Enhanced Electrochromic Performance of All-Solid-State Electrochromic Device Based on W-Doped NiO Films
Abstract
:1. Introduction
2. Materials and Methods
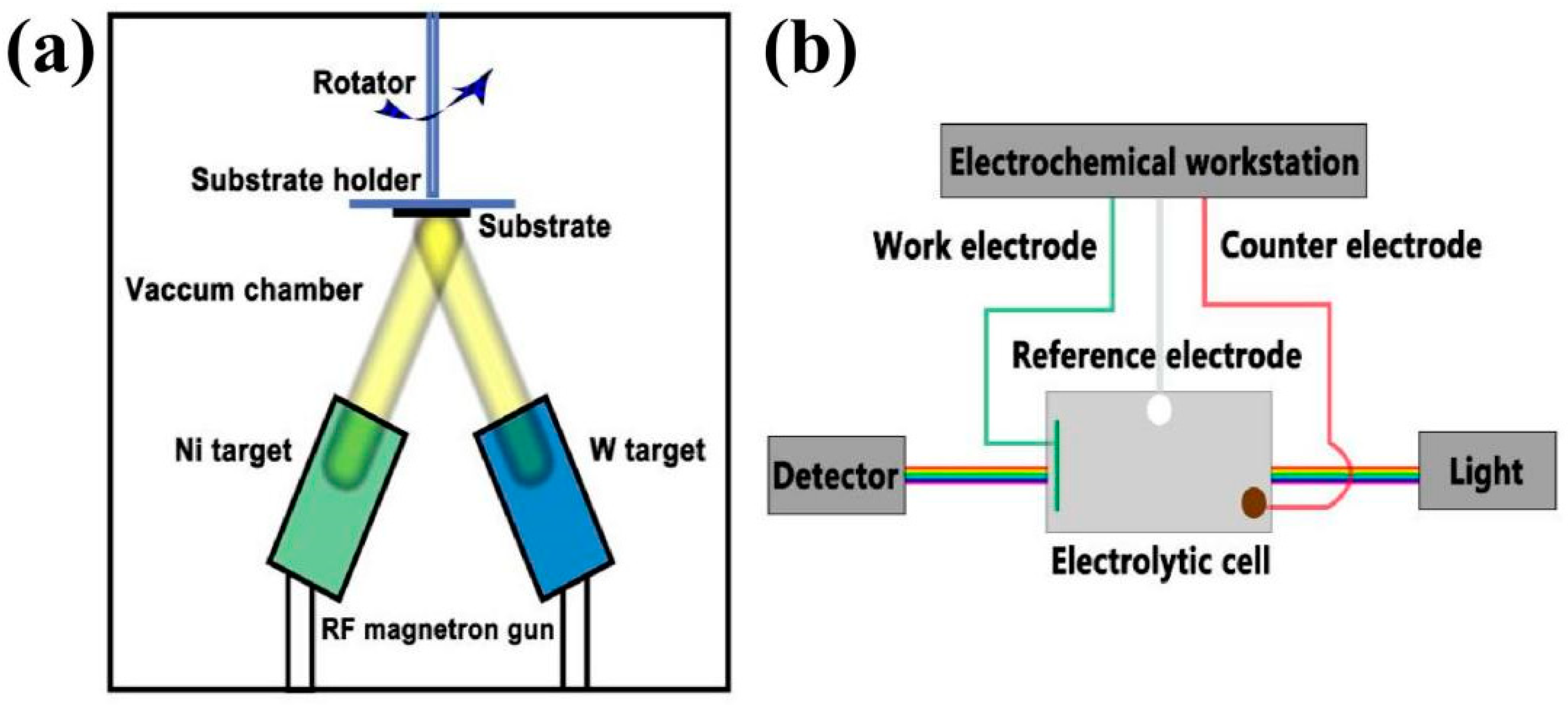
2.1. Preparation of the NiO and W-Doped NiO Films
2.2. Fabrication of the All-Solid-State ECDs
2.3. Analytical Techniques
3. Results and Discussion
3.1. NiO and W-Doped NiO Films
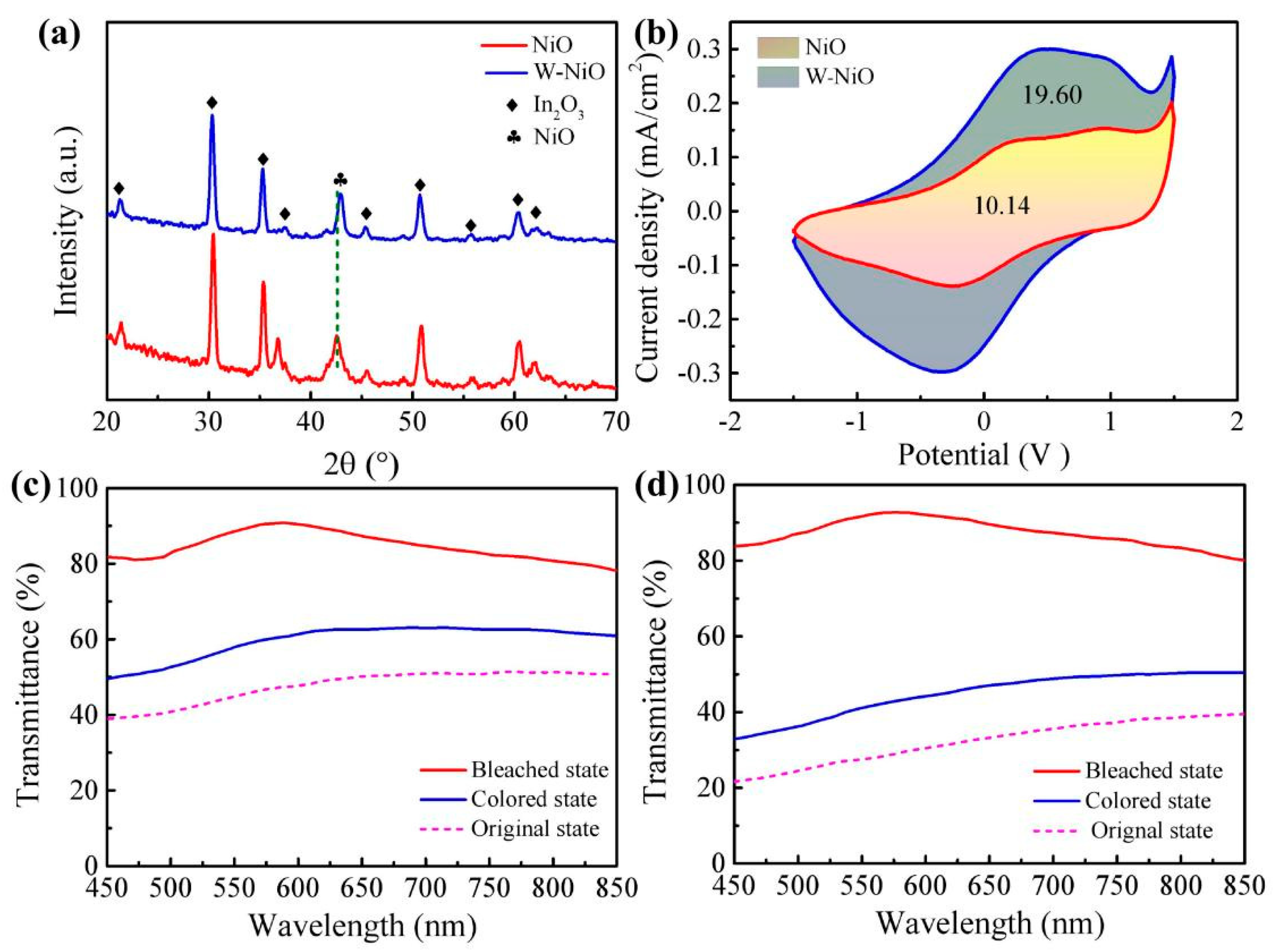
3.1.1. Structure and Composition of NiO and W-Doped NiO Films
3.1.2. EC Performances of NiO and W-Doped NiO Films
3.2. All-Solid-State ECD Based on W-NiO Film
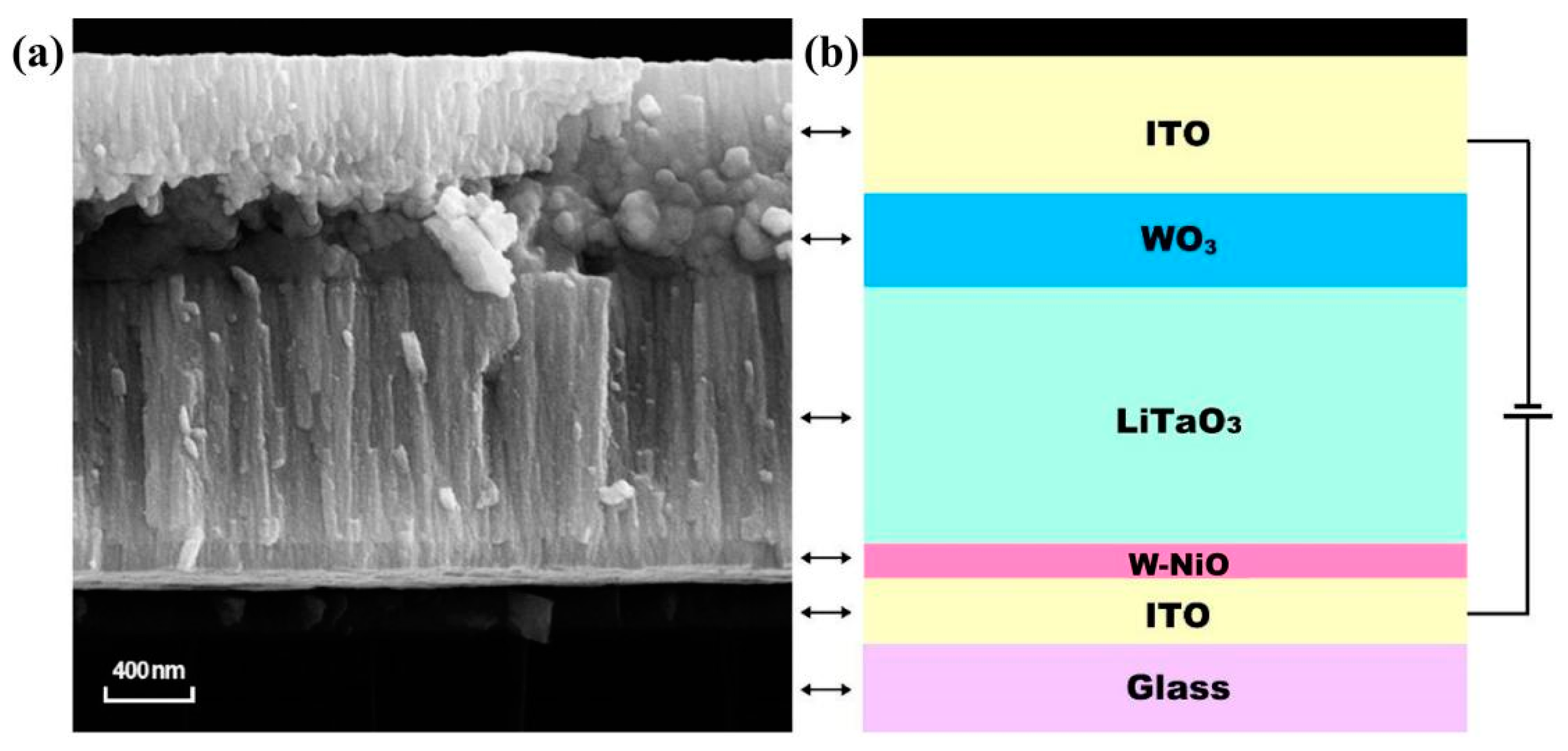
3.2.1. Morphology of the ECD
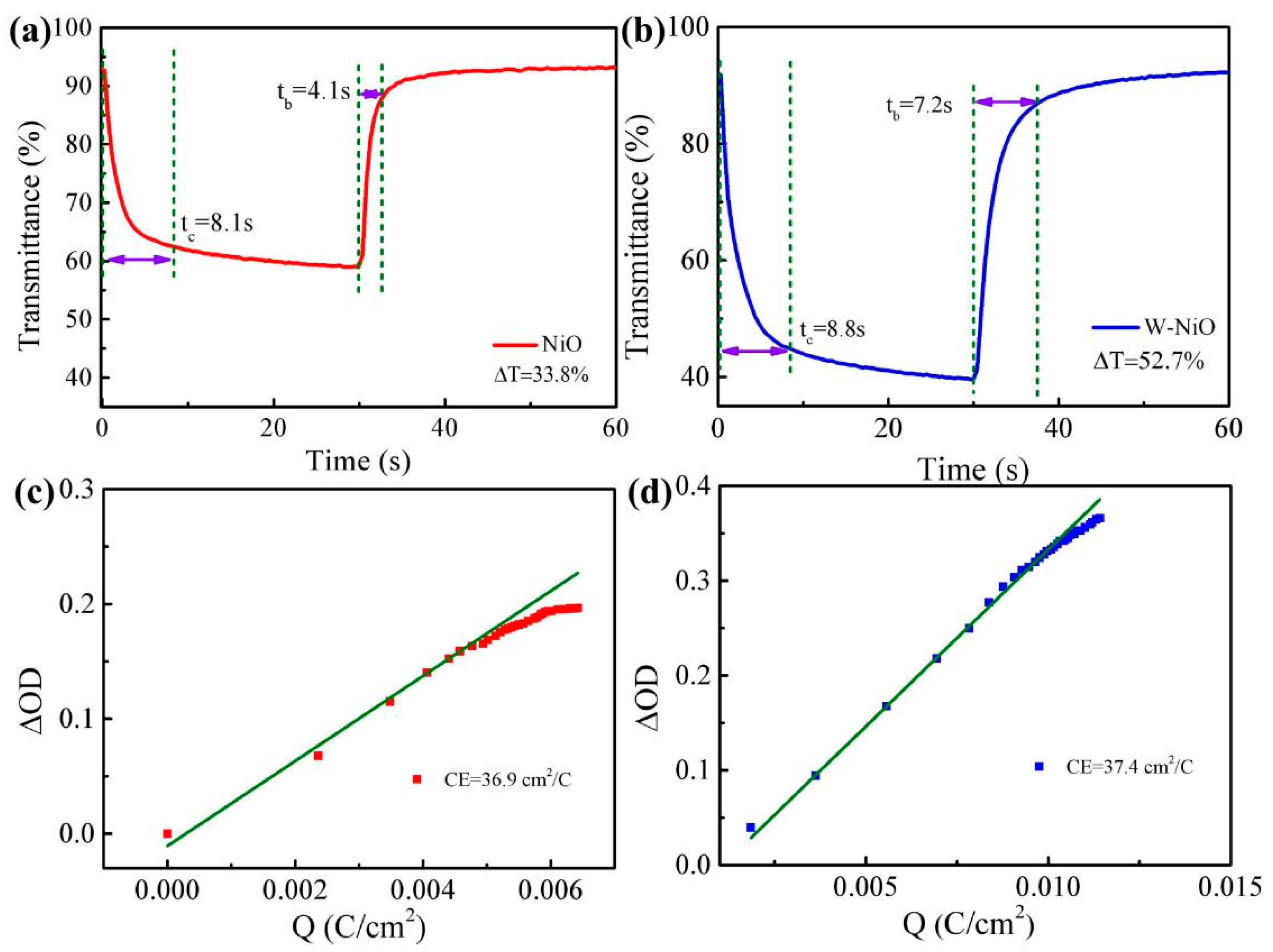
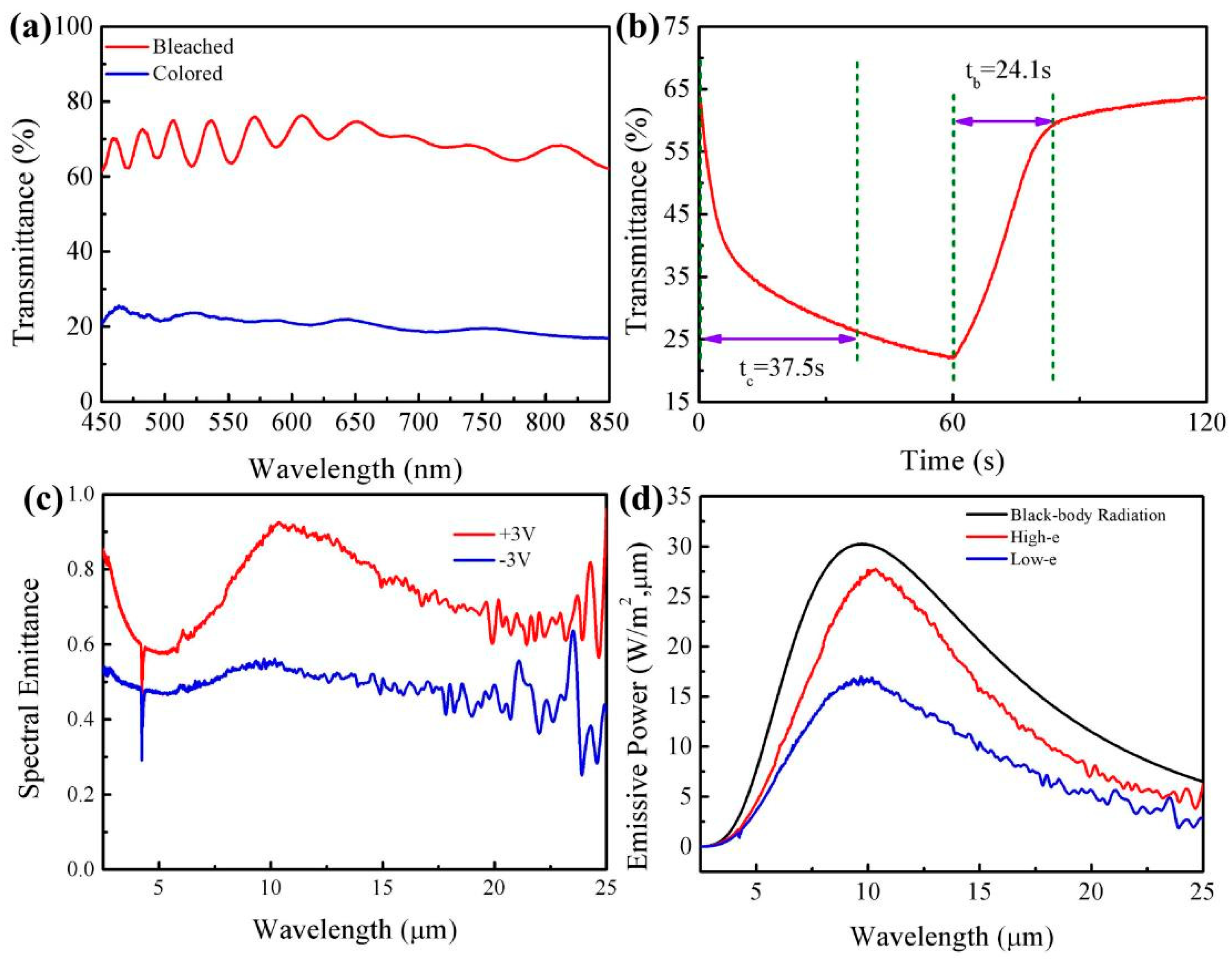
3.2.2. EC Performances of the ECD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Dong, D.; Djaoued, H.; Vienneau, G.; Robichaud, J.; Brown, D.; Brüning, R.; Djaoued, Y. Electrochromic and colorimetric properties of anodic NiO thin films: Uncovering electrochromic mechanism of NiO. Electrochim. Acta 2020, 335, 135648. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, R.; Wang, Y.; Ye, X.; Wan, Z.; Jia, C.; Weng, X.; Xie, J.; Deng, L. A high-performance electrochromic device assembled with hexagonal WO3 and NiO/PB composite nanosheet electrodes towards energy storage smart window. Sol. Energy Mater. Sol. Cells 2020, 207, 110337. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Cui, Y.; Ai, X.; Chen, Z.; Cao, C.; Xu, F.; Gao, Y. The discovery of conductive ionic bonds in NiO/Ni transparent counter electrodes for electrochromic smart windows with an ultra-long cycling life. Mater. Adv. 2021, 2, 4667–4676. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Huang, J.; Guo, X.; Liu, Q.; Yu, R.; Miao, W.; Zhou, B.; Guo, W.; Liu, X. Flexible, controllable and angle-independent photoelectrochromic display enabled by smart sunlight management. Nano Energy 2019, 63, 103830. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Li, X.; Wang, L.; Sun, W.; Zhang, H.; Wang, J.; Zhao, J.; Li, Y. Bio-inspired electrochromic skin based on tungsten oxide. Sol. Energy Mater. Sol. Cells 2021, 230, 111195. [Google Scholar] [CrossRef]
- Shah, K.W.; Wang, S.X.; Soo, D.X.Y.; Xu, J. Viologen-based electrochromic materials: From small molecules, polymers and composites to their applications. Polymers 2019, 11, 1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Qiu, W.; Fan, X.; Shi, Y.; Gong, H.; Huang, J.; Patil, A.; Li, X.; Wang, S.; Lin, H.; et al. Stretchable, stable, and degradable silk fibroin enabled by mesoscopic doping for finger motion triggered color/transmittance adjustment. ACS Nano 2021, 15, 12429–12437. [Google Scholar] [CrossRef] [PubMed]
- Mandal, J.; Du, S.; Dontigny, M.; Zaghib, K.; Yu, N.; Yang, Y. Li4Ti5O12: A visible-to-infrared broadband electrochromic material for optical and thermal management. Adv. Funct. Mater. 2018, 28, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, W.; Chen, X.; Zhao, Y.; Wang, L.; Chen, M.; Li, Z.; Li, Y. Inorganic all-solid-state electrochromic devices with reversible color change between yellow-green and emerald green. Chem. Commun. 2020, 56, 10062–10065. [Google Scholar] [CrossRef]
- Huang, H.; Tian, J.; Zhang, W.K.; Gan, Y.P.; Tao, X.Y.; Xia, X.H.; Tu, J.P. Electrochromic properties of porous NiO thin film as a counter electrode for NiO/WO3 complementary electrochromic window. Electrochim. Acta 2011, 56, 4281–4286. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Chen, X.; Zhao, Y.; Sun, W.; Xiao, Y.; Li, S.; Zhao, J.; Li, Y. Long life all-solid-state electrochromic devices by annealing. Sol. Energy Mater. Sol. Cells 2021, 224, 110992. [Google Scholar] [CrossRef]
- Green, S.; Backholm, J.; Georén, P.; Granqvist, C.G.; Niklasson, G.A. Electrochromism in nickel oxide and tungsten oxide thin films: Ion intercalation from different electrolytes. Sol. Energy Mater. Sol. Cells 2009, 93, 2050–2055. [Google Scholar] [CrossRef] [Green Version]
- Sonavane, A.C.; Inamdar, A.I.; Dalavi, D.S.; Deshmukh, H.P.; Patil, P.S. Simple and rapid synthesis of NiO/PPy thin films with improved electrochromic performance. Electrochim. Acta 2010, 55, 2344–2351. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Chen, X.; Li, W.; Wang, L.; Li, Z.; Zhao, J.; Endres, F.; Li, Y. Preparation of Sn-NiO films and all-solid-state devices with enhanced electrochromic properties by magnetron sputtering method. Electrochim. Acta 2021, 367, 137457. [Google Scholar] [CrossRef]
- Firat, Y.E.; Peksoz, A. Efficiency enhancement of electrochromic performance in NiO thin film via Cu doping for energy-saving potential. Electrochim. Acta 2019, 295, 645–654. [Google Scholar] [CrossRef]
- Choi, D.; Son, M.; Im, T.; Ahn, S.H.; Lee, C.S. Microstructure control of NiO-based ion storage layer with various sized NiO particles to evaluate the electrochromic performance. Mater. Chem. Phys. 2020, 249, 123121. [Google Scholar] [CrossRef]
- Yu, J.H.; Nam, S.H.; Gil, Y.E.; Boo, J.H. The effect of ammonia concentration on the microstructure and electrochemical properties of NiO nanoflakes array prepared by chemical bath deposition. Appl. Surf. Sci. 2020, 532, 147441. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Xia, X.H.; Wu, J.B.; Chen, Y.B.; Yang, J.L.; Guo, S.Y. Enhanced electrochromic properties of ordered porous nickel oxide thin film prepared by self-assembled colloidal crystal template-assisted electrodeposition. Electrochim. Acta 2011, 56, 1208–1212. [Google Scholar] [CrossRef]
- Penin, N.; Rougier, A.; Laffont, L.; Poizot, P.; Tarascon, J.M. Improved cyclability by tungsten addition in electrochromic NiO thin films. Sol. Energy Mater. Sol. Cells 2006, 90, 422–433. [Google Scholar] [CrossRef]
- Pereira, S.; Gonçalves, A.; Correia, N.; Pinto, J.; Pereira, L.; Martins, R.; Fortunato, E. Electrochromic behavior of NiO thin films deposited by e-beam evaporation at room temperature. Sol. Energy Mater. Sol. Cells 2014, 120, 109–115. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, X.; Zhang, H.; Lei, L.; Zhao, G. Preparation of a porous NiO array-patterned film and its enhanced electrochromic performance. J. Mater. Chem. C 2018, 6, 4952–4958. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.H.; Seo, H.J.; Jeong, R.H.; Boo, J.H. Improved electrochromic properties of nanoporous NiO film by NiO flake with thickness controlled by aluminum. Appl. Surf. Sci. 2018, 461, 88–92. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chen, Y.C.; Wang, C.M.; Chen, Y.W. Electrochromic properties of Li-Doped NiO films prepared by RF magnetron sputtering. Coatings 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, C.F.; Balboni, R.D.C.; Cholant, C.M.; Moura, E.A.; Lemos, R.M.J.; Pawlicka, A.; Gündel, A.; Flores, W.H.; Pereira, M.; Avellaneda, C.O. New thin films of NiO doped with V2O5 for electrochromic applications. J. Phys. Chem. Solids 2017, 110, 30–35. [Google Scholar] [CrossRef]
- Avendaño, E.; Azens, A.; Niklasson, G.A.; Granqvist, C.G. Electrochromism in nickel oxide films containing Mg, Al, Si, V., Zr, Nb, Ag, or Ta. Sol. Energy Mater. Sol. Cells 2004, 84, 337–350. [Google Scholar] [CrossRef]
- Yu, L.; Huang, Y.; Xiao, G.; Li, D. Application of long wavelength visible light (λ > 650 nm) in photocatalysis with a p-CuO–n-In2O3 quantum dot heterojunction photocatalyst. J. Mater. Chem. A 2013, 1, 9637–9640. [Google Scholar] [CrossRef]
- Zhou, K.; Qi, Z.; Zhao, B.; Lu, S.; Wang, H.; Liu, J.; Yan, H. The influence of crystallinity on the electrochromic properties and durability of NiO thin films. Surf. Interfaces 2017, 6, 91–97. [Google Scholar] [CrossRef]
- Hartman, P.; Chan, H.K. Application of the periodic bond chain (PBC) theory and attachment energy consideration to derive the crystal morphology of hexamethylmelamine. Pharm. Res. 1993, 10, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state Quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, J.P.; Xia, X.H.; Qiao, Y.; Lu, Y. An all-solid-state electrochromic device based on NiO/WO3 complementary structure and solid hybrid polyelectrolyte. Sol. Energy Mater. Sol. Cells 2009, 93, 1840–1845. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ko, T.F.; Hsu, S.C.; Li, K.D.; Wu, J.Y. Fast response of complementary electrochromic device based on WO3/NiO electrodes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Chen, X.; Zhao, Y.; Wang, L.; Chen, M.; Li, Z.; Zhao, J.; Li, Y. Lithiation of WO3 films by evaporation method for all-solid-state electrochromic devices. Electrochim. Acta 2020, 355, 136817. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Chen, X.; Zhao, Y.; Wang, L.; Chen, M.; Zhao, J.; Li, Y.; Zhang, Y. Effect of independently controllable electrolyte ion content on the performance of all-solid-state electrochromic devices. Chem. Eng. J. 2020, 398, 125628. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Li, W.; Dou, S.; Wang, L.; Qu, H.; Zhao, J.; Li, Y. Preparation and performances of all-solid-state variable infrared emittance devices based on amorphous and crystalline WO3 electrochromic thin films. Sol. Energy Mater. Sol. Cells 2019, 200, 109916. [Google Scholar] [CrossRef]

| Layer | O2 (%) | Pressure (Pa) | Power (W) | Time (min) |
|---|---|---|---|---|
| W-NiO | 10 | 1.0 | 10:70 | 120 |
| LiTaO3 | 5 | 1.0 | 100 | 480 |
| NiO | 50 | 1.0 | 100 | 150 |
| ITO | 1 | 0.4 | 50 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, X.; Yin, Z.; Li, W.; Yang, C.; Sun, W.; Zhang, H.; Li, Y. Enhanced Electrochromic Performance of All-Solid-State Electrochromic Device Based on W-Doped NiO Films. Coatings 2022, 12, 118. https://doi.org/10.3390/coatings12020118
Zhao X, Zhang X, Yin Z, Li W, Yang C, Sun W, Zhang H, Li Y. Enhanced Electrochromic Performance of All-Solid-State Electrochromic Device Based on W-Doped NiO Films. Coatings. 2022; 12(2):118. https://doi.org/10.3390/coatings12020118
Chicago/Turabian StyleZhao, Xin, Xiang Zhang, Zhiwei Yin, Wenjie Li, Changpeng Yang, Wenhai Sun, Hulin Zhang, and Yao Li. 2022. "Enhanced Electrochromic Performance of All-Solid-State Electrochromic Device Based on W-Doped NiO Films" Coatings 12, no. 2: 118. https://doi.org/10.3390/coatings12020118
APA StyleZhao, X., Zhang, X., Yin, Z., Li, W., Yang, C., Sun, W., Zhang, H., & Li, Y. (2022). Enhanced Electrochromic Performance of All-Solid-State Electrochromic Device Based on W-Doped NiO Films. Coatings, 12(2), 118. https://doi.org/10.3390/coatings12020118







