Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
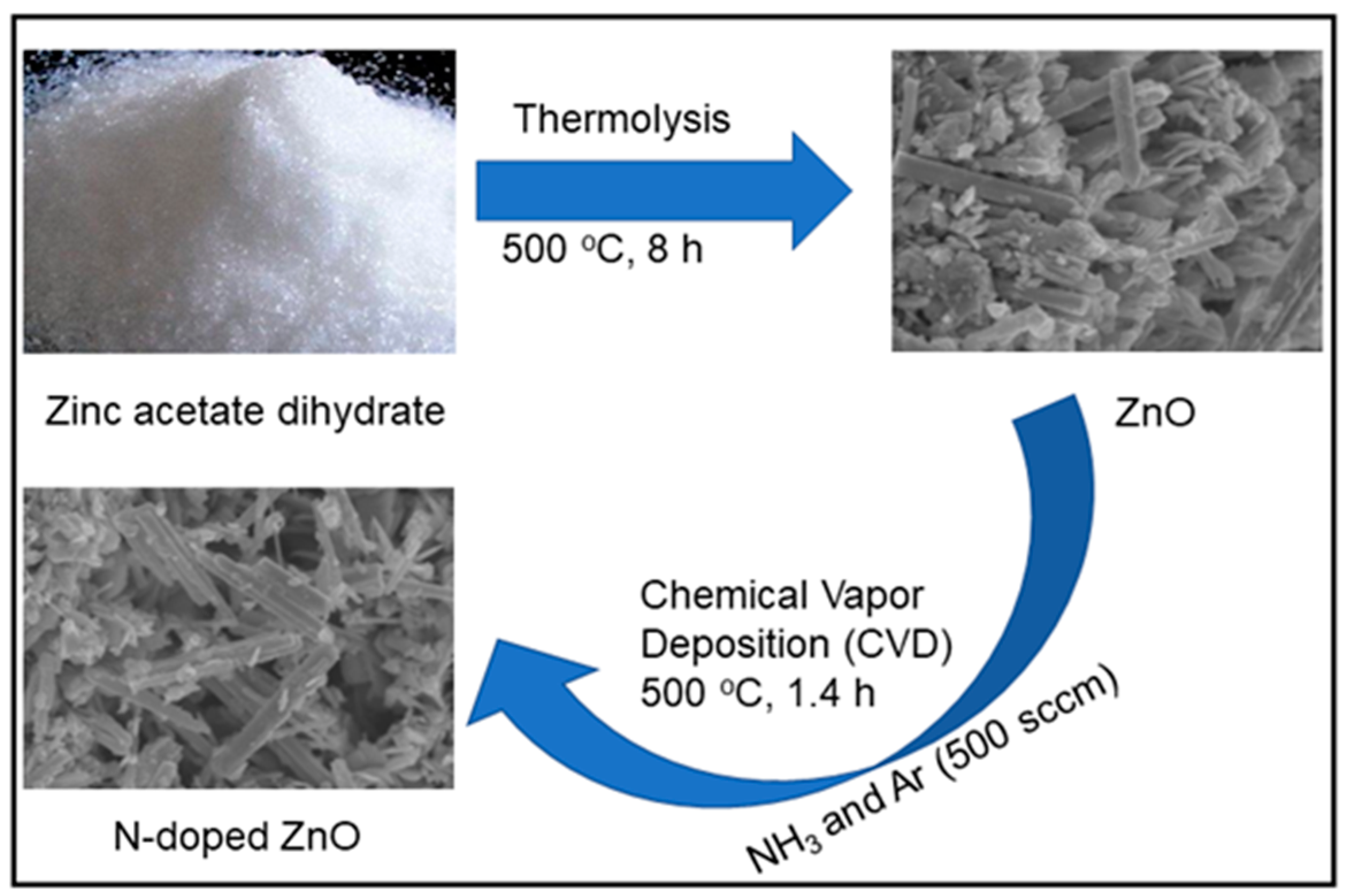
2.2. Synthesis of ZnO and N-Doped ZnO
2.3. Characterization
2.4. Photocatalytic Experiments
3. Results and Discussion
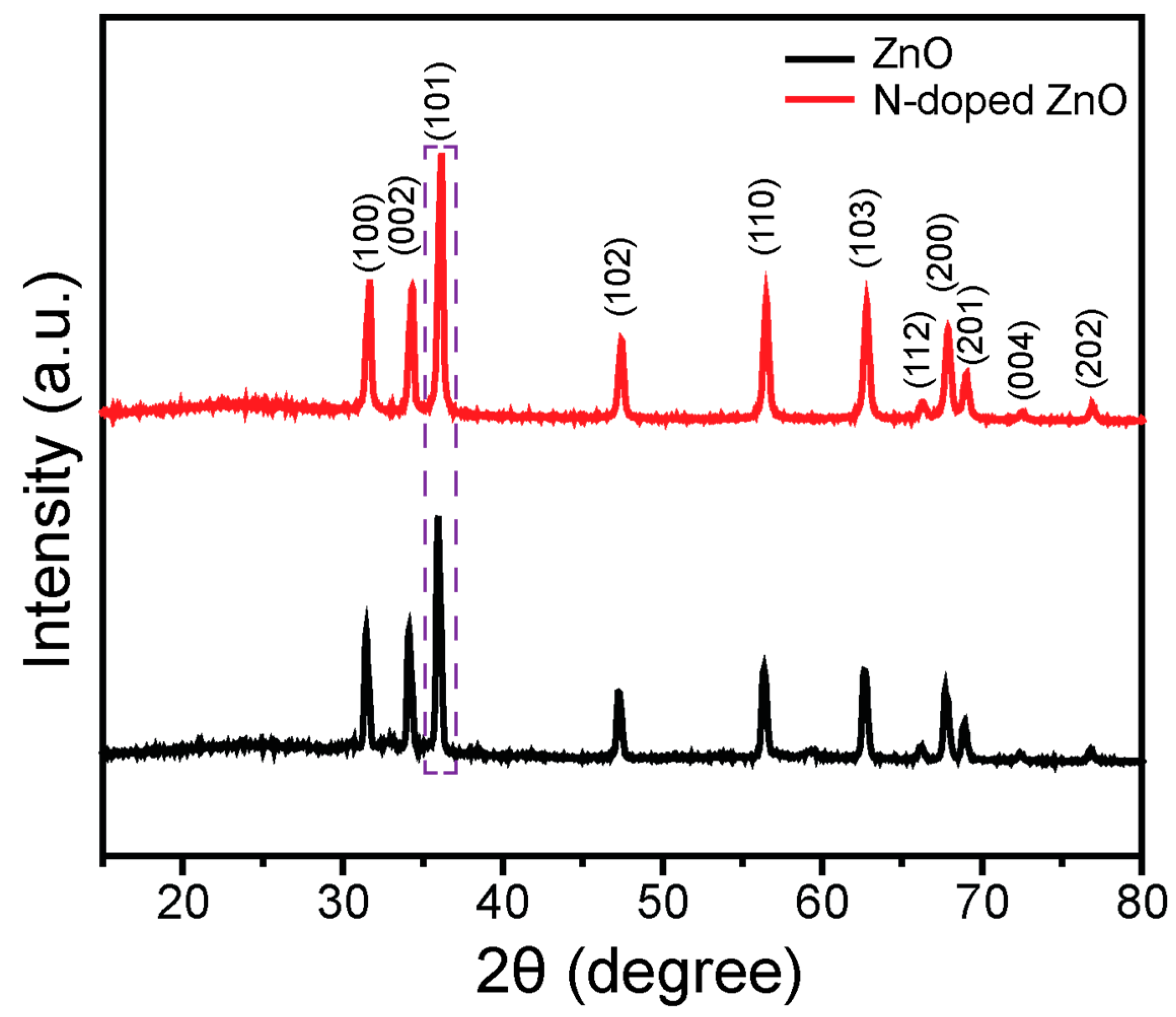
3.1. Structural Investigation
3.2. Morphological Investigation
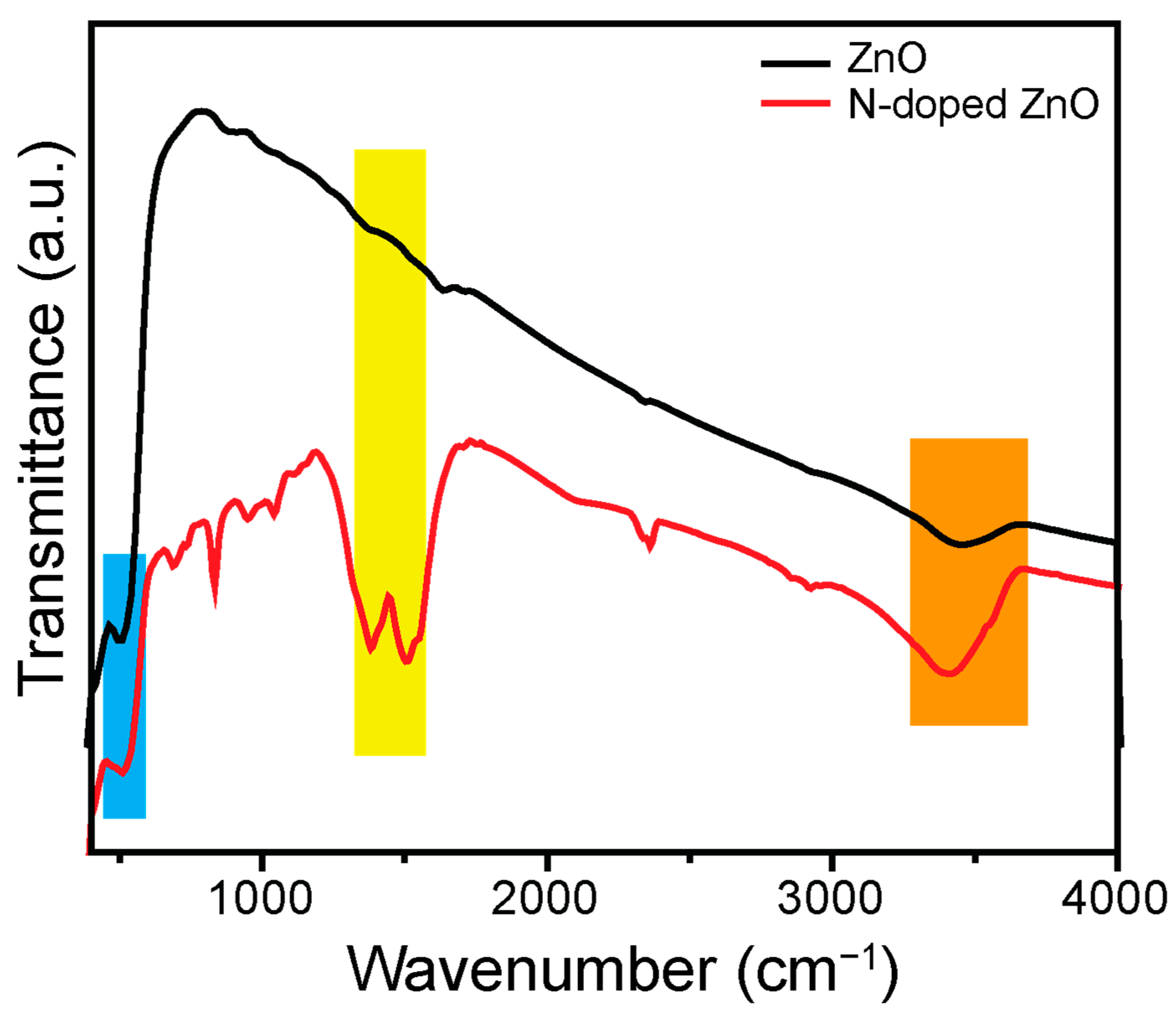
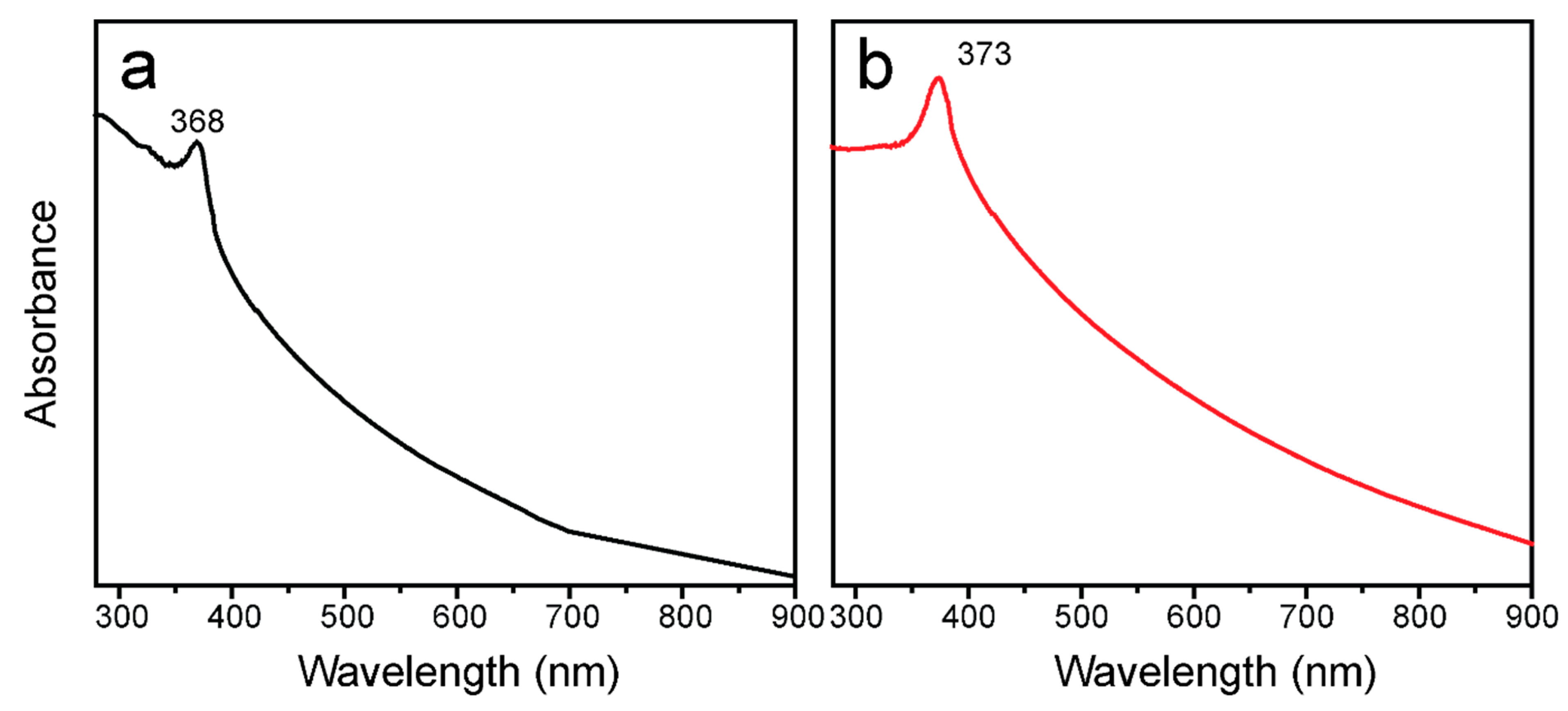
3.3. Optical Investigation
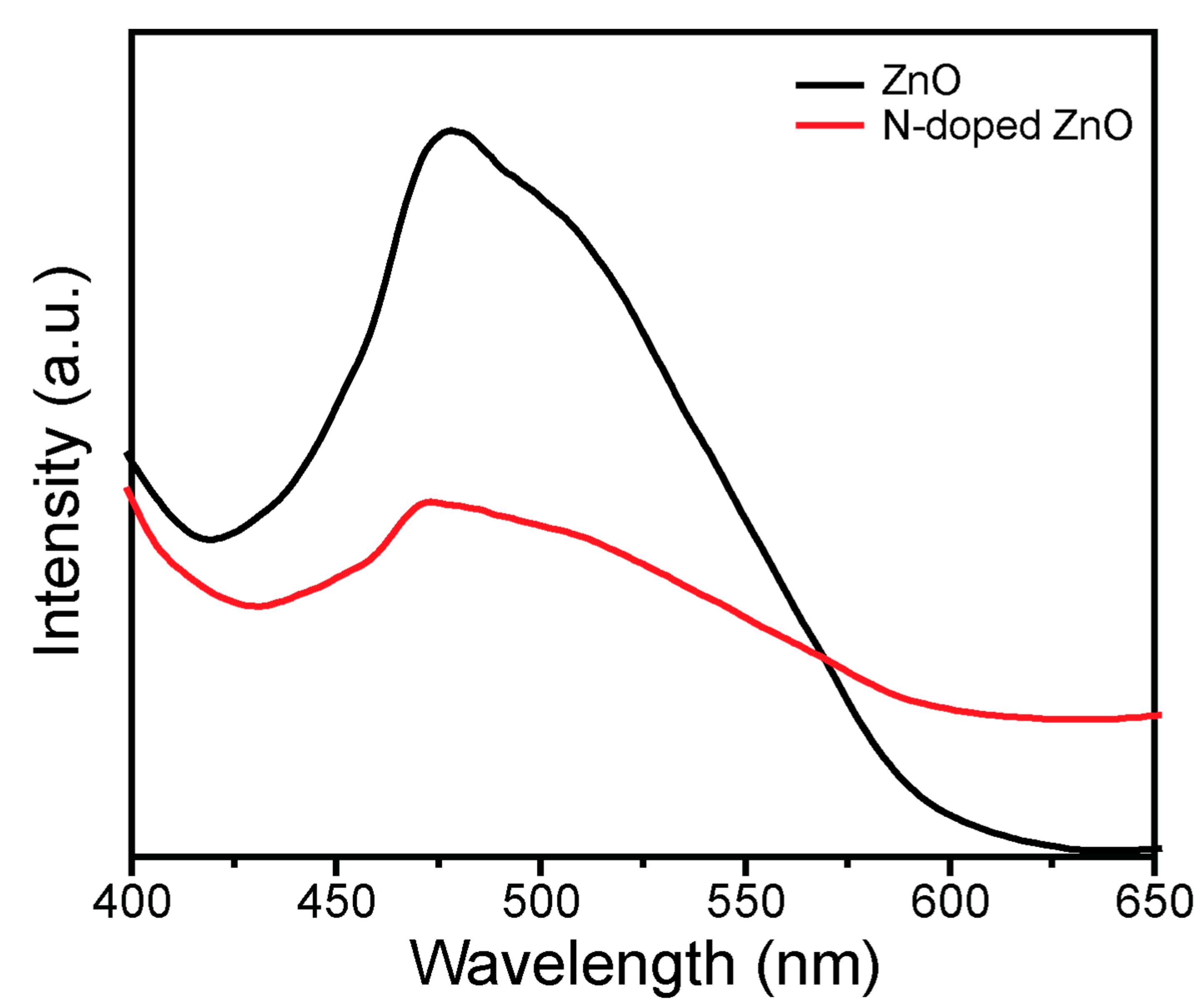
3.4. Photoluminescence (PL) Investigation
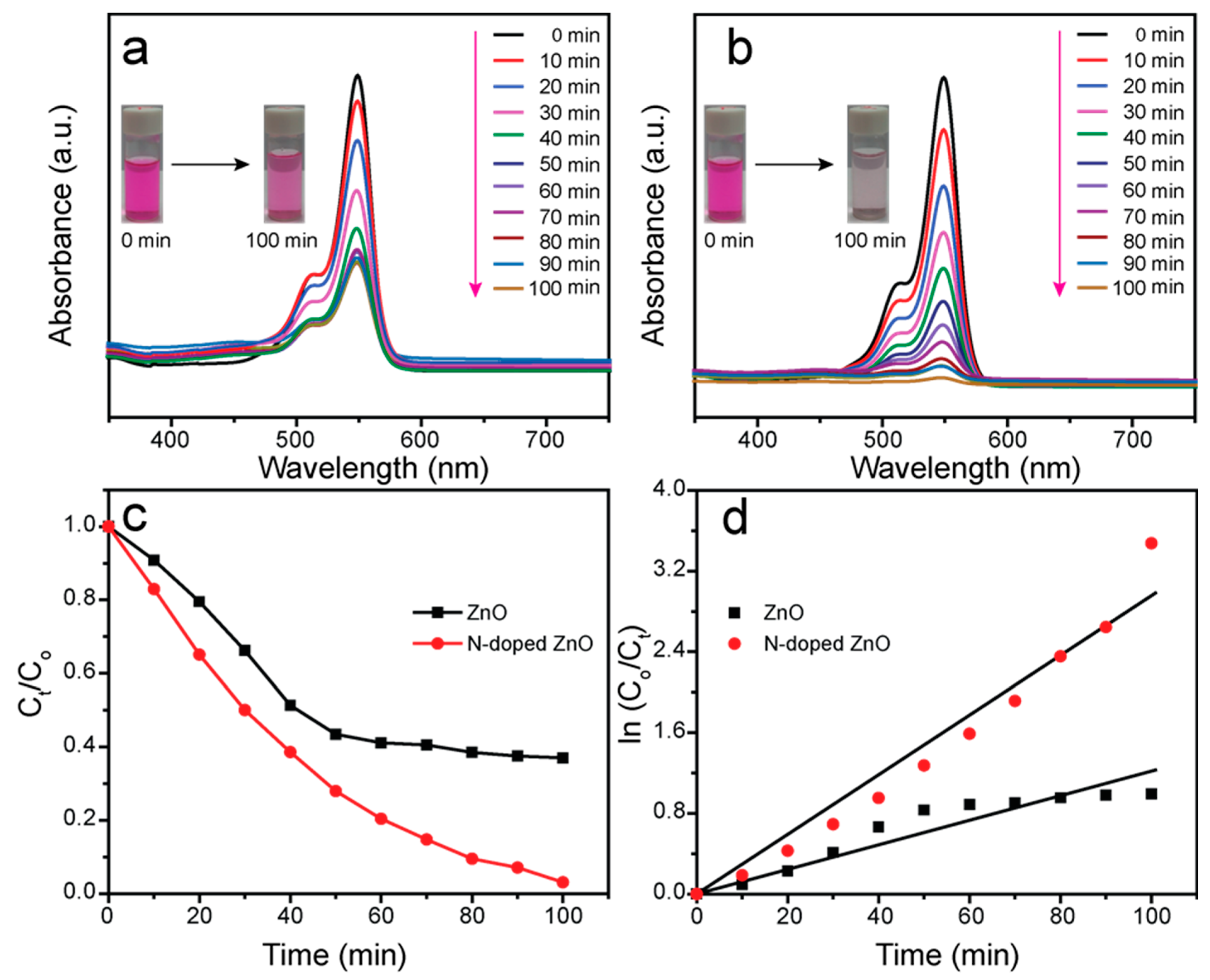
3.5. Photocatalytic Investigation
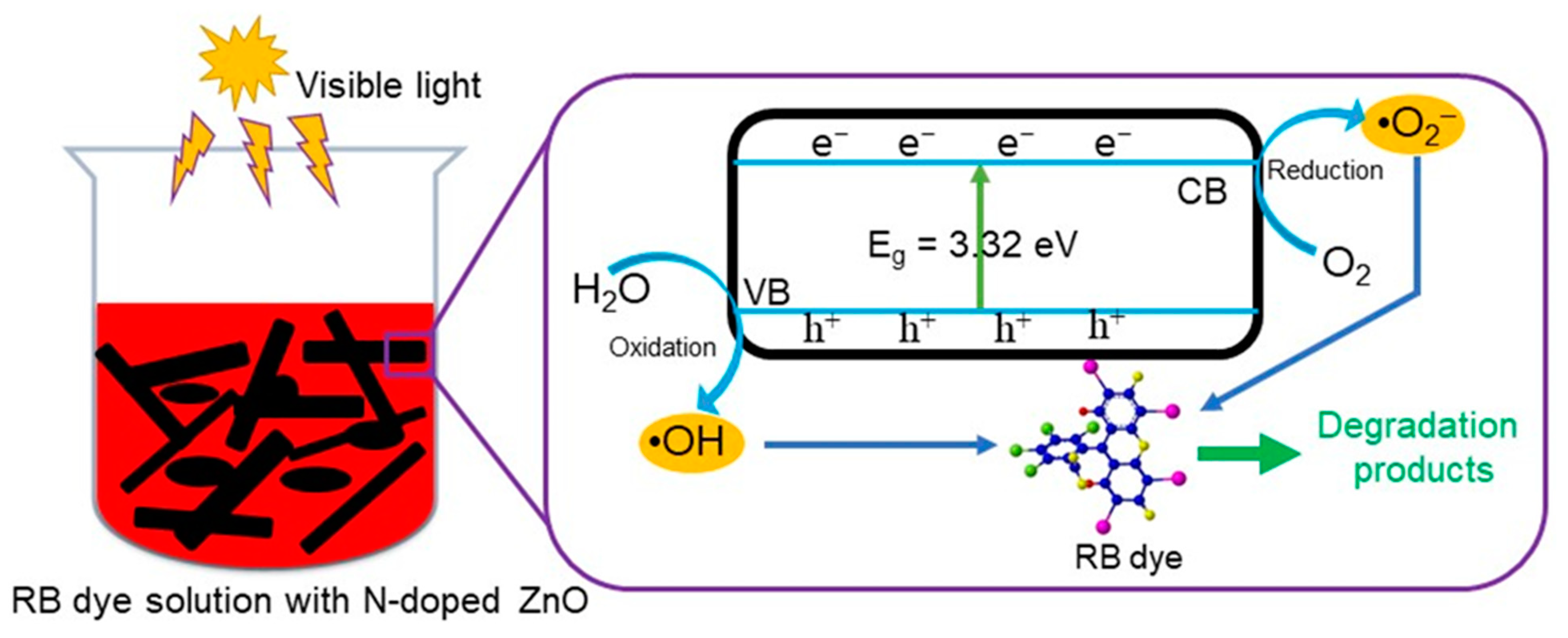
3.6. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akter, J.; Sapkota, K.P.; Hanif, M.A.; Islam, M.A.; Abbas, H.G.; Hahn, J.R. Kinetically controlled selective synthesis of Cu2O and CuO nanoparticles toward enhanced degradation of methylene blue using ultraviolet and sun light. Mater. Sci. Semicond. Process. 2021, 123, 105570. [Google Scholar] [CrossRef]
- Hanif, M.A.; Akter, J.; Lee, I.; Islam, M.A.; Sapkota, K.P.; Abbas, H.G.; Hahn, J.R. Formation of chemical heterojunctions between ZnO nanoparticles and single-walled carbon nanotubes for synergistic enhancement of photocatalytic activity. J. Photochem. Photobiol. A Chem. 2021, 413, 113260. [Google Scholar] [CrossRef]
- Japinder, K.; Sonal, S. Heterogeneous photocatalytic degradation of rose bengal: Effect of operational parameters. Phys. B 2014, 450, 49–53. [Google Scholar]
- Akter, J.; Hanif, M.A.; Islam, M.A.; Sapkota, K.P.; Hahn, J.R. Selective growth of Ti3+/TiO2/CNT and Ti3+/ TiO2/C nanocomposite for enhanced visible-light utilization to degrade organic pollutants by lowering TiO2-bandgap. Sci. Rep. 2021, 11, 9490. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Vivek, E.; Senthilkumar, N.; Pramothkumar, A.; Vimalan, M.; Potheher, I.V. Synthesis of flower-like copper oxide microstructure and its photocatalytic property. Phys. B Condens. Matter 2019, 566, 96–102. [Google Scholar] [CrossRef]
- Linhua, X.; Yang, Z.; Zijun, W.; Gaige, Z.; Jiaojiao, H.; Yanjing, Z. Improved photocatalytic activity of nanocrystalline ZnO by coupling with CuO. J. Phys. Chem. Solids 2017, 106, 29–36. [Google Scholar]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M. Enhanced visible light-active CeO2/CuO/Ag2CrO4 ternary heterostructures based on CeO2/CuO nanofber heterojunctions for the simultaneous degradation of a binary mixture of dyes. New J. Chem. 2020, 44, 5033–5048. [Google Scholar] [CrossRef]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 heterojunction flms for efficient photoelectrochemical water splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.P.; Josephine, G.A.S.; Sivasamy, A. Oxidation of organic dye using nanocrystalline rare earth metal ion doped CeO2 under UV and visible light irradiations. J. Mol. Liq. 2017, 242, 789–797. [Google Scholar] [CrossRef]
- Sharma, S.; Mehta, S.K.; Kansal, S.K. N doped ZnO/C-dots nanoflowers as visible light driven photocatalyst for the degradation of malachite green dye in aqueous phase. J. Alloys Compd. 2017, 699, 323–333. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2016, 391, 124–148. [Google Scholar] [CrossRef]
- Hanif, M.A.; Lee, I.; Akter, J.; Islam, M.A.; Zahid, A.A.S.M.; Sapkota, K.P.; Hahn, J.R. Enhanced photocatalytic and antibacterial performance of ZnO nanoparticles prepared by an efficient thermolysis method. Catalysts 2019, 9, 608. [Google Scholar] [CrossRef] [Green Version]
- Hamdah, S.A.; Naushad, A.; Fahad, A.A. Synthesis of Gd/N co-doped ZnO for enhanced UV-vis and direct solar-light-driven photocatalytic degradation. RSC Adv. 2021, 11, 10194. [Google Scholar]
- Wang, M.; Zhang, Y.; Zhou, Y.; Yang, F.; Kim, E.J.; Hahn, S.H.; Seong, S.G. Rapid room-temperature synthesis of nanosheet-assembled ZnO mesocrystals with excellent photocatalytic activity. CrystEngComm 2013, 15, 754–763. [Google Scholar] [CrossRef]
- Habib, A.; Shahadat, T.; Bahadur, N.M.; Ismail, I.M.I.; Mahmood, A.J. Synthesis and characterization of ZnO-TiO2 nanocomposites and their application as photocatalysts. Int. Nano Lett. 2013, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem. Photobiol. A Chem. 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Lavand, A.B.; Malghe, Y.S. Synthesis, characterization and visible light photocatalytic activity of nitrogen-doped zinc oxide nanospheres. J. Asian Ceram. Soc. 2015, 3, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, R.; Hossienzadeh, K.; Maleki, A.; Ghanbari, R.; Rezaee, R.; Safari, M.; Shahmoradi, B.; Daraei, H.; Jafari, A.; Yetilmezsoy, K. Effects of doping zinc oxide nanoparticles with transition metals (Ag, Cu, Mn) on photocatalytic degradation of Direct Blue 15 dye under UV and visible light irradiation. J. Environ. Health Sci. Eng. 2019, 17, 479–492. [Google Scholar] [CrossRef]
- Yousefi, R.; Jamali-Sheini, F.; Cheraghizade, M.; Khosravi-Gandomani, S.; Saáaedi, A.; Huang, N.M.; Basirun, W.J.; Azarang, M. Enhanced visible-light photocatalytic activity of strontium-doped zinc oxide nanoparticles. Mater. Sci. Semicond. Process. 2015, 32, 152–159. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, L.T.; Duong, A.T.; Nguyen, B.D.; Hai, N.Q.; Chu, V.H.; Nguyen, T.D.; Bach, L.G. Preparation, characterization and photocatalytic activity of La-doped zinc oxide nanoparticles. Materials 2019, 12, 1195. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.; Qayyum, A.; Abbas, G.; Haider, R.; Iqbal, M.; Nazir, A. Influence of different solvents on portrayal and photocatalytic activity of tin-doped zinc oxide nanoparticles. J. Mol. Liq. 2018, 260, 272–278. [Google Scholar] [CrossRef]
- Hofmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Qin, H.; Li, W.; Xia, Y.; He, T. Photocatalytic activity of heterostructures based on ZnO and N-doped ZnO. ACS Appl. Mater. Interfaces 2011, 3, 3152–3156. [Google Scholar] [CrossRef]
- Palanivel, B.; Mani, A. Conversion of a type-II to a Z scheme heterojunction by intercalation of a 0D electron mediator between the integrative NiFe2O4/g-C3N4 composite nanoparticles: Boosting the radical production for photo-fenton degradation. ACS Omega 2020, 5, 19747–19759. [Google Scholar] [CrossRef]
- Palanivel, B.; Shkir, M.; Alshahrani, T.; Mani, A. Novel NiFe2O4 deposited S-doped g-C3N4 nanorod: Visible-light driven heterojunction for photo-Fenton like tetracycline degradation. Diam. Relat. Mater. 2020, 112, 108148. [Google Scholar] [CrossRef]
- Dhonde, M.; Sahu, K.; Murty, V.V.S.; Nemala, S.S.; Bhargava, P.; Mallick, S. Enhanced photovoltaic performance of a dye sensitized solar cell with Cu/N Codoped TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 6274–6282. [Google Scholar] [CrossRef]
- Gupta, R.; Eswar, N.K.R.; Modak, J.M.; Madras, G. Visible light driven efficient N and Cu co-doped ZnO for photoinactivation of Escherichia coli. RSC Adv. 2016, 6, 85675–85687. [Google Scholar] [CrossRef]
- Hanif, M.A.; Akter, J.; Kim, Y.S.; Kim, H.G.; Hahn, J.R.; Kwac, L.K. Highly efficient and sustainable ZnO/CuO/g-C3N4 photocatalyst for wastewater treatment under visible light through heterojunction development. Catalysts 2022, 12, 151. [Google Scholar] [CrossRef]
- Buryi, M.; Remeš, Z.; Babin, V.; Novotný, M.; Vaněček, V.; Dragounová, K.A.; Mičová, J.; Landová, L.; Kučerková, R.; More-Chevalier, J.; et al. Influence of Mo doping on the luminescence properties and defect states in ZnO nanorods. Comparison with ZnO: Mo thin films. Appl. Surf. Sci. 2021, 555, 149679. [Google Scholar] [CrossRef]
- Jing, L.; Zhihui, D.; Hongbo, L. Controllable Mn-doped ZnO nanorods for direct assembly of a photoelectrochemical aptasensor. Analyst 2017, 142, 2177. [Google Scholar]
- Hanif, M.A.; Akter, J.; Islam, M.A.; Sapkota, K.P.; Hahn, J.R. Visible-light-driven enhanced photocatalytic performance using cadmium-doping of tungsten (VI) oxide and nanocomposite formation with graphitic carbon nitride disks. Appl. Surf. Sci. 2021, 565, 150541. [Google Scholar] [CrossRef]
- Akter, J.; Hanif, M.A.; Islam, M.A.; Sapkota, K.P.; Lee, I.; Hahn, J.R. Visible-light-active novel α-Fe2O3/Ta3N5 photocatalyst designed by band-edge tuning and interfacial charge transfer for effective treatment of hazardous pollutants. J. Environ. Chem. Eng. 2021, 9, 106831. [Google Scholar] [CrossRef]
- Maji, S.K.; Dutta, A.K.; Srivastava, D.N.; Paul, P.; Mondal, A.; Adhikary, B. Effective photocatalytic degradation of organic pollutant by ZnS nanocrystals synthesized via thermal decomposition of single-source precursor. Polyhedron 2011, 30, 2493. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S. Photocatalytic degradation of methylene blue using visible light active N-doped ZnO. Adv. Mater. Res. 2015, 1101, 299–302. [Google Scholar] [CrossRef]
- Suresh, M.; Sivasamy, A. Fabrication of graphene nanosheets decorated by nitrogen-doped ZnO nanoparticles with enhanced visible photocatalytic activity for the degradation of Methylene Blue dye. J. Mol. Liq. 2020, 317, 114112. [Google Scholar] [CrossRef]
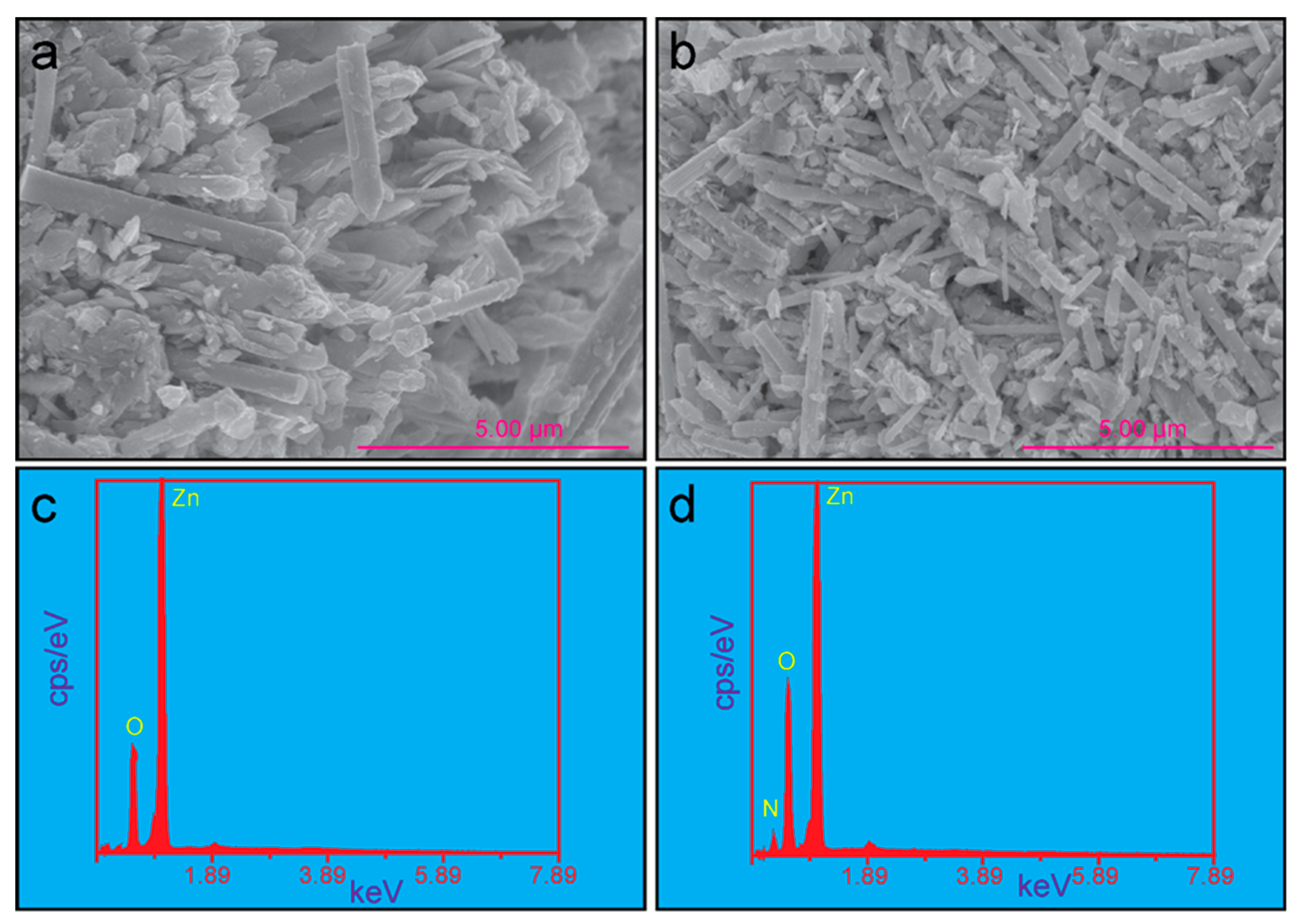
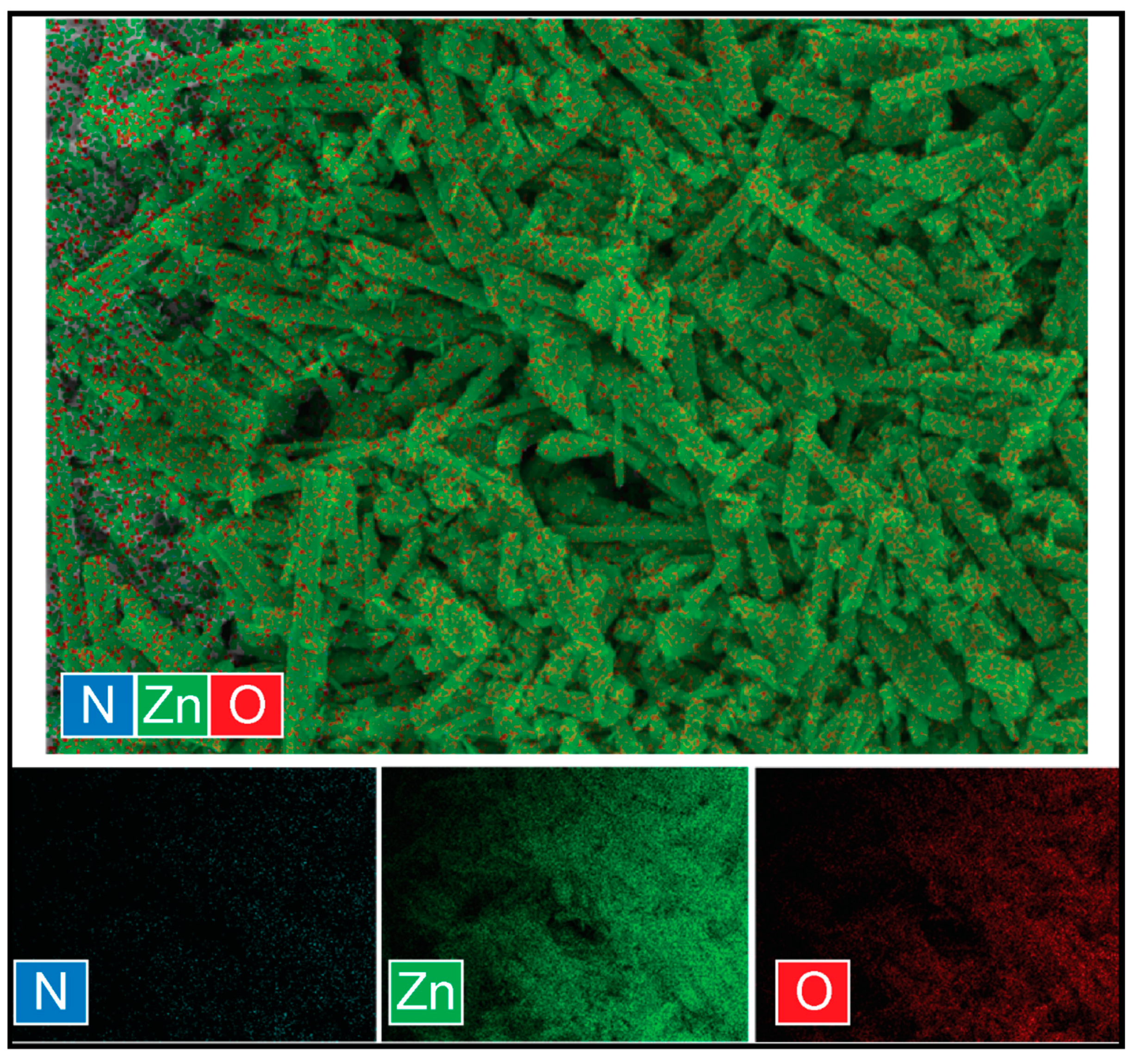
| Sample | Crystallite Size (Using Scherrer’s Equation) (nm)) | Particle Size (from HR-FE-SEM) | EDS Analysis | Bandgap (eV) | |||
|---|---|---|---|---|---|---|---|
| Average Length (μm) | Average Diameter (nm) | Element | Weight % | Atomic % | |||
| ZnO | 23.96 | 3.3 | 450 | Zn | 95.07 | 82.53 | 3.37 |
| O | 4.93 | 17.47 | |||||
| N-doped ZnO | 21.94 | 2.1 | 212 | N | 0.19 | 0.78 | 3.32 |
| Zn | 96.40 | 86.69 | |||||
| O | 3.41 | 12.53 | |||||
| Photocatalyst | Pollutant (Dye) | Light Source | Degradation (%) | Time (min) | Ref. |
|---|---|---|---|---|---|
| Cu/ZnO | DB 1 15 | Visible light | 70 | 120 | [21] |
| Sr/ZnO | MB 2 | Visible light | 50 | 120 | [22] |
| La/ZnO | MO 3 | Visible light | 85.8 | 150 | [23] |
| Sn/ZnO | MB | Visible light | 81 | 120 | [24] |
| ZnS NRs 4 | RB | Visible light | 93 | 225 | [37] |
| N-ZnO | MB | Visible light | 98 | 120 | [38] |
| 5 RGO-N-ZnO | MB | Visible light | 98.5 | 120 | [39] |
| N-ZnO/C-dots | MG 6 | Visible light | 85 | 160 | [13] |
| N-doped ZnO | RB | Visible light | 96.90 | 100 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, M.A.; Kim, Y.S.; Ameen, S.; Kim, H.G.; Kwac, L.K. Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment. Coatings 2022, 12, 579. https://doi.org/10.3390/coatings12050579
Hanif MA, Kim YS, Ameen S, Kim HG, Kwac LK. Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment. Coatings. 2022; 12(5):579. https://doi.org/10.3390/coatings12050579
Chicago/Turabian StyleHanif, Md. Abu, Young Soon Kim, Sadia Ameen, Hong Gun Kim, and Lee Ku Kwac. 2022. "Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment" Coatings 12, no. 5: 579. https://doi.org/10.3390/coatings12050579
APA StyleHanif, M. A., Kim, Y. S., Ameen, S., Kim, H. G., & Kwac, L. K. (2022). Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment. Coatings, 12(5), 579. https://doi.org/10.3390/coatings12050579









