Composition, Structure, and Properties of Ti, Al, Cr, N, C Multilayer Coatings on AISI W1-7 Alloyed Tool Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Research
3. Results and Discussion
4. Conclusions
- The possibility of titanium aluminizing of AISI W1-7 steel with precoated methods of chemical–thermal treatment is shown, including diffusion chromium with carbide layers of Cr7C3 and Cr23C6, and nitriding as well as physical gas deposition methods of TiN layer. The obtained coatings are chromium carbides Cr7C3, Cr23C6, TiC titanium carbide, TiN titanium nitride, and intermetallics with Ti, Al, Cr, and Fe. Layers of chromium carbides and TiN titanium nitride act as barriers that prevent the penetration of aluminum into the steel base and inhibit the formation of compounds under the Feα(Al) layer;
- The maximum microhardness was found for TiC layers, 30.3–35.5 GPa, and TiN layers, 22.0–22.6 GPa. The maximum microhardness of the Fe2(Ti, Al)4O layer was found for the nitrogen titanium aluminized coating, 14.0 GPa, because of the presence of nitrogen;
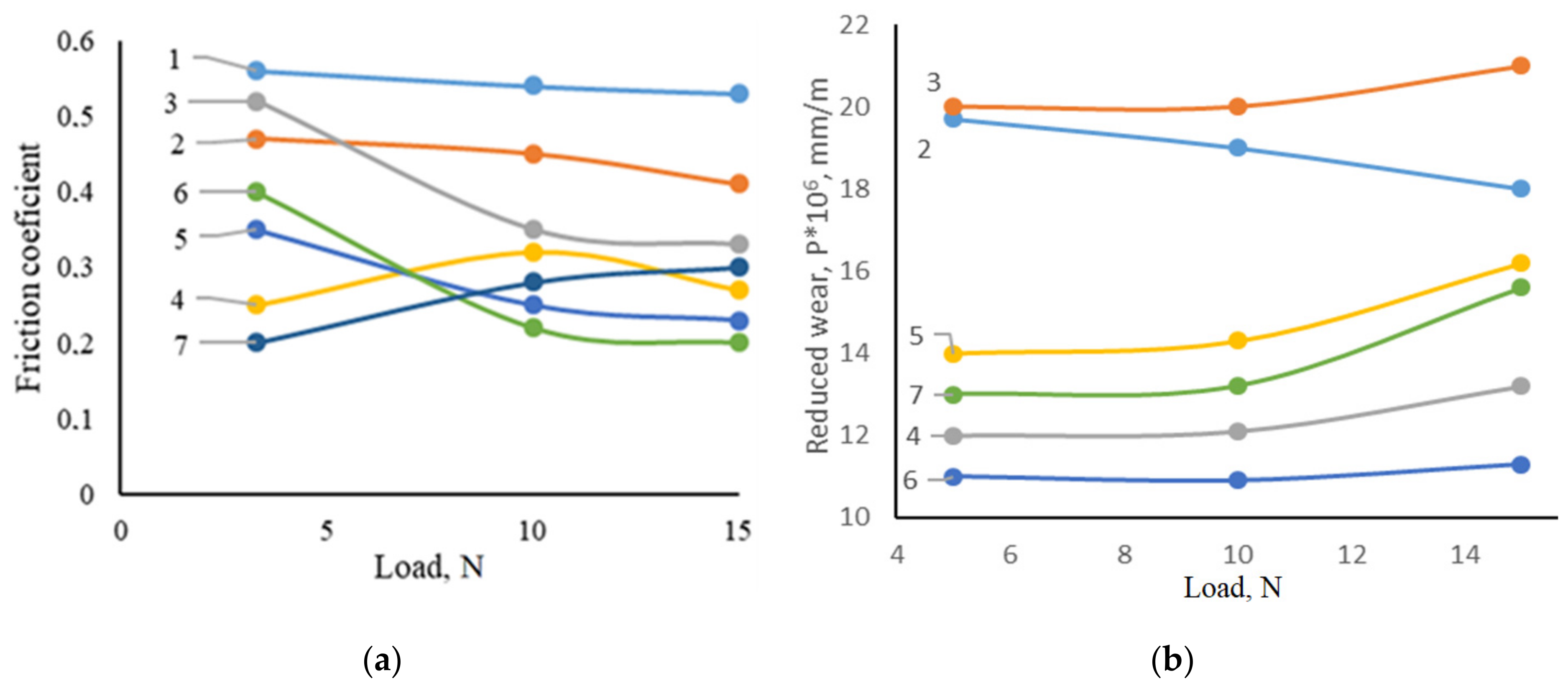
- The obtained coatings increase the heat resistance of AISI W1-7 steel at a temperature of 900 °C by 4.2–8.5 times and wear resistance under sliding friction conditions without lubrication up to 5.4 times. Maximum heat resistance showed coatings obtained by titanium alloying of chrome-plated steel. The advantage of coatings containing chromium is due to the formation during oxidation of high-quality protective film (Al, Cr)2O3. Under the same oxidation conditions, a less dense, loose film of TiO2 and Al2O3 oxides with unsatisfactory protective properties is formed on the surface of titanium alloy coatings. The high wear resistance of the coatings studied in this work is due to their structure and high microhardness of the individual components: the presence of layers TiC, TiN, Cr7C3, Cr23C6, intermetallics, TiO2, Cr2O3, and Al2O3. The properties of coatings and their structure, formed by friction layers of aluminum and titanium oxides, cause a decrease in the coefficients of friction of AISI W1-7 steel with coatings in contact with the counterweight and thus contribute to increased wear resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bitay, E.; Tóth, L.; Kovács, T.A.; Nyikes, Z.; Gergely, A.L. Experimental Study on the Influence of TiN/AlTiN PVD Layer on the Surface Characteristics of Hot Work Tool Steel. Appl. Sci. 2021, 11, 9309. [Google Scholar] [CrossRef]
- Inoue, S.; Uchida, H.; Hioki, A.; Koterazawa, K.; Howson, R.P. Structure and composition of (Ti, Al)N films prepared by r.f. planar magnetron sputtering using a composite target. Thin Solid Films 1995, 271, 15–18. [Google Scholar] [CrossRef]
- Jehn, H.A. Multicomponent and multiphase hard coatings for tribological applications. Surf. Coat. Technol. 2000, 131, 433–440. [Google Scholar] [CrossRef]
- Kohlscheen, J.; Bareiss, C. Effect of hexagonal phase content on wear behaviour of AlTiN arc PVD coatings. Coatings 2018, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Boichot, R.; Blanquet, E.; Mercier, F.; Pons, M. Chemical vapor deposition of titanium nitride thin films: Kinetics and experiments. CrystEngComm 2019, 21, 3974–3981. [Google Scholar] [CrossRef]
- Arai, T.; Moriyama, S. Growth behavior of chromium carbide and niobium carbide layers on steel substrate, obtained by salt bath immersion coating process. Thin Solid Films 1995, 259, 174–180. [Google Scholar] [CrossRef]
- Fan, X.S.; Yang, Z.G.; Zhang, C.; Zhang, Y.D.; Che, H.Q. Evaluation of vanadium carbide coatings on AISI H13 obtained by thermo-reactive deposition/diffusion technique. Surf. Coat. Technol. 2010, 20, 641–646. [Google Scholar] [CrossRef]
- Chabak, Y.G.; Fedun, V.I.; Pastukhova, T.V.; Zurnadzhy, V.I.; Berezhnyy, S.P.; Efremenko, V.G. Modification of steel surface by pulsed plasma heating. Probl. At. Sci. Technol. 2017, 110, 97–102. [Google Scholar]
- Oskolkova, T.N.; Glezer, A.M. Surface hardening of hard tungsten-carbide alloys: A review. Steel Transl. 2017, 47, 788–796. [Google Scholar] [CrossRef]
- Kostyk, K.; Kuric, I.; Saga, M.; Kostyk, V.; Ivanov, V.; Kovalov, V.; Pavlenko, I. Impact of magnetic-pulse and chemical-thermal treatment on alloyed steels’ surface layer. Appl. Sci. 2022, 12, 469. [Google Scholar] [CrossRef]
- Kowalski, S. Influence of diamond-like carbon coatings on the wear of the press joint components. Wear 2021, 486–487, 204076. [Google Scholar] [CrossRef]
- Kameneva, A.; Antonova, N.; Pesin, M.; Makarov, V.; Nikitin, S.; Bublik, N. Structural and phase transformations control in Ti and Al cathode materials, WC-Co substrate, and Ti1-xAlxN coating to improve their physico-mechanical and wear properties. Int. J. Refract. Met. Hard Mater. 2022, 102, 105726. [Google Scholar] [CrossRef]
- Kowalski, S. The influence of selected PVD coatings on fretting wear in a clamped joint based on the example of a rail vehicle wheel set. Eksploat. Niezawodn. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Von Fieandt, L.; Larsson, T.; Lindahl, E.; Bäcke, O.; Boman, M. Chemical vapor deposition of TiN on transition metal substrates. Surf. Coat. Technol. 2018, 334, 373–383. [Google Scholar] [CrossRef]
- Richter, V.; Potthoff, A.; Pompe, W.; Gelinsky, M.; Ikonomidou, H.; Bastian, S.; Schirmer, K.; Scholz, S.; Hofinger, J. Evaluation of health risks of nano- and microparticles. Powder Met. 2008, 51, 8–9. [Google Scholar] [CrossRef]
- Chen, J.-K.; Chen, S.-F.; Huang, C.-S. Formation of Al and Cr dual coatings by pack cementation on SNCM439 steel. ISIJ Int. 2012, 52, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Geib, F.D.; Rapp, R.A. Simultaneous chromizing–Aluminizing coating of low-alloy steels by a halide-activated, pack-cementation process. Oxid. Met. 1993, 40, 213–228. [Google Scholar] [CrossRef]
- Mei, S.Q.; Guryev, A.M.; Ivanov, S.G.; Lygdenov, B.D.; Tsydypov, B.S.; He, X.Z.; Liang, Q.Y. Research on the chance of increasing the wear resistance of high-speed steel using chemical thermal treatment methods. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479, 012055. [Google Scholar] [CrossRef]
- Petkov, N.; Bakalova, T.; Bahchedzhiev, H.; Louda, P.; Kejzlar, P.; Capkova, P.; Kormunda, M.; Rysanek, P. Cathodic arc deposition of TiCN coatings-influence of the C2H2/N2 ratio on the structure and coating properties. J. Nano Res. 2018, 51, 78–91. [Google Scholar] [CrossRef]
- Trotsan, A.I.; Kaverinskii, V.V.; Brodetskii, I.L. Use of fine powders of refractory carbides and nitrides for inoculation of iron-carbon alloys. Powder Metall. Met. Ceram. 2014, 52, 600–605. [Google Scholar] [CrossRef]
- Fan, Y.; Li, L.; Zhang, Y.; Zhang, X.; Geng, D.; Hu, W. Recent advances in growth of transition metal carbides and nitrides (MXenes) crystals. Adv. Funct. Mater. 2022, 2022, 2111357. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, C.; Zhu, S.; Wang, F.; Yuan, J.; Wang, P. Comparison of CrN, AlN and TiN diffusion barriers on the interdiffusion and oxidation behaviors of Ni+CrAlYSiN nanocomposite coatings. Crystals 2021, 11, 1333. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Wilkinson, D.S.; Veldhuis, S.C.; Dosbaeva, G.K.; Weatherly, G.C. Oxidation resistant Ti-Al-Cr alloy for protective coating applications. Intermetallics 2006, 14, 189–197. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Y.; Gong, S.; Xu, H. Effect of Ti–Al–Cr coatings on the high temperature oxidation behavior of TiAl alloys. Mater. Sci. Eng. A 2001, 307, 182–187. [Google Scholar] [CrossRef]
- Grachev, V.A.; Rozen, A.E.; Perelygin, Y.P.; Kireev, S.Y.; Los, I.S. Multilayer corrosion-resistant material based on iron–carbon alloys. Heliyon 2020, 6, e04039. [Google Scholar] [CrossRef]
- Joshi, A.; Hu, H.S. Oxidation behavior of titanium-aluminium nitrides. Surf. Coat. Technol. 1995, 76–77, 499–507. [Google Scholar] [CrossRef]
- Genova, V.; Paglia, L.; Pulci, G.; Bartuli, C.; Marra, F. Diffusion aluminide coatings for hot corrosion and oxidation protection of nickel-based superalloys: Effect of fluoride-based activator salts. Coatings 2021, 11, 412. [Google Scholar] [CrossRef]
- Tarelnyk, V.B.; Gaponova, O.P.; Loboda, V.B.; Konoplyanchenko, E.V.; Martsinkovskii, V.S.; Semirnenko, Y.I.; Tarelnyk, N.V.; Mikulina, M.A.; Sarzhanov, B.A. Improving ecological safety when forming wear-resistant coatings on the surfaces of rotation body parts of 12Kh18N10T steel using a combined technology based on electrospark alloying. Surf. Eng. Appl. Electrochem. 2021, 57, 173–184. [Google Scholar] [CrossRef]
- Chintha, A.R. Metallurgical aspects of steels designed to resist abrasion, and impact-abrasion wear. Mater. Sci. Technol. 2019, 35, 1133–1148. [Google Scholar] [CrossRef] [Green Version]
- Vencl, A.; Vucetic, F.; Bobic, B.; Pitel, J.; Bobic, I. Tribological characterisation in dry sliding conditions of compocasted hybrid A356/SiCp/Grp composites with graphite macroparticles. Int. J. Adv. Manuf. Technol. 2019, 100, 2135–2146. [Google Scholar] [CrossRef]
- Hovorun, T.; Khaniukov, K.; Varakin, V.; Pererva, V.; Vorobiov, S.; Burlaka, A.; Khvostenko, R. Improvement of the physical and mechanical properties of the cutting tool by applying wear-resistant coatings based on Ti, Al, Si, and N. J. Eng. Sci. 2021, 8, C13–C23. [Google Scholar] [CrossRef]
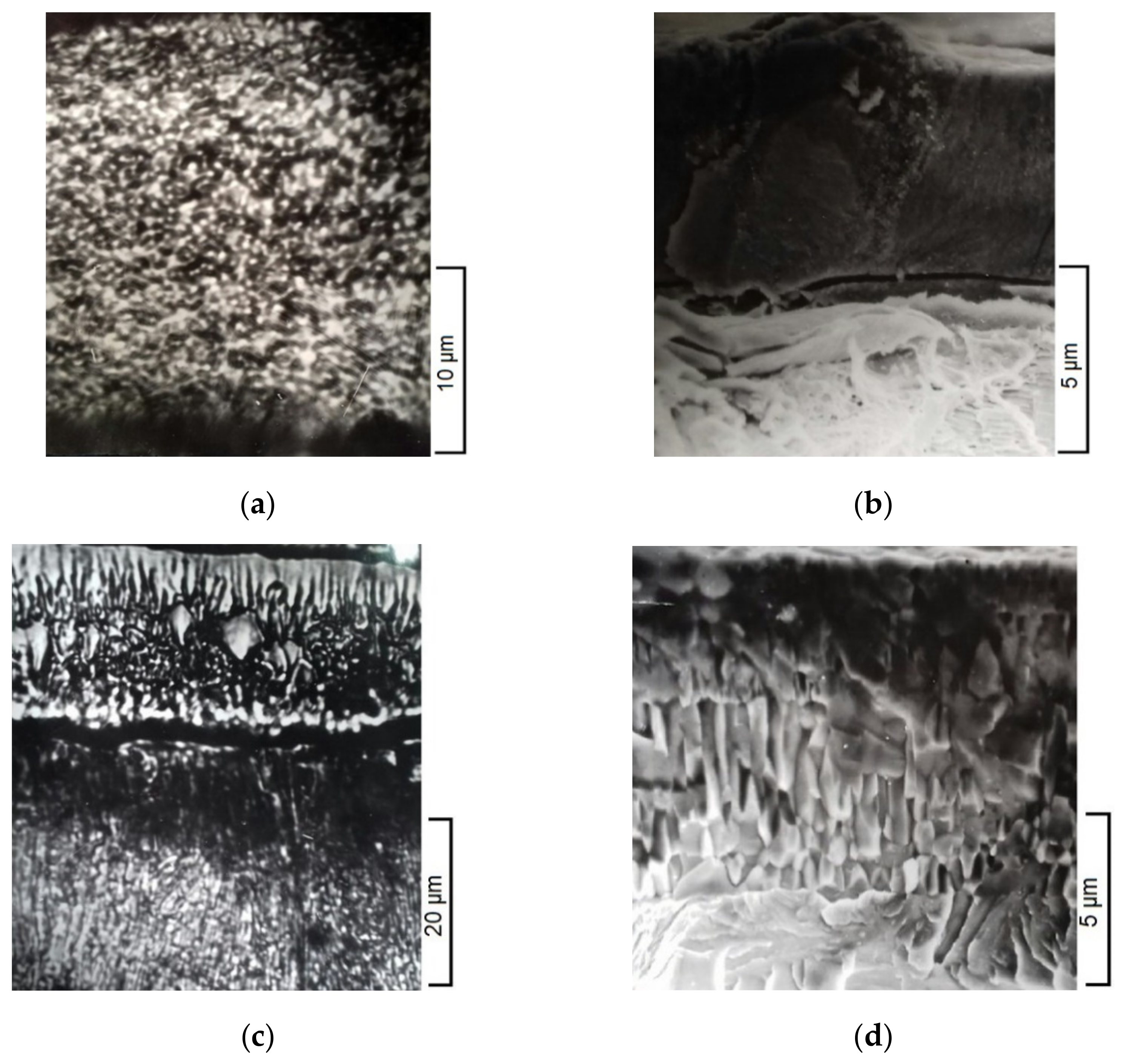
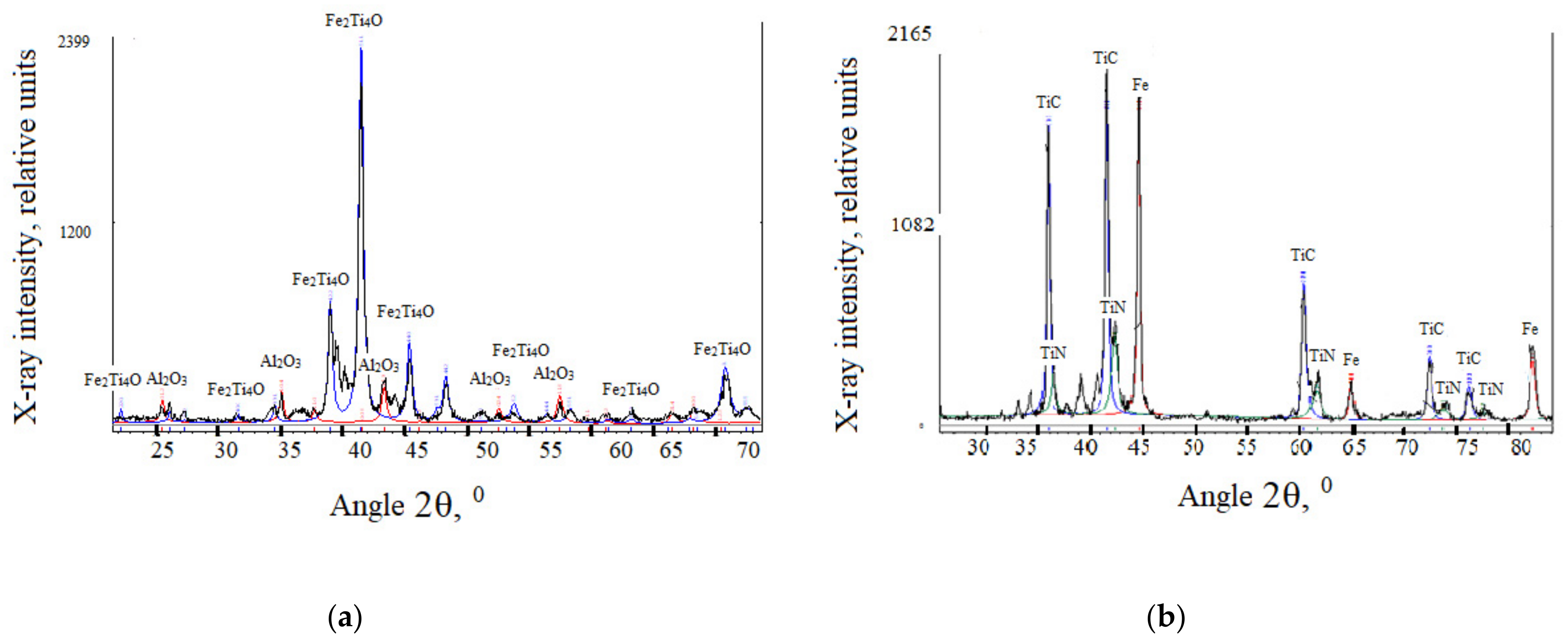

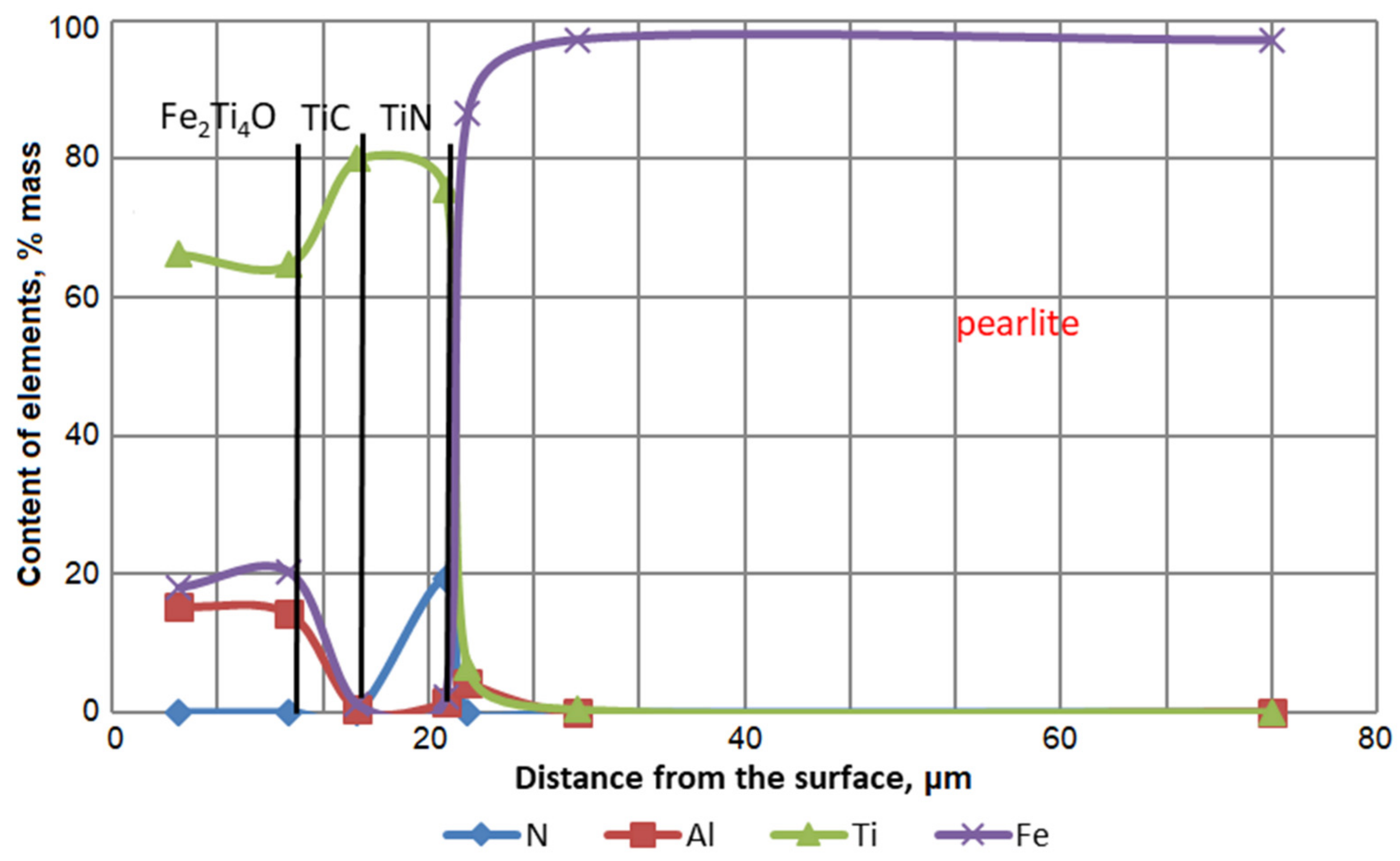
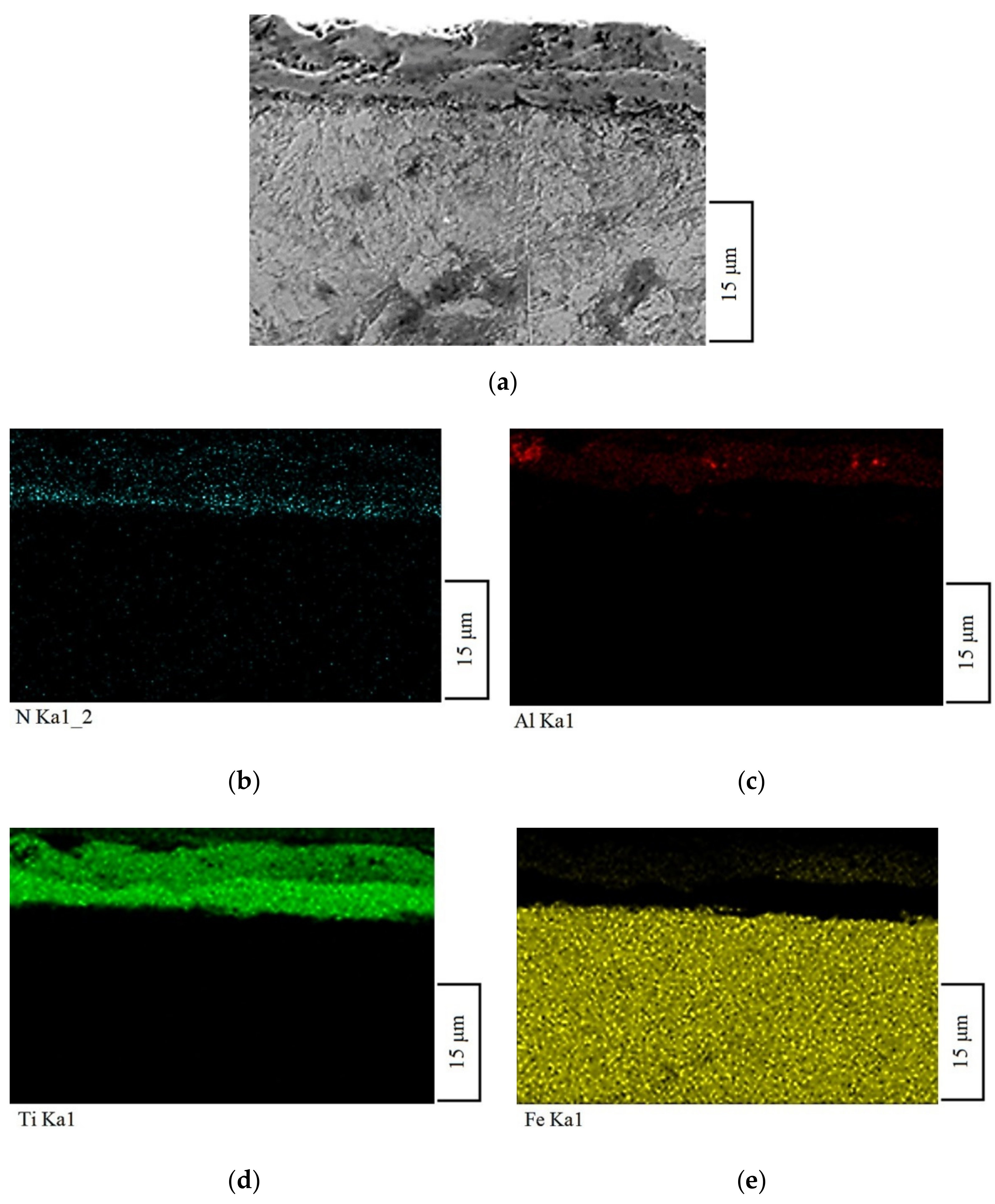
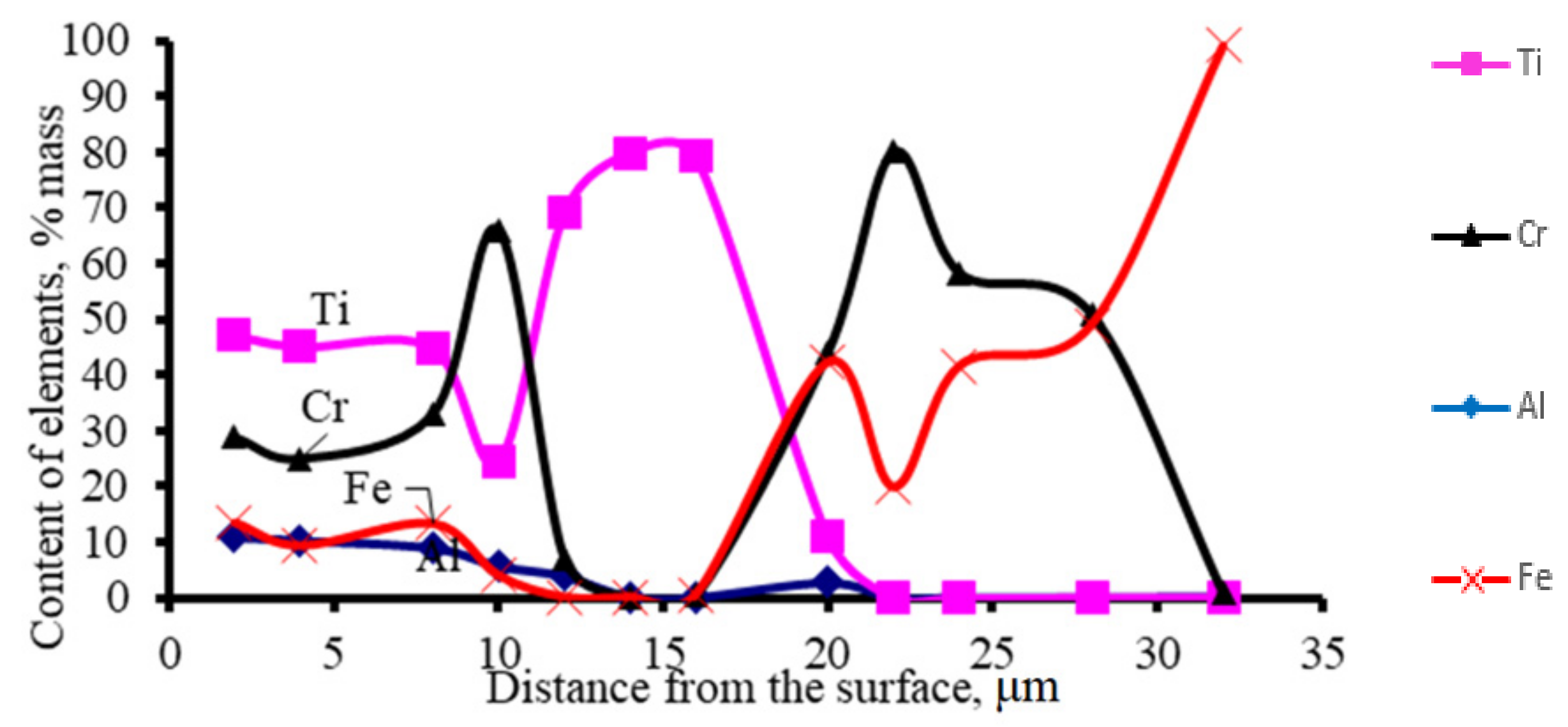

| No | The Content of Elements, % (wt.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | C | Si | Mn | Cr | S | P | Cu | Ni | Fe |
| 2 | 0.81 | 0.26 | 0.20 | 0.20 | ≤0.018 | ≤0.025 | ≤0.20 | ≤0.20 | remainder |
| No | Type of Treatment | The Phase Composition and Content of Components | |||
|---|---|---|---|---|---|
| 1 | Titanium aluminizing | Ti (Cr) | Al | NH4Cl | Al2O3 |
| 40 | 15 | 5 | 40 | ||
| 2 | Chrominizing | 55 | - | 5 | 40 |
| Coating No | Type of Treatment; Temperature, °C; Time, h | Phase Composition | Lattice Period, nm | Coating Thickness, μm | Micro-Hardness, GPa |
|---|---|---|---|---|---|
| 1 | Titanizing; 1050; 3 | FeTi | a: 0.2971 | 1.0–1.5 | 6.0 |
| Fe2Ti | a: 0.4708 | 1.5–2.0 | 8.1 | ||
| c: 0.7701 | |||||
| TiC | a: 0.4328 | 16.0 | 37.8 | ||
| 2 | Chrominizing, 1050; 4 | FeCr | a: 0.8810 | 3.5 | 6.5–7.0 |
| c: 0.5446 | |||||
| Cr23C6 | a: 1.0658 | 6.0 | 16.5 | ||
| Cr7C3 | a: 0.4528 | 9.0 | 17.2 | ||
| b: 0.7012 | |||||
| c: 1.2144 | |||||
| 3 | TiN, physical precipitation from gas phase | TiN | a: 0.4239 | 5.5–6.0 | 19.8 |
| 4 | Nitriding in ammonia; 540; 16 | Fe2N | a: 0.4799 | 11.0 | 5.6 |
| Fe4N | c: 0.4419 | 6.5 | 7.4 | ||
| a: 0.3796 | |||||
| 5 | Titanium aluminizing *, 1050; Ti (40%), Al (15%), NH4Cl (5%), Al2O3 (40%) | Fe2(Ti, Al)4O | a: 1.1309 | 8.0 | 8.0 |
| TiC | a: 0.4330 | 5.0–5.5 | 34.5 | ||
| Feα(Al) | a: 0.2800 | 35.0 | 2.0–2.9 | ||
| 6 | Titanium aluminizing * of AISI W1-7 nitric steel | Fe2(Ti, Al)4O | a: 1.1295 | 10.0 | 11.1 |
| TiC | a: 0.4329 | 6.9 | 30.3 | ||
| TiN | a: 0.4240 | 5.0 | 22.0 | ||
| 7 | Titanium aluminizing * of AISI W1-7 steel with a TiN layer | Fe2(Ti, Al)4O | a: 1.1293 | 10.0 | 11.1 |
| TiC | a: 0.4304 | 6.9 | 30.3 | ||
| TiN | a: 0.4240 | 5.0 | 22.0 | ||
| 8 | Titanium aluminizing of AISI W1-7 chromium steel | AlCrTi | a: 0.5000 | 2.0 | 6.1–7.0 |
| Cr2Ti | a: 0.6954 | 11.0 | |||
| Ti3Al | a: 0.5650 | 3.5 | |||
| b: 0.5650 | |||||
| c: 0.4576 | |||||
| TiC | a: 0.4321 | 7.0 | 35.5 | ||
| Cr23C6 | a: 1.0711 | 5.5 | 16.0 | ||
| Cr7C3 | a: 0.6890 | 4.5 | 16.5 | ||
| b: 1.2421 | |||||
| c: 0.4532 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loskutova, T.; Hatala, M.; Pogrebova, I.; Nikitina, N.; Bobina, M.; Radchenko, S.; Kharchenko, N.; Kotlyar, S.; Pavlenko, I.; Ivanov, V. Composition, Structure, and Properties of Ti, Al, Cr, N, C Multilayer Coatings on AISI W1-7 Alloyed Tool Steel. Coatings 2022, 12, 616. https://doi.org/10.3390/coatings12050616
Loskutova T, Hatala M, Pogrebova I, Nikitina N, Bobina M, Radchenko S, Kharchenko N, Kotlyar S, Pavlenko I, Ivanov V. Composition, Structure, and Properties of Ti, Al, Cr, N, C Multilayer Coatings on AISI W1-7 Alloyed Tool Steel. Coatings. 2022; 12(5):616. https://doi.org/10.3390/coatings12050616
Chicago/Turabian StyleLoskutova, Tetiana, Michal Hatala, Inna Pogrebova, Natalya Nikitina, Maryna Bobina, Svetlana Radchenko, Nadiia Kharchenko, Serhii Kotlyar, Ivan Pavlenko, and Vitalii Ivanov. 2022. "Composition, Structure, and Properties of Ti, Al, Cr, N, C Multilayer Coatings on AISI W1-7 Alloyed Tool Steel" Coatings 12, no. 5: 616. https://doi.org/10.3390/coatings12050616
APA StyleLoskutova, T., Hatala, M., Pogrebova, I., Nikitina, N., Bobina, M., Radchenko, S., Kharchenko, N., Kotlyar, S., Pavlenko, I., & Ivanov, V. (2022). Composition, Structure, and Properties of Ti, Al, Cr, N, C Multilayer Coatings on AISI W1-7 Alloyed Tool Steel. Coatings, 12(5), 616. https://doi.org/10.3390/coatings12050616








