Molecular Dynamics Simulation and Experimental Analysis of the Effect of Ultrasonic Disposal on the Compatibility of NanoAsphalt
Abstract
:1. Introduction
2. Simulation Models and Methods
2.1. Asphalt Molecular Model Construction
2.1.1. Asphalt Four-Component Model
2.1.2. Asphalt Four-Component Content
2.1.3. Asphalt Molecular Model Validation
2.2. Amorphous Nanosilica Construction
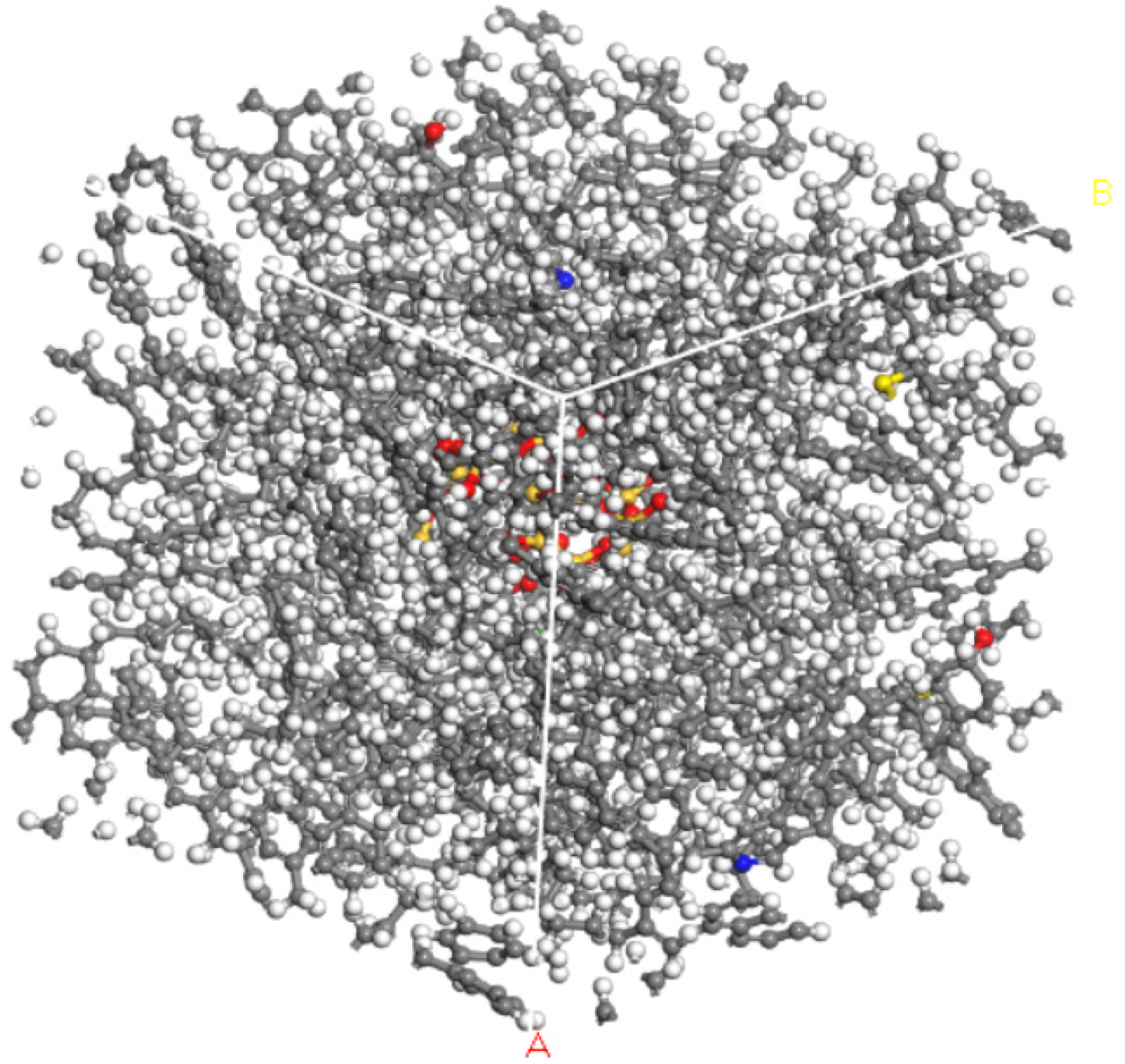
2.3. Nano-Asphalt Blend Model
3. Molecular Dynamics Simulation of Ultrasonic Field
3.1. Ultrasonic Dispersion Principle
3.2. Realization and Parameter Setting of Ultrasonic Vibration in Molecular Simulation
4. Simulation Results and Analysis
4.1. Effect of Ultrasonic Field on Solubility Parameters
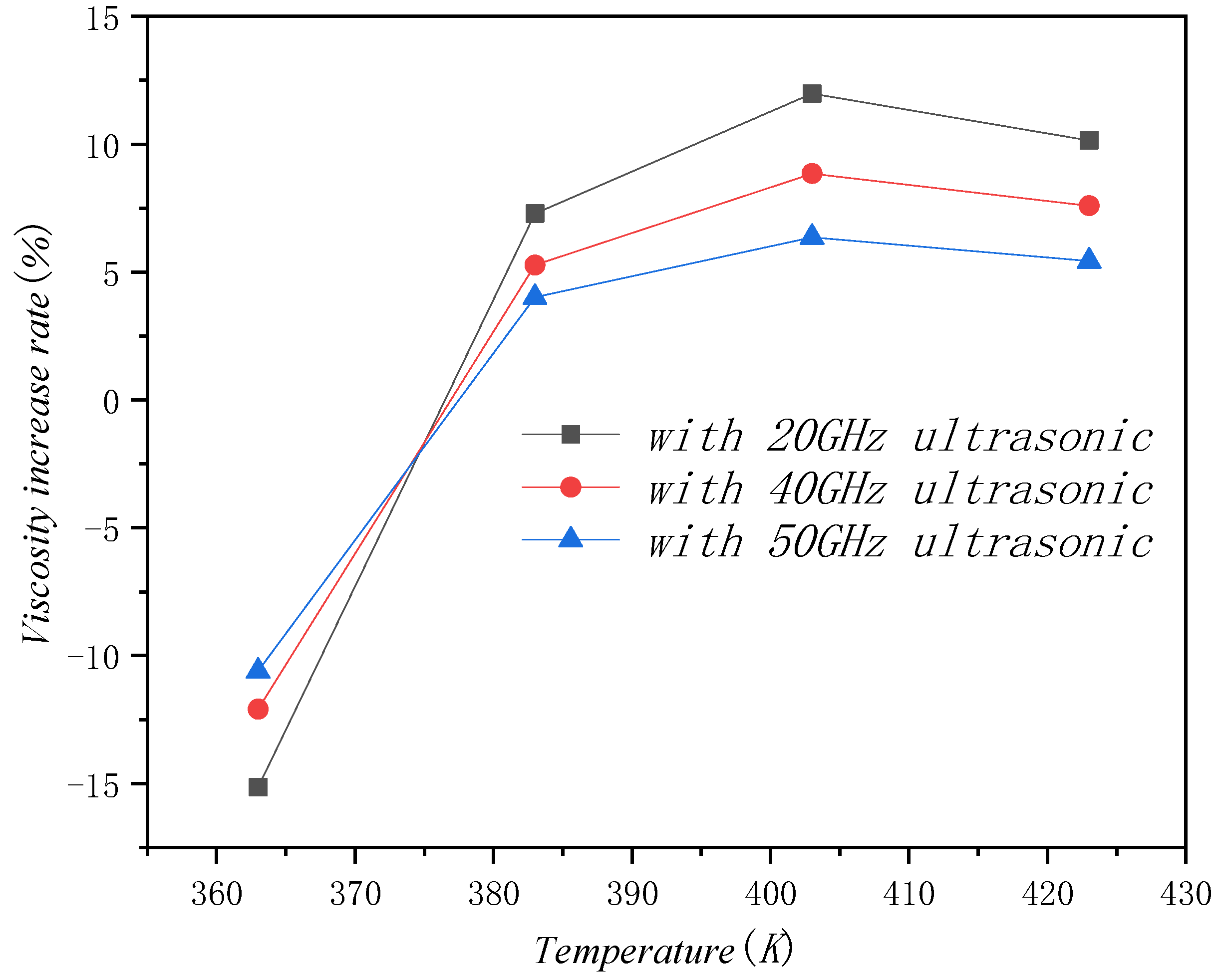
4.2. Effect of Ultrasound Field on Viscosity
5. Experiment
5.1. Test Materials and Instruments
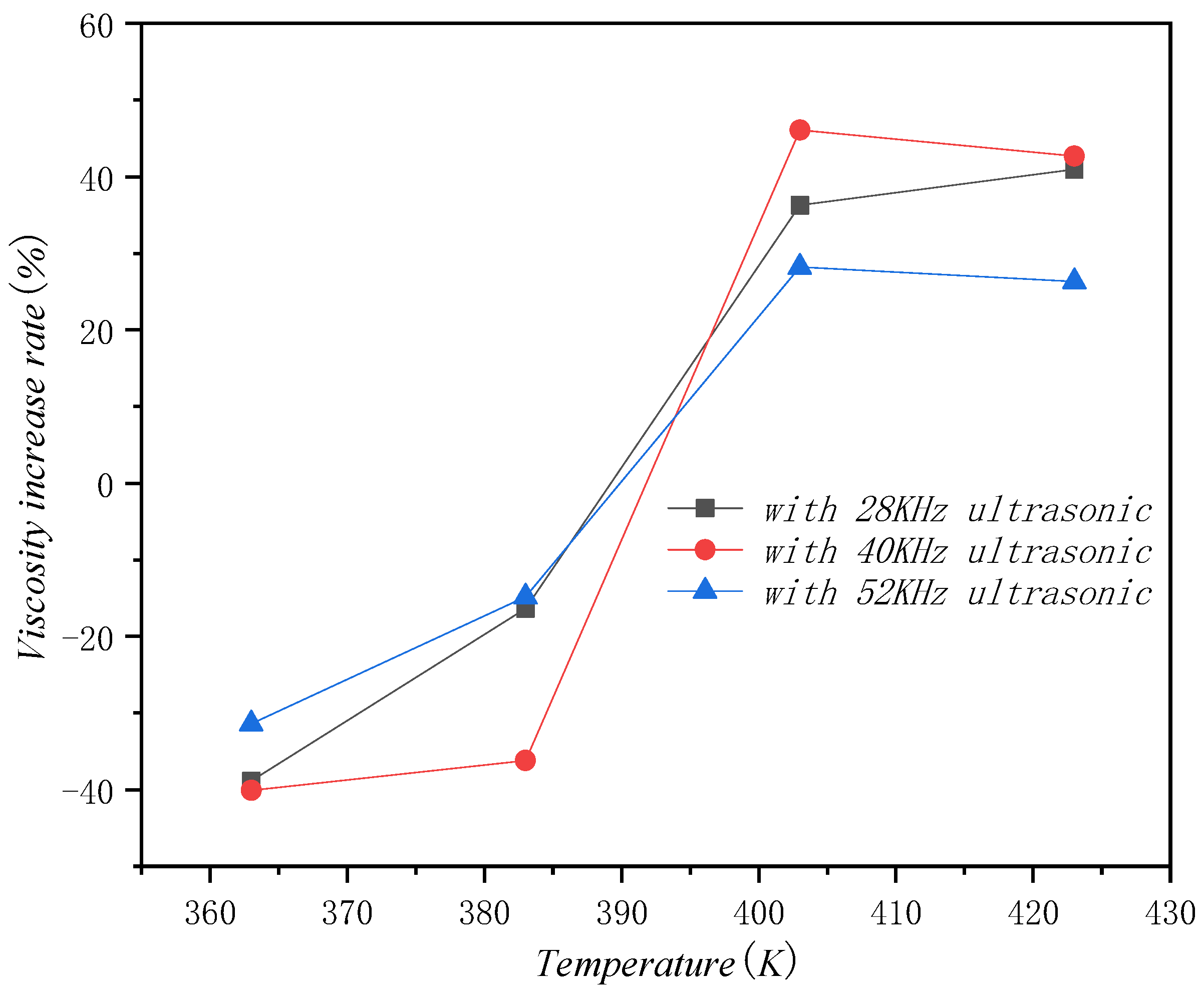
5.2. Viscosity Test and Result Analysis
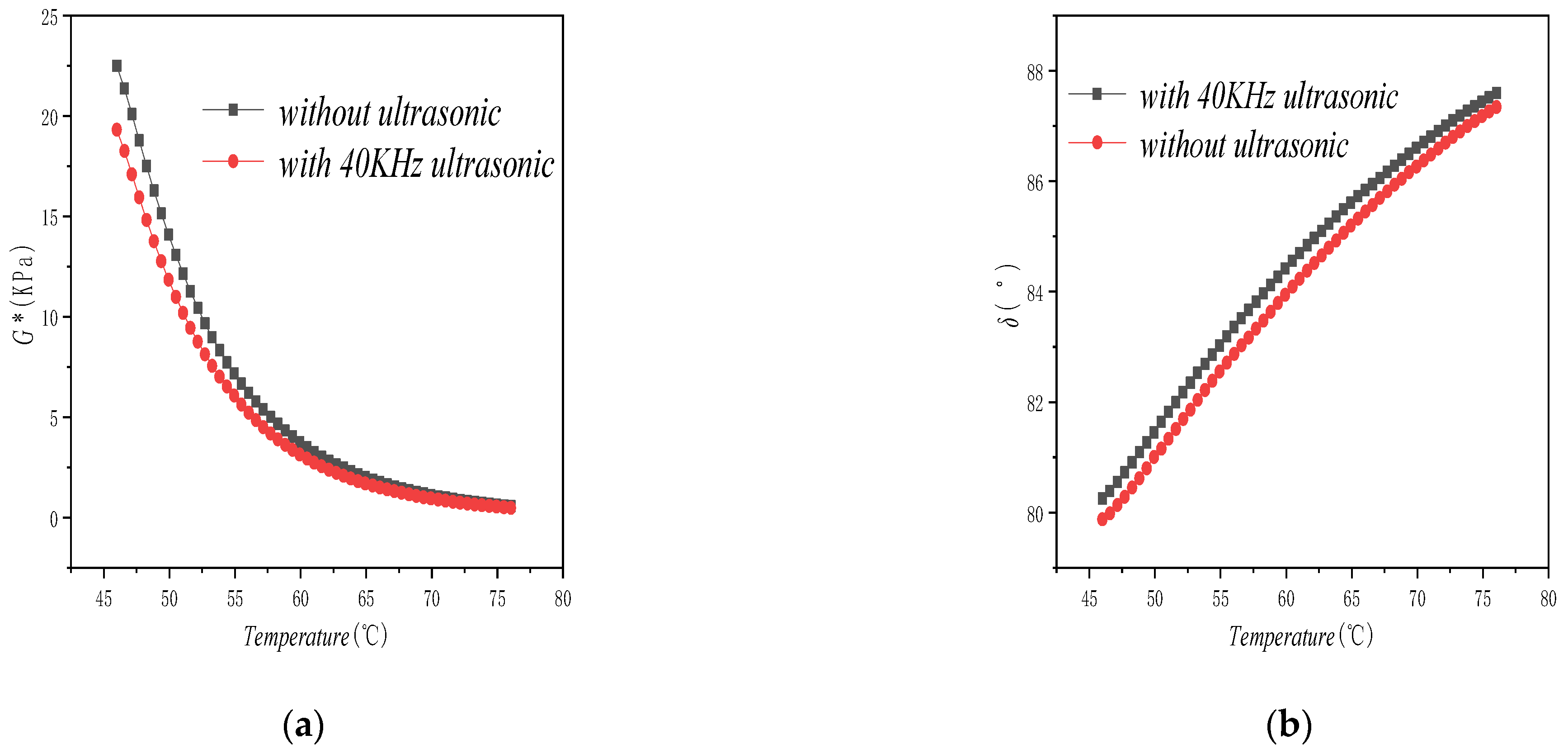
5.3. Temperature Dependence Analysis
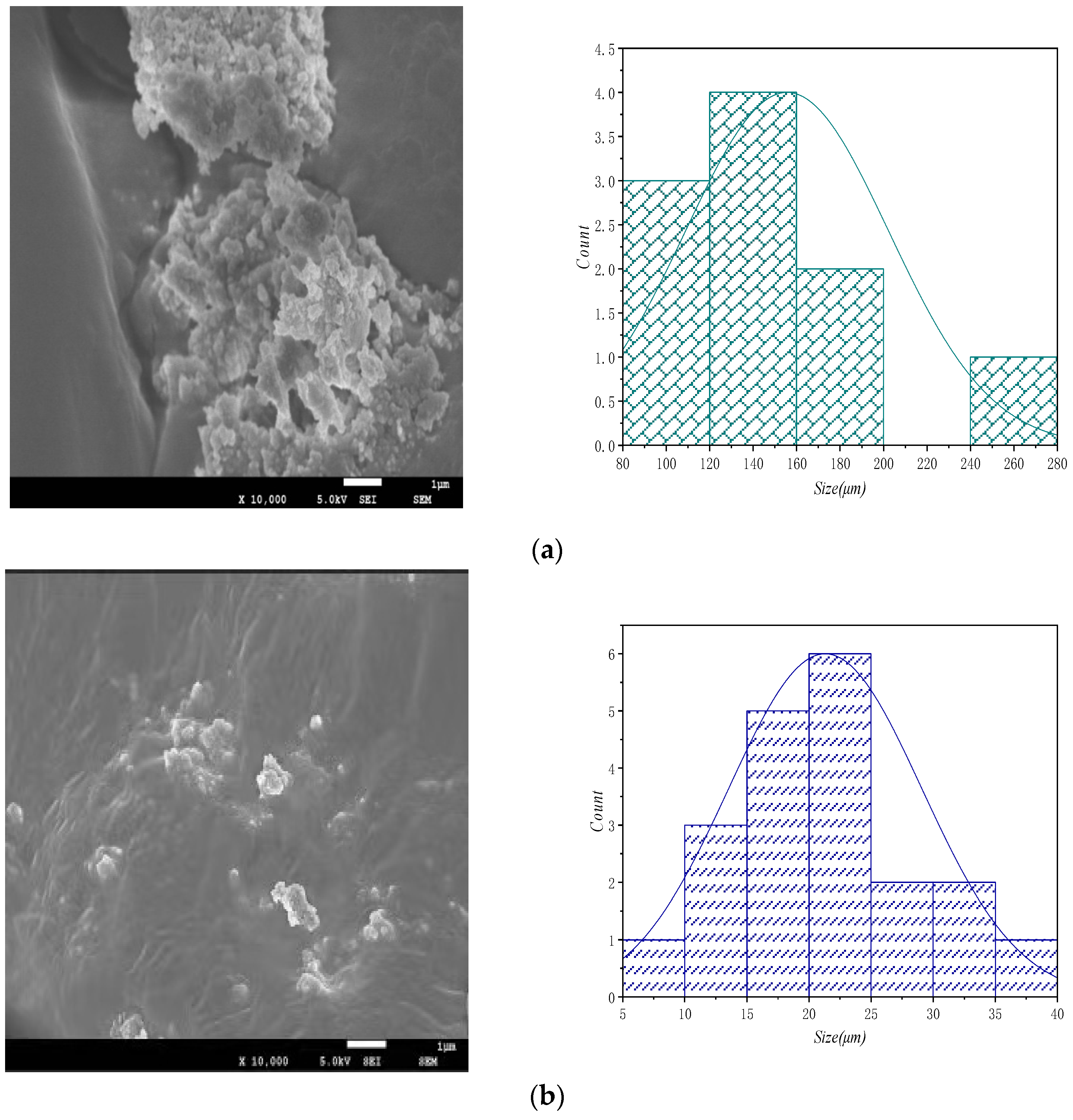
5.4. Scanning Electron Microscope Analysis
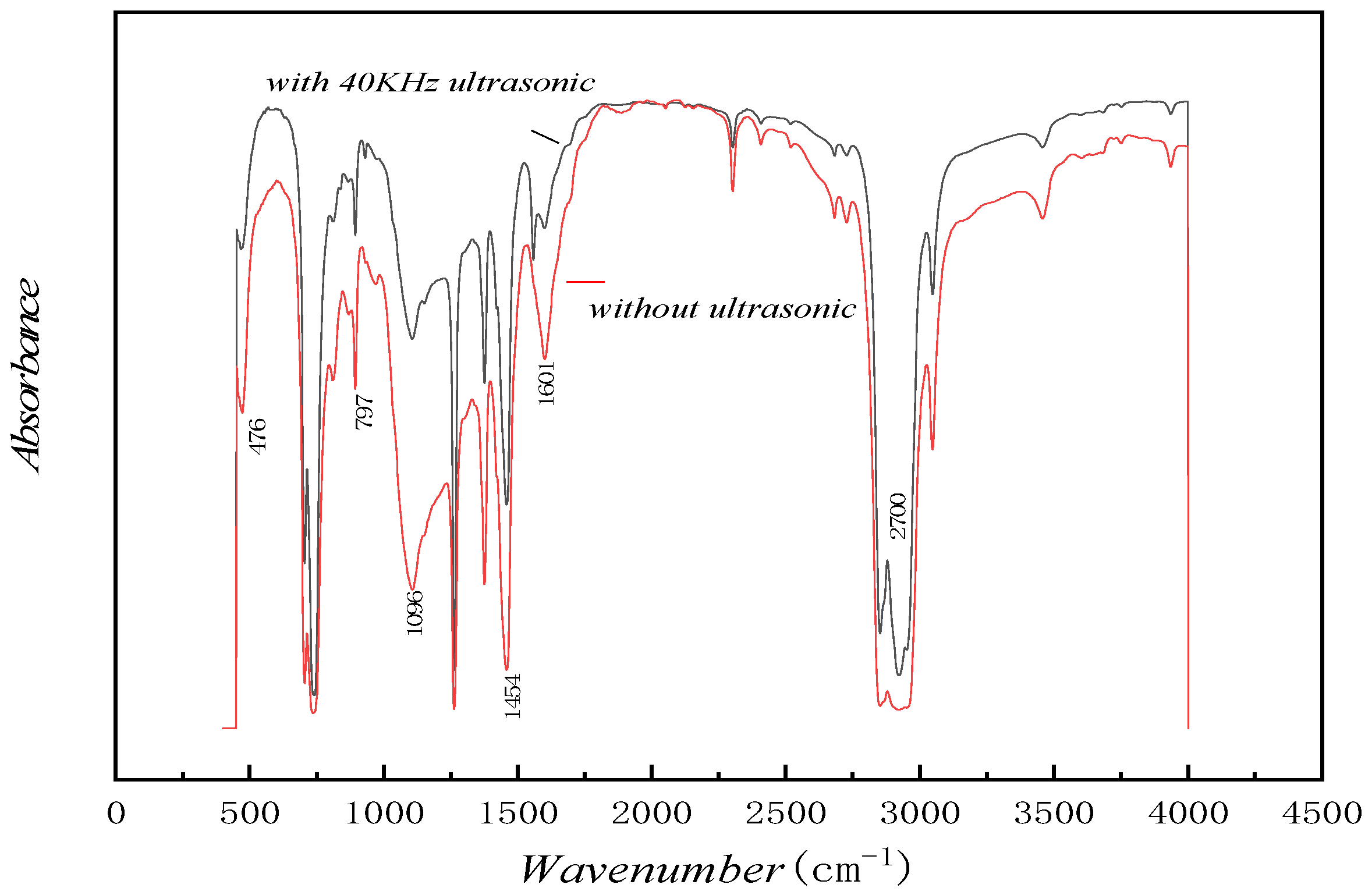
5.5. Infrared Spectroscopic Analysis
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, M.; Song, Q. Research progress of nano modified asphalt. China Highw. 2020, 4, 108–109. [Google Scholar]
- Amarnath, C.A.; Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K.; Paik, U. Nanohybridization of low-dimensional nanomaterials: Synthesis, classification, and application. Crit. Rev. Solid State Mater. Sci. 2013, 38, 1–56. [Google Scholar] [CrossRef]
- Khattak, M.J.; Khattab, A.; Rizvi, H.R.; Zhang, P. The impact of carbon nano-fiber modification on asphalt binder rheology. Constr. Build. Mater. 2012, 30, 257–264. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, Q.; Wang, D.; Wu, H.; Sun, L.; Guan, H.; Shao, C.; Su, H.; Yang, Y. Research status and progress of nanomaterial modified asphalt. J. Heilongjiang Inst. Technol. Nat. Sci. Ed. 2012, 26, 1671–4679. [Google Scholar]
- Zhang, J.; Zhang, A.; Li, M.; Xu, J.; Wang, Z.; Yin, W.; Zhao, H. Research progress of nano-modified asphalt. Mater. Rep. 2005, 19, 87–90. [Google Scholar]
- Manfred, N. Perspective on Nanotechnology in Construction Materials with a Focus on Asphaltic Materials; NSF Workshop on Nano Modifications of Cementifious Materials: Gainesville, FL, USA, 2006.
- Nanjing Forestry University. A Compatibility Evaluation Method of Asphalt and Modifier Based on Interface Interaction. CN202010853659.7, 30 October 2020. [Google Scholar]
- Zhang, J.; Li, Z.; Li, M.; Xu, J.; Yin, W.; Liu, L. Study on compatibility and dispersion stability mechanism of nano-modified asphalt. Highway 2005, 8, 142–146. [Google Scholar]
- Li, X. The compatibility of modified asphalt. Henan Sci. Technol. 2014, 21, 78–79. [Google Scholar]
- Wang, C. Preparation, Mechanism and Performance of Carbon Nanomaterial Modified Asphalt. Ph.D. Thesis, Changan University, Xi’an, China, 2017. [Google Scholar]
- Tang, X.; Han, N. Recent progress and prospect of asphalt modification technology. Mater. Bull. 2008, 22, 38–40, 49. [Google Scholar]
- Hui, X. Effect of ultrasonic treatment on physicochemical properties and rheological properties of composite asphalt. Shandong Transp. Sci. Technol. 2021, 1, 56–58. [Google Scholar]
- Zhu, H.; Wei, J.; Gong, M.; Yao, H.; Yang, J. Research progress of carbon nanotube modified asphalt. J. Pet. Process. 2017, 33, 386–394. [Google Scholar] [CrossRef]
- Hasan, Z.; Kamran, R.O.; Mohammad, F.; Ahmad, G.; Hosein, F. Evaluation of different conditions on the mixing bitumen and carbon nano-tubes. Int. J. Civ. Environ. Eng. IJCEE-IJENS 2012, 12, 53–59. [Google Scholar]
- Motlagh, A.A.; Kiasat, A.; Mirzaei, E.; Birgani, F.O. Bitumen modification using carbon nanotubes. World Appl. Sci. J. 2012, 18, 594–599. [Google Scholar]
- Santagata, E.; Baglieri, O.; Tsantilis, L.; Chiappinelli, G. Fatigue properties of bituminous binders reinforced with carbon nanotubes. Int. J. Pavement Eng. 2015, 16, 80–90. [Google Scholar] [CrossRef]
- Santagata, E.; Baglieri, O.; Tsantilis, L.; Dalmazzo, D. Rheological characterization of bituminous binders modified with carbon nanotubes. Procedia-Soc. Behav. Sci. 2012, 53, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Chen, L. Development of High Viscosity Modified Asphalt. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2012. [Google Scholar]
- Al-Adham, K.; Arifuzzaman, M. Moisture damage evaluation in carbon nanotubes reinforced asphalts. Sustain. Eco-Effic. Conserv. Transp. Infrastruct. Asset Manag. 2014, 103–109. [Google Scholar]
- Khattak, M.J.; Khattab, A.; Rizvi, H.R. Characterization of carbon nano-fiber modified hot mix asphalt mixtures. Constr. Build. Mater. 2013, 40, 738–745. [Google Scholar] [CrossRef]
- Zhu, J.; He, Z. Molecular dynamics study on compatibility of anti-stripping agent and asphalt. Highw. Transp. Technol. 2016, 33, 34–40. [Google Scholar]
- Su, M.; Zhang, H.; Zhang, Y.; Zhang, Z. Molecular dynamics simulation of compatibility and mechanical properties of SBS and asphalt. J. Chang’an Univ. Nat. Sci. Ed. 2017, 37, 28–32. [Google Scholar]
- Nesterov, P.; Shilovskikh, V.; Sokolov, A.; Gurzhiy, V.; Novikov, A.; Timralieva, A.; Belogub, E.; Kondratyuk, N.; Orekhov, N.; Skorb, E. Encapsulation of Rhodamine 6G Dye Molecules for Affecting Symmetry of Supramolecular Crystals of Melamine-Barbiturate. Symmetry 2021, 13, 1119. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, M. Halogen Bonding in Haspin-Halogenated Tubercidin Complexes: Molecular Dynamics and Quantum Chemical Calculations. Molecules 2022, 27, 706. [Google Scholar] [CrossRef]
- Zhai, W.; Yang, D.; Gong, N. Molecular Dynamics Simulation of Silicon Carbide Polishing Process under Ultrasonic Vibration. J. Shanghai Jiaotong Univ. 2018, 52, 599–603. [Google Scholar]
- Cornwell, C.F.; Welch, C.R. Very-high-strength (60-GPa) carbon nanotube fiber design based on molecular dynamics simulations. J. Chem. Phys. 2011, 134, 204708. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.B., III; Zimmerman, J.A.; Seel, S.C. Reconsideration of continuum thermomechanical quantities in atomic scale simulations. Math. Mech. Solids 2008, 13, 221–266. [Google Scholar] [CrossRef]
- Horstemeyer, M.F.; Baskes, M.I.; Plimpton, S.J. Computational nanoscale plasticity simulations using embedded atom potentials. Theor. Appl. Fract. Mech. 2001, 37, 49–98. [Google Scholar] [CrossRef]
- Jennings, P.W.; Pribanic, J.A.; Desando, M.A.; Raub, M.F.; Moats, R.; Smith, J.A.; Mendes, T.M.; McGrane, M.; Fanconi, B.; VanderHart, D.L.; et al. Binder Characterization and Evaluation by Nuclear Magnetic Resonance Spectroscopy; No. SHRP-A-335; National Research Council: Washington, DC, USA, 1993.
- Zhang, L.; Greenfield, M.L. Relaxation time, diffusion, and viscosity analysis of model asphalt systems using molecular simulation. J. Chem. Phys. 2007, 127, 194502. [Google Scholar] [CrossRef] [Green Version]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Bo, L.; Liu, Y. Molecular simulation studies the self-healing law of asphalt under different conditions. Henan Sci. 2020, 38, 243–249. [Google Scholar]
- Research of compatiblity of asphalt and anti-stripping agent using molecular dynamics. J. Highw. Transp. Res. Dev. 2016, 33, 34–40.
- Wang, L.; Zhang, L.; Liu, Y. The compatibility of rubber powder and asphalt in rubber powder is modified by molecular dynamics. J. Build. Mater. 2018, 21, 689–694. [Google Scholar]
- Dong, X.; Lei, Q.; Yu, Q. NMR determination of petroleum asphaltenes and their model molecules Evaluation. J. Fuel Chem. Technol. 2004, 32, 668–672. [Google Scholar]
- Wang, D.; Zhao, Y.; Pan, Y.; Liu, R.; Gao, J. Quantum chemistry study on the structure of petroleum gum. J. Fuel Chem. Technol. 2006, 34, 690–694. [Google Scholar]
- Kovalevsky, I.; Vandenbrook, M.; Huc, A.Y.; Taylor, M.J.; Faulon, J.L. Preliminary results of Asfarten molecular modeling using structural elucidation program and molecular simulation program. Energy Fuel. 1996, 10, 97–107. [Google Scholar]
- Qiu, Y.; Su, T.; Zheng, P.; Ding, H. Study on physical aging mechanism of asphalt binder based on molecular simulation. J. Constr. Mater. 2020, 23, 1464–1470. [Google Scholar]
- Corbett, L.W. Composition of asphalt based on generic fractionation, using solvent deasphaltening, elution-adsorption chromatography, and densimetric characterization. Anal. Chem. 1969, 41, 576–580. [Google Scholar] [CrossRef]
- Chen, M. Computational Chemistry—From Theoretical Chemistry to Molecular Simulation; Science Press: Beijing, China, 2009. [Google Scholar]
- Luo, L.; Chu, L.J.; Fwa, T.F. Molecular dynamics analysis of moisture effect on asphalt -aggregate adhesion considering anisotropic mineral surfaces. Appl. Surf. Sci. 2020, 527, 146830. [Google Scholar] [CrossRef]
- Feng, P.; Wang, H.; Ding, H.; Xiao, J.; Hassan, M. Effects of surface texture and its mineral composition on interfacial behavior between asphalt binder and coarse aggregate. Constr. Build. Mater. 2020, 262, 120869. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, S.; Liu, G.; Pan, P. Molecular simulations and experimental evaluation on the curing of epoxy bitumen. Mater. Struct. 2016, 49, 241–247. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, S. Ultrasonic cavitation effect and its application. J. Water Resour. Water Eng. 2009, 1, 136–138. [Google Scholar]
- Sato, K.; Li, J.G.; Kamiya, H.; Ishigaki, T. Ultrasonic dispersion of TiO2 nanoparticles in aqueous suspension. J. Am. Ceram. Soc. 2008, 91, 2481–2487. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M.R. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment–issues and recommendations. Nanotoxicology 2011, 5, 711–729. [Google Scholar] [CrossRef]
- Bittmann, B.; Haupert, F.; Schlarb, A.K. Ultrasonic dispersion of inorganic nanoparticles in epoxy resin. Ultrason. Sonochem. 2009, 16, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Okumura, H.; Itoh, S.G. Amyloid fibril disruption by ultrasonic cavitation: Nonequilibrium molecular dynamics simulations. J. Am. Chem. Soc. 2014, 136, 10549–10552. [Google Scholar] [CrossRef] [PubMed]
- de Arenaza, I.M.; Meaurio, E.; Coto, B.; Sarasua, J.R. Molecular dynamics modelling for the analysis and prediction of miscibility in polylactide/polyvinilphenol blends. Polymer 2010, 51, 4431–4438. [Google Scholar] [CrossRef]
- Fallah, F.; Khabaz, F.; Kim, Y.R.; Kommidi, S.R.; Haghshenas, H.F. Molecular dynamics modeling and simulation of bituminous binder chemical aging due to variation of oxidation level and saturate-aromatic-resin-asphaltene fraction. Fuel 2019, 237, 71–80. [Google Scholar] [CrossRef]
- Tian, Y. Compatibility Study of Modified Asphalt. Ph.D. Thesis, China University of Petroleum (East China), Dongying, China, 2002. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Ramazani, A.; Najafi, I.; Davachi, S.M. Effect of ultrasonic irradiation on rheological properties of asphaltenic crude oils. Pet. Sci. 2012, 9, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Nevins, D.; Spera, F.J. Accurate computation of shear viscosity from equilibrium molecular dynamics simulations. Mol. Simul. 2007, 33, 1261–1266. [Google Scholar] [CrossRef]
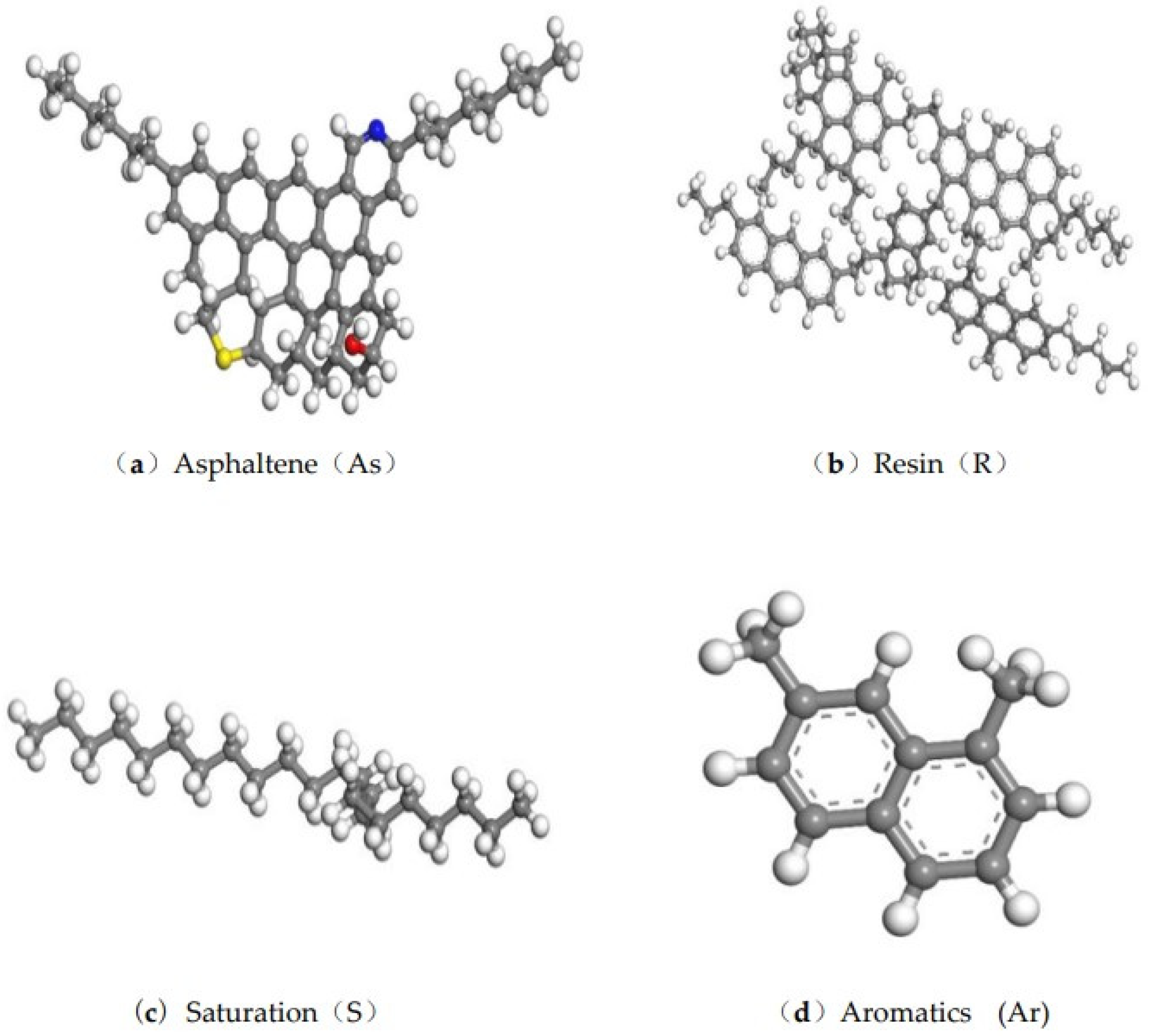


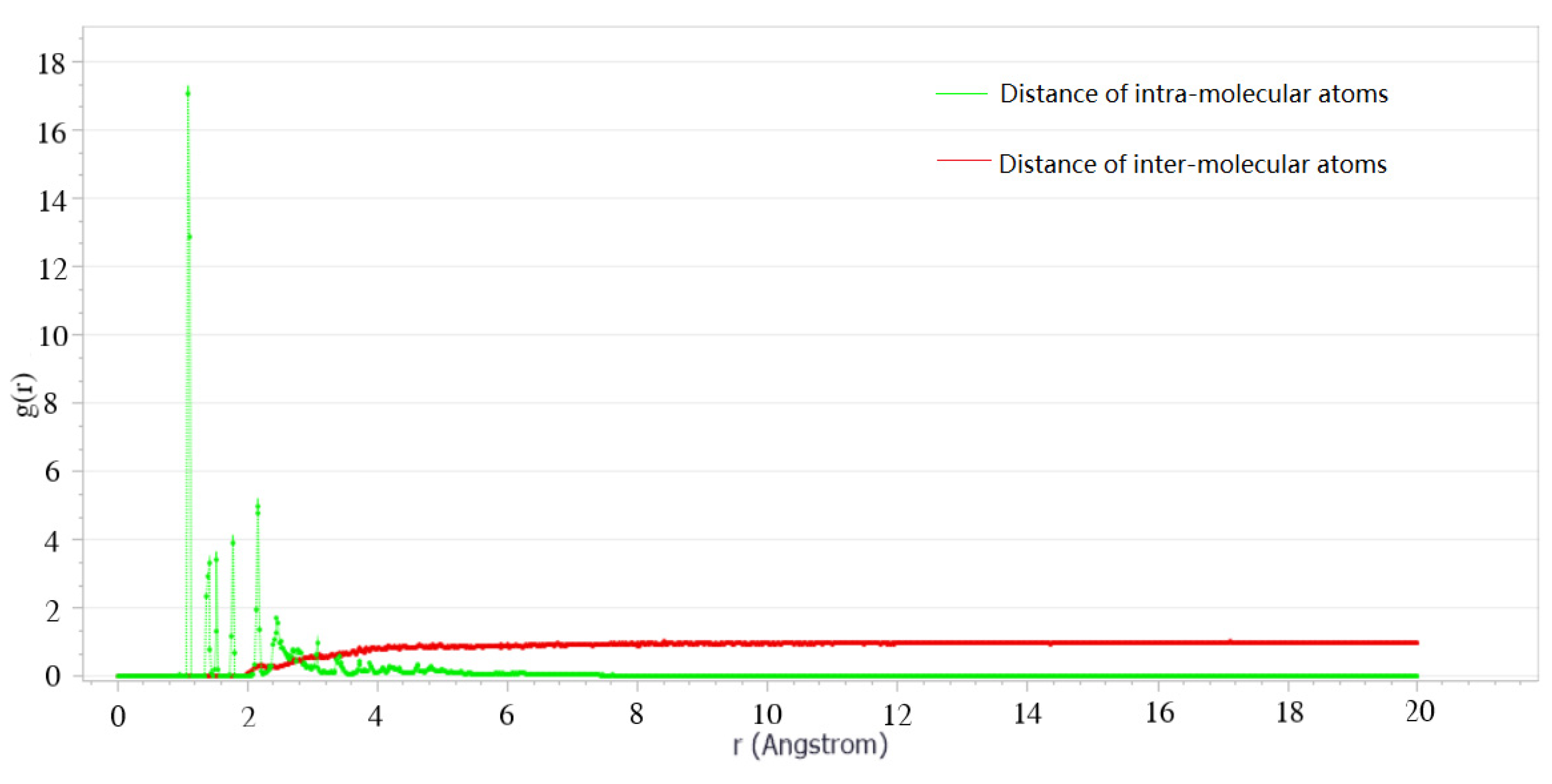
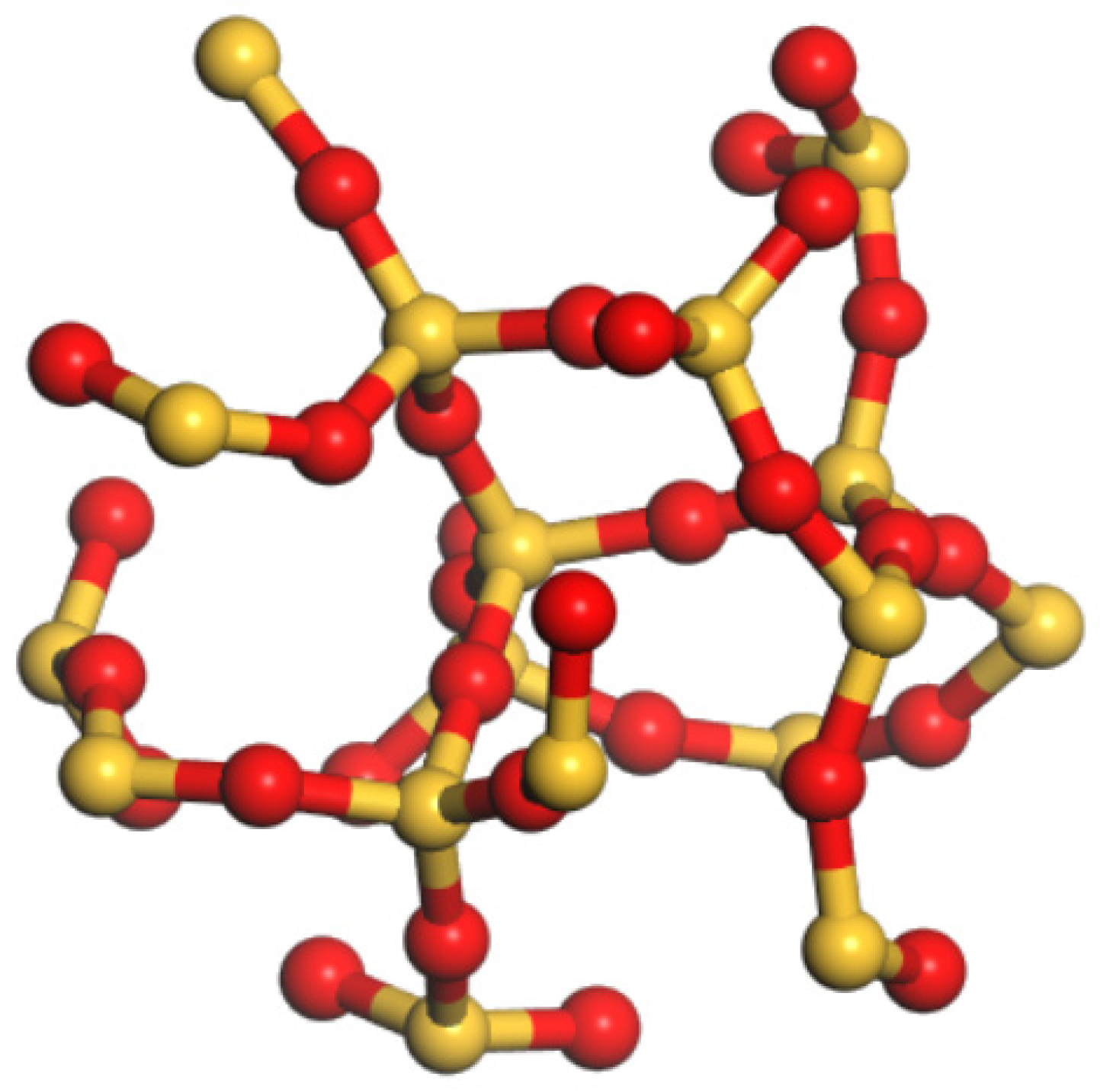
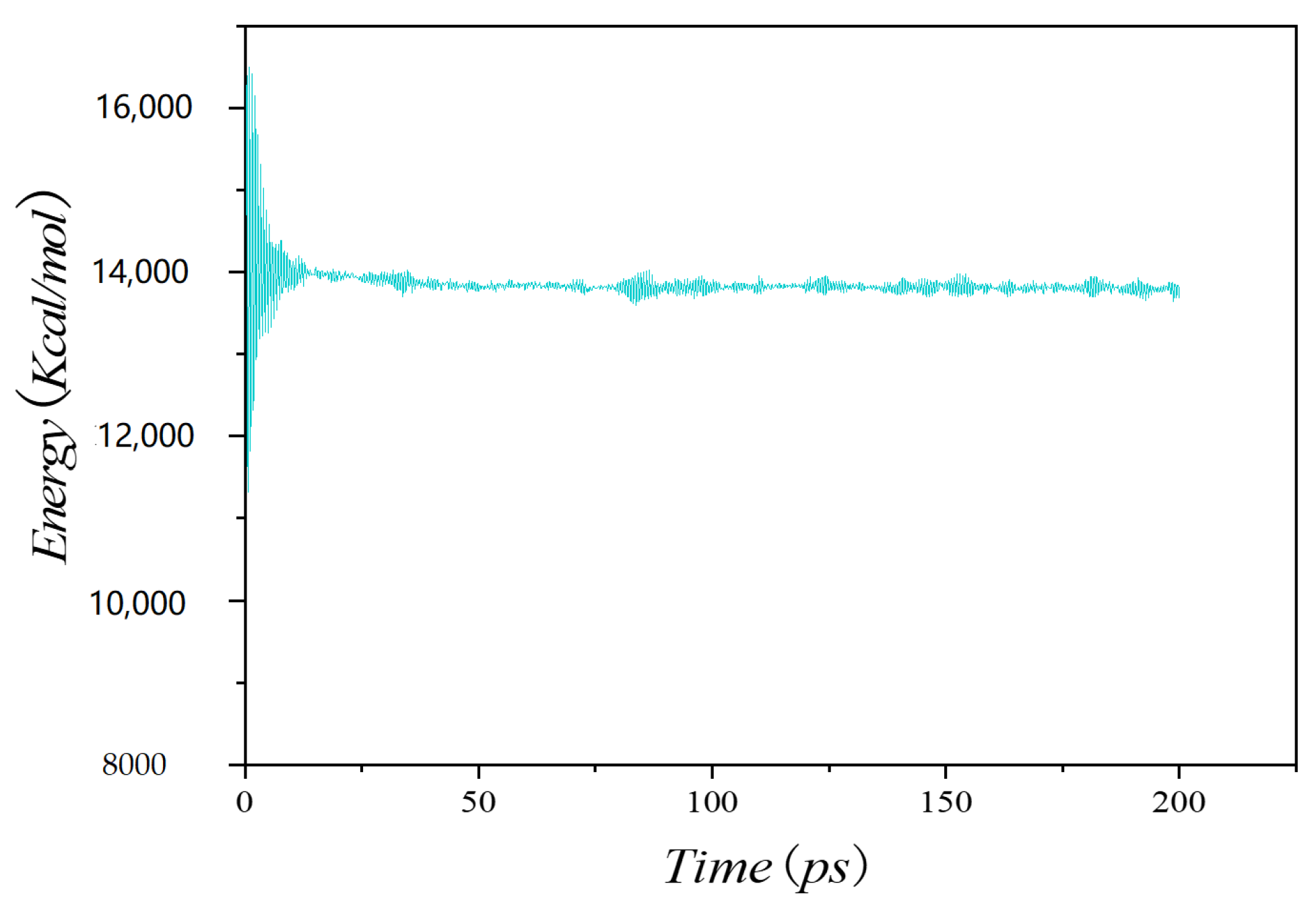




| Author | Time | Asphalt System |
|---|---|---|
| Jennings [29] | 1993 | AAA-1, AAB-1, AAC-1, AAD-1, AAF-1, AAG-1, AAK-1, AAM-1 |
| Zhang [30] | 2007 | Asphaltene: C64H52S2 Saturation: C22H46 Resin: 1,7-dimethylnaphthalene |
| Zhang [30] | 2007 | Asphaltene: C72H98S Saturation: C22H46 Resin: 1,7-dimethylnaphthalene |
| Li [31] | 2014 | Asphaltene A: C42H54O Asphaltene B: C66H81N Asphaltene C: C51H62S Saturation A: C30H62 Saturation B: C35H62 Aromatics A: C35H44 Aromatics B: C30H46 Resin A: C40H59N Resin B: C40H60N Resin C: C18H10S2 Resin D: C36H57N Resin E: C29H50O |
| Zhu [21] | 2016 | Asphaltene: C53H57NOS Saturation: C22H46 Aromatics: 1,7-dimethylnaphthalene Resin: C100H106 |
| Bo [32] | 2020 | Asphaltene: C63H76S2N2 Saturation: C22H46 Aromatics: 1,7-dimethylnaphthalene Resin: C100H106 |
| Sample | As | S | Ar | R | Total |
|---|---|---|---|---|---|
| Matrix asphalt | 10.1 | 23.7 | 53.7 | 12.5 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, Z. Molecular Dynamics Simulation and Experimental Analysis of the Effect of Ultrasonic Disposal on the Compatibility of NanoAsphalt. Coatings 2022, 12, 424. https://doi.org/10.3390/coatings12040424
Wang L, Li Z. Molecular Dynamics Simulation and Experimental Analysis of the Effect of Ultrasonic Disposal on the Compatibility of NanoAsphalt. Coatings. 2022; 12(4):424. https://doi.org/10.3390/coatings12040424
Chicago/Turabian StyleWang, Liming, and Zhuying Li. 2022. "Molecular Dynamics Simulation and Experimental Analysis of the Effect of Ultrasonic Disposal on the Compatibility of NanoAsphalt" Coatings 12, no. 4: 424. https://doi.org/10.3390/coatings12040424
APA StyleWang, L., & Li, Z. (2022). Molecular Dynamics Simulation and Experimental Analysis of the Effect of Ultrasonic Disposal on the Compatibility of NanoAsphalt. Coatings, 12(4), 424. https://doi.org/10.3390/coatings12040424





