WO3 Films Grown by Spray Pyrolysis for Smart Windows Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WO3 Samples
2.3. Characterization Methods
3. Results and Discussion
3.1. Electrochemical Characterization
3.2. Electrochromic Characterization
3.3. Morphological Characterization
3.4. Structural Characterization
3.5. Raman Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega, J.M.; Martínez, A.I.; Acosta, D.R.; Magaña, C.R. Structural and Electrochemical Studies of WO3 Films Deposited by Pulsed Spray Pyrolysis. Sol. Energy Mater. Sol. Cells 2006, 90, 2471–2479. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Kubota, T.; Tajima, K. Flexible Electrochromic Devices Based on Tungsten Oxide and Prussian Blue Nanoparticles for Automobile Applications. RSC Adv. 2021, 11, 28614–28620. [Google Scholar] [CrossRef]
- Yao, M.; Li, T.; Long, Y.; Shen, P.; Wang, G.; Li, C.; Liu, J.; Guo, W.; Wang, Y.; Shen, L.; et al. Color and Transparency-Switchable Semitransparent Polymer Solar Cells towards Smart Windows. Sci. Bull. 2020, 65, 217–224. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, S.; Choi, J.O.; Song, J.H.; Taya, M.; Ahn, S.H. Low-Cost Fabrication of WO3 Films Using a Room Temperature and Low-Vacuum Air-Spray Based Deposition System for Inorganic Electrochromic Device Applications. Thin Solid Films 2015, 589, 412–418. [Google Scholar] [CrossRef]
- Dhandayuthapani, T.; Sivakumar, R.; Zheng, D.; Xu, H.; Ilangovan, R.; Sanjeeviraja, C.; Lin, J. WO3/TiO2 Hierarchical Nanostructures for Electrochromic Applications. Mater. Sci. Semicond. Process. 2021, 123, 105515. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Liveri, M.; Galanopoulou, M.; Manariotis, I.D.; Yianoulis, P. A Simple Method for the Fabrication of WO3 Films with Electrochromic and Photocatalytic Properties. Thin Solid Films 2014, 573, 6–13. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Muralidharan, G.; Vijayakumar, S. Sol-Gel Coated WO3 Thin Films Based Complementary Electrochromic Smart Windows. Mater. Lett. 2021, 296, 129881. [Google Scholar] [CrossRef]
- Xue, S.; Gao, G.; Zhang, Z.; Jiang, X.; Shen, J.; Wu, G.; Dai, H.; Xu, Y.; Xiao, Y. Nanoporous WO3 Gasochromic Films for Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 8368–8375. [Google Scholar] [CrossRef]
- Chang-Jian, C.W.; Cho, E.C.; Yen, S.C.; Ho, B.C.; Lee, K.C.; Huang, J.H.; Hsiao, Y.S. Facile Preparation of WO3/PEDOT:PSS Composite for Inkjet Printed Electrochromic Window and Its Performance for Heat Shielding. Dye. Pigment. 2018, 148, 465–473. [Google Scholar] [CrossRef]
- Parshina, L.S.; Novodvorsky, O.A. Low-Temperature Laser Synthesis of LiCoO2 and WO3 Films for Electrochromic Application. Russ. J. Inorg. Chem. 2021, 66, 1234–1238. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Jang, S.-H.; Shchegolkov, A.V.; Rodionov, Y.V.; Sukhova, A.O.; Lipkin, M.S. A Brief Overview of Electrochromic Materials and Related Devices: A Nanostructured Materials Perspective. Nanomaterials 2021, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier: Amsterdam, The Netherlands, 1995; p. 633. [Google Scholar]
- Bertus, L.M.; Enesca, A.; Duta, A. Influence of Spray Pyrolysis Deposition Parameters on the Optoelectronic Properties of WO3 Thin Films. Thin Solid Films 2012, 520, 4282–4290. [Google Scholar] [CrossRef]
- González-Borrero, P.P.; Sato, F.; Medina, A.N.; Baesso, M.L.; Bento, A.C.; Baldissera, G.; Persson, C.; Niklasson, G.A.; Granqvist, C.G.; Ferreira Da Silva, A. Optical Band-Gap Determination of Nanostructured WO3 Film. Appl. Phys. Lett. 2010, 96, 061909. [Google Scholar] [CrossRef]
- Jayatissa, A.H.; Cheng, S.T.; Gupta, T. Annealing Effect on the Formation of Nanocrystals in Thermally Evaporated Tungsten Oxide Thin Films. Mater. Sci. Eng. B 2004, 109, 269–275. [Google Scholar] [CrossRef]
- Porqueras, I.; Bertran, E. Optical Properties of Li+ Doped Electrochromic WO3 Thin Films. Thin Solid Films 2000, 377–378, 8–13. [Google Scholar] [CrossRef]
- Subrahmanyam, A.; Karuppasamy, A. Optical and Electrochromic Properties of Oxygen Sputtered Tungsten Oxide (WO3) Thin Films. Sol. Energy Mater. Sol. Cells 2007, 91, 266–274. [Google Scholar] [CrossRef]
- Washizu, E.; Yamamoto, A.; Abe, Y.; Kawamura, M.; Sasaki, K. Optical and Electrochromic Properties of RF Reactively Sputtered WO3 Films. Solid State Ion. 2003, 165, 175–180. [Google Scholar] [CrossRef]
- Gesheva, K.A.; Ivanova, T.M.; Bodurov, G.K. APCVD Transition Metal Oxides—Functional Layers in “Smart Windows”. J. Phys. Conf. Ser. 2014, 559, 012002. [Google Scholar] [CrossRef] [Green Version]
- Deepa, M.; Srivastava, A.K.; Saxena, T.K.; Agnihotry, S.A. Annealing Induced Microstructural Evolution of Electrodeposited Electrochromic Tungsten Oxide Films. Appl. Surf. Sci. 2005, 252, 1568–1580. [Google Scholar] [CrossRef]
- Livage, J.; Ganguli, D. Sol–Gel Electrochromic Coatings and Devices: A Review. Sol. Energy Mater. Sol. Cells 2001, 68, 365–381. [Google Scholar] [CrossRef]
- Bathe, S.R.; Patil, P.S. Electrochromic Characteristics of Fibrous Reticulated WO3 Thin Films Prepared by Pulsed Spray Pyrolysis Technique. Sol. Energy Mater. Sol. Cells 2007, 91, 1097–1101. [Google Scholar] [CrossRef]
- Spanu, D.; Recchia, S.; Schmuki, P.; Altomare, M. Thermal-Oxidative Growth of Substoichiometric WO3–x Nanowires at Mild Conditions. Phys. Status Solidi RRL 2020, 14, 20002352000235. [Google Scholar] [CrossRef]
- Gutpa, J.; Shaik, H.; Kumar, K.N.; Sattar, S.A. PVD techniques proffering avenues for fabrication of porous tungsten oxide (WO3) thin films: A review. Mater. Sci. Semicond. Processing 2022, 143, 106534. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Kharade, R.R.; Mali, S.S.; Mane, R.M.; Patil, P.B.; Patil, P.S.; Choudhury, S.; Bhosale, P.N. Nanobrick-like WO3 thin films: Hydrothermal synthesis and electrochromic application. Superlattices Microstruct. 2014, 73, 290–295. [Google Scholar] [CrossRef]
- Chatzikyriakou, D.; Maho, A.; Cloots, R.; Henrist, C. Ultrasonic Spray Pyrolysis as a Processing Route for Templated Electrochromic Tungsten Oxide Films. Microporous Mesoporous Mater. 2017, 240, 31–38. [Google Scholar] [CrossRef]
- Liao, C.C.; Chen, F.R.; Kai, J.J. Electrochromic Properties of Nanocomposite WO3 Films. Sol. Energy Mater. Sol. Cells 2007, 91, 1282–1288. [Google Scholar] [CrossRef]
- Regragui, M.; Addou, M.; Outzourhit, A.; El Idrissi, E.; Kachouane, A.; Bougrine, A. Electrochromic Effect in WO3 Thin Films Prepared by Spray Pyrolysis. Sol. Energy Mater. Sol. Cells 2003, 77, 341–350. [Google Scholar] [CrossRef]
- Bertus, L.M.; Faure, C.; Danine, A.; Labrugere, C.; Campet, G.; Rougier, A.; Duta, A. Synthesis and Characterization of WO3 Thin Films by Surfactant Assisted Spray Pyrolysis for Electrochromic Applications. Mater. Chem. Phys. 2013, 140, 49–59. [Google Scholar] [CrossRef]
- Sivakumar, R.; Raj, A.M.E.; Subramanian, B.; Jayachandran, M.; Trivedi, D.C.; Sanjeeviraja, C. Preparation and Characterization of Spray Deposited N-Type WO3 Thin Films for Electrochromic Devices. Mater. Res. Bull. 2004, 39, 1479–1489. [Google Scholar] [CrossRef]
- Buch, V.R.; Chawla, A.K.; Rawal, S.K. Review on Electrochromic Property for WO3 Thin Films Using Different Deposition Techniques. Mater. Today Proc. 2016, 3, 1429–1437. [Google Scholar] [CrossRef]
- Mouratis, K.; Tudose, V.; Romanitan, C.; Pachiu, C.; Tutunaru, O.; Suchea, M.; Couris, S.; Vernardou, D.; Emmanouel, K. Electrochromic Performance of V2O5 Thin Films Grown by Spray Pyrolysis. Materials 2020, 13, 3859. [Google Scholar] [CrossRef] [PubMed]
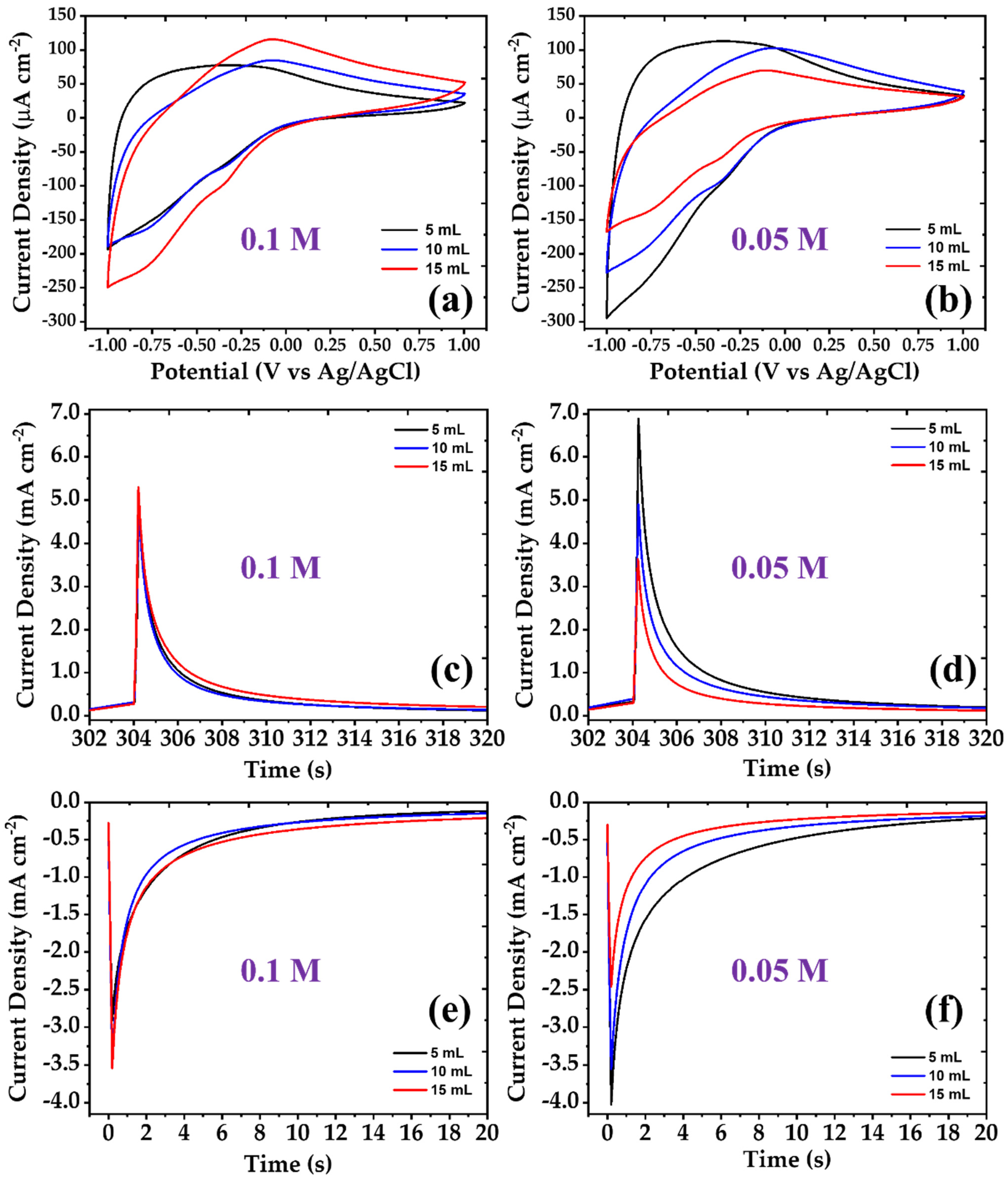


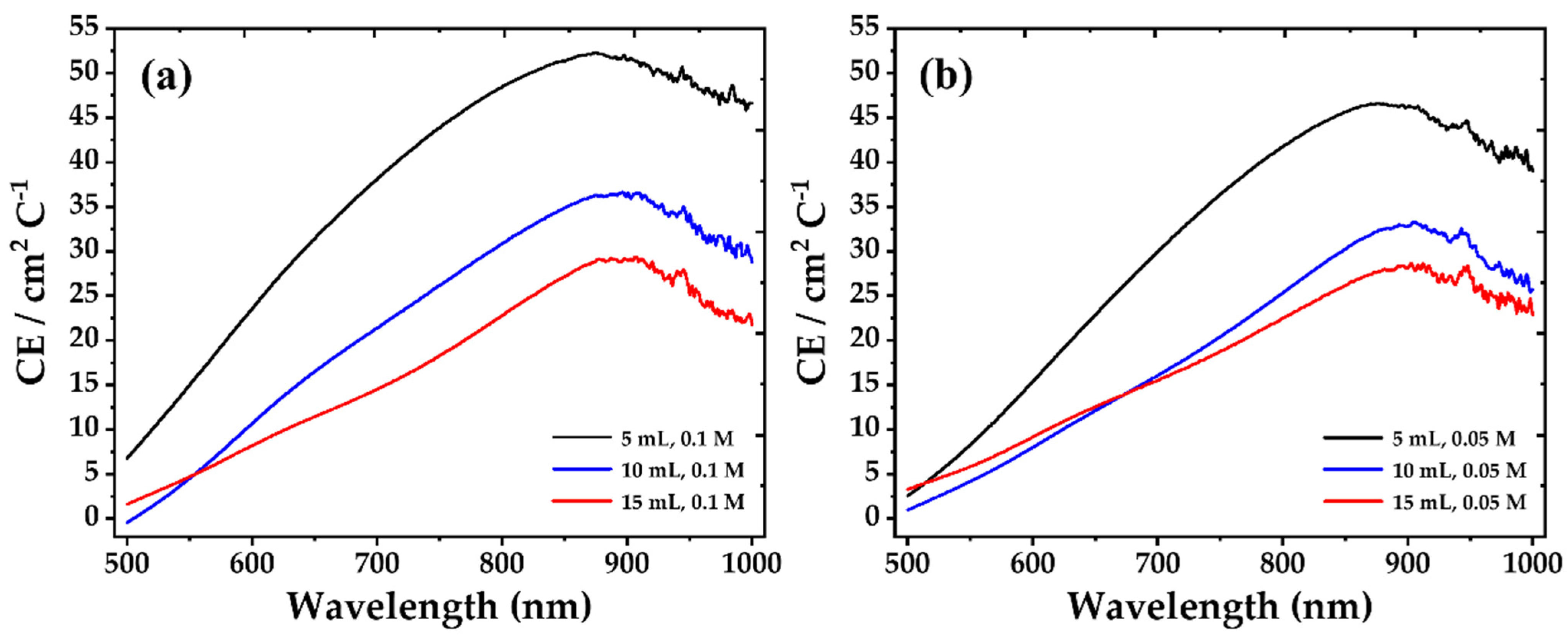
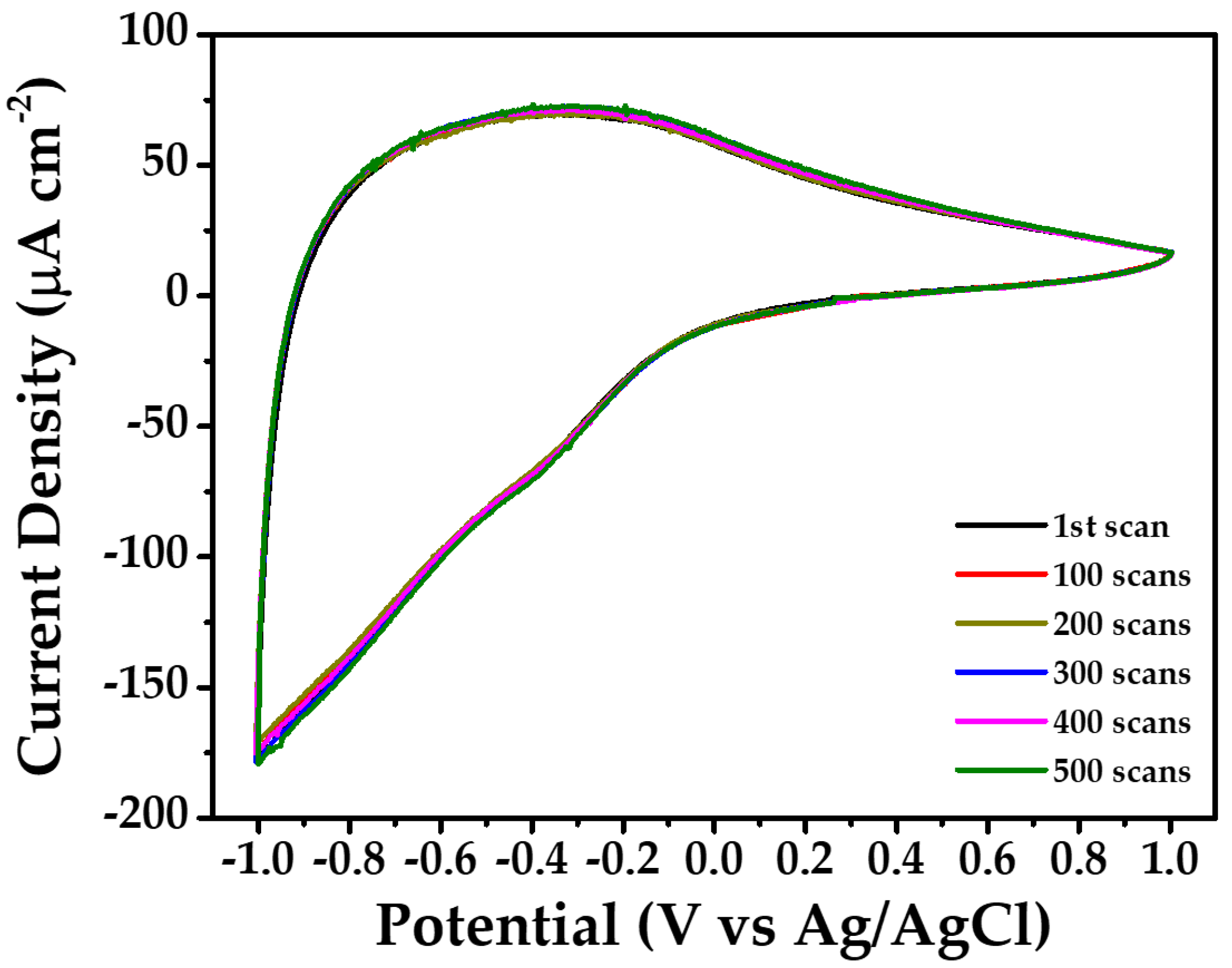

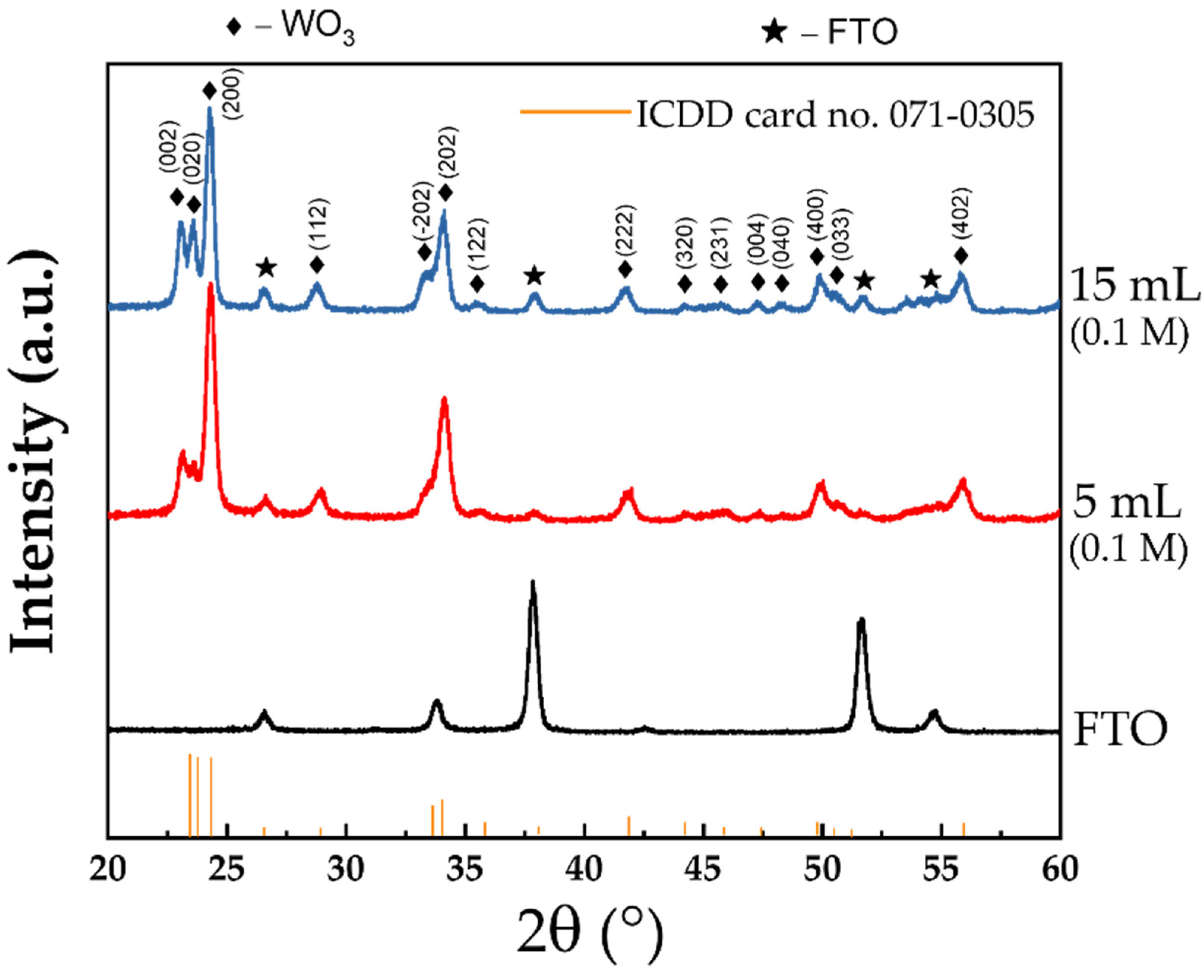

| Concentration | Precursor Volume (mL) | Ip,a (μA cm−2) | Ip,c (μA cm−2) | Qd (mC cm−2) | Qi (mC cm−2) | tb (sec) | tc (sec) |
|---|---|---|---|---|---|---|---|
| 0.1 M | 5 | 77.82 | −193.45 | 14.04 | −15.23 | 4.22 | 9.22 |
| 10 | 84.68 | −189.10 | 16.65 | −18.47 | 3.75 | 6.84 | |
| 15 | 115.43 | −249.37 | 23.26 | −25.76 | 5.20 | 9.77 | |
| 0.05 M | 5 | 112.99 | −294.70 | 21.45 | −23.14 | 4.66 | 11.58 |
| 10 | 102.61 | −227.56 | 20.04 | −22.29 | 5.25 | 8.40 | |
| 15 | 69.89 | −167.61 | 14.03 | −16.84 | 4.26 | 8.05 |
| Concentration | Precursor Volume (mL) | Tb(λ) (%) | Tc(λ) (%) | ΔΤ(λ) (%) |
ΔOD (λ) | CE(λ) (cm2 C−1) |
|---|---|---|---|---|---|---|
| 0.1 M | 5 | 40.65 | 22.74 | 17.90 | 0.580 | 38.11 |
| 10 | 26.77 | 18.04 | 8.73 | 0.394 | 21.37 | |
| 15 | 10.35 | 7.12 | 3.22 | 0.373 | 14.48 | |
| 0.05 M | 5 | 28.39 | 14.19 | 14.20 | 0.693 | 29.95 |
| 10 | 25.42 | 17.77 | 7.65 | 0.358 | 16.06 | |
| 15 | 10.78 | 8.31 | 2.47 | 0.260 | 15.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouratis, K.; Tudose, I.V.; Romanitan, C.; Pachiu, C.; Popescu, M.; Simistiras, G.; Couris, S.; Suchea, M.P.; Koudoumas, E. WO3 Films Grown by Spray Pyrolysis for Smart Windows Applications. Coatings 2022, 12, 545. https://doi.org/10.3390/coatings12040545
Mouratis K, Tudose IV, Romanitan C, Pachiu C, Popescu M, Simistiras G, Couris S, Suchea MP, Koudoumas E. WO3 Films Grown by Spray Pyrolysis for Smart Windows Applications. Coatings. 2022; 12(4):545. https://doi.org/10.3390/coatings12040545
Chicago/Turabian StyleMouratis, Kyriakos, Ioan Valentin Tudose, Cosmin Romanitan, Cristina Pachiu, Marian Popescu, Georgios Simistiras, Stelios Couris, Mirela Petruta Suchea, and Emmanuel Koudoumas. 2022. "WO3 Films Grown by Spray Pyrolysis for Smart Windows Applications" Coatings 12, no. 4: 545. https://doi.org/10.3390/coatings12040545
APA StyleMouratis, K., Tudose, I. V., Romanitan, C., Pachiu, C., Popescu, M., Simistiras, G., Couris, S., Suchea, M. P., & Koudoumas, E. (2022). WO3 Films Grown by Spray Pyrolysis for Smart Windows Applications. Coatings, 12(4), 545. https://doi.org/10.3390/coatings12040545










