Abstract
Oral mucosal diseases account for an increasing proportion of hμMan diseases. Among the many common risk factors that cause oral diseases and systemic diseases, dietary factors, especially high sugar, are particularly prominent. Exhibiting therapeutic potential in treating certain inflammation-related diseases, platycodin D (PD) has been known to possess anti-inflammatory benefits in cases of cytokine-induced inflammation, a fact that has been widely docμMented. However, there are few studies about PD in the oral mucosal disease. Investigating the effect of PD on high-glucose (HG)-induced inflammatory responses in oral mucosal cells was the endeavor of this study. The results revealed that HG induced cell mortality, promoted activity of inflammatory factor (TNF-α, IL-1β, IL-6, and IL-8), and increased ROS production in oral mucosal cells. Interestingly, PD obviously alleviated HG-induced oral mucosal cells inflammatory response. Simultaneously, the expressions of PI3K and mTOR were inhibited by PD. In addition, the activation of PI3K and mTOR decreased the protective effect of PD on oral mucosal cells. To conclude, the PI3K/mTOR signaling pathway was found to be inactivated, thereby restraining the activation of the full immune cell by inhibition of the pro-inflammatory cytokines, as revealed by the results indicating the prevention of the HG-induced inflammation response by PD.
1. Introduction
The oral cavity is an indispensable organ for people to engage in various social activities in daily life. Healthy oral tissue is the foundation of our happy life, while any impairment would lead to reducing the quality of life vastly [1]. A major global health concern having significant economic and social impact is represented by oral inflammatory diseases [2]. In the research progress of autoinflammatory diseases, the oral inflammatory diseases act as potential factors in modifying the severity. Besides, chronic inflammatory illnesses such as heart diseases, arthritis, and diabetes are known to affect the oral cavity [3]. The oral mucosal cells are exposed directly to hypertonic environments consisting of saline, glucose, and such other elements in vivo, while intake of added sugars in excessive quantities has been known to be associated with several health disorders including cardiovascular disease, diabetes, dental caries, and obesity, since the mouth is the first part of the digestive canal that receives food and produces saliva [4]. Cell deaths of cultured epithelial cells in vitro have been associated with inducement of high glucose [5]. Moreover, oral complications have been indicated in more than 90% of diabetic patients [6]. Besides, weakened protection against inflammation and infections with damaged small blood vessels have been known to delay wound healing in cases of diabetes mellitus [7]. Therefore, it is particularly important to understand the molecular mechanism of oral cavity cells under high glucose.
The expression of inflammatory cytokines such as interleukin (IL)-1β and IL-6 and tμMor necrosis factor-α (TNF-α) has been known to be increased in the case of inflammation. The cellular pathways engaged in the protection of the organs need to be regulated by these inflammatory regulators. Nevertheless, severe inflammatory disorders could occur in the case of excessive inflammatory responses resulting in overexpression of pro-inflammatory factors [8]. On a global scale, over 80 to 90% of adults are afflicted by oral inflammation diseases, with oral cavity diseases being the most chronic diseases in adults and children prevalent worldwide [9]. If oral inflammation is not treated in time, it may lead to oral cancer and other lesions in the body [3].
Isolated from the root of the plant, Platycodon grandiflorus platycodin D (PD) is a triterpenoid saponin, and has been known to possess hepatoprotective, anti-cancer, immune-enhancing, and anti-inflammatory properties [10]. The two major cellular signaling pathways affecting the cellular functions, including growth, survival, and metabolism, are the mammalian target of rapamycin (mTOR) and the phosphatidylinositol-3 kinases (PI3Ks) pathways [11]. LPS-stimulated inflammatory response could cause the suppression of the PI3K/AKT and MAPK signaling pathways, and NF-κB activation has been found [8]. Moreover, in the primary rat microglia cells, PD was observed to significantly inhibit the production of the LPS-induced inflammatory factors and ROS [12]. However, PD in the treatment of diabetes is unclear.
In this study, our main goal is to investigate the effects of PD on inflammatory responses in high-glucose-induced oral mucosal cells, as well as uncover whether high glucose can induce activation of PI3K/mTOR signaling pathway involved in oral mucosal cell inflammatory responses. These research results will provide solutions for oral disease therapy.
2. Materials and Methods
2.1. Cell Culture and Treatment
Normal oral mucosal cells were purchased from Shanghai Anwei Biotechnology Co., Ltd. (Shanghai, China); the cells that were cultured contained 5% CO2 at 37 °C, in a basal culture mediμM DEME (Gibco, New York, NY, USA, dulbecco’s modified eagle medium) containing 100 μg/mL streptomycin, 100 U/mL penicillin, pH 7.2, and 10% fetal bovine serμM (FBS). Then, oral mucosal cells were seeded in 6-well dishes and treated with glucose (10, 20, 35, 50, 100 mM) at 37 °C for 48 h with 5% CO2. Moreover, before the HG treatment, the oral mucosal cells were predisposed by PD for 6 h from 0–10 μM. The methanol treatment was the same as in the control group. The PI3K/AKT activator (740-YP, 20 μM, Sigma, New York, NY, USA, Phosphoinositide-3-Kinase/Threonine Kinase) and mTOR activator (3BDO, 60 μM, Sigma, New York, NY, USA, Mechanistic target of rapamycin kinase) predisposed the oral mucosal cells for 24 h before the HG treatment in the follow-up correlative experiments.
2.2. Cell Counting Kit-8 (CCK-8) Assay
Following the instructions of the manufacturer, using a CCK-8 assay kit (Beyotime Biotechnology Co., Ltd., Shanghai, China), the cell viability of oral mucosal cells was detected. Briefly, in 96-well plates, the oral mucosal cells were cultured. The cells were treated with drugs at 37 °C for 24 h when the confluence of the cells grew to 80%. The control group was constituted by the cells treated with 0.05% DMSO. Prior to being incubated for 2 h at 37 °C, 10 μL CCK-8 solution was added to the well after treatment. The measurement of absorbance was 450 nm. The percentage of the drug group to the control group (100%) expressed the cell viability. The mean of three independent experiments constituted the data.
2.3. Reactive Oxygen Species (ROS)
Based on the instructions of the manufacturer, the ROS production in the oral mucosal cells was measured with the cell permeant probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA, Beyotime). Briefly, in a 6-well plate, the oral mucosal cells were cultured. The cells were treated with drugs at 37 °C for 24 h as the confluence of the cells reached 80%. The cells were cultured for 30 min in the dark at 37 °C, after adding 10 μM DCFH-DA to the well. After being detected using a BD FACSAria III flow cytometer (BD Biosciences, franklin, New York, NY, USA), the cells were subsequently resuspended using PBS. Around 10,000 events were collected for every sample. The mean DCF fluorescence represented the production of ROS.
2.4. Real-Time Quantitative PCR (RT-qPCR)
According to the instructions of the manufacturer, total RNA was extracted from the treated oral mucosal cells with TRIzol reagent (Invitrogen, New York, NY, USA). Synthesis of the cDNA was performed using the First Strand gDNA Synthesis Kit (Takara, Kusatsu, Japan) with 500 ng of total RNA. Based on the recommendations of the manufacturer, using the SYBR dye (Takara Kusatsu, Japan), with an ABI 7500 thermal cycler (Applied Biosystems, Foster City, CA, USA), the determination of the mRNA expression level was achieved through quantitative real-time PCR (qRT-PCR). While the relative quantification was calculated applying the 2−ΔΔCt method, the relative expression of the target was normalized by β-actin. The representative results were indicated after performing three independent RT-PCR experiments.
2.5. Enzyme-Linked Immunosorbent (ELISA) Assay
Oral mucosal cells were cultured in a 6-well plate for 24 h in DMEM with 10% FBS, and thereafter, the supernatant was collected to measure the levels of TNF-α, IL-1β, IL-6, and IL-8 using kits according to the manufacturer’s instructions (Invitrogen, New York, NY, USA).
2.6. Western Blot
The cell protein was extracted with 100 μL of lysis buffer at 12,000 g for 30 min at 4 °C. Samples (35 μg protein/well) were loaded on 6% SDS-PAGE and electro-transferred to PVDF membranes for 3 h. Then, the membranes were blocked with TBS (containing 5% non-fat dry milk) for 1 h at room temperature. Subsequently, the membranes were incubated with primary antibodies of mTOR (Abcam ab134903, 1:8000, Cambridge, UK), PI3K (Abcam ab32089, 1:10,000), and β-actin (Abcam ab8226, 1:5000) overnight at 4 °C. Afterwards, they were washed 5 times with TBST. The membranes were incubated with HRP IgG for 2 h at room temperature. After, they were washed 5 times with TBST. The membranes were visualized using ECL reagent.
2.7. Statistical Analysis
We used SPSS (version 20.0 for Windows) for all statistical analyses. For quantitation of gene expression, we used the comparative CT method (2−ΔΔCT). All results are expressed as means ± SD. Logarithmic transformations of the data were made when necessary to fulfil the conditions of the analysis of variance, but data are shown in their decimal values for clarity. Using the one-way ANOVA to detect the multiple comparisons and the LSD post hoc comparisons, the differences between the groups were analyzed. Results were considered statistically significant when p < 0.05.
3. Results
3.1. HG Induced Oral Mucosal Cells Inflammatory Responses
For observing the change of oral mucosal cells viability, various concentrations of glucose (0, 10, 20, 35, 50, and 100 mM) were used to expose the oral mucosal cell in the present study. Cell viability decreased significantly for oral mucosal cells at the concentrations of 35 mM (p < 0.05), 50 mM (p < 0.01), and 100 mM (p < 0.01) (Figure 1A). Later, HG concentration was chosen as 50 mM for the following experiments. The concentration of pro-inflammatory cytokines, TNF-α, IL-1β, IL-6, and IL-8 in oral mucosal cells was measured by ELISA; under HG-stimulated condition, the TNF-α, IL-1β, IL-6, and IL-8 secretion was significantly increased (Figure 1B). Meanwhile, a significant increase of the level of ROS by HG in the oral mucosal cells was observed (Figure 1C,D). Taken together, the above results suggested that HG could induce oral mucosal cell damage and arouse inflammatory responses.
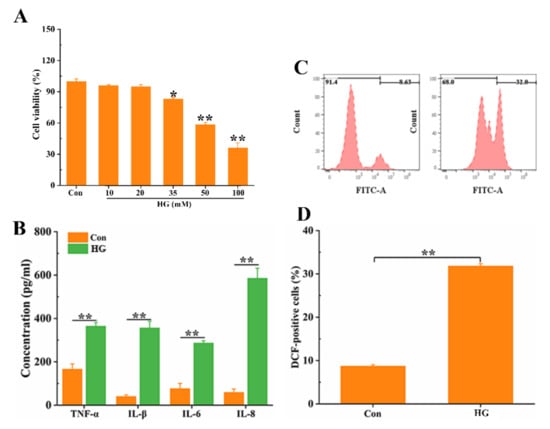
Figure 1.
HG-induced oral mucosal cell inflammatory responses. (A) HG-induced oral mucosal cell viability. The oral mucosal cells were treated with different concentrations of glucose (0, 10, 20, 35, 50, and 100 mM) for 48 h, and cell viability was examined by CCK-8 assay. After treatment with 50 mM glucose, inflammatory (B) and ROS (C,D) were detected. * p < 0.05, ** p < 0.01.
3.2. PD Inhibits HG-Induced Oral Mucosal Cells Inflammatory Responses
Whether PD could affect the inflammatory responses induced by HG in oral mucosal cells when the cells were treated with platycodin D at the doses of 0–80 μg/mL, no toxicity was observed in oral mucosal cells (Figure 2A). Then, when oral mucosal cells were co-treated with different concentrations of PD (20, 40, 80 μg/mL) and HG (50 mM), oral mucosal cell viability was significantly increased in HG-injured oral mucosal cells when the concentration of PD was increased (Figure 2B). Hence, PD was used at the doses of 80 μg/mL in the subsequent experiments. We measured the concentration of pro-inflammatory cytokines in oral mucosal cells treated with HG and PD; the results showed that PD significantly reduced TNF-α, IL-1β, IL-6, and IL-8 levels in HG-treated cells (Figure 2C). Meanwhile, the level of ROS in HG-treated oral mucosal cells was decreased significantly by PD (Figure 2D). In the oral mucosal cells, the inflammatory responses and the HG-induced injury were alleviated by PD, as suggested by the data.
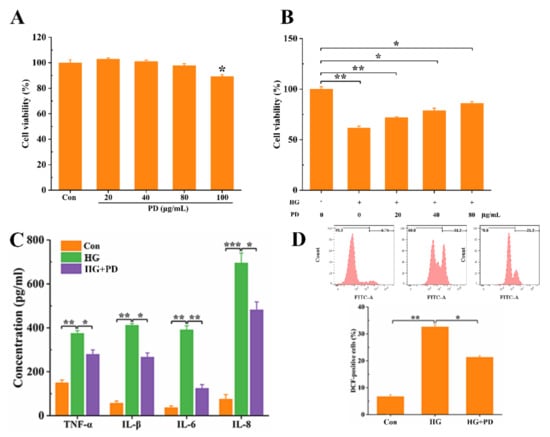
Figure 2.
PD inhibits HG-induced oral mucosal cell inflammatory responses. (A) The oral mucosal cells were treated with different concentrations of PD (0, 20, 40, 80, and 100 ug/mL) for 48 h, and cell viability was examined by CCK-8 assay. After co-treatment with HG (50 mM) and PD (0, 20, 40, 80 ug/mL), cell viability (B), inflammatory response (C), and ROS (D) were detected. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. PD Suppresses the Expression of PI3K/mTOR Pathway
We determined the possible involvement of this signaling pathway to understand in a better manner the molecular mechanisms of the PD-inhibited inflammatory responses. 740YP is a PI3K activator, which can increase the expression of PI3K (Figure 3A and Figure S1). As shown in Figure 3B, there was a significant upregulation of the PI3K level (p < 0.01) in HG-treated oral mucosal cells. Meanwhile, we observed that the increased PI3K level in HG-treated oral mucosal cells was reduced by the treatment of PD (Figure 3B and Figure S1). Compared with the HG + PD group, the PI3K levels in the HG + PD + 740YP group increased (Figure 3B). Further, the increase of mTOR expression in HG-induced oral mucosal cells was significantly inhibited by PD treatment; 3BDO as an activator of mTOR activated mTOR expression in co-treatment with HG and PD oral mucosal cells (Figure 3D and Figure S1). Collectively, PD suppressed HG-induced expression of PI3K and mTOR in oral mucosal cells.
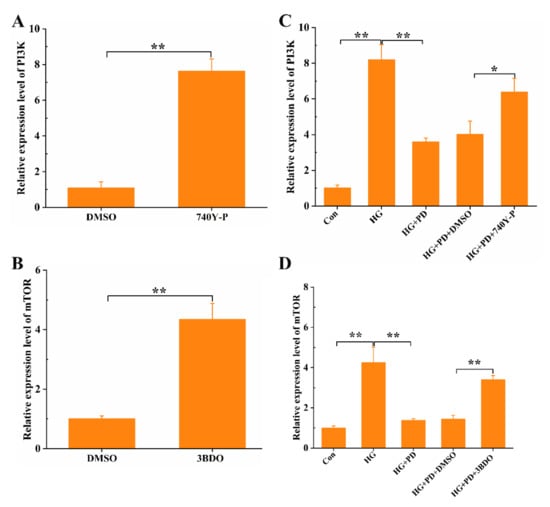
Figure 3.
PD suppresses the expression of PI3K/mTOR pathway. The oral mucosal cells were treated with 740-YP (20 μM), 3BDO (60 μM), HG (50 mM), and PD (80 μg/mL); the expression of PI3K (A,C) and mTOR (B,D) was detected by RT-PCR. * p < 0.05, ** p < 0.01.
3.4. PD Downregulated Pro-Inflammatory Cytokines through the PI3K/mTOR Pathway
The production of pro- and anti-inflammatory cytokines was regulated by mammalian target of rapamycin (mTOR) and phosphoinositide 3-kinase (PI3K). To further clarify the nature of PD-inhibited inflammatory responses, ELISA was used to detect pro-inflammatory cytokines of oral mucosal cells treated in different conditions. The detection of secretion showed that PD decreased TNF-α, IL-1β, IL-6, and IL-8 secretion in HG-treated oral mucosal cells; however, through combined use of 740-YP and 3BDO, TNF-α, IL-1β, IL-6, and IL-8 secretion was increased (Figure 4). These results confirm that the reduction of TNF-α, IL-1β, IL-6, and IL-8 mediated by PD in HG-treated oral mucosal cells was associated with PI3K/mTOR pathway.
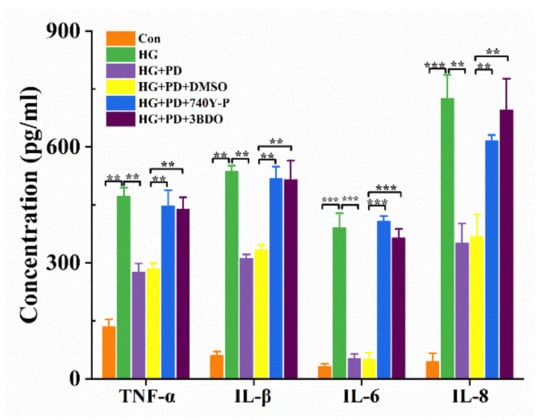
Figure 4.
PD downregulated pro-inflammatory cytokines through the PI3K/mTOR pathway. ** p < 0.01, *** p < 0.001. The oral mucosal cells were treated with 740-YP (20 μM), 3BDO (60 μM), HG (50 mM), and PD (80 μg/mL); the concentration of TNF-α, IL-1β, IL-6, and IL-8 was detected by ELISA.
3.5. PD Improves Cell Anti-Inflammatory Response by Inhibiting PI3K/mTOR Pathway
Although the uncontrolled inflammatory response would lead to cell damage with even more severe consequences, the inflammation mechanism of self-protection of the cells ensures the removal of harmful stimuli. Studies and experiments have shown that HG exposure to oral mucosal cells increases ROS stress and decreases cell viability. Oral mucosal cell damage caused by high glucose can be alleviated by PD. However, the co-treatment of PD + 740-YP and PD + 3BDO in HG-induced oral mucosal cells reduced cellular activity and improved ROS level compared with only treated PD (Figure 5). All these outcomes suggested that PD inhibited inflammatory response in HG-treated oral mucosal cells through inactivation of PI3K and mTOR.
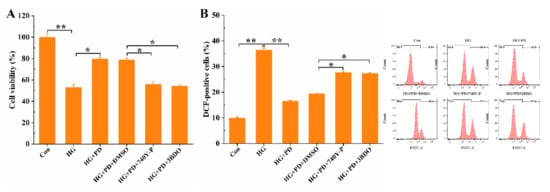
Figure 5.
PD improves cell anti-inflammatory response by inhibiting PI3K/mTOR pathway. * p < 0.05, ** p < 0.01. The oral mucosal cells were treated with 740-YP (20 μM), 3BDO (60 μM), HG (50 mM), and PD (80 μg/mL); the cell viability (A) and ROS (B) were detected by flow cytometer. * p < 0.05, ** p < 0.01.
4. Discussion
The physiological response of the body to any injury is in the form of inflammation. Not being resolved and/or leading to pathology, the inflammatory response could be of short duration, acute, or chronic [3]. Chronic inflammation due to the presence of persistent chemical, bacterial, or viral agents could be a risk factor for cancer, as indicated by evidence. Diabetes is a chronic disease, and oral disease is one of its complications. Previous investigations have demonstrated that diabetes is a hyperglycemic state with elevated levels of oxidative stress directly caused by hyperglycemia [13]. In endothelial cells [14] and seminiferous epitheliμM cells [13], glucose overload triggers the formation of ROS, which in turn damages cells. In inflamed oral tissues, the local concentrations of the cytokines were found to be elevated in studies involving both hμMans and animals. The production of cytokines is induced by the presence of an oral injury. The expression of IL-6, IL-8, and TNF-α in saliva [15], IFN-γ, IL-3, IL-4, and IL-15 in gingival crevicular fluid [16], and IL-1b, IL-6, IL-8, and TNF-a in peri-implant crevicular fluid [17] are the commonly elevated cytokine levels in oral diseases. Our data support the conclusions that high glucose induces excessive ROS production in oral mucosal cells, and induces apoptosis to cause cell damage. In addition, damaged cells produce a large nμMber of inflammatory factors, including TNF-α, IL-1β, IL-6, and IL-8. These results reveal that high glucose can induce oral mucosal cell damage.
Widely distributed in Asian countries, the Platycodon grandiflorus (Jacq.) A. DC. is a perennial herbaceous flowering plant from the genus Platycodon of the Campanulaceae family [18]. Displaying anti-oxidant, anti-inflammatory, anti-tμMor, and anti-obesity activities, platycodin D is the major compound active in the Platycodon grandiflorus extract, as confirmed by nμMerous studies [19]. Previous studies have reported LPS-induced nitric oxide production, pro-inflammatory cytokines (IL-1β, TNF-α) release, and prostaglandin E2 expression, via suppressing NF-κB pathway in BV2 murine microglial cells, and RAW 264.7 macrophages were reported to have been decreased by the purified compounds polygalacin D, platycodin A, and PD [8]. Platycodin D also alleviates acute kidney injury in septic rats by regulating the MAPK pathway [20]. In our study, PD obviously recovered cell viability, suppressed the production of ROS, and decreased the expression level of NF-α, IL-1β, IL-6, and IL-8 in oral mucosal cells. These results showed that PD has a protective effect on oral mucosal cells with HG-induced injury.
Previous studies have reported that activating the Akt/mTOR signaling pathway promotes inflammation and apoptosis; on the contrary, inhibiting the Akt/mTOR signaling pathway can alleviate the occurrence of inflammation in hμMan liver cancer cells [21]. Data show that platycodin D treatment diminished phosphorylation of PI3K/Akt/mTOR signaling pathway in A549 and the NCI-H460 cells [22]. We speculated that PD alleviates high-glucose-induced cytotoxicity by inhibiting the PI3K/Akt/mTOR signaling pathway. Our results show that the expression levels of PI3K and mTOR were found to be decreased significantly by PD. To further clarify the role of the PD-mediated inactivation of the PI3K/mTOR pathway, we subsequently used 740-YP and 3BDO. This signaling pathway was found to be inactivated by PD, leading to inflammatory response suppression; as evidenced by our results, the effect of PD on HG-induced oral mucosal cell injury and inflammatory responses was abolished by 740Y-P and 3BDO addition. These results suggest that platycodin D might inhibit activation of PI3K/Akt/mTOR signaling pathway to attenuate HG-induced oral mucosal cell injury.
Overall, through the downregulation of PI3K and mTOR, PD was found to be able to mitigate the inflammatory responses of the HG-induced oral mucosal cells, as demonstrated by the results. Albeit further studies would still be needed to investigate the exact role of PD in oral inflammatory responses, these findings could, as of now, provide a novel strategy in the treatment of oral diseases.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/coatings12040444/s1, Figure S1: PD suppresses the expression of PI3K/mTOR pathway. The Oral Mucosal Cells were treatment with 740-YP (20 μM), 3BDO (60 μM), HG (50 mM), and PD (80 μg/mL), the expression of PI3K (A,C) and mTOR (B,D) was detected by western blot.
Author Contributions
Funding acquisition, J.D.; Investigation, Y.H.; Methodology, Z.L.; Resources, Z.L.; Software, Z.L.; Validation, Y.H.; Writing—original draft, B.L.; Writing—review & editing, J.D. and and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical Scientific Research Foundation of Guangdong Province, Grant No. B2019157. And Scientific research start-up project of Stomatological Hospital, Southern Medical University, Grant No. PY2018038.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Edmans, J.G.; Clitherow, K.H.; Murdoch, C.; Hatton, P.V.; Spain, S.G.; Colley, H.E. Mucoadhesive Electrospun Fibre-Based Technologies for Oral Medicine. Pharmaceutics 2020, 12, 504. [Google Scholar] [CrossRef]
- Tonoyan, L.; Vincent-Bugnas, S.; Olivieri, C.V.; Doglio, A. New Viral Facets in Oral Diseases: The EBV Paradox. Int. J. Mol. Sci. 2019, 20, 5861. [Google Scholar] [CrossRef] [Green Version]
- Hasturk, H.; Kantarci, A.; Van Dyke, T.E. Oral Inflammatory Diseases and Systemic Inflammation: Role of the Macrophage. Front. Immunol. 2012, 3, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cock, P. Erythritol Functional Roles in Oral-Systemic Health. Adv. Dent. Res. 2018, 29, 104–109. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Sun, S.; Wang, X.; Guan, Y.; Mi, Q.; Zeng, W.; Xiang, H.; Zhu, H.; Zou, X.; et al. Autophagy and Hsp70 activation alleviate oral epithelial cell death induced by food-derived hypertonicity. Cell Stress Chaperones 2020, 25, 253–264. [Google Scholar]
- Nazir, M.A.; AlGhamdi, L.; AlKadi, M.; AlBeajan, N.; AlRashoudi, L.; AlHussan, M. The burden of Diabetes, Its Oral Complications and Their Prevention and Management. Open Access Maced. J. Med Sci. 2018, 6, 1545–1553. [Google Scholar]
- Mauri-Obradors, E.; Estrugo-Devesa, A.; Jane-Salas, E.; Vinas, M.; Lopez-Lopez, J. Oral manifestations of Diabetes Mellitus. A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e586–e594. [Google Scholar] [CrossRef]
- Jang, K.J.; Kim, H.K.; Han, M.H.; Oh, Y.N.; Yoon, H.M.; Chung, Y.H.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Choi, Y.H. Anti-inflammatory effects of saponins derived from the roots of Platycodon grandiflorus in lipopolysaccharidestimulated BV2 microglial cells. Int. J. Mol. Med. 2013, 31, 1357–1366. [Google Scholar]
- Ramirez, J.H.; Arce, R.; Contreras, A. Why must physicians know about oral diseases? Teach. Learn Med. 2010, 22, 148–155. [Google Scholar]
- Wang, G.; Guo, H.; Wang, X. Platycodin D protects cortical neurons against oxygen-glucose deprivation/reperfusion in neonatal hypoxic-ischemic encephalopathy. J. Cell Biochem. 2019, 120, 14028–14034. [Google Scholar]
- Bai, D.; Zhao, Y.; Zhu, Q.; Zhou, Y.; Zhao, Y.; Zhang, T.; Guo, Q.; Lu, N. LZ205, a newly synthesized flavonoid compound, exerts anti-inflammatory effect by inhibiting M1 macrophage polarization through regulating PI3K/AKT/mTOR signaling pathway. Exp. Cell Res. 2018, 364, 84–94. [Google Scholar] [PubMed]
- Fu, Y.; Xin, Z.; Liu, B.; Wang, J.; Wang, J.; Zhang, X.; Wang, Y.; Li, F. Platycodin D Inhibits Inflammatory Response in LPS-Stimulated Primary Rat Microglia Cells through Activating LXRalpha-ABCA1 Signaling Pathway. Front Immunol. 2017, 8, 1929. [Google Scholar] [PubMed] [Green Version]
- Tian, Y.; Song, W.; Xu, D.; Chen, X.; Li, X.; Zhao, Y. Autophagy Induced by ROS Aggravates Testis Oxidative Damage in Diabetes via Breaking the Feedforward Loop Linking p62 and Nrf2. Oxidative Med. Cell. Longev. 2020, 9, 7156579. [Google Scholar]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Gornowicz, A.; Bielawska, A.; Bielawski, K.; Grabowska, S.Z.; Wójcicka, A.; Zalewska, M.; Maciorkowska, E. Pro-inflammatory cytokines in saliva of adolescents with dental caries disease. Ann. Agric. Environ. Med. 2012, 19, 711–716. [Google Scholar]
- Thunell, D.H.; Tymkiw, K.D.; Johnson, G.K.; Joly, S.; Burnell, K.K.; Cavanaugh, J.E.; Brogden, K.A.; Guthmiller, J.M. A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J. Periodontal Res. 2010, 45, 148–152. [Google Scholar]
- Javed, F.; Al-Hezaimi, K.; Salameh, Z.; Almas, K.; Romanos, G.E. Proinflammatory cytokines in the crevicular fluid of patients with peri-implantitis. Cytokine 2011, 53, 8–12. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandiflorus-an ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar]
- Zhang, L.L.; Huang, M.Y.; Yang, Y.; Huang, M.Q.; Shi, J.J.; Zou, L.; Lu, J.J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. 2020, 327, 127029. [Google Scholar]
- Wang, J.; Yu, X. Platycodin D Ameliorates Acute Kidney Injury in Septic Rats by Regulating Mitogen Activated Protein Kinase Pathway. Curr. Top. Nutraceut. Res. 2021, 19, 270–276. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Xiao, B.; Jiao, W.; Zhang, S.; Zhang, X. TTF1NP induces protective autophagy during apoptosis by inhibiting the Akt/mTOR pathway and activating JNK in hμMan liver cancer cells. Oncol. Rep. 2018, 39, 1423–1431. [Google Scholar] [PubMed] [Green Version]
- Zhao, R.; Chen, M.; Jiang, Z.; Zhao, F.; Xi, B.; Zhang, X.; Fu, H.; Zhou, K. Platycodin-D Induced Autophagy in Non-Small Cell Lung Cancer Cells via PI3K/Akt/mTOR and MAPK Signaling Pathways. J. Cancer 2015, 6, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).