Influence of Graphene Oxide Additions on the Corrosion Resistance of a Rust Converter Primer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
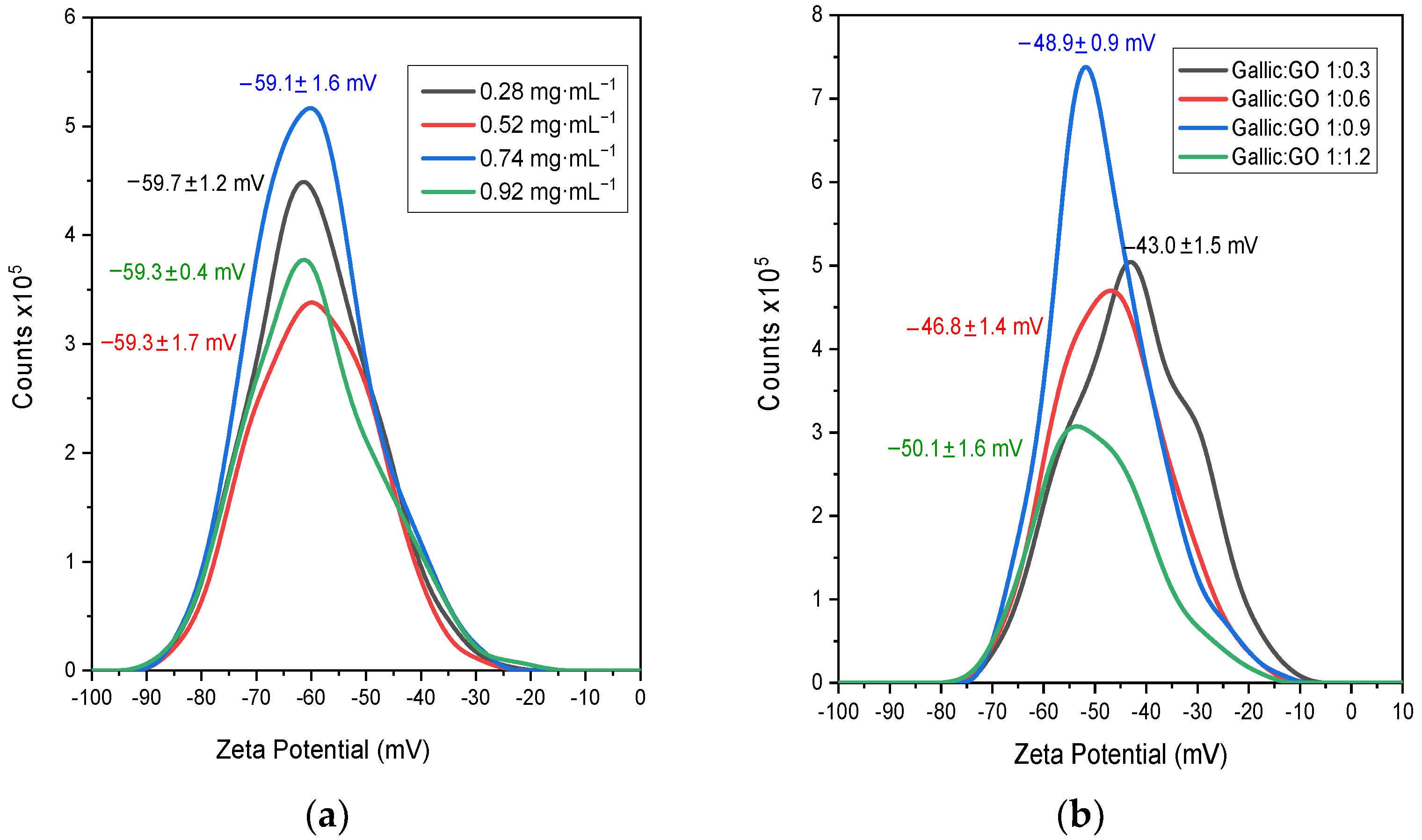
3.1. Surface Charge of the Graphene Oxide Particles
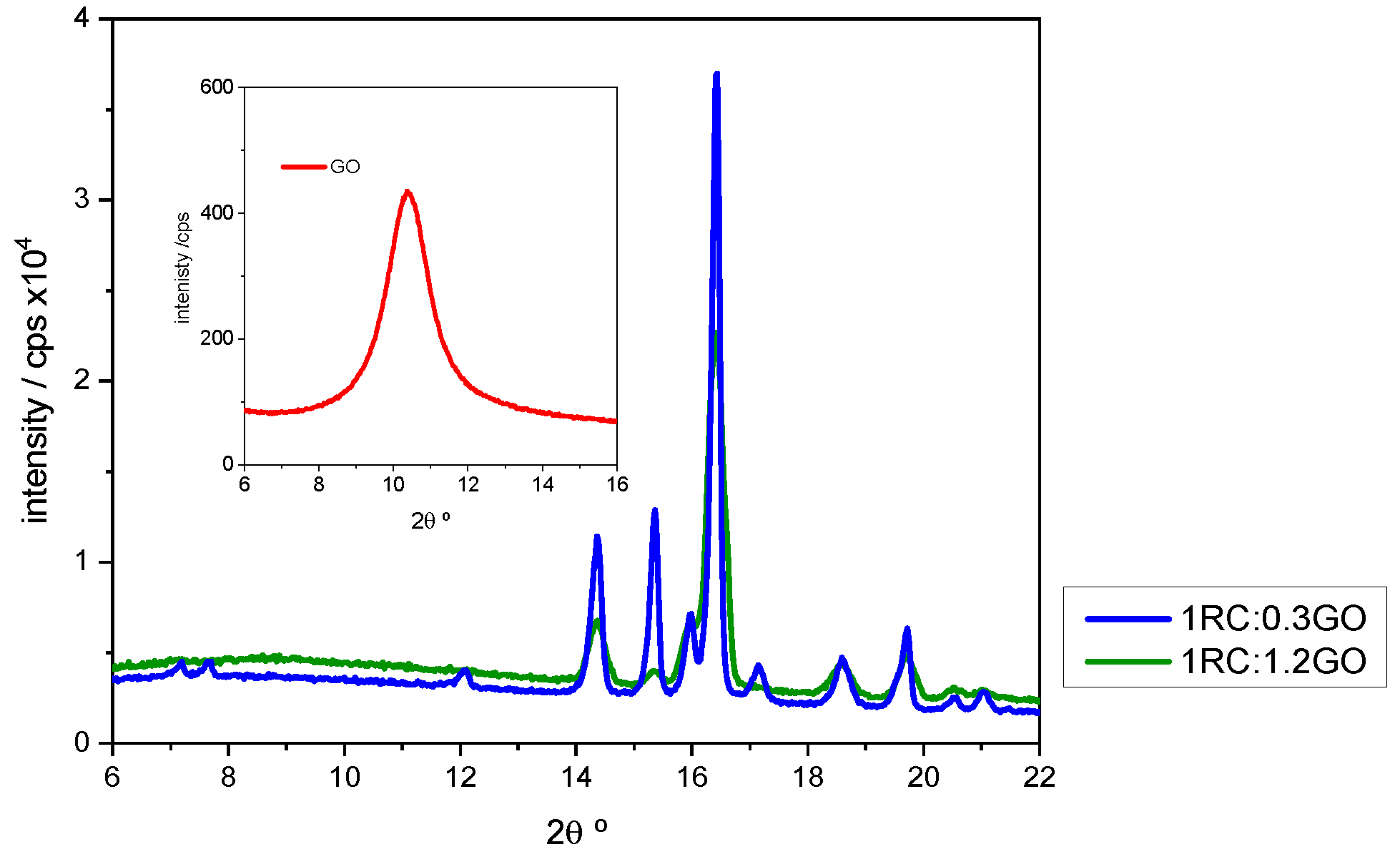
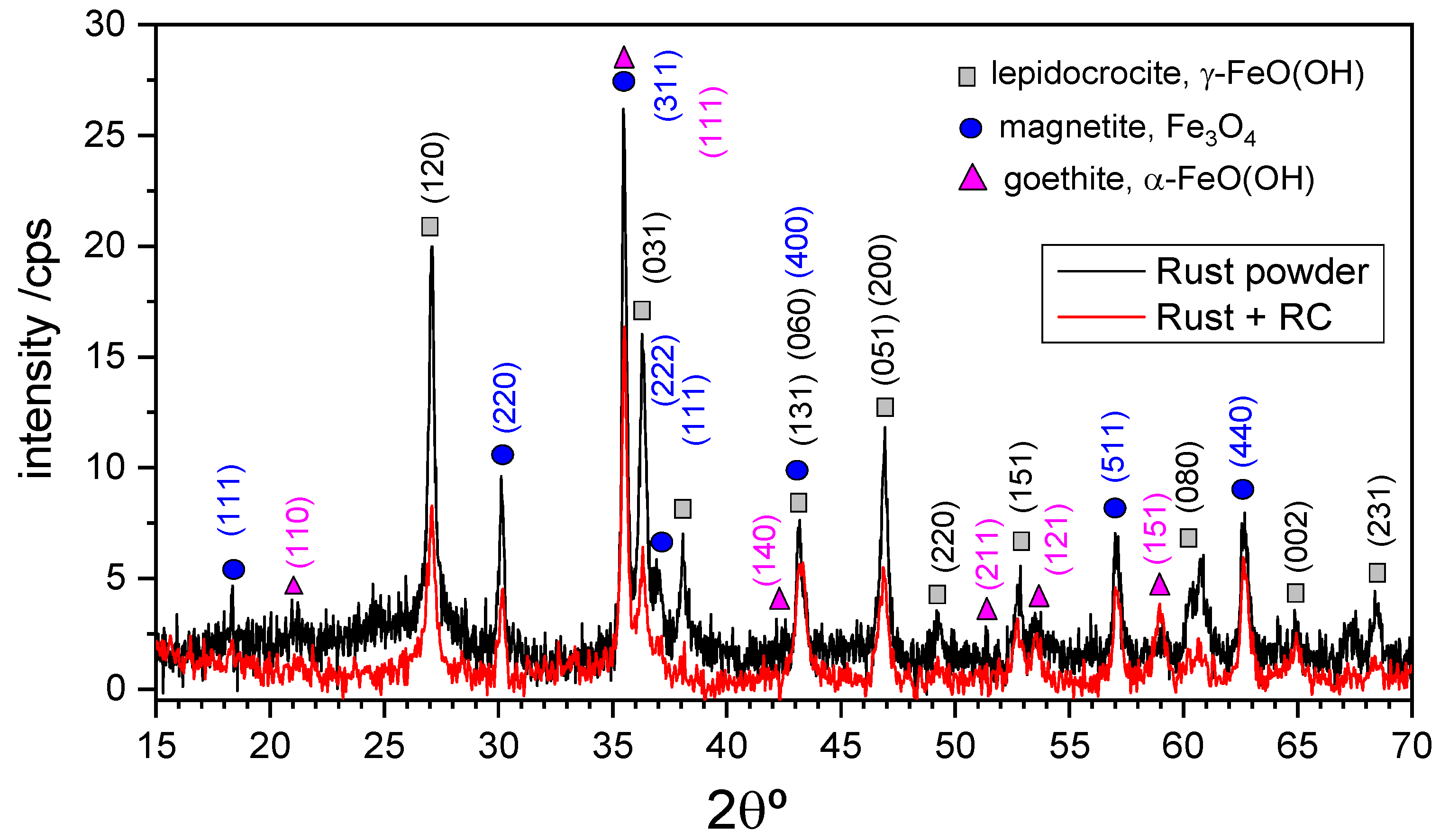
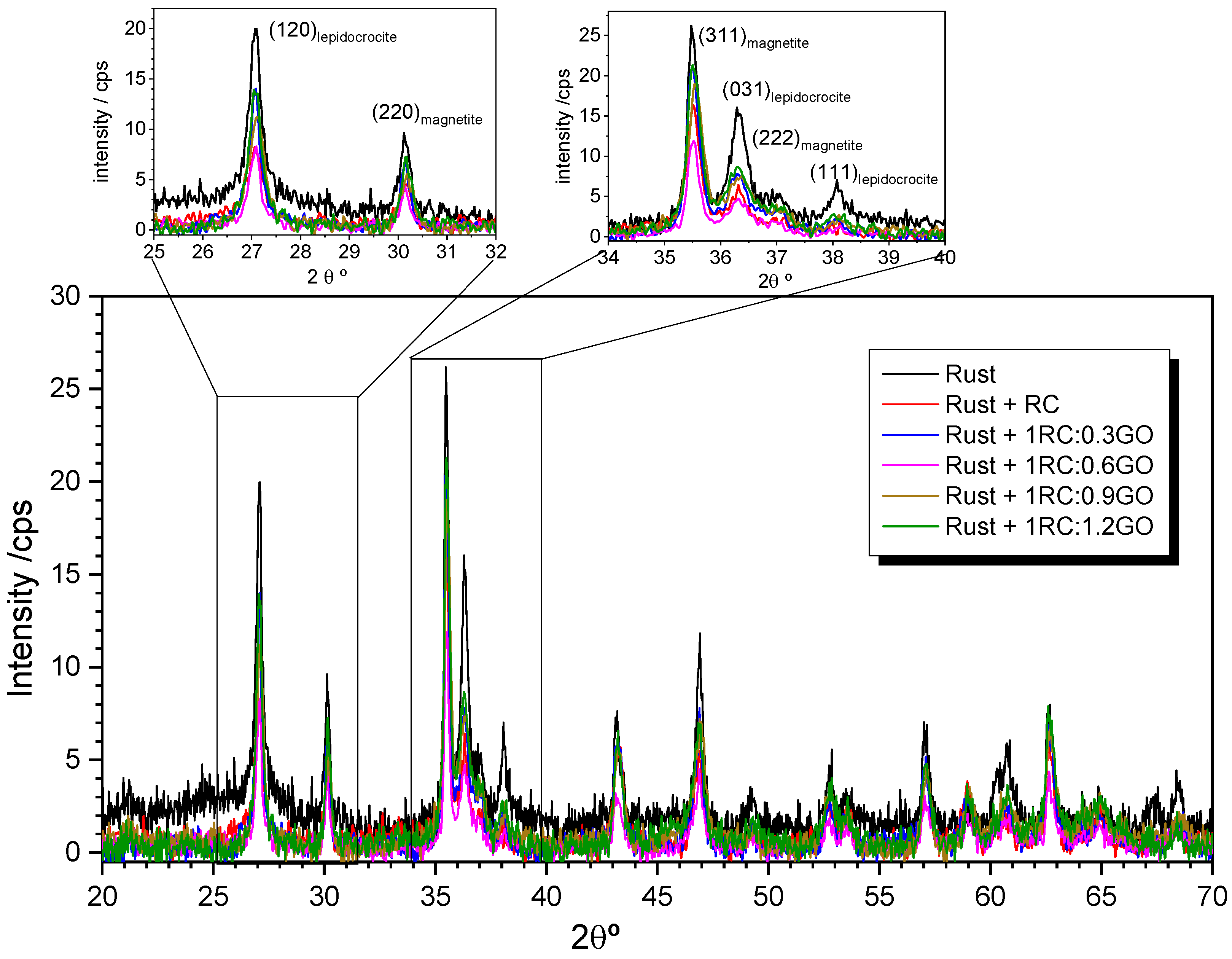
3.2. X-ray Characterization
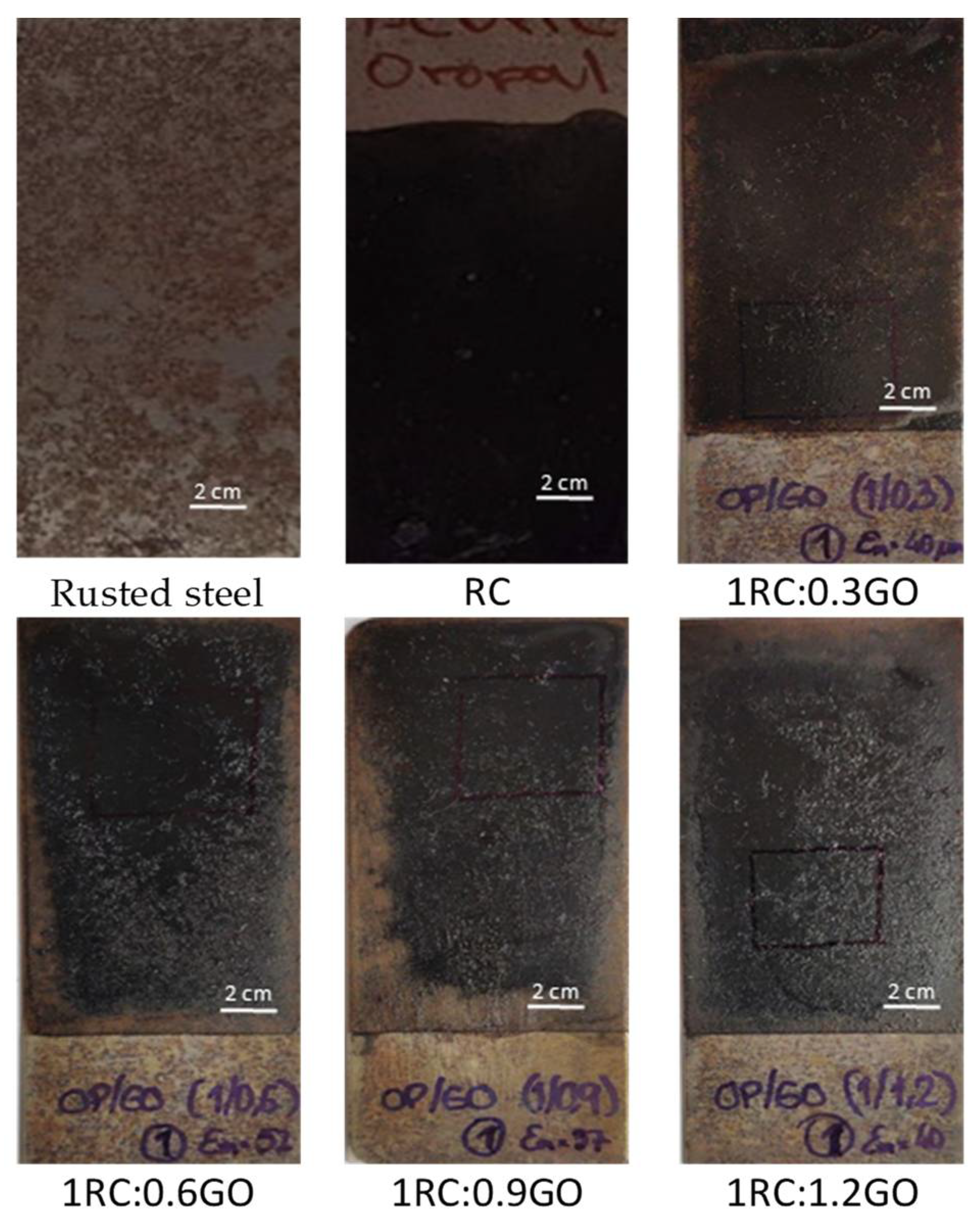
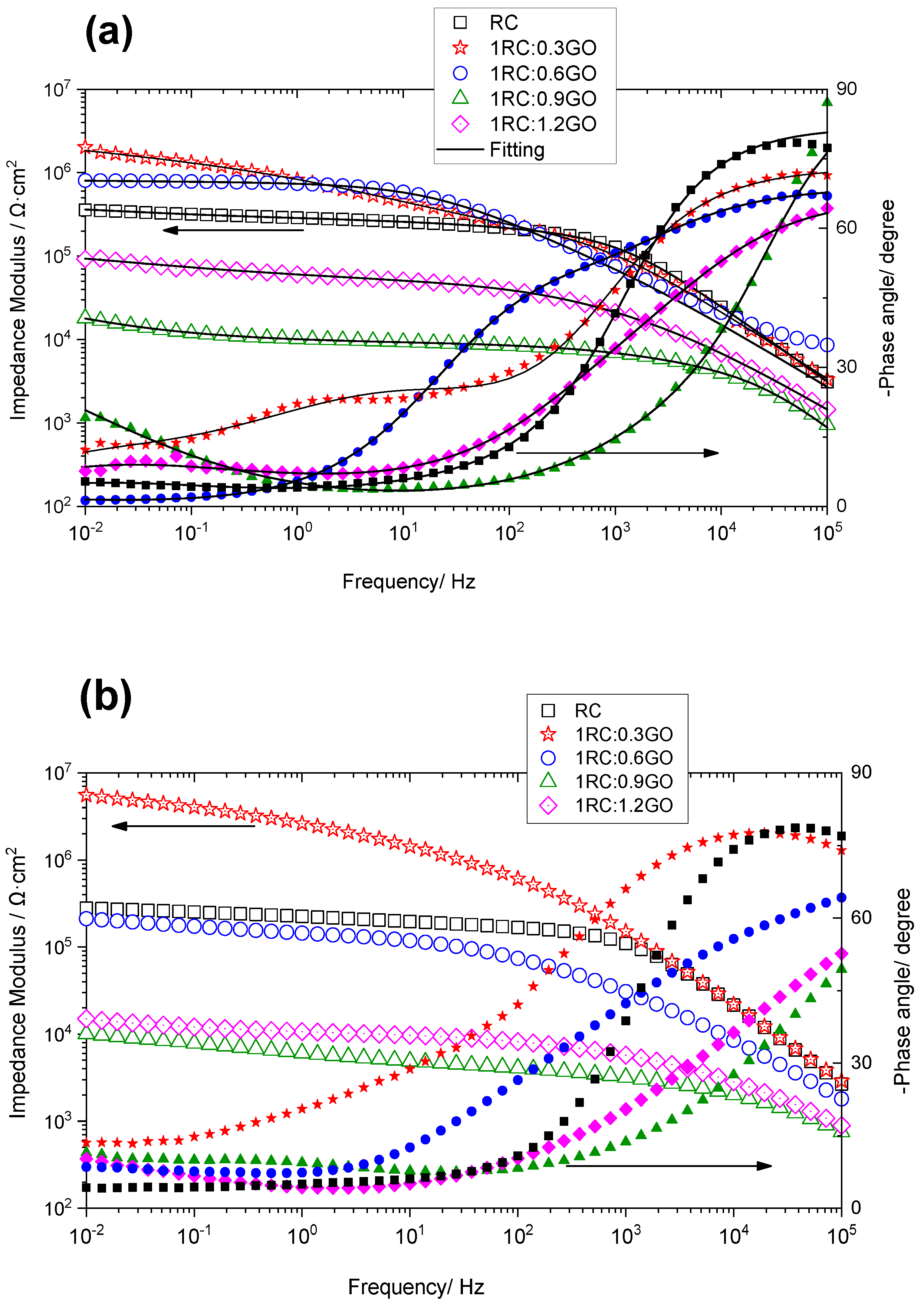
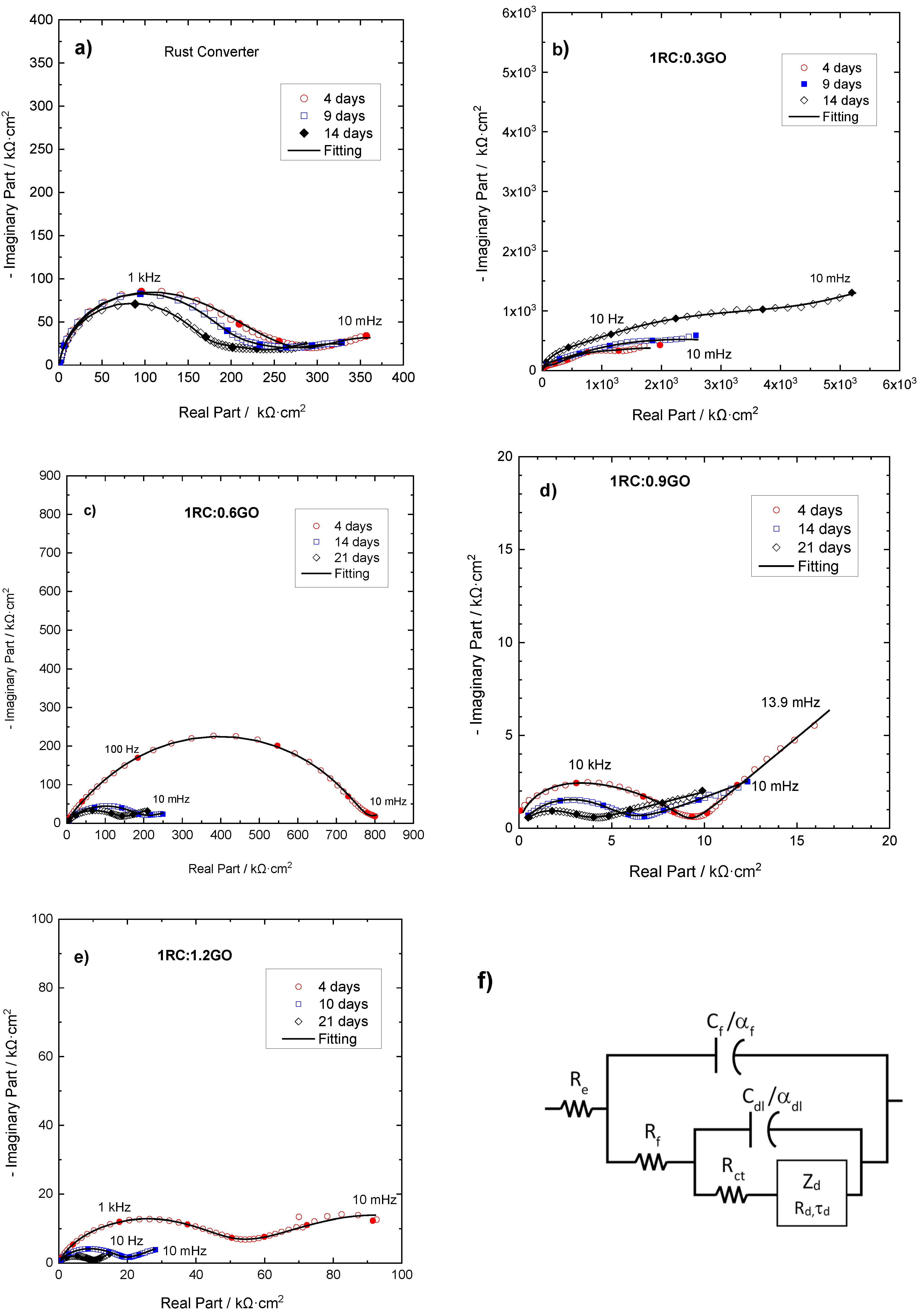
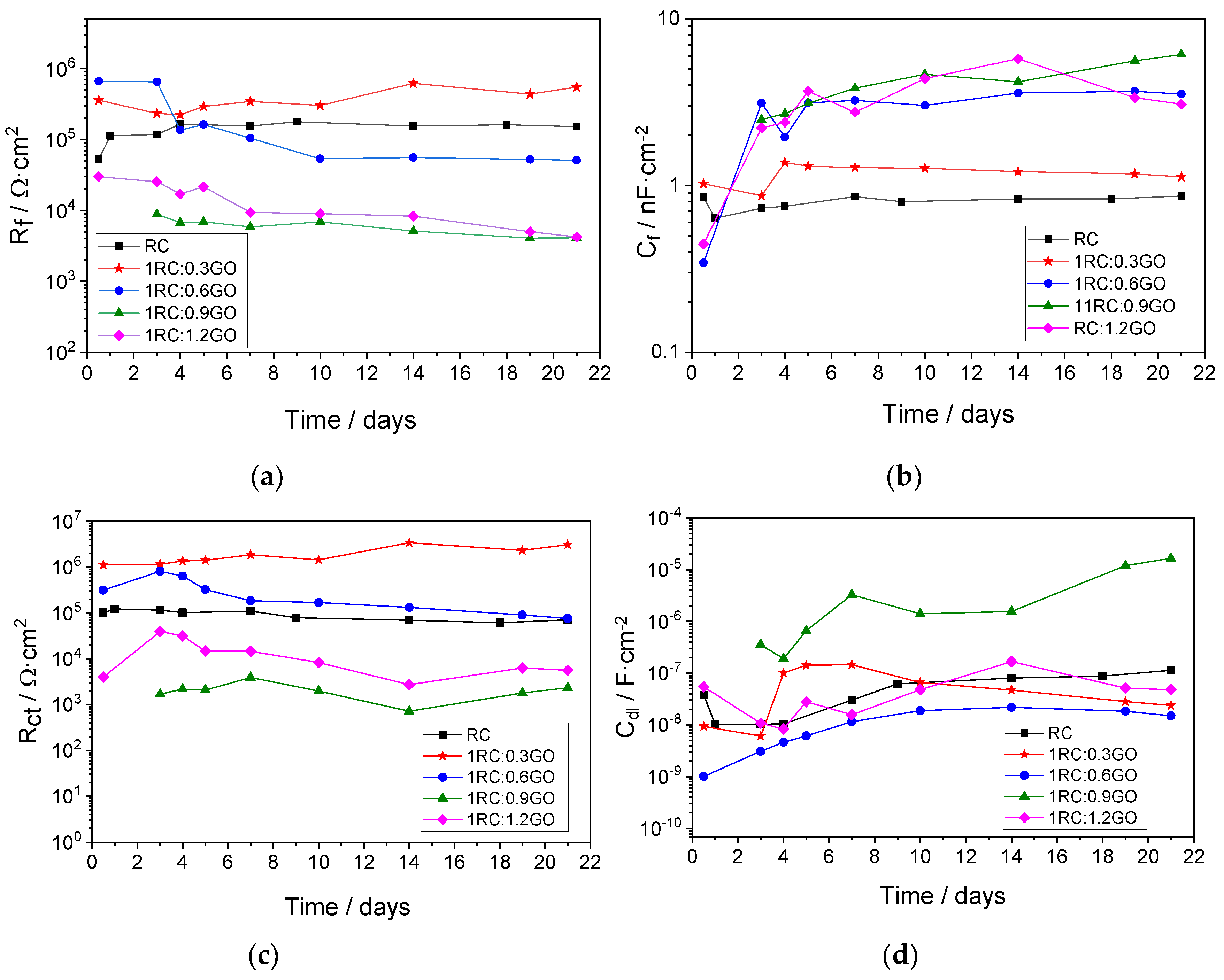
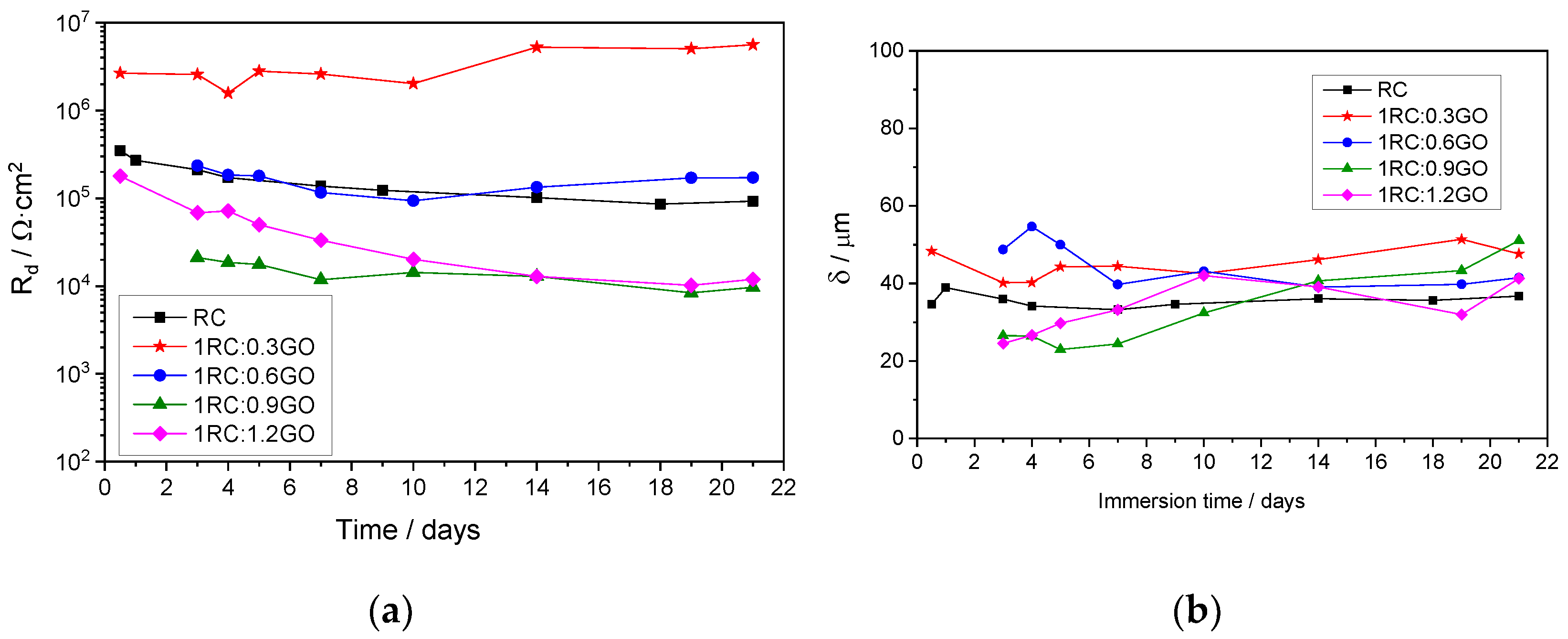
3.3. Electrochemical Study of Rusted Steel Coated with the RC/GO Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ocampo, L.M.; Margarit, I.C.P.; Mattos, O.R.; Córdoba-de-Torresi, S.I.; Fragata, F.L. Performance of rust converter based in phosphoric and tannic acids. Corros. Sci. 2004, 46, 1515–1525. [Google Scholar] [CrossRef]
- Feliu, S.; Galván, J.C.; Feliu, S.; Bastidas, J.M.; Simancas, J.; Morcillo, M.; Almeida, E.M. An electrochemical impedance study of the behaviour of some pretreatments applied to rusted steel surfaces. Corros. Sci. 1993, 35, 1351–1358. [Google Scholar] [CrossRef]
- Saji, V.S. Progress in rust converters. Prog. Org. Coat. 2019, 127, 88–99. [Google Scholar] [CrossRef]
- Morcillo, M.; Feliu, S.; Simancas, J.; Bastidas, J.M.; Galvan, J.C.; Feliu, S.; Almeida, E.M. Corrosion of Rusted Steel in Aqueous Solutions of Tannic Acid. Corrosion 1992, 48, 1032–1039. [Google Scholar] [CrossRef]
- Nasrazadani, S. The application of infrared spectroscopy to a study of phosphoric and tannic acids interactions with magnetite (Fe3O4), goethite (α-FEOOH) and lepidocrocite (γ-FeOOH). Corros. Sci. 1997, 39, 1845–1859. [Google Scholar] [CrossRef]
- Qian, B.; Hou, B.; Zheng, M. The inhibition effect of tannic acid on mild steel corrosion in seawater wet/dry cyclic conditions. Corros. Sci. 2013, 72, 1–9. [Google Scholar] [CrossRef]
- Gust, J. Application of infrared spectroscopy for investigation of rust phase component conversion by agents containing oak tannin and phosphoric acid Indexed keywords. Corrosion. 1991, 47, 453–457. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Jain Kassim, M. Inhibitive action of mangrove tannins and phosphoric acid on pre-rusted steel via electrochemical methods. Corros. Sci. 2008, 50, 1546–1550. [Google Scholar] [CrossRef]
- Jia, Y.; Ren, N.; Yue, H.; Deng, J.; Liu, Y. Preparation and properties of natural gallic acid based rust conversion emulsion. Pigment Resin Technol. 2016, 45, 191–198. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Liu, X.; Hou, B. Rust Conversion Performance of Phosphoric Acid-Gallic Acid in Vinyl Chloride Acrylic Emulsion. Coatings 2021, 11, 152. [Google Scholar] [CrossRef]
- Díaz, B.; Figueroa, R.; Nóvoa, X.R.; Pérez, C.; Pintos, A. The corrosion protection afforded by a commercial rust converter doped with graphene oxide. Electrochim. Acta 2020, 342, 136096. [Google Scholar] [CrossRef]
- Xu, W.; Han, E.H.; Wang, Z. Effect of tannic acid on corrosion behavior of carbon steel in NaCl solution. J. Mater. Sci. Technol. 2019, 35, 64–75. [Google Scholar] [CrossRef]
- Collazo, A.; Nóvoa, X.R.R.; Pérez, C.; Puga, B. EIS study of the rust converter effectiveness under different conditions. Electrochim. Acta 2008, 53, 7565–7574. [Google Scholar] [CrossRef]
- Barrero, C.A.; Ocampo, L.M.; Arroyave, C.E. Possible improvements in the action of some rust converters. Corros. Sci. 2001, 43, 1003–1018. [Google Scholar] [CrossRef]
- Favre, M.; Landolt, D. The influence of gallic acid on the reduction of rust on painted steel surfaces. Corros. Sci. 1993, 34, 1481–1494. [Google Scholar] [CrossRef]
- Gust, J.; Bobrowicz, J. Sealing and Anti-Corrosive Action of Tannin Rust Converters. Corrosion 1993, 49, 24–30. [Google Scholar] [CrossRef]
- Favre, M.; Landolt, D.; Hoffman, K.; Stratmann, M. Influence of gallic acid on the phase transformation in iron oxide layers below organic coatings studied with Moessbauer spectroscopy. Corros. Sci. 1998, 40, 793–803. [Google Scholar] [CrossRef]
- Galván, J.C.; Simancas, J.; Morcillo, M.; Bastidas, J.M.; Almeida, E.; Feliu, S. Effect of treatment with tannic, gallic and phosphoric acids on the electrochemical behaviour of rusted steel. Electrochim. Acta 1992, 37, 1983–1985. [Google Scholar] [CrossRef]
- Li, J.; Ge, S.; Wang, J.; Du, H.; Song, K.; Fei, Z.; Shao, Q.; Guo, Z. Water-based rust converter and its polymer composites for surface anticorrosion. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 537, 334–342. [Google Scholar] [CrossRef]
- Xue, B.; Yu, M.; Liu, J.; Li, S.; Xiong, L.; Kong, X. Corrosion Protective Properties of Silane Functionalized Graphene Oxide Film on AA2024-T3 Aluminum Alloy. J. Electrochem. Soc. 2016, 163, C798–C806. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Mohamadzadeh Moghadam, M.H. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; He, Y.; Liang, L.; Lv, L.; Ran, X.; Pan, Y.; Luo, Z. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings. Surf. Coat. Technol. 2015, 276, 471–478. [Google Scholar] [CrossRef]
- Cui, J.; Xiong, Z.; Qiu, H.; LI, J.; Yang, J. Functionalized graphene oxide: Carrier for corrosion inhibitor and barrier in waterborne epoxy coatings. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106354. [Google Scholar] [CrossRef]
- Li, M.J.; Liu, C.M.; Xie, Y.B.; Cao, H.B.; Zhao, H.; Zhang, Y. The evolution of surface charge on graphene oxide during the reduction and its application in electroanalysis. Carbon N. Y. 2014, 66, 302–311. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Hou, W.; Zhou, J.; Song, L. Effects of particle size and pH value on the hydrophilicity of graphene oxide. Appl. Surf. Sci. 2013, 273, 118–121. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Li, J.; Xiao, G.; Chen, C.; Li, R.; Yan, D. Superior dispersions of reduced graphene oxide synthesized by using gallic acid as a reductant and stabilizer. J. Mater. Chem. A 2013, 1, 1481–1487. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, Z.; Liao, R.; Guo, B. Hydrolysable tannin as environmentally friendly reducer and stabilizer for graphene oxide. Green Chem. 2011, 13, 1655–1658. [Google Scholar] [CrossRef]
- Ross, T.K.; Francis, R.A. The treatment of rusted steel with mimosa tannin. Corros. Sci. 1978, 18, 351–361. [Google Scholar] [CrossRef]
- de la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Li, F.A. Kinetic study on removal of copper(II) using goethite and hematite nano-photocatalysts. J. Colloid Interface Sci. 2010, 347, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Compeán-Jasso, M.E.; Ruiz, F.; Martínez, J.R.; Herrera-Gómez, A. Magnetic properties of magnetite nanoparticles synthesized by forced hydrolysis. Mater. Lett. 2008, 62, 4248–4250. [Google Scholar] [CrossRef]
- Antony, H.; Legrand, L.; Maréchal, L.; Perrin, S.; Dillmann, P.; Chaussé, A. Study of lepidocrocite γ-FeOOH electrochemical reduction in neutral and slightly alkaline solutions at 25 °C. Electrochim. Acta 2005, 51, 745–753. [Google Scholar] [CrossRef]
- Chaudhry, A.U.; Mittal, V.; Mishra, B. Inhibition and promotion of electrochemical reactions by graphene in organic coatings. RSC Adv. 2015, 5, 80365–80368. [Google Scholar] [CrossRef]
- MacDonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; John Wiley & Sons, Inc.: New York, NY, USA, 1987; ISBN 0-471-83122-0. [Google Scholar]
- Pérez, C.; Collazo, A.; Izquierdo, M.; Merino, P.; Nóvoa, X.R. Electrochemical impedance spectroscopy study of the corrosion process on coated galvanized steel in a salt spray fog chamber. Corrosion 2000, 56, 1220–1232. [Google Scholar] [CrossRef]
- Amirudin, A.; Thierry, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Lorenz, W.J.; Mansfeld, F. Determination of corrosion rates by electrochemical DC and AC methods. Corros. Sci. 1981, 21, 647–672. [Google Scholar] [CrossRef]
- McIntyre, J.M.; Pham, H.Q. Electrochemical impedance spectroscopy; a tool for organic coatings optimizations. Prog. Org. Coat. 1996, 27, 201–207. [Google Scholar] [CrossRef]
- Cabanelas, I.; Collazo, A.; Izquierdo, M.; Nóvoa, X.R.; Pérez, C. Influence of galvanised surface state on the duplex systems behaviour. Corros. Sci. 2007, 49, 1816–1832. [Google Scholar] [CrossRef]
- Thomas, N.L. The Barrier Properties of Paint Coatings. Prog. Org. Coat. 1991, 19, 101–121. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, B.; Nóvoa, X.R.; Pérez, C.; Rodríguez-Morgado, M. Influence of Graphene Oxide Additions on the Corrosion Resistance of a Rust Converter Primer. Coatings 2022, 12, 345. https://doi.org/10.3390/coatings12030345
Díaz B, Nóvoa XR, Pérez C, Rodríguez-Morgado M. Influence of Graphene Oxide Additions on the Corrosion Resistance of a Rust Converter Primer. Coatings. 2022; 12(3):345. https://doi.org/10.3390/coatings12030345
Chicago/Turabian StyleDíaz, Belén, Xosé Ramón Nóvoa, Carmen Pérez, and Miguel Rodríguez-Morgado. 2022. "Influence of Graphene Oxide Additions on the Corrosion Resistance of a Rust Converter Primer" Coatings 12, no. 3: 345. https://doi.org/10.3390/coatings12030345
APA StyleDíaz, B., Nóvoa, X. R., Pérez, C., & Rodríguez-Morgado, M. (2022). Influence of Graphene Oxide Additions on the Corrosion Resistance of a Rust Converter Primer. Coatings, 12(3), 345. https://doi.org/10.3390/coatings12030345








