiY coating in 3.5 wt.% NaCl solution. (a) suevey spectrum; (b) Cr 2p3/2; (c) Co 2p3/2; (d) Fe 2p3/2; (e) Ni 2p3/2; (f) O 1s detail spectrums.
Figure 9b and Figure 10b show the binding energies (576.8 eV and 577.3 eV) of the high-resolution spectra of Cr 2p3/2 on the surface passivation films of FeCoNiCrBSiNb and FeCoNiCrBSiY coatings. The chemical species corresponding to the two binding energies are Cr2O3 and Cr(OH)3, respectively [33]. Figure 9c and Figure 10c show the Co 2p3/2 spectra of the two coatings, the peak at 780.7 eV corresponds to Co3+ and the peak at 785 eV corresponds to Cosat (an unanalyzed companion peak) [41,42]. Figure 9d and Figure 10d show the binding energy components of the Fe 2p3/2 spectrum at 710 eV and 711.8 eV on the passivation films for the two coatings, representing Feox3+ and Fehy3+, respectively. This is similar to the results reported in CoCrFeMnNi high-entropy alloys [39]. The deconvolution results of iron species show that the main component in the passivation films of the two coatings is iron hydroxide.
Figure 9e and Figure 10e show the Ni 2p3/2 spectra of the passivation films of the two coatings, the peak at 855.7 eV corresponds to Ni(OH)2 and the peak at 859.2 eV corresponds to Nisat (Unanalyzed companion peak) [43]. The O1s spectra obtained from the passivation films on FeCoNiCrBSiNb and FeCoNiCrBSiY coatings are divided into 2 components (Figure 9f and Figure 10f). O2− species (530.2 eV) mainly exist in the form of Cr and Fe oxides. The peak at 531.8 eV represents OH− species, which exist in the passive film as metal hydroxides.
Figure 11 shows the atomic percentages of Fe, Co, Cr and Ni in the passivation films of FeCoNiCrBSiNb and FeCoNiCrBSiY coatings. It is obviously found that the passive film on the surface of FeCoNiCrBSiY coating contains lower Fe content and higher Cr content. The Cr/Fe ratios in the passivation films which formed on the FeCoNiCrBSiNb and FeCoNiCrBSiY coating are 0.15 and 1.8, respectively. This means that the passivation film on the surface of FeCoNiCrBSiY coating contains high content of Cr oxide or hydroxide, which is beneficial to improve its compactness and stability.
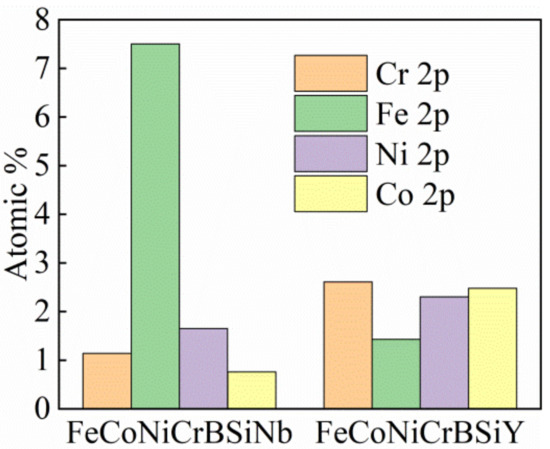
Figure 11.
Atomic percentage of each element in passivation films on the FeCoNiCrBSiNb and FeCoNiCrBSiY coatings.
3.5.2. Mott-Schottky Curves
In general, passivation films on metal surfaces exhibit semiconducting behavior [44]. By adjusting the applied voltage, the majority carriers in the oxide space charge layer can be taken out to make the layer in a depleted state. Figure 12 shows the Mott-Schottky plots of the passivation films on the surfaces of FeCoNiCrBSiNb and FeCoNiCrBSiY coatings. According to the mott-schottky theory, the capacitance of the p-type semiconductor space charge layer can be calculated according to formula (2) [45].
where NA is the doping density; εr is the dielectric constant of the passive film, which usually takes a value of 12 [46]; ε0 is the permittivity of the vacuum (8.854 × 10−14 F·m−1); k is the Boltzmann’s constant; e is the electron charge; EFB is the flat band potential; T is the absolute temperature; and kT/e is negligible (about 25 mV at room temperature).
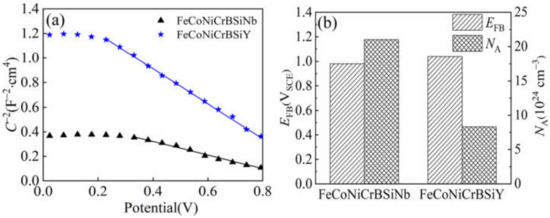
Figure 12.
(a) Mott-Schottky plots for the passive films formed on the FeCoNiCrBSiNb coating and FeCoNiCrBSiY coating in 3.5 wt% NaCl solution after film formation at 1 V for 1 h; (b) flat band potential and accepter densities of the passive films.
It can be seen from Figure 12a that the Mott-Schottky curves of FeCoNiCrBSiNb and FeCoNiCrBSiY coatings exhibit linear forms in the range of 0.33 VSCE~0.80 VSCE and 0.21 VSCE~0.80 VSCE, respectively. The Mott-Schottky linear relationship with negative slope indicates that the passivation films formed by both coatings in 3.5 wt.% NaCl solution have p-type semiconductor properties. The main carriers of p-type semiconductors are cation vacancies [47]. Similar phenomena have been reported in other studies on the properties of metal-semiconductors [48,49,50]. The carrier concentration (acceptor concentration, NA) and flat band potential (EFB) can be calculated according to the linear equation fitted in Figure 12a. Figure 12b shows the flat band potential values and acceptor concentration values of the passivation films of the two coatings. The acceptor concentration (NA = 8.3 × 1024 cm−3) of the passivation film of FeCoNiCrBSiY coating is lower than that of FeCoNiCrBSiNb coating (NA = 2.1 × 1025 cm−3), indicating that the passivation film of FeCoNiCrBSiY coating has fewer defects. In addition, the flat band potential (1.04 V) of the passivation film of FeCoNiCrBSiY coating is higher than that of FeCoNiCrBSiNb coating (0.98 V), indicating that the space charge layer of the passivation film on the surface of FeCoNiCrBSiY coating has a more stable structure and is not easily broken down.
4. Discussion
4.1. Phase Structure of the Coating
It can be seen from Figure 3 that both coatings have crystal phases formed during the laser cladding process. Studies have shown that crystallization is difficult to avoid in laser cladding because the cooling rate in the heat-affected zone is lower than the critical cooling rate required to avoid crystallization [51,52]. The results show that the phases formed by the two coatings are mainly borides, such as Cr2B, Fe2B, Fe3B, CoB and Ni4B3 phases. Similar phenomena have been reported in a large number of literatures [53,54,55,56]. This is related to the binding energy of boron and other metal elements [4]. Table 7 lists the mixing enthalpies of some atom pairs. The mixing enthalpy of Cr-B and Fe-B is relatively negative, which means that Cr and Fe can easily combine with B to form compounds. The phase structures of FeCoNiCrBSiNb and FeCoNiCrBSiY coatings are roughly similar. The difference is that the Laves phase exists in the FeCoNiCrBSiNb coating. According to literature reports [11,19], the Laves phase is (Co,Cr)Nb with a hexagonal close-packed structure. According to literature reports, borides and Laves phase can significantly improve the hardness of the coatings, but they are easy to cause galvanic corrosion and lead to a decrease in the corrosion resistance of the coatings [53,54,55,56].

Table 7.
Mixing enthalpy of different atom-pair [4] (KJ/mol).
4.2. Influence of Passive Film on Corrosion Resistance of Coatings
4.2.1. The Composition of the Passivation Film
A large number of studies have shown that Cr is a key element to form a passivation film on the surface of the alloy and improve the corrosion resistance of the alloy [35,36,39,57]. The ratio of Cr/Fe can characterize the chromium enrichment rate of the passive film [58]. It can be seen from Figure 11 that the Cr/Fe ratio (1.8) in the FeCoNiCrBSiY coating passivation film is significantly higher. This shows that there is a higher content of Cr in the passivation film of FeCoNiCrBSiY coating, and Cr exists in the form of oxide and hydroxide. Both Zhou et al. [43] and Zheng et al. [59] reported that a higher Cr/Fe ratio is beneficial for enhancing the compactness and stability of passivation films. Wang et al. [33] and Liu et al. [57] reported that both Cr(OH)3 and Cr2O3 in the passivation film are beneficial to improve the corrosion resistance of coatings.
According to the Pilling-Bedworth theory, the PBR value of Cr2O3 is greater than 1, resulting in compressive stress in the film [58]. The residual compressive stress can improve the stability of the passive film, resulting in improved corrosion resistance of the coating. In addition, Cr(OH)3 is beneficial to improve the activity of micropores in the passivation film and its repassivation ability, thereby improving its corrosion resistance [60]. The “water” in the hydrated oxide is believed to maintain the amorphous state of the film, thus maintaining the high corrosion resistance of the passivation film [59].
4.2.2. The Semiconducting Properties of the Passivation Film
Figure 12 shows that the passivation films of both coatings exhibit p-type semiconductor characteristics. According to the point defect model, chloride ions are transferred from the electrolyte solution to the oxygen vacancies of the passivation layer and reduce their concentration, thereby increasing the number of cation vacancies in the passivation layer and making the passivation film exhibit p-type semiconductor characteristics [61,62]. It can be seen from Figure 12a that in the potential range of 0.4–0.8 V, the capacitance of the passivation film formed on the surface of the FeCoNiCrBSiNb coating in the chloride-containing environment is significantly higher. This indicates that the porosity of the passivation film is increased due to the diffusion of chloride ions in the passivation layer and causing it to dissolve [63]. Studies have shown that chloride ions in the passivation film accelerate the dissolution of the passivation film through the adsorption and permeation of the acceptor (cation vacancies), thereby reducing the compactness and stability of the passivation film [50]. According to the calculation results in Figure 12b, the FeCoNiCrBSiNb coating passivation film contains more cation vacancies. Anion absorption leads to an increase in the number of cation vacancies at the passivation film/solution interface, which will pass through the passivation film and reach the passivation film/coating interface. If the diffusion rate of cation vacancies is high, the cations generated at the coating/passivation film interface cannot accommodate cation vacancies, so that a large number of cation vacancies aggregate, which will easily lead to local thinning of the passivation film or detachment from the coating [44].
Figure 12b also compares the flat band potential (EFB) of the passivation films of the two coatings. The Fermi level (EF) is inversely proportional to the flat band potential (EFB). Therefore, the Fermi level (EF) can be easily obtained by the value of EFB [64]. It is well known that the corrosion process is accompanied by the transfer of electrons. In general, the transfer of electrons becomes easier with the increase of the EF value and reduces the corrosion resistance of the passivation film [65]. It can also be seen from Figure 12b that the FeCoNiCrBSiY coating passivation film has a higher flat band potential (1.04 V), which indicates that the FeCoNiCrBSiY coating passivation film has better protection performance. Similar findings were also reported by Zhou et al. [43]. In addition, they also reported that a higher flat-band potential would increase the stability of the space charge layer of the passivation film and make the passivation film more difficult to destroy.
5. Conclusions
- (1)
- FeCoNiCrBSiNb powder is composed of face-centered cubic solid solution (FCC), Laves and Cr2B. FeCoNiCrBSiY powder is composed of face-centered cubic solid solution (FCC) and Cr2B. Due to the remelting and multiple heat treatments during the coating preparation, various borides such as Cr2B, Fe2B, Fe3B, CoB and Ni4B3 phases were precipitated in the coating. The amorphous or nanocrystalline content of the top layer of the coatings increases significantly due to the faster cooling rate.
- (2)
- Both coatings exhibited dendritic structures in the bonding area with the substrate. In the FeCoNiCrBSiNb coating, the size of the dendrites on top of the coating is significantly reduced. A large number of elongated borides precipitated in the FeCoNiCrBSiY coating. Due to the faster cooling rate of the top layer of the coating, the content of the borides decreases and the size becomes smaller.
- (3)
- FeCoNiCrBSiY coating exhibits high corrosion potential (Ecorr = −340 mV), low corrosion current density (icorr = 1.2 × 10−6 A/cm2), large polarization resistance (Rp = 20,000 Ohm·cm2) and high passivation film resistance (Rfilm = 1977 Ohm·cm2) in 3.5 wt.% NaCl solution, showing better corrosion resistance. The passivation film formed on the FeCoNiCrBSiY coating contains higher Cr/Fe ratio, indicating that the passivation film has better compactness.
Author Contributions
Conceptualization, W.G. and B.L.; methodology, H.Z.; software, H.Z., J.Z. and W.L.; validation, B.L., W.G. and Z.D.; formal analysis, Z.D.; investigation, H.Z., W.L., H.X. and L.C.; resources, W.G. and B.L.; data curation, W.L., H.X. and L.C.; writing—original draft preparation, H.Z.; writing—review and editing, B.L. and W.G.; visualization, Z.D.; supervision, B.L.; project administration, W.G. and B.L.; funding acquisition, W.G. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Postdoctoral Science Foundation (2019M662798), National Natural Science Foundation of China (52101208), Research Foundation of Education Bureau of Hunan Province, China (21A0465) and Postgraduate Scientific Research Innovation Project Shaoyang University (CX2020SY028).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, M.X.; Melchers, R.; Chaves, L. Corrosion and pitting of 6060 series aluminium after 2 years exposure in seawater splash, tidal and immersion zones. Corros. Sci. 2018, 140, 286–296. [Google Scholar] [CrossRef]
- Chen, M.D.; Pang, K.; Liu, Z.Y.; Wu, J.S.; Li, X.G. Influence of rust permeability on corrosion of E690 steel in industrial and non-industrial marine splash zones. Mater. Eng. Perform. 2018, 27, 3742–3749. [Google Scholar] [CrossRef]
- Si, C.R.; Duan, B.B. Microstructure, corrosion-resistance, and wear-resistance properties of subsonic flame sprayed amorphous Fe-Mo-Cr-Co coating with extremely high amorphous rate. J. Mater. Res. Technol. 2020, 9, 3292–3303. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, Q.; Li, M. New ferromagnetic (Fe1/3Co1/3Ni1/3)80(P1/2B1/2)20 high entropy bulk metallic glass with superior magnetic and mechanical properties. J. Alloys Compd. 2019, 791, 947–951. [Google Scholar] [CrossRef]
- Ding, J.; Inoue, A.; Han, Y.; Kong, F.; Zhu, S.; Wang, Z.; Shalaan, E.-S.; Al-Marzouki, F. High entropy effect on structure and properties of (Fe,Co,Ni,Cr)-B amorphous alloys. J. Alloys Compd. 2017, 696, 345–352. [Google Scholar] [CrossRef]
- Shu, F.Y.; Zhang, B.L.; Liu, T.; Sui, S.H.; Liu, Y.X.; He, P.; Liu, B.; Xu, B.S. Effects of laser power on microstructure and properties of laser cladded CoCrBFeNiSi high-entropy alloy amorphous coatings. Surf. Coat. Technol. 2019, 358, 667–675. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y. Solid-Solution CrCoCuFeNi High-Entropy Alloy Thin Films Synthesized by Sputter Deposition. Mater. Res. Lett. 2015, 34, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Cui, R.; Cheng, Y.H.; Meng, X.L.; Feng, S.Z.; Han, Z.T. Microstructure and properties of heat treated 1Cr17Ni4MoB steel fabricated by laser melting deposition. Mater. Process. Technol. 2017, 108, 59–68. [Google Scholar]
- Olakanmi, E.O.; Sepako, M.; Morake, J.; Hoosain, S.E.; Pityana, S.L. Microstructural characteristics, crack frequency and diffusion kinetics of functionally graded Ti-Al composite coatings: Effects of laser energy density (LED). Met. Mater. Soc. 2018, 71, 900–911. [Google Scholar] [CrossRef]
- Cheng, J.B.; Sun, B.; Ge, Y.Y.; Hu, X.L. Nb doping in laser-cladded Fe25Co25Ni25(B0.7Si0.3)25 high entropy alloy coatings: Microstructure evolution and wear behavior. Surf. Coat Technol. 2020, 402, 126321. [Google Scholar] [CrossRef]
- Shu, F.Y.; Yang, B.; Dong, S.Y.; Zhao, H.Y.; Xu, B.S.; Xu, F.J.; Liu, B.; He, P.; Feng, J.C. Effects of Fe-to-Co ratio on microstructure and mechanical properties of laser cladded FeCoCrBNiSi high-entropy alloy coatings. Appl. Surf. Sci. 2018, 450, 538–544. [Google Scholar] [CrossRef]
- Cheng, J.B.; Sun, B.; Ge, Y.; Hu, X.; Zhang, L.; Liang, X.; Zhang, X. Effect of B/Si ratio on structure and properties of high-entropy glassy Fe25Co25Ni25(BxSi1-x)25 coating prepared by laser cladding. Surf. Coat. Technol. 2020, 402, 126320. [Google Scholar] [CrossRef]
- Shu, F.Y.; Wu, L.; Zhao, H.Y.; Sui, S.H.; Zhou, L.; Zhang, J.; He, W.X.; He, P.; Xu, B.S. Microstructure and high-temperature wear mechanism of laser cladded CoCrBFeNiSi high-entropy alloy amorphous coating. Mater. Lett. 2018, 211, 235–238. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Z.W.; Jiang, J.Z. High entropy metallic glasses: Glass formation, crystallization and properties. J. Alloys Compd. 2021, 866, 158852. [Google Scholar] [CrossRef]
- Shu, F.Y.; Liu, S.; Zhao, H.Y.; He, W.X.; Sui, S.H.; Zhang, J.; He, P.; Xu, B.S. Structure and high-temperature property of amorphous composite coating synthesized by laser cladding FeCrCoNiSiB high-entropy alloy powder. J. Alloys Compd. 2018, 731, 662–666. [Google Scholar] [CrossRef]
- Koga, G.; Otani, L.; Silva, A.; Roche, V.; Nogueira, R.; Jorge, A.; Bolfarini, C.; Kiminami, C.; Botta, W. Characterization and corrosion resistance of boron-containing-austenitic stainless steels produced by rapid solidification techniques. Materials 2018, 11, 2189. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.L.; Zhang, Z.Y.; Gong, Y.B.; Nie, G.M. Microstructures and corrosion resistance of Fe-based amorphous/nanocrystalline coating fabricated by laser cladding. J. Alloys Compd. 2017, 728, 1116–1123. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, D.; Liang, X.; Xu, B. Microstructure and electrochemical properties of CoCrCuFeNiNb high-entropy alloys coatings. Acta. Metall. Sin.-Engl. 2014, 27, 1031–1037. [Google Scholar] [CrossRef]
- Xiao, M.Y.; Gao, H.B.; Sun, L.B.; Wang, Z. Microstructure and mechanical properties of Fe-based amorphous alloy coatings prepared by ultra-high speed laser cladding. Mater. Lett. 2021, 297, 130002. [Google Scholar] [CrossRef]
- Milanti, A.; Matikainen, V.; Koivuluoto, H.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. Effect of spraying parameters on the microstructural and corrosion properties of HVAF-sprayed Fe-Cr-Ni-B-C coatings. Surf. Coat. Technol. 2015, 277, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Z.; Cheng, Y.H.; Yang, J.Y.; Wang, Q.Q. Influence of laser remelting on organization, mechanical properties and corrosion resistance of Fe-based amorphous composite coating. Surf. Coat. Technol. 2021, 414, 127081. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Corrosion behavior of (Ti-Al-Cr-Si-V)xNy coatings on mild steels derived from RF magnetron sputtering. Surf. Coat. Technol. 2008, 203, 558–561. [Google Scholar] [CrossRef]
- Bijalwan, P.; Kumar, A.; Nayak, S.K.; Banerjee, A.; Laha, T. Microstructure and corrosion behavior of Fe-based amorphous composite coatings developed by atmospheric plasma spraying. J. Alloys Compd. 2019, 796, 47–54. [Google Scholar] [CrossRef]
- Huang, F.; Kang, J.J.; Yue, W.; Fu, Z.Q.; Zhu, L.N.; She, D.S.; Liang, J.; Wang, C.B. Corrosion Behavior of FeCrMoCBY Amorphous Coating Fabricated by High-Velocity Air Fuel Spraying. Therm. Spray Technol. 2019, 28, 842–850. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Y.; Zhang, J.; Hong, S.; Chen, L.; Qin, Y. A Comparative study of cyclic oxidation and sulfates-Induced hot corrosion behavior of arc-sprayed Ni-Cr-Ti coatings at moderate temperatures. Therm. Spray Technol. 2015, 24, 789–797. [Google Scholar] [CrossRef]
- Guo, W.M.; Zhang, H.L.; Zhao, S.; Ding, Z.B.; Liu, B.; Li, W.J.; Xu, H.H.; Liu, H.Y. Corrosion behavior of the CoNiCrAlY-Al2O3 composite coating based on core-shell structured powder design. Materials 2021, 14, 7093. [Google Scholar] [CrossRef]
- Berger, J.; Jorge, A.M., Jr.; Koga, G.Y.; Roche, V.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Influence of chromium concentration and partial crystallization on the corrosion resistance of FeCrNiB amorphous alloys. Mater. Charact. 2021, 179, 111369. [Google Scholar] [CrossRef]
- Liu, W.H.; Shieu, F.S.; Hsiao, W.T. Enhancement of wear and corrosion resistance of iron-based hard coatings deposited by high-velocity oxygen fuel (HVOF) thermal spraying. Surf. Coat. Technol. 2014, 249, 24–41. [Google Scholar] [CrossRef]
- Kiminami, C.S.; Souza, C.; Bonavina, L.F. Partial crystallization and corrosion resistance of amorphous Fe-Cr-M-B (M = Mo, Nb) alloys. J. Non-Cryst Solids 2010, 356, 2651–2657. [Google Scholar] [CrossRef]
- Botta, W.J.; Berger, J.E.; Kiminami, C.S.; Roche, V.; Nogueira, R.P.; Bolfarini, C. Corrosion resistance of Fe-based amorphous alloys. J. Alloys Compd. 2014, 586, 105–110. [Google Scholar] [CrossRef]
- Hao, E.K.; Liu, X.; An, Y.L.; Zhou, H.D.; Yan, F.Y. The coupling effect of immersion corrosion and cavitation erosion of NiCoCrAlYTa coatings in artificial seawater. Corros. Sci. 2020, 169, 108635. [Google Scholar] [CrossRef]
- Wang, W.R.; Qi, W.; Zhang, X.L.; Yang, X.; Xie, L.; Li, D.Y.; Xiang, Y.H. Superior corrosion resistance-dependent laser energy density in (CoCrFeNi)95Nb5 high entropy alloy coating fabricated by laser cladding. Int. J. Min. Met. Mater. 2021, 28, 888–897. [Google Scholar] [CrossRef]
- Feng, K.; Zhang, Y.; Li, Z.G.; Yao, C.W.; Yao, L.; Fan, C.Y. Corrosion properties of laser cladded CrCoNi medium entropy alloy coating. Surf. Coat. Technol. 2020, 397, 126004. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhou, Z.H.; Wang, Q.J. Role of passive film in dominating the electrochemical corrosion behavior of FeCrMoCBY amorphous coating. J. Alloys Compd. 2019, 811, 151962. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, W.H.; Yang, H.L.; Huang, H.; Ji, S.X.; Ruan, J.M.; Liu, Z.L. Corrosion behavior of CoCrNi medium-entropy alloy compared with 304 stainless steel in H2SO4 and NaOH solutions. Corros. Sci. 2020, 177, 108973. [Google Scholar] [CrossRef]
- Antou, G.; Montavon, G.; Hlawka, F.; Cornet, A.; Coddet, C. Exploring thermal spray gray alumina coating pore network architecture by combining stereological protocols and impedance electrochemical spectroscopy. Therm. Spray Technol. 2006, 15, 765–772. [Google Scholar] [CrossRef]
- Gong, X.; Cui, Y.; Wei, D.; Liu, B.; Liu, R.; Nie, Y.; Li, Y. Building direction dependence of corrosion resistance property of Ti-6Al-4V alloy fabricated by electron beam melting. Corros. Sci. 2017, 127, 101–109. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Benoit, M.; Bataillon, C.; Gwinner, B.; Miserque, F.; Vivier, V. Comparison of different methods for measuring the passive film thickness on metals. Electrochim. Acta 2016, 201, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Qian, W.X.; Ma, H.F.; Zhang, H.T.; Ying, W.Y. Highly selective production of heavy hydrocarbons over cobalt–graphene–silica nanocomposite catalysts. Rsc Adv. 2017, 7, 33441–33449. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.; Celik, G.; Gunduz, S.; Dogu, D.; Zhang, S.; Shan, J.; Tao, F.F.; Ozkan, U.S. Oxygen mobility in prereduced nano- and macro-ceria with Co loading: An AP-XPS, In-Situ DRIFTS and TPR Study. Catal. Lett. 2017, 147, 2863–2876. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, B.; Guo, W.; Fu, A.; Duan, H.; Li, W. Corrosion behavior and mechanism of FeCrNi medium entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2021, 867, 159094. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Jiang, L.L. Comparison of the pitting susceptibility and semiconducting properties of the passive films on carbon steel in chromate and bicarbonate solutions. Appl. Surf. Sci. 2000, 167, 113–121. [Google Scholar] [CrossRef]
- Li, G.; Gan, Y.; Liu, C.; Shi, Y.; Zhao, Y.; Kou, S. Corrosion and Wear Resistance of Fe-Based Amorphous Coatings. Coatings 2020, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Belo, M.; Hakiki, N.E.; Ferreira, M. Semiconducting properties of passive films formed on nickel-base alloys type Alloy 600: Influence of the alloying elements. Electrochim. Acta 1999, 44, 2473–2481. [Google Scholar] [CrossRef]
- De Oliveira, M.C.L.; Pereira, V.S.M.; Correa, O.V.; de Lima, N.B.; Antunes, R.A. Correlation between the corrosion resistance and the semiconducting properties of the oxide film formed on AZ91D alloy after solution treatment. Corros. Sci. 2013, 69, 311–321. [Google Scholar] [CrossRef]
- Elzbieta, S.; Macdonald, D.D. Nature of the passive film on nickel. Electrochim. Acta 2002, 48, 69–77. [Google Scholar]
- Oje, A.M.; Ogwu, A.A.; Rahman, S.U.; Tsendzughul, N. Effect of temperature variation on the corrosion behaviour and semiconducting properties of the passive film formed on chromium oxide coatings exposed to saline solution. Corros. Sci. 2019, 154, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.D.; Wei, X.S.; Wang, Y.; Jiang, H.; Shen, J. Electrochemical behaviors and passive film properties of Fe-based bulk metallic glass in Cl-containing acetic acid solutions under high temperature. J. Alloys Compd. 2018, 766, 964–972. [Google Scholar] [CrossRef]
- Yang, G.; Lin, X.; Liu, F.; Hu, Q.; Ma, L.; Li, J.; Huang, W. Laser solid forming Zr-based bulk metallic glass. Intermetallics 2012, 22, 110–115. [Google Scholar] [CrossRef]
- Pauly, S.; Löber, L.; Petters, R.; Stoica, M.; Scudino, S.; Kühn, U.; Eckert, J. Processing metallic glasses by selective laser melting. Mater. Today 2013, 16, 37–41. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.J.; Dai, P.Q. Evolution of the microstructure and properties of laser-clad FeCrNiCoBx high-entropy alloy coatings. Mater. Sci. Technol. 2016, 32, 1666–1672. [Google Scholar] [CrossRef]
- Berger, J.E.; Schulz, R.; Savoie, S.; Gallego, J.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Wear and corrosion properties of HVOF coatings from Superduplex alloy modified with addition of boron. Surf. Coat. Technol. 2017, 309, 911–919. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, Y.; Liao, B.; Sun, Y.; Dai, X.; Yang, J.; Li, Z. Effect of laser remelting on microstructure and properties of WC reinforced Fe-based amorphous composite coatings by laser cladding. Opt. Laser Technol. 2018, 103, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Sudha, C.; Shankar, P.; Rao, R.V.; Thirumurugesan, R.; Vijayalakshmi, M.; Raj, B. Microchemical and microstructural studies in a PTA weld overlay of Ni-Cr-Si-B alloy on AISI 304L stainless steel. Surf. Coat. Technol. 2008, 202, 2103–2112. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, P.J.; Hao, J.B. Microstructural characterization and corrosion behaviour of AlCoCrFeNiTix high-entropy alloy coatings fabricated by laser cladding. Surf. Coat. Technol. 2019, 361, 63–74. [Google Scholar] [CrossRef]
- Cui, Z.; Qin, Z.; Dong, P.; Mi, Y.; Gong, D.; Li, W. Microstructure and corrosion properties of FeCoNiCrMn high entropy alloy coatings prepared by high speed laser cladding and ultrasonic surface mechanical rolling treatment. Mater. Lett. 2019, 259, 126769. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G. Effects of surface treatments on the corrosion and erosion-corrosion of 304 stainless steel in 3.5% NaCl solution. Corros. Sci. 2016, 112, 657–668. [Google Scholar] [CrossRef]
- Rossi, A.; Elsener, B. Role of the interface oxide film/alloy composition and stability of stainless steels. Mater. Corros. 2012, 63, 1188–1193. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.D.; Chen, L.X.; Yuan, J.; Zhang, Z.H.; Wang, Q.D. The effect of partial substitution of Zr for Ti on the electrochemical properties and surface passivation film of Mg35Ti10−xZrxNi55 (x = 1, 3, 5, 7, 9) electrode alloys. J. Alloys Compd. 2002, 337, 296–302. [Google Scholar] [CrossRef]
- Amri, J.; Souier, T.; Malki, B.; Baroux, B. Effect of the final annealing of cold rolled stainless steels sheets on the electronic properties and pit nucleation resistance of passive films. Corros. Sci. 2008, 50, 431–435. [Google Scholar] [CrossRef]
- Cardoso, M.V.; Amaral, S.T.; Martini, E. Temperature effect in the corrosion resistance of Ni–Fe–Cr alloy in chloride medium. Corros. Sci. 2008, 50, 2429–2436. [Google Scholar] [CrossRef]
- Lu, W.; Wang, D.; Wang, Q.; Yang, F.; Li, T.; Shi, Y.; Zhang, S.; Yang, B. Sensitivity of corrosion behavior for Fe-based amorphous coating to temperature and chloride concentration. Coatings 2021, 11, 331. [Google Scholar] [CrossRef]
- Wang, Z.M.; Ma, Y.T.; Zhang, J.; Hou, W.; Chang, X.; Wang, J. Influence of yttrium as a minority alloying element on the corrosion behavior in Fe-based bulk metallic glasses. Electrochim. Acta 2009, 54, 261–269. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).