Abstract
Wood modification is an excellent and increasingly used method to expand the application of woody materials. Traditional methods, such as chemical or thermal, have been developed for the targeted improvement of some selected properties, unfortunately typically at the expense of others. These methods generally alter the composition of wood, and thus its mechanical properties, and enhance dimensional stability, water resistance, or decrease its susceptibility to microorganisms. Although conventional methods achieve the desired properties, they require a lot of energy and chemicals, therefore research is increasingly moving towards more environmentally friendly processes. The advantage of modern methods is that in most cases, they only modify the surface and do not affect the structure and mechanical properties of the wood, while reducing the amount of chemicals used. Cold plasma surface treatment is one of the cheapest and easiest technologies with a limited burden on the environment. In this review, we focus on cold plasma treatment, the interaction between plasma and wood compounds, the advantages of plasma treatment compared to traditional methods, and perspectives.
1. Introduction
In the history of humankind, people all over the world have used wood [1,2]. Due to its numerous advantageous properties, it is one of the most widely used structural materials [3]. At the very beginning, people used it in the form it was available in nature, but over time, people began to tailor the properties of wood for particular purposes. As science and technology advanced, people expected wood products to have more and more advanced functions and superior qualities, similarly to other modern products. Durability, flame retardancy, electrical conductivity, improved adhesion, water repellence, and self-cleaning are just a few of the properties that different industries require from wood products. Properties can be tailored with different chemical methods but they are not environmentally friendly and sometimes impair the properties of the bulk wood [4,5,6,7,8,9].
Fortunately, in the last few years, cost effective and environmentally friendly, green technologies have come to the forefront and technologies with a high consumption of chemicals and energy, and those posing health and environmental hazards are slowly losing importance.
Plasma treatment has become more and more widely used for the modification of polymer surfaces [10,11,12,13]. In some areas, it has even substituted traditional methods, because it is safe, environmentally friendly, does not use or release toxic chemicals, and is fast and easy to control [14]. However, adequate methods to completely replace current wood modification methods have not been developed yet. Plasma treatment is limited to the top layers of a sample, which is appropriate for establishing a coating film or form anchoring points for subsequent reaction, but it cannot change the whole structure of the wood, which is essential sometimes [15]. The penetration depth of plasma cannot be increased by changing the reaction parameters. Moreover, during plasma treatment of wood, all components will be affected by plasma simultaneously, and it is difficult to decide what causes chemical or physical changes.
In this review, we focus on the advantages of plasma treatments and its effect of the different components of wood, and try to show which components are involved in the chemical reaction during plasma treatment, which and which degrade first. We also briefly describe the most commonly used types of plasma and possible plasma–surface interactions.
2. Modification of Wood—Traditional Methods
During the long-term use of wood in many applications, it became clear that untreated wood is perishable over time [16]. Besides its strengths, wood has a number of weaknesses [17], including poor weather resistance (especially to humidity and UV radiation), or its susceptibility of microorganisms, which considerably limits its application. Its hygroscopicity has a significant impact on its dimensional stability—swelling and shrinkage are inevitable.
With the development of science, the disadvantageous properties of wood can be significantly improved [18,19,20]. Some properties can be improved by changing the genetics of the tree or plant, but this is not applicable in most cases or the method is still in the experimental stage [3]. For example, modifying the lignin biosynthesis gene can result in lower lignin content with increased pulp yield, which is desirable in paper production [21]. Therefore, wood with a higher cellulose content could make paper production cheaper.
However, modification of the properties usually means the alteration of the chemical composition of the wood after preliminary processing [22,23]. Hill [17] classifies modifications into two groups. According to him, there is passive modification or impregnation, where the chemistry of the material does not change considerably, and active modification (chemical, thermal, and/or enzymatic) which alters the chemical nature of wood.
2.1. Impregnation and Coating
One of the oldest methods of conserving some of the properties (composition, dimensions or color) of wood is impregnation [24]. The principle of the method is to fill the cell walls and lumen with different organic or inorganic chemicals or a mixture of them [25]. During the process, intermolecular forces such as dipole–dipole or hydrogen bonds may occur between the impregnant and the cell wall components and this results in a dimensionally stable wood with enhanced resistance to various forms of degradation. Usually, the impregnation agents are resins, polymers, oils, and inorganic salts which can penetrate deep into the structure of the wood. Pressure or vacuum is often used to achieve a near-perfect effect [26]. In terms of mechanism, there are two types of impregnation: either a monomer is introduced into the cell wall and the subsequent polymerization step fixes it, or a soluble chemical diffuses into the cell walls and some chemical treatment makes it insoluble [27]. As a matter of fact, the goal of impregnation is to make the cell wall permanently swollen so that the hydroxyl groups are physically or chemically unavailable to water or other chemicals. The most frequently used impregnants are resins (phenol, urea, or melamine formaldehyde resin, etc.), polymers, compounds containing silicon, or inorganic salts.
Coating is in fact impregnation but only applied on the top of the wood to create a protective layer [28]. The coating agent can be water-based, solvent-based, or a powder. A volatile compound or a pigment is dissolved or dispersed in the solvent, and so it can be applied to the surface with conventional painting methods. There is powder coating as well, when the surface is coated with a powder and a subsequent heat or radiation treatment produces a continuous layer [29].
2.2. Thermal Modification
The heat treatment of wood is a well-established commercial technology to improve the dimensional stability and durability of wood. There are many excellent review articles on this topic [30,31]. The technology has been used since the 1920s, when Tiemann [31] showed that the drying of wood at elevated temperature improves its dimensional stability. The process has been refined a great deal since then, but the principle remains the same: wood is exposed to elevated temperature (150–250 °C) without the presence of oxygen and undergoes a chemical transformation. Outside this temperature range, unwanted changes occur: below 150 °C, water loss typically occurs and above 250 °C, carbonization starts. As the temperature rises, hemicellulose begins to decompose and undergoes dehydration, so hydrophilic OH groups disappear [32]. Later, cellulose degrades, but firstly its amorphous part. Cellulose is more resistant to heat due to its crystalline structure, so the crystallinity of the sample will increase and the accessibility of the hydroxyl groups to water and other chemicals will decrease [33]. During the degradation of lignin, polycondensation occurs and additional crosslinks are formed in the already cross-linked macromolecule. This increases the lignin content of the wood and hence its dimensional stability due to the destruction of the OH groups and the concomitant decreased affinity to water. Overall, on one hand, thermal treatment reduces the number and availability of hydroxyl groups, which improves the durability of wood, on the other hand, the degradation of hemicellulose and the decrease in amorphous cellulose content significantly impair its mechanical properties. Sometimes thermal methods are combined with water or external forces in order to achieve better results in terms of properties. These are thermo-mechanical or thermo-hydro-mechanical methods [34,35,36,37,38].
2.3. Enzymatic Modification
In the last decades, the application of biotechnology in pulp and paper industry has been intensively investigated and some enzymatic processes (e.g., bio-pulping, bio-bleaching) have already become industrial technologies. However, there is still little interest in biotechnological treatments in the wood or wood-based industry [39]. This could be attributed the fact that enzymes cannot penetrate deeper layers of wood; they only affect the surface. Therefore, this technique is real surface modification. There is increasing pressure on the industry to reduce the amount of chemicals used, and enzymatic processing is a promising and sustainable alternative to conventional methods as this method usually operates under milder reaction conditions [40].
Enzymatic treatment of wood aims to (1) improve the properties of the wood by modifying the lignocellulose system, (2) activate the surface by creating new properties by enzymatic pre-treatment, (3) simplify and/or make conventional processing technologies more environmentally friendly. Since enzymes have high substrate specificity, the literature focuses on the enzymatic modification, activation and reactions of cellulose, hemicelluloses, and lignin. An important area of research focuses on wood protection aiming to increase the interaction between wood and preservatives, and replace chemical agents with “bioagents”, to improve the finishing of wood surfaces. Others focus on replacing conventional chemical adhesives by enzymatic treatment in the production of veneers. Most commonly, the effect of laccase enzymes is investigated. Kudanga and Katrin [41,42] formed a hydrophobic surface in a laccase-mediated coupling reaction. In this research, authors grafted different hydrophobic molecules on the wood surface and stable covalent bonds were justified between the functional groups on the surface and the compounds, resulting in a tenacious hydrophobic layer on the wood surface. Müller [43], using similar methods, also formed successfully covalently bonded wood preservatives to the surface.
2.4. Chemical Modification
Chemical modification means that a chemical reagent forms a covalent bond with any functional group (mostly –OH groups) of some constituent of the wood to change the selected properties [44]. Wood is traditionally modified by a reaction through its hydroxyl groups, since this is the only available functional group in cellulose, hemicellulose, and lignin. The modification reaction is limited to esterification and etherification [45]. The accessibility of OH groups is highly influenced by their reactivity which is different even within one anhydroglucose unit, which can be explained with intra- and intermolecular hydrogen bonds. Moreover, there are amorphous and crystalline regions in the cellulose chains, which also affect the accessibility of hydroxyl groups. Among the many chemical modification reactions, acetylation has been studied the most and this method has given the most consistent results [44]. The procedure has been considerably developed with respect to catalysts, reactor type, state of the matter of the reactant, and is still commonly used. Figure 1 shows the different types of traditional wood modification procedures according to Teaca and Tanasa [46].
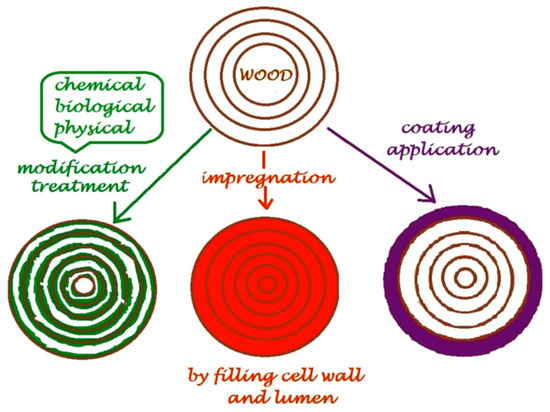
Figure 1.
Schematic representation of the methods used for wood modification [46].
2.5. Efficiency and Environmental Impact of Methods
Each of the methods that can be considered traditional has its drawbacks. The impregnants, especially inorganic ones, can be environmental hazards after the disposal of scrap wood [47,48] or the combustibility of wood can change because of different resins [49]. There are evidences that copper based nanoparticles preservatives during the decomposition of treated wood may accumulate in the mycelium of Cu-tolerant fungi and end up in their spores getting out to the environment and become a potential rick by inhaling [50]. Wastewaters from pulp and paper industry can contain more than 250 identified chemicals, including harmful components, such as resin acids and sterols. Most of them can removed with modern wastewater purification technologies, but some of them are still released into the environment [51,52].
Painting can trap moisture in the structure of the wood, and it then soaks in water [53]. If the coating is damaged, there is nothing left that would protect the wood, so the whole object must be re-coated [54]. Impregnation focuses changing the surface, as this is in contact with the environment and possible reactions take place here. Methods that modify the bulk besides the surface result in the degradation of bulk properties, which is to be avoided if possible.
Moreover, the previously mentioned technologies have been known for a long time, but, in the past, there was no economic or environmental urgency to make these technologies less hazardous. Now, there is an increasing pressure to improve or replace them with environmentally friendly technologies because of the high consumption of chemicals, water, and energy. Plasma surface modification can be a solution as its burden on the environment is considerably less. There are attempts to replace conventional wood treatment methods with plasma treatment [55,56,57]. However, the impact of plasma treatment is still being explored and we believe that the complete elimination of traditional methods is yet to be seen. Moreover, when the purpose of wood modification is to create structural changes in wood, improve its mechanical properties or change the crystal allomorph of cellulose, plasma treatment will not achieve the desired goal. In such cases, traditional methods are essential [58].
3. Modification of the Surface of Wood with Plasma
Surface treatment is an important area of surface engineering, which is the targeted modification of surface properties. The goal is to open new possibilities of application. Surface treatment is widely used in the industry and a wide range of relevant processes are known, which can be sorted in several ways, such as chemical or physical methods, wet or dry processes, and chemical or physical vapor deposition [59]. Although traditional methods of surface modification (oxidation, fire enameling, and cementation) are useful in many areas, they are less and less used. In addition, they often produce pollutants and toxic by-products. Modern surface modification processes eliminate these drawbacks. For wood, the same surface treatment methods are usually used as for other materials. The main purpose of these treatments is to establish anchoring sites for additional reactions or to reduce the surface polarity of wood to improve compatibility with low surface energy materials for the preparation of composites [60]. In contrast to chemical surface treatment processes, cold plasma treatment is an environmentally friendly, solvent-free method requiring relatively little energy and material [61,62]. It can also be used to treat heat-sensitive materials [63]. Because of these advantages, cold plasma surface modification is becoming increasingly popular in several industries and the tailoring of surface properties is intensively researched.
In many cases, using plasma treatment before conventional techniques is beneficial. It creates additional active points besides the not very reactive hydroxyl groups, which can lead to much more efficient surface coating, painting or gluing [64]. Furthermore, extractives, which can be aromatic or aliphatic compounds (fats and waxes) and terpenes or terpenoids, are always present on the wood surface [39]. These can reduce the effectiveness of the chemicals, so they must be removed. To prepare an extractive-free wood surface, plasma treatment is the best way in terms of efficiency, cost, time, energy consumption, and environmental footprint. However, Avramidis et al. [65] found that only this layer of extractives was removed during the plasma treatment of wood.
3.1. Plasma
Plasma is a partially or fully ionized gas that can be considered the fourth state of matter based on thermodynamic considerations. It is characterized by the presence of neutral and electrically charged particles such as atoms, radicals free electrons, ions, and photons (including UV photons) [66,67]. Plasma is generally macroscopically neutral in terms of electrostatic charge. Plasmas can be produced over a wide range of pressures and temperatures, but two basic types are known: equilibrium (thermal) and non-equilibrium (cold) plasmas. In the former, the kinetic energy (temperature) of atoms and electrons is in thermodynamic equilibrium, therefore they cannot be used for the surface modification of heat-sensitive materials, while in the latter, the temperature of electrons is significantly higher than that of heavy particles. Plasmas and their applications are described in detail in several excellent review papers [68,69,70,71].
In cold plasma the heavy particles are at or at near room temperature, while the electron temperatures can be higher by orders of magnitude. High electron temperatures lead to high chemical reactivity, so cold plasma is suitable for surface modification of solids [72,73], even if they have otherwise low chemical reactivity in the conventional sense. Cold plasma can be formed and maintained in several ways, even thermally, but due to its efficiency, it is advisable to use an electrostatic or electromagnetic field for this purpose. Cold plasma treatment can be performed under reduced pressure or even at atmospheric pressure. Although both are viable pathways and the former has a larger scientific literature, the latter has significant advantages. Atmospheric methods are extensively developed and used; therefore, we will focus on describing them below.
3.1.1. Atmospheric Plasma Treatment Applications
Atmospheric plasmas have a wide range of applications. Plasmas are widely used in treatments involving hazardous or undesirable gases or wastes [74]. Volatile organic compounds (VOCs) or inorganic gases such as nitrogen oxides (NOx) or sulfur dioxide, pollute the environment when released into the atmosphere. These toxic molecules can collide with the reactive particles in the plasma and form free radicals that can assemble into harmless molecules. Plasma can also be used to produce various gases in the industry, such as acetylene or conversion of methane to higher hydrocarbon [75]. Various atmospheric plasmas are also widely used in medicine (dentistry, dermatology, sterilization) and even to increase biocompatibility [76]. Atmospheric plasma methods (corona, dielectric barrier discharge, plasma jet) are more attractive since on-line treatment is possible without the use of vacuum [77,78]. Moreover, these methods are cost effective, fast and easy to handle, and do not need expensive equipment, or cooling water [79,80]. Below are some atmospheric pressure plasma sources but the list is not exhaustive, only the most frequently used plasma sources are mentioned. By using specially designed electrodes, or changing the dielectric, individual plasmas can even be converted into each other (Figure 2).
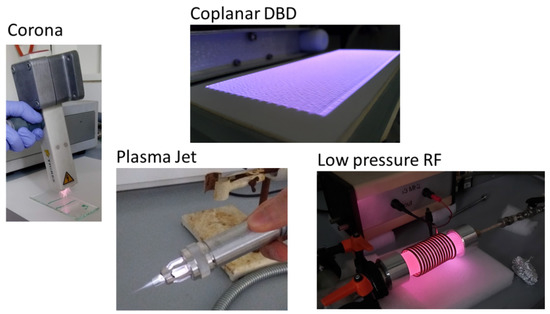
Figure 2.
Some examples of atmospheric or low-pressure plasma from the author’s laboratory.
- Corona
Electrical discharges are generated with relatively low current (μA to mA), usually in an air atmosphere. It typically develops between asymmetric (e.g., point-like) metal electrodes with the formation of an electron avalanche. It takes place under conditions in which a continuous arc discharge does not yet occur. Corona discharges have a small active volume and a fibrous structure. It is widely used for the surface treatment of polymeric materials. It is most commonly used to produce or remove surface electrostatic charges [81].
- Dielectric Barrier Discharge (DBD)
It is also called silent discharge. It is not a new method, but it has only recently been used for surface treatment. Several types are known: volumetric (tubular or planar), and surface or coplanar DBD. It operates with alternating current (50–10 MHz), and in practice, it is a large number of micro-discharges (in pulse mode). It is suitable for forming relatively homogeneous plasmas with high energy density, low contact time and can be used together with continuous technologies. Two types of homogeneous atmospheric DBD plasmas are known: atmospheric pressure glow discharge (APGD) and Townsend DBD plasma (atmospheric pressure Townsend discharges, (APTS)) [82]. Diffuse Coplanar Surface Barrier Discharge (DCSBD) is a special type of surface DBD and easy to apply in industry (Figure 2) [83].
- Plasma jet
Plasma jets are produced by generating non-thermal plasma in various gases or gas mixtures with a direct or alternating current with a wide frequency range (up to microwaves) and blowing it out of the source. The treatment is usually in the afterglow range. They are suitable for local treatment, e.g., to improve adhesion between two parts to be joined. Sometimes, with a suitable electrode design, a plasma jet can even be considered a DBD plasma [84].
- Radio frequency (RF) and microwave (MW) plasmas
They are produced and sustained by high-frequency electromagnetic fields. RF and MW discharges are typically generated with a frequency of 1–100 MHz, and 0.3–10 GHz, respectively. Both are frequently used for etching, activation, and plasma polymerization. RF plasma can be capacitively or inductively coupled; in the latter case, the plasma is in no contact with the induction coil [85].
3.1.2. Low-Pressure Plasma Treatment Applications
Low-pressure plasmas, which were invented earlier, are widely used in materials processing and surface treatment. They have some advantages over atmospheric plasmas, for example, a relatively high concentration of ions and radicals and uniform glow over a large gas volume, but they require vacuum during operation, which limits their use. Creating vacuum makes the whole process time and energy consuming, and requires expensive equipment. Therefore, some authors believe [64] that low-pressure plasma systems are only appropriate for the preparation of value-added materials while atmospheric equipment is suitable for mass production.
3.2. Plasma Surface Interaction
Modification is usually limited to the outermost surface or some of its atomic layers, and bulk properties usually remain unchanged. The depth of treatment can be changed with the use of different types of plasma and different energy densities.
In the case of a polyethylene model material, in inductively coupled radiofrequency oxygen plasma a single monolayer is modified. Using microwave oxygen plasma, the modified layer is 5 nm thick, while in corona discharge air plasma it is thicker than 10 nm. The very slight difference can be attributed to the different reactivities of the excited particles (oxygen atom, singlet oxygen molecule, ions containing oxygen) in the plasmas [86]. The depth of plasma treatment cannot be increased considerably by varying the parameters of the plasma, such as time, power, or gas. Therefore, reactions will not occur in the micrometer range.
During plasma treatment, a number of processes takes place on the surfaces. These processes are highly dependent on the selected gas, the operating parameters and reactor design. Nevertheless, plasma–surface interactions are quite complex and not well understood [87]. We present the current scientific knowledge with the details needed to understand this article. In general, the interaction between plasma and surface can be divided into two different types [88]. One of them is when plasma is generated from non-polymerizing gases (e.g., N2, O2, Ar, air, and NH3) and the major effect can be surface cleaning, etching, cross-linking, or formation of new functional groups and radicals simultaneously, because of the reactive particles in the plasma [89,90] (Figure 3).
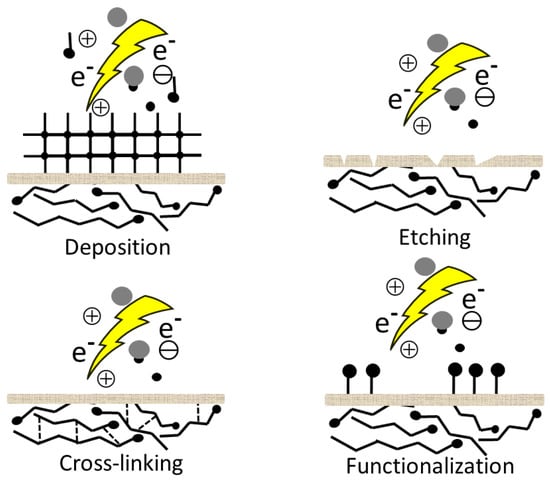
Figure 3.
Plasma surface interaction.
In this case, the surface becomes more hydrophilic mostly due to the newly formed functional groups or radicals. Since the hydrophilic surface itself is not desirable for the preservation of wood, in most cases, activation is followed by another reaction to tailor the properties of the surface. In the other case, the plasma gases are different polymerizable monomers or precursors (fluorocarbons, hydrocarbons, silicone-containing monomers, ethylene, or acetylene, etc.), which are converted by the plasma into reactive fragments. During recombination, a hydrophobic nanocoating will be deposited on the surface in a one-step reaction without any subsequent reaction. Sometimes a noble gas is used as a carrier gas during plasma polymerization. A schematic representation of the plasma treatment with two different types of gas is shown in Figure 4.
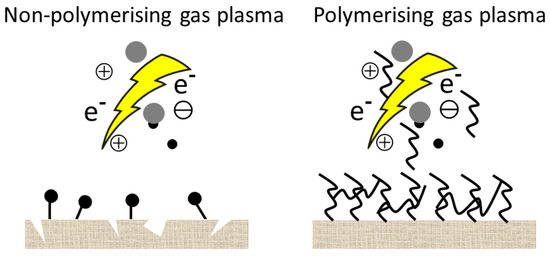
Figure 4.
A schematic representation of the plasma treatment with two different types of gas.
3.2.1. Surface Cleaning
Plasma surface cleaning has already been used for a long time in industries such as metallurgy, and the production of microchips, semiconductors and medical plastics, etc. [91]. During manufacture, organic contamination is always deposited on surfaces in layers of a few nanometers thick. It can hinder subsequent bonding or processing and can be easily removed by cold plasma. However, even if the aim is not to clean the surface, this cleaning process always occurs during plasma treatment
3.2.2. Functionalization
Low-pressure non-thermal discharges are usually applied for polymer surface functionalization. Air, O2, N2, NH3, or CF4 gases can generate functional groups containing O, N, H, and F (C=O, –COOH, –OH, –NH2, etc.) on the surface [92]. The resulting groups can produce hydrophobic or hydrophilic properties or promote the subsequent binding of molecules to establish the desired properties.
3.2.3. Radicals, Double Bonds and Cross-Linking
When the plasma is generated by noble gases, it produces active radicals on the surface by breaking the C–H bonds. The recombination of these radicals leads to the formation of double bonds or crosslinking of the molecular chains. The radicals and double bonds play an important role in anchoring molecules for grafting. Cross-linking is sometimes just an undesired side reaction, but there are cases where the goal is specifically to create a cross-linked surface [93].
3.2.4. Etching
The objective of etching is to increase roughness by removing materials from the outmost layer generating volatile or low molecular weight by-products that desorb from the surface. It is a non-equilibrium process and often used in microelectronic industry [94].
Most such processes are effective in the long term, but the formation of functional groups is only temporary and either a partial or complete hydrophobic recovery (Figure 5) is usually observed [95]. A possible explanation is the thermodynamically driven reorientation of the polar groups from the surface into the bulk. Therefore, plasma treatment is just the first step, and it is followed by a subsequent grafting reaction to obtain the desired surface property permanently. Usually, the plasma-activated surface can induce the grafting reactions, so this step does not require a solvent or a catalyst, either.
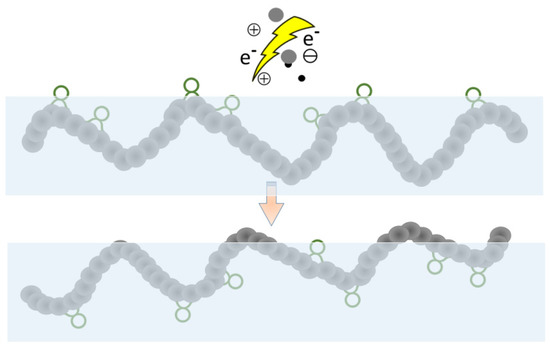
Figure 5.
Hydrophobic recovery.
An additional advantage of plasma treatment is that not only esterification and etherification reactions take place. The active particles in the plasma can react oxygen and carbon atoms by the extraction of hydrogen, which results in oxidation, reduction, substitution, or establishment of an active radical, which is ready for a subsequent grafting [96].
3.3. Characterization of the Effectiveness of Plasma Treatment
By systematically varying the plasma parameters, many researchers have tried to find the best settings to achieve the desired surface effect. Such parameters can be the choice of plasma gas, treatment time, or plasma power. With the choice of plasma gas, the incorporation of new functional groups can be planned in advance. For the incorporation of hydroxyl or carbonyl groups, oxygen or air plasma is required. De Farias [97] found that oxygen plasma was far more effective than air plasma in terms of morphology and chemical changes with the same treatment time and power. They attributed this result to the stronger etching effect of oxygen plasma, as nitrogen is less reactive than oxygen in this environment. Treatment time is also a key parameter and not easy to determine. Some authors use a few seconds of treatment [98], claiming that longer treatment times did not produce a more significant effect, or the sample was damaged seriously [99], while others choose very long treatment times, sometimes up to 1 hour, without the destruction of the sample [97]. It is very difficult to compare the different treatments, as in most cases, the plasmas are different even if produced in the same device. Plasma treatment does not only influence the chemistry of the materials but also results in surface roughening due to the etching effect of plasma. Both changes, morphological and chemical, play an important role in a further reaction on the surface, mostly grafting, and these changes can be examined by modern surface characterization methods. These methods apply photon (X-ray photoelectron spectroscopy (XPS)), electron (Auger electron spectroscopy (AES)) or ion beam (Ion Scattering Spectroscopy (ISS), Rutherford Backscattering Spectroscopy (RBS), Secondary Ion Mass Spectrometry (SIMS)) and can show the chemical composition of the surface, while surface imaging techniques (Scanning Electron Microscopy (SEM), Atomic Force Microscopy (AFM), Scanning Probe Microscopy (SPM), profilometry, as well as imaging XPS and AES) reveal morphological changes [100].
3.3.1. XPS, Fourier Transform Infrared Spectroscopy (FTIR)
Researchers usually monitor changes in surface chemistry by XPS [101] and FTIR. Plasma surface modification typically induces chemical changes in the outermost surface layer with a thickness of a few nanometers. XPS is considered the best technique for both the qualitative and quantitative chemical analysis of such thin layers because the penetration depth of XPS is roughly the same as that of plasma treatment [102,103,104]. If a layered structure is developed during the treatment, the thickness of the layers can also be estimated from XPS data. In contrast, FTIR is less suitable for the analysis of a surface layer because FTIR provides information about the bulk material due to its large penetration depth (approximately a thousand times greater than that of XPS). This way, the signal from the surface is less intense, due to the limited number of functional groups. FTIR can give reliable results if bonds are formed during plasma treatment not present in the initial sample [105].
3.3.2. SEM
Scanning electron microscopy is extensively used in the study of structural micro-morphological details on the surface of a sample [106]. The high-resolution SEM images usually provide information about the topography, morphology, and composition of the surface. It is difficult to follow the elemental composition of wood by SEM as the components of wood (cellulose, lignin and hemicellulose) consist of the same elements (C, N, O). If the amount of one or more components changes, the elemental composition of the surface still remains the same.
3.3.3. Imaging XPS and Scanning AES
These techniques give structural information similar to SEM, although with limited lateral resolution (approx. 1 μm for XPS and 20 nm for AES). As extra advantage, elemental, in case of XPS also bonding state, composition data are supplied at the same time.
3.3.4. AFM
Morphological changes in the nanometer range can be monitored by atomic force microscopy [107]. It is a very sensitive method for following surface changes, but over a far smaller magnification range than SEM [108]. It is one of the best methods to monitor a polished plastic surface after plasma treatment, but examining wood is not easy. Owing to its composite nature, wood often exhibits macroscopic roughness, which is not in the AMF measurement range, so further increase in roughness can be measured with uncertainty.
3.4. What Really Happens during the Plasma Treatment of Wood
Wood or wood fiber is a natural composite material which consists of a mixture of semicrystalline cellulose in the form of fibrils, in an amorphous matrix of hemicellulose, lignin and lower molecular weight substances or extractives. These polymeric components are not uniformly distributed within the cell walls, and their structure, composition, and concentration change, even in the same tree [109]. As the plasma treatment is performed in the outmost layer, these components are not treated with the same condition, due to the physical accessibility and the different chemical composition and structure. Furthermore, the origin, age, moisture content, and geometry of the wood are parameters that can seriously affect the effect of plasma treatment. Thus, examining the effect of surface treatment is much more complex than in the case of a monocomponent material. Another complicating factor is that the resistance of aliphatic and aromatic polymers to plasma is different [110]—aromatic polymers are more resistant to plasma etching than aliphatic ones. Since wood contains both kinds of polymers, the different effects of plasma can be attributed to the difference in physical accessibility (which compound is closer to the plasma on the surface) and chemical composition as well. In addition, cellulose has amorphous and crystalline regions and according to Warner [111], in semi-crystalline polymers, the crystalline components are more resistant to plasma. It is almost impossible to decide which effect is stronger. In general, when treating a wood surface with a non-polymerizing gas-generated plasma, the surface becomes more hydrophilic than before due to surface activation and formation of new functional groups, with an increase in roughness owing to etching regardless of the exact composition of the wood surface. Therefore, if we want to improve the durability and water resistance of the wood, plasma treatment alone is not only not useful, but can make things worse. In these cases, after plasma activation a subsequent chemical reaction particularly grafting to be proposed to achieve the desired surface properties. During grafting or graft copolymerization, monomers are covalently bonded and even polymerized as side chains onto the main backbone [112].
Plasma activation, which improves adhesion to other materials/chemicals, could be also very useful and cost saving before gluing or coating of wooden samples [113]. Changing the composition of plasma gas or using different polymerizing gases, the surface can be hydrophobic, due to the formed coating layer with decreased surface roughness [114].
3.4.1. Chemical Changes
As we wrote earlier, during the plasma treatment of wood, we treat all the components simultaneously, so it is difficult to distinguish exactly what happens to each component separately. The methods used to examine the effectiveness of plasma cannot determine which component is responsible for the changes. Chemical changes are perhaps even more difficult to identify, as the chemical composition and the existing functional groups of the constituents of wood are quite similar. The fastest and simplest technique to follow both chemical and physical changes is contact angle measurement. It describes the wettability of a material and can be determined by the direct measurement of contact angle by viewing the drop profile [115]. Wettability shows the hydrophobic or hydrophilic attitude of a surface. It is influenced by the chemical and physical structure of the surface. Contact angle measurement is very fast and simple, but the least accurate in showing chemical and even physical properties.
Although it is very rare, there are examples in the literature when wood components are treated separately, which give idea of what happens when an individual component of wood is exposed to plasma. There have been experiments to determine which ingredients of wood are most sensitive to plasma. During the chemical removal of hemicelluloses used for this purpose before plasma treatment any other component can damaged [105].
- Hemicelluloses
Hemicelluloses are the most hydrophilic wood constituents with a composition of various sugar units such as pentoses, hexoses, hexuronic acids and deoxy hexoses and with a significantly lower degree of polymerization than cellulose. They are considered a sugar source for the bioethanol industry. Hemicelluloses are also used as a filler in polymer composites as part of wood flour, but their presence is a disadvantage for the properties of the composites [116]. Talviste [117] and coworker treated European beech by DCSBD in different atmosphere. They found that changes in the surface chemistry can be attributed to the degradation of hemicellulose on the surface (which degrade first during heat treatment [118]). Changes were detected by the increased water contact angle. Altgen et al. [31] modified different thermally pretreated wood samples in a DBD reactor and the reaction caused hydrophilization of the surfaces. They related it to the formation of carboxyl groups in the lignin network in the absent of hemicellulose which has been removed during heat pretreatment.
- Cellulose
Cellulose is a linear (1-4)-linked β-D-glucoglycan and one of the most abundant polymers in the world, making up 40%–50% of wood. Cellulose is responsible for the flexibility and strength of wood. It has three hydroxyl groups per glucose unit with different reactivities. These can form strong inter- and intra- hydrogen bonding with each other and result in different structure of cellulose [44]. Unmodified cellulose is used in the paper industry, but for a more widespread application of cellulose, the accessibility of hydroxyl groups must be improved in the hydrogen bonded network [119]. Cotton is the purest source of cellulose in nature—after scouring and bleaching, it contains 99% cellulose, so studies on the plasma treatment of cotton sometimes give an idea of what happens to cellulose during plasma treatment. Calvimontes [120] treated cellophane foils with a low-pressure oxygen plasma and the treatment changed cellulose chemically and physically. XPS analysis showed that cellulose was oxidized and formed aldehyde and carboxylate groups. However, in ammonia and nitrogen plasma Flynn [121] did not find any evidence for ring cleavage only building in of N-containing functional groups. Kolářová [122] used cotton as a cellulose source and treated it with argon plasma at low pressure. The investigation of the chemistry of the surface showed that fiber surface oxidized and D-glucose circle degraded moreover dramatic morphological changes were observed on the fiber surface. It seems that if hemicellulose is not present, the cellulose itself can undergo significant chemical and physical changes.
The authors also proved with XRD that plasma treatment did not change the crystallinity of the cellulose. This is difficult to declare for certain because the plasma treatment only affects a layer a few nanometers thick, but X-rays can penetrate to a depth of up to 10 microns. Therefore, minimal surface changes may not be detectable by XRD. The situation is very similar to FTIR analysis.
- Lignin
The lignin content of an average tree is usually around 15%–35%, which means that this is the second most common biomass on earth after cellulose. It is a highly aromatic cross-linked complex phenolic polymer and contains a number of functional groups such as aliphatic, phenolic hydroxyl, and carbonyl groups [123]. The available lignin on the market is mainly produced in the bioethanol and the paper industries, as a by-product of the extraction of the much more valuable cellulose. It is estimated that 98% of the world’s lignin production is immediately burned to generate the heat and electricity needed to produce cellulose [124]. The use of lignin as an additive in polymers is worth exploring because it contains phenolic hydroxyl groups, which are able to scavenge free radicals and stabilize the polymer (mostly polyolefin) matrix against oxidation, which improves the properties of the plastic. Moreover, it is very economical because of the large available quantity and low price [125]. Although the modification of lignin particles has been extensively studied, the modification of its surface by plasma has not been thoroughly investigated in spite of its many advantages over chemical treatment—this is because the method requires a reactor specially designed for powder. Klarhöfer et. al. [126] treated cellobiose and lignin in synthetic air and argon in a DBD apparatus. They found that the surface of lignin oxidized in oxygen plasma atmosphere creating hydroxyl, carbonyl, and carboxyl groups while in an Ar-plasma the reduction of the surface was observed. Sokolov [127] used pulsed corona discharge (PCD) for the modification of lignin and showed that during treatment, lignin oxidized to aldehydes, and oxidative cleavage of aromatic rings occurred. However, the precise nature of the changes in the structure remained unclear. Atz Dick [128] treated lignin in an oxygen RF plasma reactor under low pressure at room temperature, then grafted lactide covalently onto the surface of the lignin to prepare a reinforced PLA-based composite. The originality of his work was that the radical produced by the plasma on the surface of the lignin-initiated ring opening polymerization and this produced the reaction on the surface of the lignin.
3.4.2. Morphological Changes—Microscopically Visible Changes
Sometimes microscopically visible changes make it easier to understand what is happening, how the reactions can be imagined. Together with the chemical reactions that can take place, we can obtain a clear picture of what can happen during plasma treatment. Morphological changes usually result in increased roughness sometimes in the nanometer scale and it may not be visible on regular SEM micrographs. It can be revealed only at higher magnification or with other microscope techniques, based on different principle [129]. It is also worth noting that when treated with a polymerizing gas, the layer formed tends to mask the irregularities on the surface, so in this case the surface roughness reduced indeed. Ražić [130] experienced than in low pressure oxygen plasma the surface of cellulose will be etched and becomes more accessible for subsequent grafting. However, treatment with acrylic acid as a polymerizing agent surface becomes cleaner and smooth, compared to the untreated one. Jamali and Evans [131] exposed wood to water vapor glow discharge plasma and examined the etching effect by SEM and confocal profilometry. They concluded that the result of plasma treatment is very similar to the physical degradation achieved with gamma rays and lignin-rich cell wall layers were less affected by the plasma treatment than other parts of the cell wall. Galmiz et al. [132] examined the nanostructure produced by air DCSBD treatment of aspen, which is the result of plasma etching, and also determined the optimal distance (0.2–0.3 mm) between wood and DBD for etching. Talviste and co-workers [133] treated different thermally modified woods by DCSBD in air. SEM images showed that on the micrometer scale, plasma did not change the morphology significantly. Altgen et al. [98] studied the effect of air plasma by filamentary DBD and determined contact angle and morphological changes besides surface chemistry. They determined from SEM images that the treatment produced a hydrophobic surface but no changes in the morphology. Avramidis et al. [134] prepared both hydrophobic and hydrophilic surfaces with plasma on different woods and wood-based materials. Hydrophilization was carried out with a DBD air plasma while hydrophilization or plasma deposition in a plasma jet. The plasma polymerization reaction produced a water-repellent layer on the surfaces. The carrier gas was argon and the precursor was hexamethyldisiloxane. The surface changes were detected by contact angle measurement, XPS and AFM. The authors found that the polarity of the surfaces changed as expected, and AFM confirmed that a compact layer covered the surface. Košelová at al. [135] used DCSBD at atmospheric pressure with different gases for activation of spruce and finding the proper treatment distance and studied chemical and micro-morphological changes. Their observations are useful for industrial plasma application as regards the precise position of the sample during treatment and cannot find morphological changes by SEM during short plasma treatment.
4. Perspectives
The surface modification of wood by plasma still has much unexploited potential. Perhaps the most dynamically developing application of plasma is the production of composites with enhanced adhesion, and superhydrophobic cellulose-based materials. One of the key research areas of recent decades is the production of composites and endowing them with unique properties. Reinforced polymer composites are the most dynamically developing and most researched type of composites. Fillers, especially fibers can increase stiffness, strength, and heat resistance. The basic principle of fiber-reinforced composites is that the stiff and strong fibers carry the load, while the polymer matrix transfers it between the fibers. Natural fibers are not as stiff and strong as glass or carbon ones, but they have a number of advantages, including their natural origin, low density and low cost [136]. The properties of heterogeneous polymer composites are determined by different factors, including interfacial interactions between the components. These interactions can be influenced by the plasma treatment of the fiber/filler. Improper interfacial adhesion between the matrix and the filler leads to worse mechanical properties than could be achieved with good interfacial adhesion [137]. Mostly wood-based reinforcements are added to polyolefins [138], but nowadays natural-based biodegradable plastics (e.g., polylactic acid (PLA)) come to the fore to preserve the biodegradability of the composite [139]. For both types of polymer matrix, either the filler is treated with a chemical or an interfacial adhesion is increased with a compatibilizer, which creates a chemical bond between the filler and the matrix. For example, in case of polypropylene, this compatibilizer is maleinated polypropylene [140]. The plasma treatment of wood fibers can improve interfacial interactions, due to the newly formed functional groups and the enhanced roughness. Yuan [138] found that air and argon plasma treatment improved the tensile strength and modulus of wood fiber-PP composites, which could be attributed to the enhanced roughness, which promoted mechanical interlocking [141] (Figure 6) between the components. Ragoubi [142] studied both PP and PLA with corona-treated miscanthus fibers and stated that in both composites, mechanical and thermal properties were greatly enhanced. In PP composites, the mechanical interlocking were dominant, but in PLA, both the chemical and physical changes improve adhesion. Although plasma treatment can significantly improve the properties of a wood-polymer composite, the treatment of a fiber or powder (wood flour) by plasma on an industrial scale is not a mature technology and needs to be further developed.
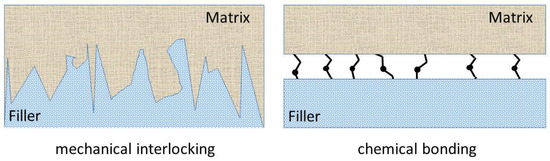
Figure 6.
Mechanical interlocking and chemical bonding.
Another promising area is the formation of superhydrophobic surfaces on a cellulose-based material [143]. Superhydrophobicity was first observed in nature on lotus leaves, which are not moistened by water, forming a droplet on it with a static contact angle above 150°, which does not adhere to the surface of the plant, but rolls off, and may even remove dirt as well [144]. Microscopic studies have shown that the regular pattern on the surface makes leaves extraordinary water-repellent, and since then a wide range of techniques have been used to mimic the fine micro- and nanoscale fractal structure of lotus leaves and other naturally occurring super-hydrophobic constructions. There are two basic ways to make a surface superhydrophobic. One is to make a rough surface from a low surface energy material, the other is to modify a rough surface with a material of low surface energy [145]. Usually, chemical methods are used to create a superhydrophobic surface [146,147,148], but roughness often needs to be changed as well [149]. During plasma treatment, with the right parameters, both processes can occur simultaneously, which is a significant step forward in improving the thermally and chemically unstable cellulose [114,150,151].
5. Conclusions
There are many wood finishing techniques, and nearly all of them have a long history. They are generally well-established technologies, all of which have, of course, been improved over the years as science has developed to meet the needs of industry. The aim of the treatments is to protect wood from environmental influences, prolonging its lifetime. Conventional treatments change the bulk properties of the wood, changing its structure, which is necessary in some cases, but often it is enough to change just the surface where the reactions take place. Each of these methods has its advantages and disadvantages but the disadvantages have come to the fore due to increasing environmental awareness. These include lengthy, high-temperature, chemical-intensive reactions that are a considerable burden on the environment. Any development or innovation that helps to reduce this burden is useful. Modern surface modification processes are more environmentally friendly, as much less of the wood needs to be modified, and this is beneficial as the consumption of energy and chemicals is greatly reduced. Among surface modification methods, cold plasma treatment is undoubtedly the most promising one. Compared to purely chemical methods, cold plasma processes offer an economical and more environmentally friendly solution for cleaning, etching, and modifying surfaces from a few atomic layers up to a thickness of 10 nm. Research is active in this field and plasma treatment of wood is gaining ground. It will not, however, completely replace chemical methods for changing the structure of wood. The other advantage of plasma treatment is that one can create active, anchoring points on the surface for subsequent grafting without catalysts or solvents, and endow wood with the desired properties
Author Contributions
Conceptualization, writing—original draft preparation, S.K.; Conceptualization, writing—review and editing, E.C.; writing—review and editing, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Attila Balaskó, Budapest University of Technology and Economics, for the careful proofreading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sandberg, D. Additives in wood products—Today and future development. In Environmental Impacts of Traditional and Innovative Forest-Based Bioproducts; Kutnar, A., Muthu, S.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–172. [Google Scholar]
- Gellerstedt, G. Wood chemistry and biotechnology. In Pulp and Paper Chemistry and Technology; Monica, E., Göran, G., Henriksson, U., Eds.; De Gruyter: Berlin, Germany, 2016. [Google Scholar]
- Wegner, T.; Skog, K.E.; Ince, P.J.; Michler, C.J. Uses and desirable properties of wood in the 21st century. J. For. 2010, 108, 165–173. [Google Scholar] [CrossRef]
- Petrič, M. Surface modification of wood: A critical review. Rev. Adhes. Adhes. 2013, 1, 216–247. [Google Scholar] [CrossRef]
- Thang, N.H.; Huyen, N.T.B. Fabrication of transparent composites from pinaceae wood packaging residues. Period. Polytech. Chem. Eng. 2022, 66, 135–146. [Google Scholar] [CrossRef]
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood modification technologies—A review. iForest-Biogeosci. For. 2017, 10, 895–908. [Google Scholar] [CrossRef] [Green Version]
- Bartos, A.; Anggono, J.; Farkas, Á.E.; Kun, D.; Soetaredjo, F.E.; Móczó, J.; Antoni; Purwaningsih, H.; Pukánszky, B. Alkali treatment of lignocellulosic fibers extracted from sugarcane bagasse: Composition, structure, properties. Polym. Test. 2020, 88, 106549. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, K.; Li, J.; Zhang, S.; Shi, S.Q. Environmentally Benign Wood Modifications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 3532–3540. [Google Scholar] [CrossRef]
- Militz, H.; Lande, S. Challenges in wood modification technology on the way to practical applications. Wood Mater. Sci. Eng. 2009, 4, 23–29. [Google Scholar] [CrossRef]
- Černák, M.; Kováčik, D.; Ráhel, J.; St’Ahel, P.; Zahoranová, A.; Kubincová, J.; Tóth, A.; Černková, L. Generation of a high-density highly non-equilibrium air plasma for high-speed large-area flat surface processing. Plasma Phys. Control. Fusion 2011, 53, 124031. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Mozetič, M.; Primc, G. Surface modification of PS polymer by oxygen-atom treatment from remote plasma: Initial kinetics of functional groups formation. Appl. Surf. Sci. 2021, 561, 150058. [Google Scholar] [CrossRef]
- Sundriyal, P.; Pandey, M.; Bhattacharya, S. Plasma-assisted surface alteration of industrial polymers for improved adhesive bonding. Int. J. Adhes. Adhes. 2020, 101, 102626. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma surface modification of biodegradable polymers: A review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- López Durán, V.; Larsson, P.A.; Wågberg, L. Chemical modification of cellulose-rich fibres to clarify the influence of the chemical structure on the physical and mechanical properties of cellulose fibres and thereof made sheets. Carbohydr. Polym. 2018, 182, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, F.W.M.R. Wood decay under the microscope. Fungal Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Spear, M.J.; Curling, S.F.; Dimitriou, A.; Ormondroyd, G.A. Review of functional treatments for modified wood. Coatings 2021, 11, 327. [Google Scholar] [CrossRef]
- Broda, M.; Majka, J.; Olek, W.; Mazela, B. Dimensional stability and hygroscopic properties of waterlogged archaeological wood treated with alkoxysilanes. Int. Biodeterior. Biodegrad. 2018, 133, 34–41. [Google Scholar] [CrossRef]
- Acosta, A.P.; Barbosa, K.T.; Amico, S.C.; Missio, A.L.; de Avila Delucis, R.; Gatto, D.A. Improvement in mechanical, physical and biological properties of eucalyptus and pine woods by raw pine resin in situ polymerization. Ind. Crops Prod. 2021, 166, 113495. [Google Scholar] [CrossRef]
- Verma, S.R.; Dwivedi, U.N. Lignin genetic engineering for improvement of wood quality: Applications in paper and textile industries, fodder and bioenergy production. S. Afr. J. Bot. 2014, 91, 107–125. [Google Scholar] [CrossRef] [Green Version]
- Corleto, R.; Gaff, M.; Niemz, P.; Sethy, A.K.; Todaro, L.; Ditommaso, G.; Razaei, F.; Sikora, A.; Kaplan, L.; Das, S.; et al. Effect of thermal modification on properties and milling behaviour of African padauk (Pterocarpus soyauxii Taub.) wood. J. Mater. Res. Technol. 2020, 9, 9315–9327. [Google Scholar] [CrossRef]
- Gaff, M.; Kačík, F.; Sandberg, D.; Babiak, M.; Turčani, M.; Niemz, P.; Hanzlík, P. The effect of chemical changes during thermal modification of European oak and Norway spruce on elasticity properties. Compos. Struct. 2019, 220, 529–538. [Google Scholar] [CrossRef]
- Özçifçi, A.; Okçu, O. Impacts of some chemicals on combustion properties of impregnated laminated veneer lumber (LVL). J. Mater. Process. Technol. 2008, 199, 1–9. [Google Scholar] [CrossRef]
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Walsh-Korbs, Z.; Avérous, L. Recent developments in the conservation of materials properties of historical wood. Prog. Mater. Sci. 2019, 102, 167–221. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Cabane, E.; Gierlinger, N.; Koetz, J.; Burgert, I. Improvement of wood material properties via in situ polymerization of styrene into tosylated cell walls. RSC Adv. 2014, 4, 12981–12988. [Google Scholar] [CrossRef] [Green Version]
- Mader, A.; Schirò, A.; Brischetto, M.; Pizzo, B. Interactions and penetration of polymers and nanolatexes into wood: An overview. Prog. Org. Coat. 2011, 71, 123–135. [Google Scholar] [CrossRef]
- Bulian, F.; Graystone, J. Wood Coatings: Theory and Practice; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Hill, C.; Altgen, M.; Rautkari, L. Thermal modification of wood—A review: Chemical changes and hygroscopicity. J. Mater. Sci. 2021, 56, 6581–6614. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural changes of oak wood main components caused by thermal modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef] [Green Version]
- Sikora, A.; Kačík, F.; Gaff, M.; Vondrová, V.; Bubeníková, T.; Kubovský, I. Impact of thermal modification on color and chemical changes of spruce and oak wood. J. Wood Sci. 2018, 64, 406–416. [Google Scholar] [CrossRef]
- Bekhta, P.; Salca, E.A.; Lunguleasa, A. Some properties of plywood panels manufactured from combinations of thermally densified and non-densified veneers of different thicknesses in one structure. J. Build. Eng. 2020, 29, 101116. [Google Scholar] [CrossRef]
- Bekhta, P.; Proszyk, S.; Krystofiak, T.; Mamonova, M.; Pinkowski, G.; Lis, B. Effect of thermomechanical densification on surface roughness of wood veneers. Wood Mater. Sci. Eng. 2014, 9, 233–245. [Google Scholar] [CrossRef]
- Khademi Bami, L.; Mohebby, B. Bioresistance of poplar wood compressed by combined hydro-thermo-mechanical wood modification (CHTM): Soft rot and brown-rot. Int. Biodeterior. Biodegrad. 2011, 65, 866–870. [Google Scholar] [CrossRef]
- Laskowska, A.; Sobczak, J.W. Surface chemical composition and roughness as factors affecting the wettability of thermo-mechanically modified oak (Quercus robur L.). Holzforschung 2018, 72, 993–1000. [Google Scholar] [CrossRef]
- Antikainen, T.; Paajanen, O.; Rautkari, L.; Kutnar, A.; Kamke, F.A.; Hughes, M. Simultaneous drying and densification of silver birch (Betula pendula L.) veneers: Analysis of morphology, thickness swelling, and density profile. Wood Sci. Technol. 2014, 48, 325–336. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H.; Kües, U. Biotechnology in the wood industry. Appl. Microbiol. Biotechnol. 2004, 63, 477–494. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Nielsen, P.H. Environmental assessment of enzyme use in industrial production-a literature review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Kudanga, T.; Prasetyo, E.N.; Sipilä, J.; Nousiainen, P.; Widsten, P.; Kandelbauer, A.; Nyanhongo, G.S.; Guebitz, G. Laccase-mediated wood surface functionalization. Eng. Life Sci. 2008, 8, 297–302. [Google Scholar] [CrossRef]
- Greimel, K.J.; Kudanga, T.; Nousiainen, P.; Sipilä, J.; Herrero Acero, E.; Nyanhongo, G.S.; Guebitz, G.M. Two distinct enzymatic approaches for coupling fatty acids onto lignocellulosic materials. Process Biochem. 2017, 59, 111–115. [Google Scholar] [CrossRef]
- Muller, C.; Euring, M.; Kharazipour, A. Enzymatic modification of wood fibres for activating their ability of self bonding. Int. J. Mater. Prod. Technol. 2009, 36, 189. [Google Scholar] [CrossRef]
- Rowell, R.M. Chemical modification of wood: A short review. Wood Mater. Sci. Eng. 2006, 1, 29–33. [Google Scholar] [CrossRef]
- Papadopoulos, A.N. Chemical modification of solid wood and wood raw material for composites production with linear chain carboxylic acid anhydrides: A brief review. BioResources 2010, 5, 499–506. [Google Scholar] [CrossRef]
- Teacă, C.A.; Tanasa, F. Wood surface modification-classic and modern approaches in wood chemical treatment by esterification reactions. Coatings 2020, 10, 629. [Google Scholar] [CrossRef]
- Freeman, M.H.; Shupe, T.F.; Vlosky, R.P.; Barnes, H.M. Past, Present and Future of The Wood Preservation Industry. For. Prod. J. 2003, 53, 8–15. [Google Scholar]
- Helsen, L.; Van Den Bulck, E. Review of disposal technologies for chromated copper arsenate (CCA) treated wood waste, with detailed analyses of thermochemical conversion processes. Environ. Pollut. 2005, 134, 301–314. [Google Scholar] [CrossRef]
- Cesprini, E.; Resente, G.; Causin, V.; Urso, T.; Cavalli, R.; Zanetti, M. Energy recovery of glued wood waste–A review. Fuel 2020, 262, 116520. [Google Scholar] [CrossRef]
- Civardi, C.; Schwarze, F.W.M.R.; Wick, P. Micronized copper wood preservatives: An efficiency and potential health risk assessment for copper-based nanoparticles. Environ. Pollut. 2015, 200, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.K.; Bhardwaj, N.K. Biobleaching-An ecofriendly and environmental benign pulp bleaching technique: A review. J. Carbohydr. Chem. 2019, 38, 87–108. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Knuutinen, J.S.; Ahkola, H.S.J.; Herve, S.H. Refractory organic pollutants and toxicity in pulp and paper mill wastewaters. Environ. Sci. Pollut. Res. 2015, 22, 6473–6499. [Google Scholar] [CrossRef] [Green Version]
- Wagle, P.G.; Tamboli, S.S.; More, A.P. Peelable coatings: A review. Prog. Org. Coat. 2021, 150, 106005. [Google Scholar] [CrossRef]
- De Meijer, M. Review on the durability of exterior wood coatings with reduced VOC-content. Prog. Org. Coat. 2001, 43, 217–225. [Google Scholar] [CrossRef]
- Wolkenhauer, A.; Avramidis, G.; Hauswald, E.; Militz, H.; Viöl, W. Plasma treatment of wood-plastic composites to enhance their adhesion properties. J. Adhes. Sci. Technol. 2008, 22, 2025–2037. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, W.; Lin, H.; Yu, J.; Yang, W.; Zhang, X. Plasma treatments to improve the bonding of thermo-treated cherry wood. Coatings 2019, 9, 656. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Jakob, M.; Mahendran, A.R.; Gindl-Altmutter, W.; Bliem, P.; Konnerth, J.; Müller, U.; Veigel, S. The strength and stiffness of oriented wood and cellulose-fibre materials: A review. Prog. Mater. Sci. 2022, 125, 100916. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Surface engineering. In Tribology. Friction and Wear of Engineering Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 237–281. ISBN 0858253593. [Google Scholar]
- Väisänen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef]
- Samyn, P. Plasma-assisted fibrillation and surface-modification of microfibrillar cellulose. Mater. Lett. 2021, 304, 130615. [Google Scholar] [CrossRef]
- Klébert, S.; Károly, Z.; Késmárki, A.; Domján, A.; Mohai, M.; Keresztes, Z.; Kutasi, K. Solvent- and catalysts-free immobilization of tannic acid and polyvinylpyrrolidone onto PMMA surface by DBD plasma. Plasma Process. Polym. 2017, 14, 1600202. [Google Scholar] [CrossRef] [Green Version]
- Tajima, S.; Komvopoulos, K. Effect of reactive species on surface crosslinking of plasma-treated polymers investigated by surface force microscopy. Appl. Phys. Lett. 2006, 89, 124102. [Google Scholar] [CrossRef]
- Riedl, B.; Angel, C.; Prégent, J.; Blanchet, P.; Stafford, L. Effect of wood surface modification by atmospheric pressure plasma on waterborne coating adhesion. BioResources 2014, 9, 4908–4923. [Google Scholar] [CrossRef] [Green Version]
- Avramidis, G.; Klarhöfer, L.; Maus-Friedrichs, W.; Militz, H.; Viöl, W. Influence of air plasma treatment at atmospheric pressure on wood extractives. Polym. Degrad. Stab. 2012, 97, 469–471. [Google Scholar] [CrossRef]
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A. Recent advances of cold plasma technology for water and soil remediation: A critical review. Chem. Eng. J. 2022, 428, 131657. [Google Scholar] [CrossRef]
- Rane, R.; Ranjan, M.; Mukherjee, S. Basics of plasma and its industrial applications in textiles. In Plasma Technologies for Textile and Apparel; Nema, S.K., Jhala, P.B., Eds.; Taylor & Francis: New Delhi, India, 2015; ISBN 9789380308951. [Google Scholar]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, P. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; Van der Mullen, J. Gas discharge plasmas and their applications. Spectrochim. Acta-Part B At. Spectrosc. 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Kócs, L.; Késmárki, A.; Klébert, S.; Madarász, J.; Hórvölgyi, Z. Plasma-assisted template removal and consolidation of silica coatings on polycarbonate. Thin Solid Films 2021, 738, 138976. [Google Scholar] [CrossRef]
- Károly, Z.; Románszki, L.; Weltz, G.; Mohai, M.; Móczó, J.; Klébert, S. Comparison of dielectric barrier discharge and radio-frequency plasma processing of carbon fibers. Express Polym. Lett. 2021, 15, 1004–1017. [Google Scholar] [CrossRef]
- Gomez, E.; Rani, D.A.; Cheeseman, C.R.; Deegan, D.; Wise, M.; Boccaccini, A.R. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626. [Google Scholar] [CrossRef]
- Okumoto, M.; Mizuno, A. Conversion of methane for higher hydrocarbon fuel synthesis using pulsed discharge plasma method. Catal. Today 2001, 71, 211–217. [Google Scholar] [CrossRef]
- Plattfaut, I.; Besser, M.; Severing, A.L.; Stürmer, E.K.; Opländer, C. Plasma medicine and wound management: Evaluation of the antibacterial efficacy of a medically certified cold atmospheric argon plasma jet. Int. J. Antimicrob. Agents 2021, 57, 106319. [Google Scholar] [CrossRef]
- Demir, A. Atmospheric plasma advantages for mohair fibers in textile applications. Fibers Polym. 2010, 11, 580–585. [Google Scholar] [CrossRef]
- Pavliňák, D.; Galmiz, O.; Pavliňáková, V.; Poláček, P.; Kelar, J.; Stupavská, M.; Černák, M. Application of dielectric barrier plasma treatment in the nanofiber processing. Mater. Today Commun. 2018, 16, 330–338. [Google Scholar] [CrossRef]
- Pykönen, M.; Johansson, K.; Dubreuil, M.; Vangeneugden, D.; Ström, G.; Fardim, P.; Toivakka, M. Evaluation of plasma-deposited hydrophobic coatings on pigment-coated paper for reduced dampening water absorption. J. Adhes. Sci. Technol. 2010, 24, 511–537. [Google Scholar] [CrossRef]
- Radetić, M.; Marković, D. A review on the role of plasma technology in the nano-finishing of textile materials with metal and metal oxide nanoparticles. Plasma Process. Polym. 2022, e2100197. [Google Scholar] [CrossRef]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold plasma in food processing: Design, mechanisms, and application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Černák, M.; Černáková, L.; Hudec, I.; Kováčik, D.; Zahoranová, A. Diffuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. EPJ Appl. Phys. 2009, 47, 22806. [Google Scholar] [CrossRef] [Green Version]
- Galmiz, O.; Tucekova, Z.K.; Kelar, J.; Zemanek, M.; Stupavska, M.; Kovacik, D.; Cernak, M. Effect of atmospheric pressure plasma on surface modification of paper. AIP Adv. 2019, 9, 105013. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio 2022, 13, 100200. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Microwave- and radio-frequency-powered cold plasma applications for food safety and preservation. In Advances in Cold Plasma Applications for Food Safety and Preservation; Bermudez-Aguirre, D., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 309–329. [Google Scholar]
- Dilks, A. Polymer surfaces. Anal. Chem. 1981, 53, 802A–816A. [Google Scholar] [CrossRef]
- Lafleur, T.; Schulze, J.; Donkó, Z. Plasma-surface interactions. Plasma Sources Sci. Technol. 2019, 28, 040201. [Google Scholar] [CrossRef]
- Kale, K.H.; Desai, A.N. Atmospheric pressure plasma treatment of textiles using non-polymerising gases. Indian J. Fibre Text. Res. 2011, 36, 289–299. [Google Scholar]
- George, J.; Sreekala, M.S.; Thomas, S. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001, 41, 1471–1485. [Google Scholar] [CrossRef]
- Karaca, B.; Csiszár, E.; Bozdogan, F. Effects of atmospheric plasma pretreatments on pectinase efficiency in bioscouring of linen fabrics. Plasma Chem. Plasma Process. 2011, 31, 623–633. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Gogolides, E. Atmospheric plasma etching of polymers: A palette of applications in cleaning/ashing, pattern formation, nanotexturing and superhydrophobic surface fabrication. Microelectron. Eng. 2018, 194, 109–115. [Google Scholar] [CrossRef]
- Alanis, A.; Valdés, J.H.; María Guadalupe, N.V.; Lopez, R.; Mendoza, R.; Mathew, A.P.; Díaz De León, R.; Valencia, L. Plasma surface-modification of cellulose nanocrystals: A green alternative towards mechanical reinforcement of ABS. RSC Adv. 2019, 9, 17417–17424. [Google Scholar] [CrossRef] [Green Version]
- Bertóti, I.; Mohai, M.; Tóth, A.; Ujvári, T. Nitrogen-PBII modification of ultra-high molecular weight polyethylene: Composition, structure and nanomechanical properties. Surf. Coat. Technol. 2007, 201, 6839–6842. [Google Scholar] [CrossRef]
- Fridman, A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Bormashenko, E.; Chaniel, G.; Grynyov, R. Towards understanding hydrophobic recovery of plasma treated polymers: Storing in high polarity liquids suppresses hydrophobic recovery. Appl. Surf. Sci. 2013, 273, 549–553. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Kung, F.C.; Ko, C.L.; Okino, A.; Chiang, T.C.; Guo, J.Y.; Chen, S.Y. Tailoring surface properties of polyethylene terephthalate by atmospheric pressure plasma jet for grafting biomaterials. Thin Solid Films 2020, 709, 138152. [Google Scholar] [CrossRef]
- de Farias, J.G.G.; Cavalcante, R.C.; Canabarro, B.R.; Viana, H.M.; Scholz, S.; Simão, R.A. Surface lignin removal on coir fibers by plasma treatment for improved adhesion in thermoplastic starch composites. Carbohydr. Polym. 2017, 165, 429–436. [Google Scholar] [CrossRef]
- Altgen, D.; Avramidis, G.; Viöl, W.; Mai, C. The effect of air plasma treatment at atmospheric pressure on thermally modified wood surfaces. Wood Sci. Technol. 2016, 50, 1227–1241. [Google Scholar] [CrossRef]
- Szabo, O.E.; Csiszar, E.; Koczka, B.; Toth, A.; Klebert, S. Enhancing the accessibility of starch size and cellulose to enzymes in raw cotton woven fabric by air-plasma pretreatment. Text. Res. J. 2016, 86, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Kale, D.P.; Bansal, A.K. Surface characterization of pharmaceutical solids. Trends Anal. Chem. 2021, 138, 116228. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; László, K. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon 2015, 84, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Tóth, A.; Černáková, L.; Černák, M.; Kunovská, K. Surface analysis of groundwood paper treated by diffuse coplanar surface barrier discharge (DCSBD) type atmospheric plasma in air and in nitrogen. Holzforschung 2007, 61, 528–531. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. Probing the nano- and micro-scales of reverse osmosis membranes-A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements. J. Memb. Sci. 2007, 287, 146–156. [Google Scholar] [CrossRef]
- Vandencasteele, N.; Reniers, F. Plasma-modified polymer surfaces: Characterization using XPS. J. Electron Spectros. Relat. Phenom. 2010, 178–179, 394–408. [Google Scholar] [CrossRef]
- Pejić, B.M.; Kramar, A.D.; Obradović, B.M.; Kuraica, M.M.; Žekić, A.A.; Kostić, M.M. Effect of plasma treatment on chemical composition, structure and sorption properties of lignocellulosic hemp fibers (Cannabis sativa L.). Carbohydr. Polym. 2020, 236, 116000. [Google Scholar] [CrossRef]
- Hansmann, C.; Weichslberger, G.; Gindl, W. A two-step modification treatment of solid wood by bulk modification and surface treatment. Wood Sci. Technol. 2005, 39, 502–511. [Google Scholar] [CrossRef]
- Ţălu, Ş. Micro and Nanoscale Characterization of Three Dimensional Surfaces: Basics and Applications; Napoca Star: Cluj-Napoca, Romania, 2015. [Google Scholar]
- Baldelli, A.; Trivanovic, U.; Sipkens, T.A.; Rogak, S.N. On determining soot maturity: A review of the role of microscopy- and spectroscopy-based techniques. Chemosphere 2020, 252, 126532. [Google Scholar] [CrossRef]
- Kollmann, F.F.P.; Cote, W.A.J. Chemical Composition of Wood. In Principles of Wood Science and Technology; Springer: Berlin/Heidelberg, Germany, 1968. [Google Scholar]
- Pederson, L.A. Structural composition of polymers relative to their plasma etch characteristics. J. Electrochem. Soc. 1982, 129, 205. [Google Scholar] [CrossRef]
- Warner, S.B.; Uhlmann, D.R.; Peebles, L.H. Ion etching of amorphous and semicrystalline fibres. J. Mater. Sci. 1975, 10, 758–764. [Google Scholar] [CrossRef]
- Olsén, P.; Herrera, N.; Berglund, L.A. Polymer grafting inside wood cellulose fibers by improved hydroxyl accessibility from fiber swelling. Biomacromolecules 2020, 21, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Acda, M.N.; Devera, E.E.; Cabangon, R.J.; Ramos, H.J. Effects of plasma modification on adhesion properties of wood. Int. J. Adhes. Adhes. 2012, 32, 70–75. [Google Scholar] [CrossRef]
- Matouk, Z.; Torriss, B.; Rincón, R.; Dorris, A.; Beck, S.; Berry, R.M.; Chaker, M. Functionalization of cellulose nanocrystal films using Non-Thermal atmospheric –Pressure plasmas. Appl. Surf. Sci. 2020, 511, 145566. [Google Scholar] [CrossRef]
- Románszki, L.; Mohos, M.; Telegdi, J.; Keresztes, Z.; Nyikos, L. A comparison of contact angle measurement results obtained on bare, treated, and coated alloy samples by both dynamic sessile drop and Wilhelmy method. Period. Polytech. Chem. Eng. 2014, 58, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Hosseinaei, O.; Wang, S.; Enayati, A.A.; Rials, T.G. Effects of hemicellulose extraction on properties of wood flour and wood-plastic composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 686–694. [Google Scholar] [CrossRef]
- Talviste, R.; Galmiz, O.; Stupavská, M.; Ráhel’, J. Effect of DCSBD plasma treatment distance on surface characteristics of wood and thermally modified wood. Wood Sci. Technol. 2020, 54, 651–665. [Google Scholar] [CrossRef]
- Gérardin, P. New alternatives for wood preservation based on thermal and chemical modification of wood— a review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Arumughan, V.; Nypelö, T.; Hasani, M.; Larsson, A. Fundamental aspects of the non-covalent modification of cellulose via polymer adsorption. Adv. Colloid Interface Sci. 2021, 298, 102529. [Google Scholar] [CrossRef]
- Calvimontes, A.; Mauersberger, P.; Nitschke, M.; Dutschk, V.; Simon, F. Effects of oxygen plasma on cellulose surface. Cellulose 2011, 18, 803–809. [Google Scholar] [CrossRef]
- Flynn, C.N.; Byrne, C.P.; Meenan, B.J. Surface modification of cellulose via atmospheric pressure plasma processing in air and ammonia-nitrogen gas. Surf. Coat. Technol. 2013, 233, 108–118. [Google Scholar] [CrossRef]
- Kolářová, K.; Vosmanská, V.; Rimpelová, S.; Švorčík, V. Effect of plasma treatment on cellulose fiber. Cellulose 2013, 20, 953–961. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kun, D. Polymer/Lignin Blends: Interactions, Structure, Properties. Budapest University of Technology and Economics, Budapest, Hungary, 2017. [Google Scholar]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Klarhöfer, L.; Viöl, W.; Maus-Friedrichs, W. Electron spectroscopy on plasma treated lignin and cellulose. Holzforschung 2010, 64, 331–336. [Google Scholar] [CrossRef]
- Sokolov, A.; Lagerquist, L.; Eklund, P.; Louhi-Kultanen, M. Non-thermal gas-phase pulsed corona discharge for lignin modification. Chem. Eng. Process.-Process Intensif. 2018, 126, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Atz Dick, T.; Couve, J.; Gimello, O.; Mas, A.; Robin, J.J. Chemical modification and plasma-induced grafting of pyrolitic lignin. Evaluation of the reinforcing effect on lignin/poly(L-lactide) composites. Polymer 2017, 118, 280–296. [Google Scholar] [CrossRef]
- Románszki, L.; Klébert, S.; Héberger, K. Estimating nanoscale surface roughness of polyethylene terephthalate fibers. ACS Omega 2020, 5, 3670–3677. [Google Scholar] [CrossRef]
- Ražić, S.E.; Čunko, R.; Bautista, L.; Bukošek, V. Plasma effect on the chemical structure of cellulose fabric for modification of some functional properties. Procedia Eng. 2017, 200, 333–340. [Google Scholar] [CrossRef]
- Jamali, A.; Evans, P.D. Etching of wood surfaces by glow discharge plasma. Wood Sci. Technol. 2011, 45, 169–182. [Google Scholar] [CrossRef]
- Galmiz, O.; Talviste, R.; Panáček, R.; Kováčik, D. Cold atmospheric pressure plasma facilitated nano-structuring of thermally modified wood. Wood Sci. Technol. 2019, 53, 1339–1352. [Google Scholar] [CrossRef]
- Talviste, R.; Galmiz, O.; Stupavská, M.; Tučeková, Z.; Kaarna, K.; Kováčik, D. Effect of DCSBD plasma treatment on surface properties of thermally modified wood. Surf. Interfaces 2019, 16, 8–14. [Google Scholar] [CrossRef]
- Avramidis, G.; Hauswald, E.; Lyapin, A.; Militz, H.; Viöl, W.; Wolkenhauer, A. Plasma treatment of wood and wood-based materials to generate hydrophilic or hydrophobic surface characteristics. Wood Mater. Sci. Eng. 2009, 4, 52–60. [Google Scholar] [CrossRef]
- Košelová, Z.; Ráhel, J.; Galmiz, O. Plasma treatment of thermally modified and unmodified norway sprucewood by diffuse coplanar surface barrier discharge. Coatings 2021, 11, 40. [Google Scholar] [CrossRef]
- Várdai, R. Polypropylene Hybrid Composites: Structure and Properties. Budapest University of Technology and Economics, Budapest, Hungary, 2021. [Google Scholar]
- Müller, P.; Renner, K.; Móczó, J.; Fekete, E.; Pukánszky, B. Thermoplastic starch/wood composites: Interfacial interactions and functional properties. Carbohydr. Polym. 2014, 102, 821–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Jayaraman, K.; Bhattacharyya, D. Effects of plasma treatment in enhancing the performance of woodfibre-polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1363–1374. [Google Scholar] [CrossRef]
- Gibeop, N.; Lee, D.W.; Prasad, C.V.; Toru, F.; Kim, B.S.; Song, J. Il Effect of plasma treatment on mechanical properties of jute fiber/poly (lactic acid) biodegradable composites. Adv. Compos. Mater. 2013, 22, 389–399. [Google Scholar] [CrossRef]
- Dányádi, L.; Janecska, T.; Szabó, Z.; Nagy, G.; Móczó, J.; Pukánszky, B. Wood flour filled PP composites: Compatibilization and adhesion. Compos. Sci. Technol. 2007, 67, 2838–2846. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fan, M.; Chen, L. Interface and bonding mechanisms of plant fibre composites: An overview. Compos. Part B Eng. 2016, 101, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Ragoubi, M.; George, B.; Molina, S.; Bienaimé, D.; Merlin, A.; Hiver, J.M.; Dahoun, A. Effect of corona discharge treatment on mechanical and thermal properties of composites based on miscanthus fibres and polylactic acid or polypropylene matrix. Compos. Part A Appl. Sci. Manuf. 2012, 43, 675–685. [Google Scholar] [CrossRef]
- Shateri Khalil-Abad, M.; Yazdanshenas, M.E. Superhydrophobic antibacterial cotton textiles. J. Colloid Interface Sci. 2010, 351, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Hu, Z.; Dzapata, R.L.; Xu, Y.; Christie, P.; Guo, D.; Li, J. Facile method for the preparation of superhydrophobic cellulosic paper. Appl. Surf. Sci. 2019, 496, 143648. [Google Scholar] [CrossRef]
- Li, E.; Pan, Y.; Wang, C.; Liu, C.; Shen, C.; Pan, C.; Liu, X. Multifunctional and superhydrophobic cellulose composite paper for electromagnetic shielding, hydraulic triboelectric nanogenerator and Joule heating applications. Chem. Eng. J. 2021, 420, 129864. [Google Scholar] [CrossRef]
- Ahuja, D.; Dhiman, S.; Rattan, G.; Monga, S.; Singhal, S.; Kaushik, A. Superhydrophobic modification of cellulose sponge fabricated from discarded jute bags for oil water separation. J. Environ. Chem. Eng. 2021, 9, 105063. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic coatings on cellulose-based materials: Fabrication, properties, and applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Balu, B.; Breedveld, V.; Hess, D.W. Fabrication of “roll-off” and “sticky” superhydrophobic cellulose surfaces-via plasma processing. Langmuir 2008, 24, 4785–4790. [Google Scholar] [CrossRef]
- Yao, M.Z.; Liu, Y.; Qin, C.N.; Meng, X.J.; Cheng, B.X.; Zhao, H.; Wang, S.F.; Huang, Z.Q. Facile fabrication of hydrophobic cellulose-based organic/inorganic nanomaterial modified with POSS by plasma treatment. Carbohydr. Polym. 2021, 253, 117193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).