Numerical Modelling on the Effect of Temperature on MOCVD Growth of ZnO Using Diethylzinc and Tertiarybutanol
Abstract
:1. Introduction
2. Numerical Model and Boundary Conditions
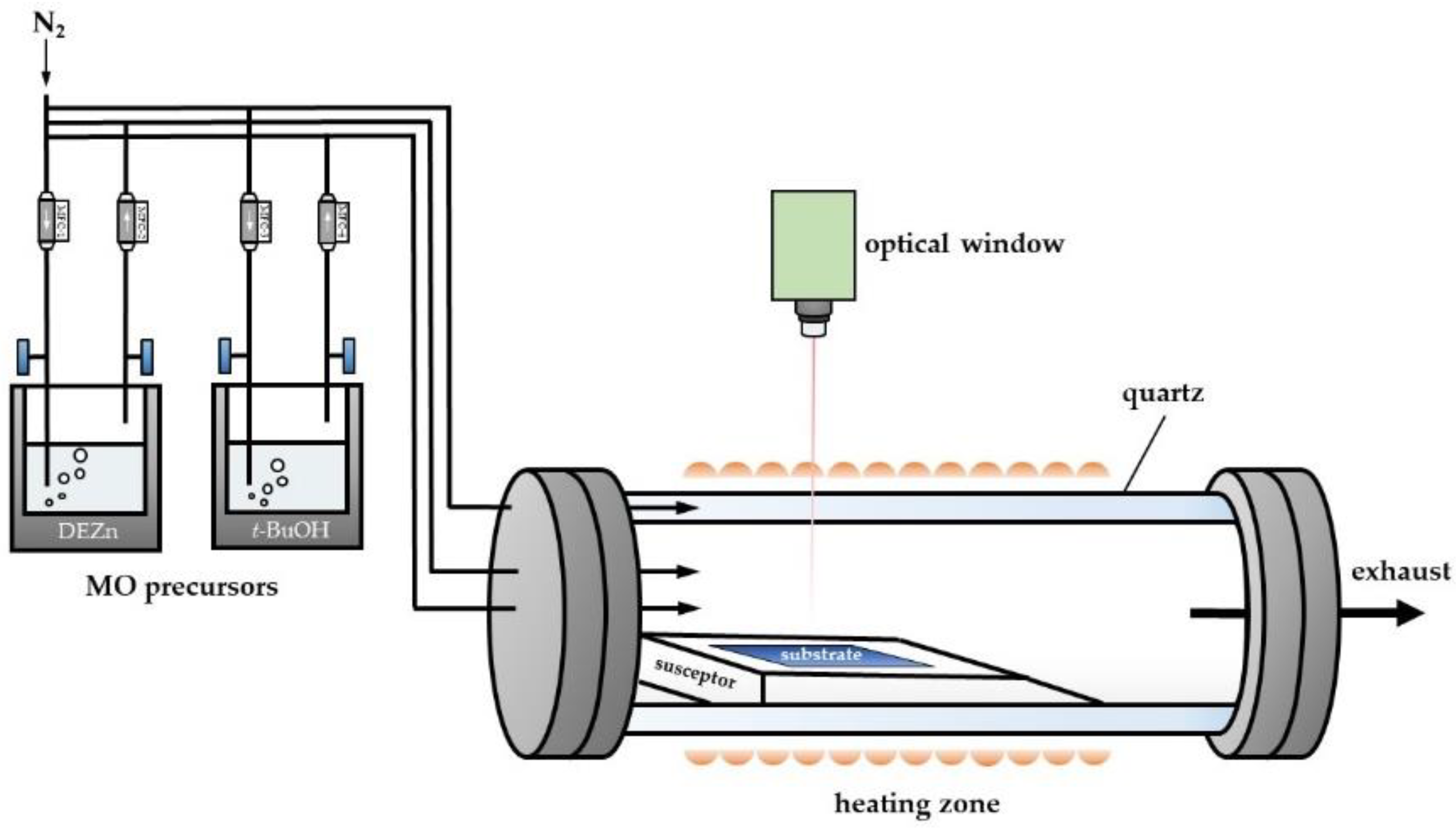
2.1. Physical Model
2.2. Governing Equations
2.3. Boundary Conditions
2.4. Numerical Procedure
3. Experimental Details
3.1. Growth of ZnO
3.2. Deposition Rate and Characterization of ZnO
4. Results and Discussion
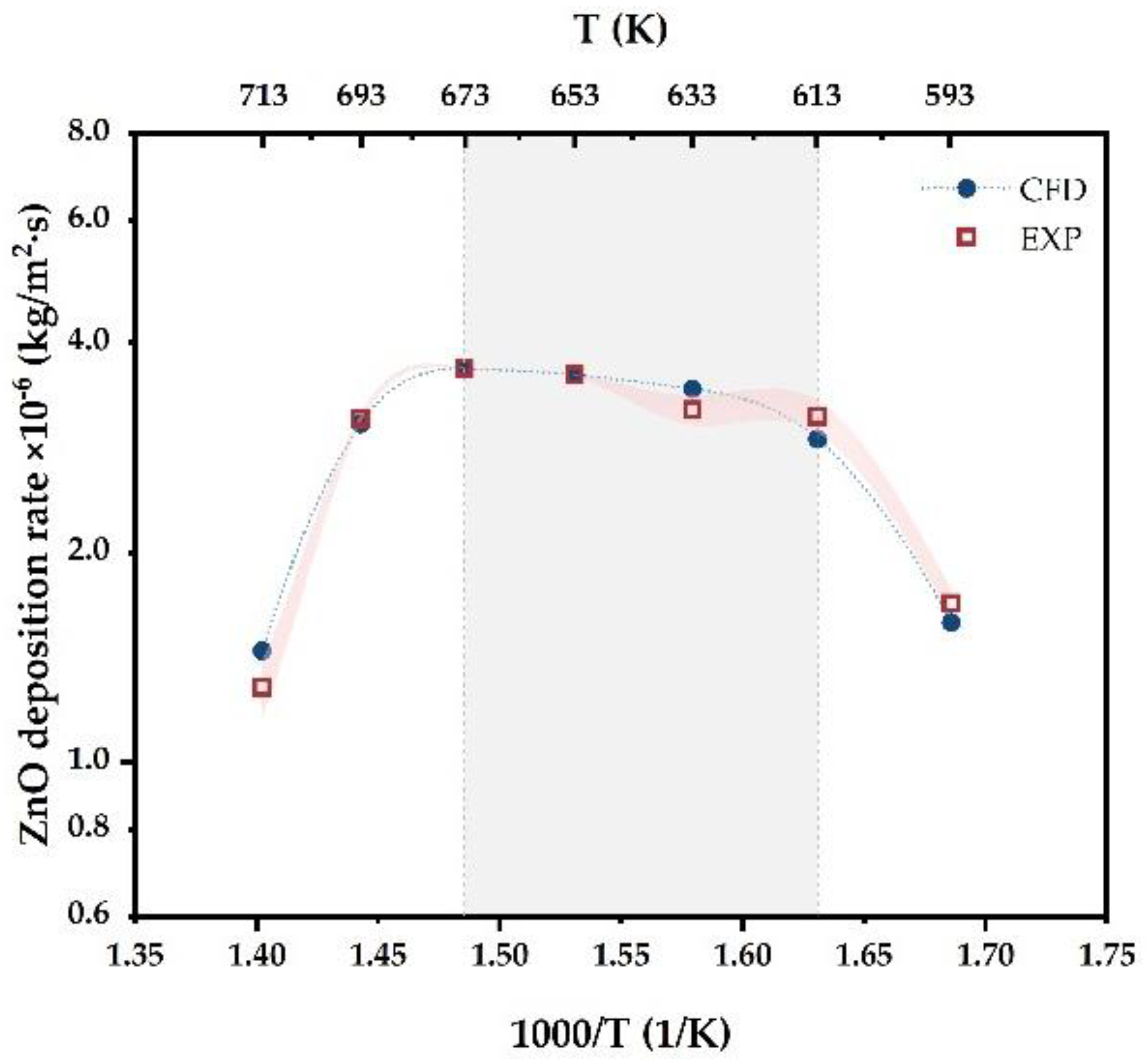
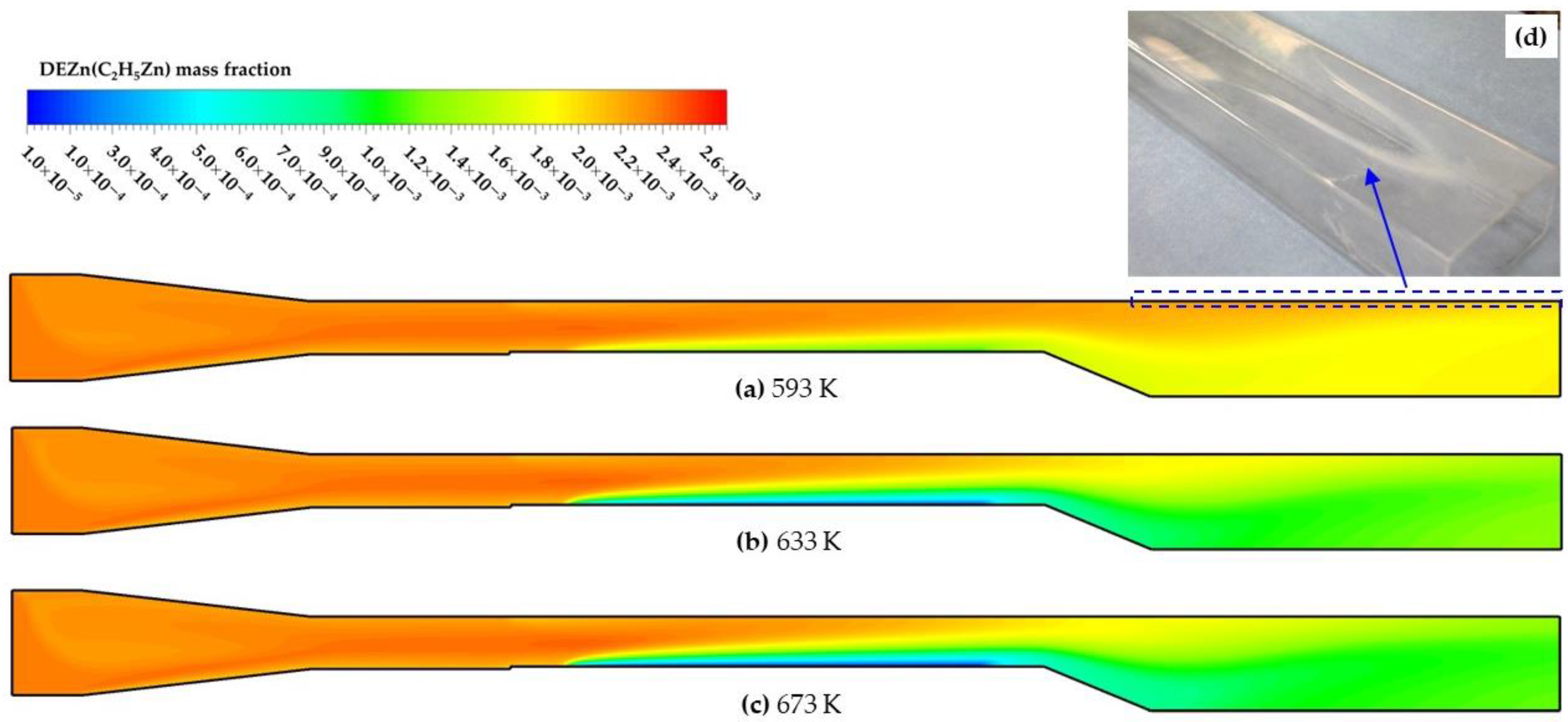
4.1. Model Validation and Temperature Effects
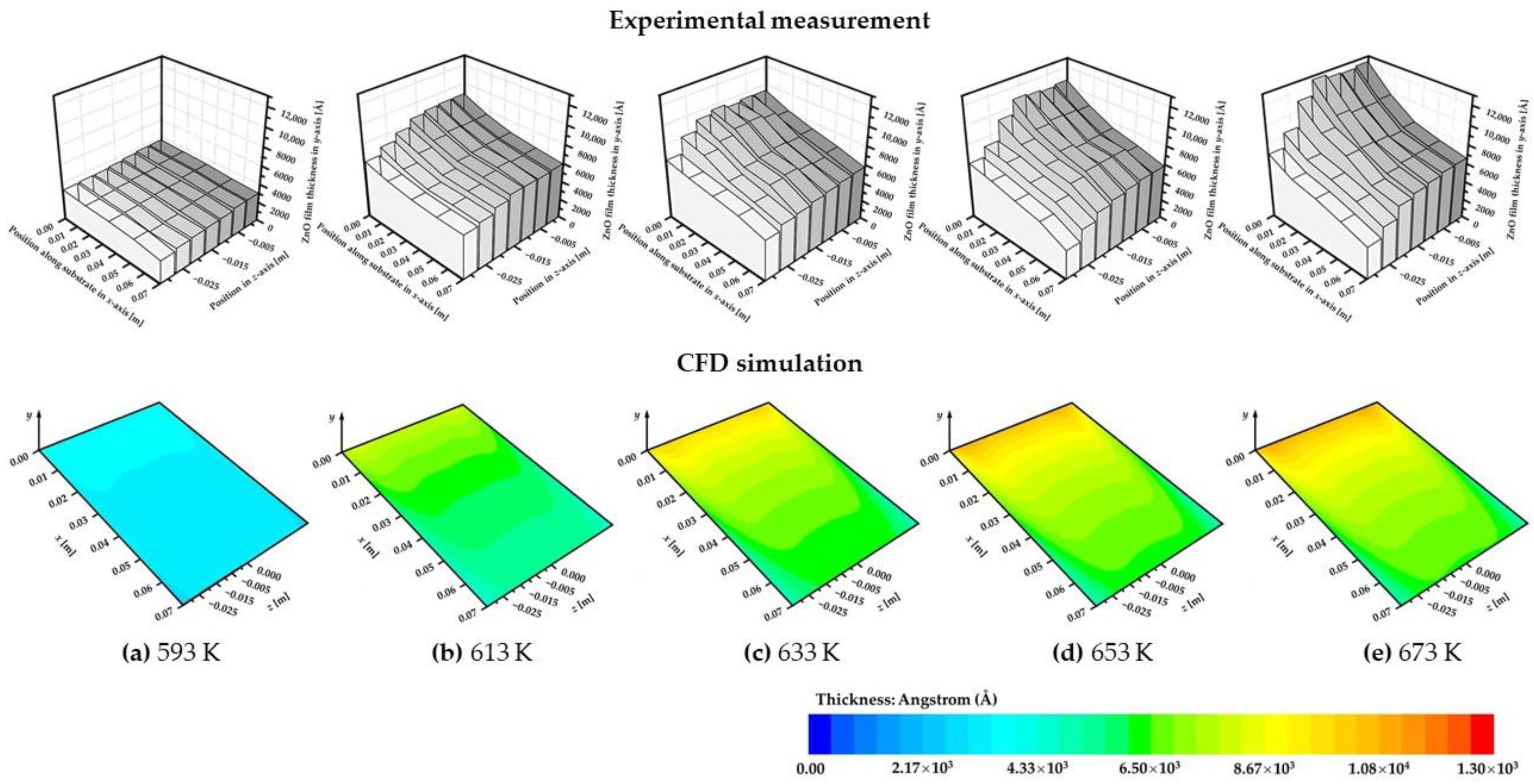
4.2. Thin Film Thickness and Uniformity
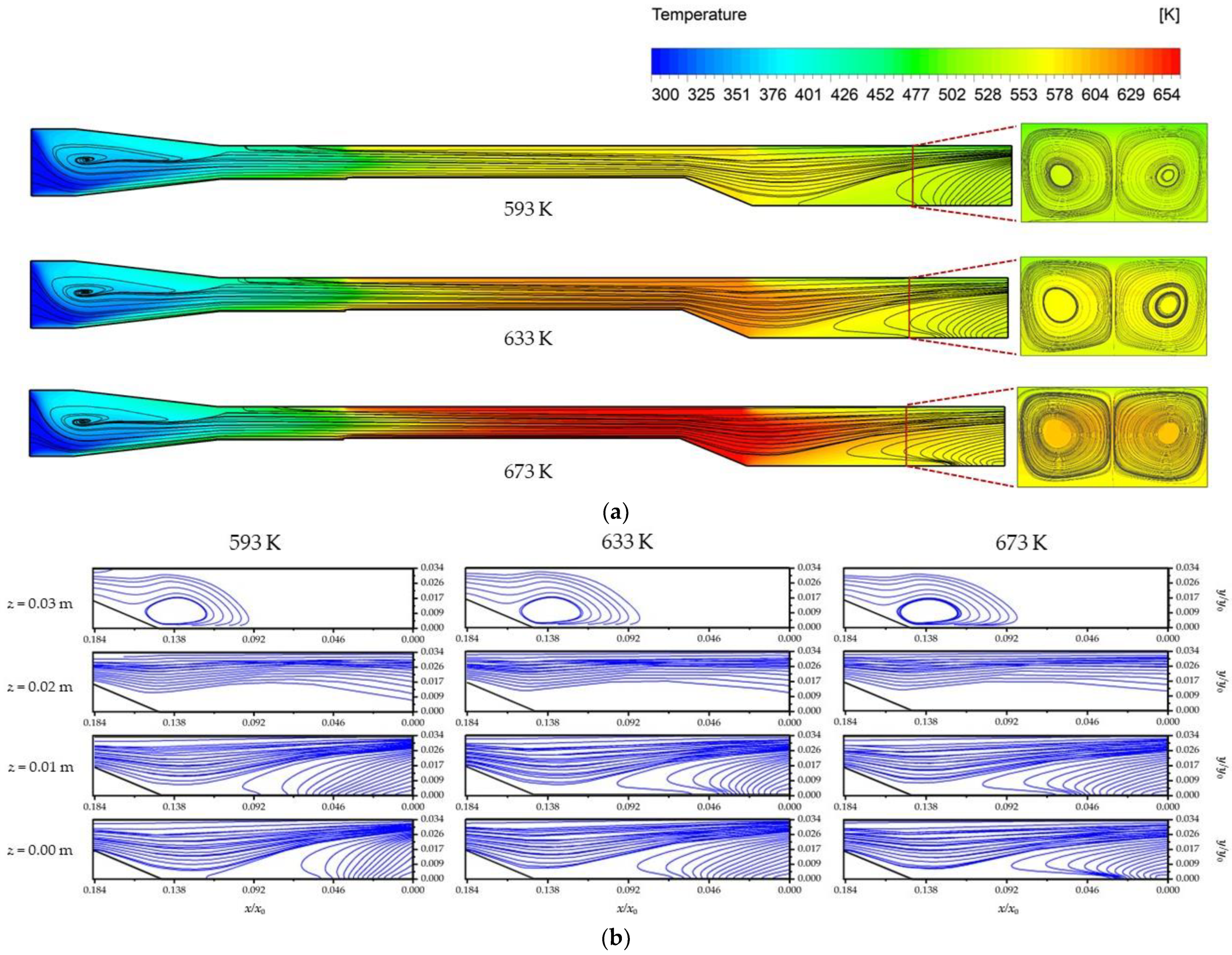
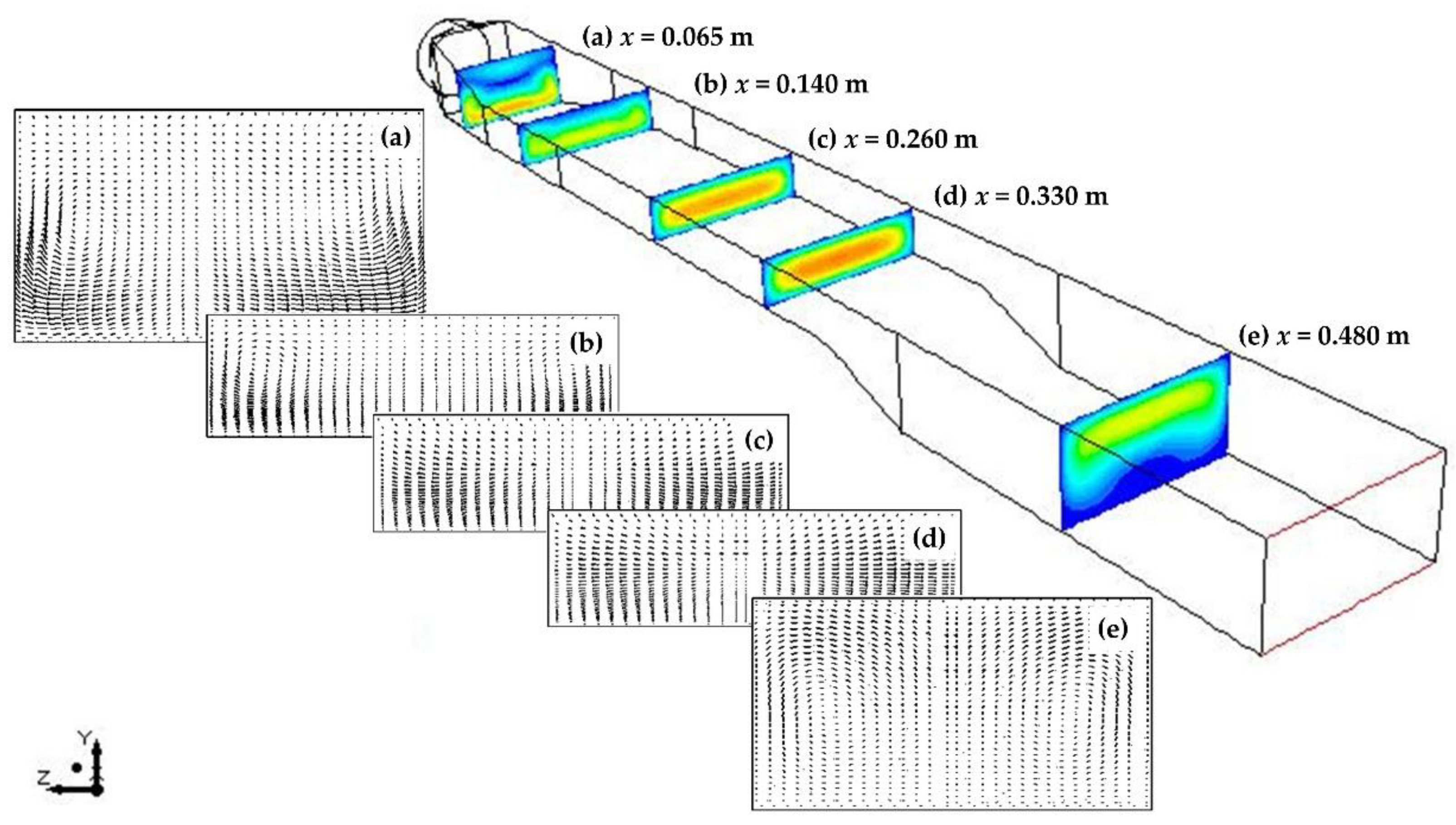
4.3. Flow, Thermal and Concentration Fields
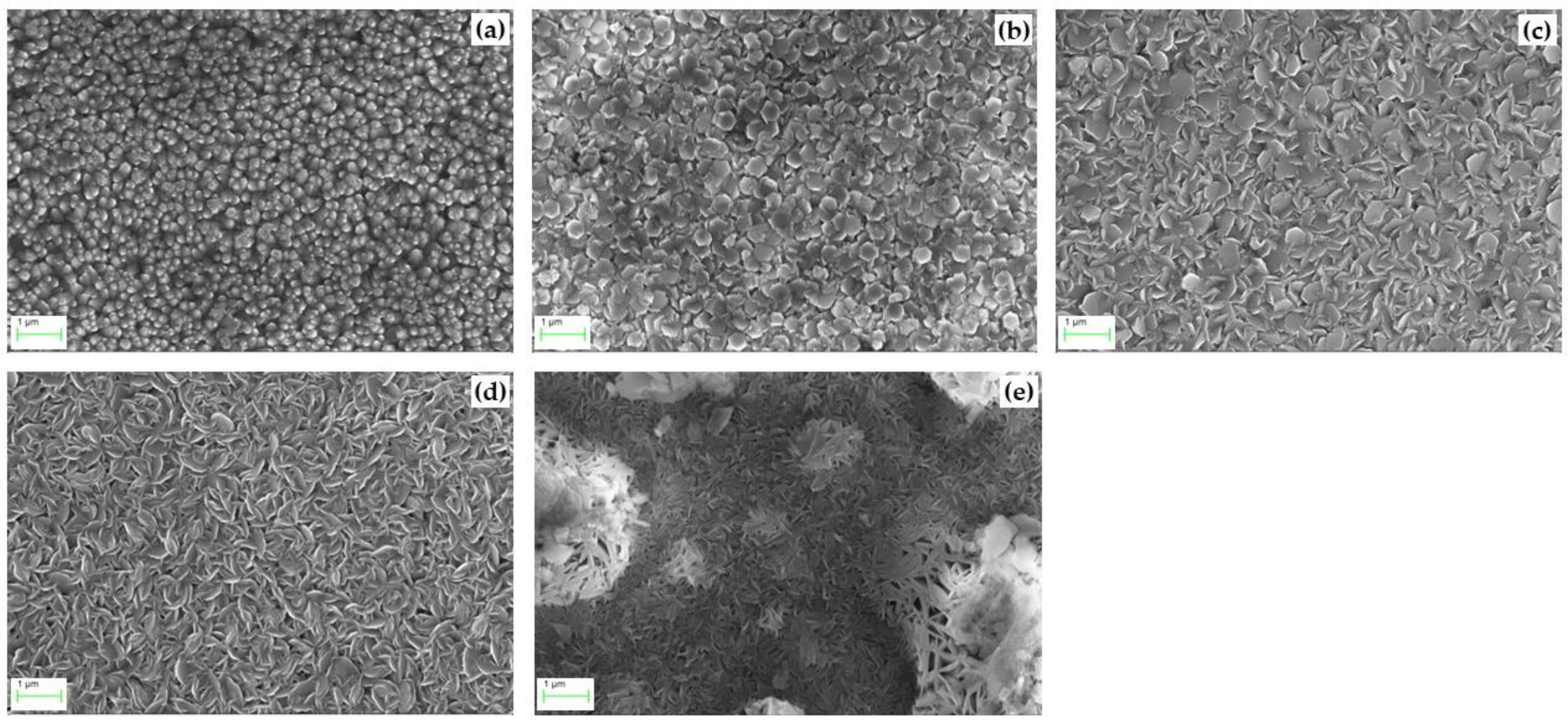
4.4. The Characterization of ZnO
5. Conclusions
- The increasing temperature contributed to ZnO growth below 673 K. It was confirmed that the mass transfer-dominated deposition process was more suitable for high-quality ZnO growth. Where the deposition temperature varied in the range from 633 K to 673 K, the highest deposition rate 3.7 × 10−6 kg/m2·s was obtained with an appropriate homogeneity at 673 K;
- For the flow conditions studied (1290 < Ra < 3000 and 15 < Re < 60), the admixture of transverse and longitudinal rolls was observed in the upstream and downstream of the reactor. The size and location of transverse rolls was found to be almost independent of temperature variations; thus, it had a minor effect on the heat flux and hence on the deposition rate. However, the size of longitudinal rolls had a direct impact on the axial and lateral uniformity of the deposition. As the temperature decreased, the mixed convection rolls in the upstream had the drawback of lower growth rates, but it could enhance the precursors supply near the susceptor;
- The crystallinity of ZnO films was enhanced by the increasing temperature. XRD analysis revealed that the ZnO preferential orientation was (002) at 593 K and 673 K, and the peak intensity increased with increasing temperature. Taking the growth rate and uniformity of the ZnO thin films into consideration, the preferrable deposition temperature is 673 K.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| bi″ | — | stoichiometric coefficients for reactant of species i |
| bi′ | — | stoichiometric coefficients for product of species i |
| cp | J/(kg·K) | specific heat capacity at constant pressure |
| g | m/s2 | gravitational acceleration |
| Gi | mol/m3 | molar concentration of gas-phase species i |
| Ji | mol/s | diffusion flux of species i |
| k | mol/(m3·s) | rate constant of chemical reaction |
| p | Pa | pressure |
| Ri | mol/s | chemical reaction rate of species i |
| SE | — | internal heat source term |
| Si | — | combined source term |
| t | s | time |
| T | K | temperature |
| U | m/s | velocity of gas flow |
| η | — | rate exponent of gaseous species i |
| κ | W/m·K | thermal conductivity |
| ρ | kg/m3 | density of gas flow |
| τ | Pa | viscous stress tensor |
| ωi | — | mass fraction of species i |
| Φ | — | dissipation function |
| CFD | computational fluid dynamics | |
| DEZn | diisopropyltelluride | |
| DR | deposition rate | |
| Gr | Grashof number | |
| M | mean thickness | |
| MOCVD | metal-organic chemical vapour deposition | |
| Ra | Rayleigh number | |
| Re | Renoylds number | |
| SD | standard deviation | |
| SLM | standard litre per minute | |
| SIMPLE | semi-implicit method for pressure-linked equations | |
| t-BuOH | tertiarybutanol | |
| ZnO | zinc oxide |
References
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today: Proc. 2020, 49, 3028–3035. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Y.; Wu, D.; Jiang, G. Preparation and gas sensing properties of ZnO/MXene composite nanomaterials. Sensors Actuators A Phys. 2022, 344, 113740. [Google Scholar] [CrossRef]
- Sulaiman, A.A.; Ali, G.G.; Thanon, A.I. Synthesis and Study of ZnO Thin Films Using CVD Technique For Waveguide Sensor Applications. J. Nanostructures. 2022, 12, 1–11. [Google Scholar]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Sandeep, K.M.; Bhat, S.; Dharmaprakash, S.M. Structural, optical, and LED characteristics of ZnO and Al doped ZnO thin films. J. Phys. Chem. Solids 2017, 104, 36–44. [Google Scholar] [CrossRef]
- Vinitha, V.; Preeyanghaa, M.; Vinesh, V.; Dhanalakshmi, R.; Neppolian, B.; Sivamurugan, V. Two is better than one: Catalytic, sensing and optical applications of doped zinc oxide nanostructures. Emergent Mater. 2021, 4, 1093–1124. [Google Scholar] [CrossRef]
- Müller, R.; Huber, F.; Töws, M.; Mangold, M.; Madel, M.; Scholz, J.-P.; Thonke, K. High-Quality ZnO Layers Grown by CVD on Sapphire Substrates with an AlN Nucleation Layer. Cryst. Growth Des. 2020, 20, 3918–3926. [Google Scholar] [CrossRef]
- Mao, H.; Gu, S.; Ye, J.; Tang, K.; Gu, R.; Zhu, S.; Huang, S.; Yao, Z.; Zheng, Y. Comparative study of the effect of H2 addition on ZnO films grown by different zinc and oxygen precursors. J. Mater. Res. 2015, 30, 935–945. [Google Scholar] [CrossRef]
- Muñoz-Sanjosé, V.; Irvine, S.J.C. ZnO and Related Materials. In Metalorganic Vapor Phase Epitaxy (MOVPE) Growth, Materials Properties, and Applications; Irvine, S.J.C., Capper, P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 355–422. [Google Scholar]
- Tena-Zaera, R.; Zuniga-Perez, J.; Martínez-Tomás, C.; Muñoz-Sanjosé, V. Numerical study of the ZnO growth by MOCVD. J. Cryst. Growth 2004, 264, 237–245. [Google Scholar] [CrossRef]
- Ye, J.; Gu, S.; Zhu, S.; Liu, S.; Liu, W.; Zhou, X.; Hu, L.; Zhang, R.; Shi, Y.; Zheng, Y. Comparative study of diethylzinc and dimethylzinc for the growth of ZnO. J. Cryst. Growth 2004, 274, 489–494. [Google Scholar] [CrossRef]
- Lamb, D.A.; Irvine, S.J.C. Growth properties of thin film ZnO deposited by MOCVD with n-butyl alcohol as the oxygen precursor. J. Cryst. Growth 2004, 273, 111–117. [Google Scholar] [CrossRef]
- Kirchner, C.; Gruber, T.; Reuß, F.; Thonke, K.; Waag, A.; Gießen, C.; Heuken, M. MOVPE growth of ZnO using various oxygen precursors. J. Cryst. Growth 2003, 248, 20–24. [Google Scholar] [CrossRef]
- Oleynik, N.; Adam, M.; Krtschil, A.; Bläsing, J.; Dadgar, A.; Bertram, F.; Forster, D.; Diez, A.; Greiling, A.; Seip, M.; et al. Metalorganic chemical vapor phase deposition of ZnO with different O-precursors. J. Cryst. Growth 2003, 248, 14–19. [Google Scholar] [CrossRef]
- Ellingsen, J.; Venkatachalapathy, V.; Azarov, A.; Nilsen, O.; Kuznetsov, A. Al incorporation during metal organic chemical vapour deposition of aluminium zinc oxide. Thin Solid Films 2020, 709, 138245. [Google Scholar] [CrossRef]
- Narin, P.; Kutlu-Narin., E.; Kayral., S.; Tulek., R.; Gokden., S.; Teke., A.; Lisesivdin., S.B. Morphological and optical characterizations of different ZnO nanostructures grown by mist-CVD. J. Lumin. 2022, 251, 119158. [Google Scholar] [CrossRef]
- Bhutto, W.A.; Soomro, A.M.; Nizamani, A.H.; Saleem, H.; Khaskheli, M.A.; Sahito, A.G.; Das, R.; Khan, U.A.; Saleem, S. Controlled growth of zinc oxide nanowire arrays by chemical vapor deposition (CVD) method. Int. J. Comput. Sci. Netw. Secur. 2019, 19, 135–141. [Google Scholar]
- Wen, T.; Lu, L.; Zhang, S.; Zhong, H. Experimental study and CFD modelling on the thermal and flow behavior of EG/water ZnO nanofluid in multiport mini channels. Appl. Therm. Eng. 2021, 182, 116089. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Yang, X.; Huang, C. Numerical Analysis of an Inline Metal-Organic Chemical Vapour Deposition Process Based on Sliding-Mesh Modelling. Coatings 2020, 10, 1198. [Google Scholar] [CrossRef]
- Shinde, V.M.; Deivendran, B.; Kumar, H.; Prasad, N.E. Investigation of transport processes in a commercial hot wall CVD reactor with multi-substrates for high-quality pyrocarbon deposition. Surf. Coatings Technol. 2021, 425, 127685. [Google Scholar] [CrossRef]
- Li, X.-G.; Xiao, W.-D. Model on transport phenomena and control of rod growth uniformity in siemens CVD reactor. Comput. Chem. Eng. 2018, 117, 351–358. [Google Scholar] [CrossRef]
- Li, J.; Luo, T.; Wang, J.; Wang, G.; Pei, Y.; Deng, J. Design of a new MD-600C ZnO-MOCVD reaction cavity and study of process parameters. Int. Commun. Heat Mass Transf. 2020, 110, 104394. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, H.; Yao, Q.; Yan, H.; Gan, Z. Species transport and chemical reaction in a MOCVD reactor and their influence on the GaN growth uniformity. J. Cryst. Growth 2016, 454, 87–95. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.C.; Luo, T.C.; Li, Y.; Zhou, Z.; Fan, B.F.; Li, J.; Wang, G. Simulation and experimental verification study on the process parameters of ZnO-MOCVD. Ceram. Int. 2021, 47, 15471–15482. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; He, Y.-C.; Luo, T.-C.; Li, Y.; Fan, B.-F.; Wang, G. High-speed flow field prediction and process parameters optimization in a vertical MOCVD reactor based on a hybrid RSM-KNN model. Int. Commun. Heat Mass Transf. 2021, 129, 105741. [Google Scholar] [CrossRef]
- Fauzi, F.B.; Ismail, E.; Osman, M.N.; Rosli, M.S.; Ismail, A.F.; Mohamed, M.A.; Abu Bakar, S.N.S.; Ani, M.H. Influence of mixed convection in atmospheric pressure chemical vapor deposition of graphene growth. Mater. Today Proc. 2019, 7, 638–645. [Google Scholar] [CrossRef]
- Yousefian, P.; Pimputkar, S. Computational fluid dynamics modeling of a new high-pressure chemical vapor deposition reactor design. J. Cryst. Growth 2021, 566, 126155. [Google Scholar] [CrossRef]
- Li, J.; Cai, J.; Wu, Z.; Wang, J.; Pei, Y.; Wang, G. Numerical simulation and study of the metal-organic chemical vapor deposition growth of ZnO film. Phys. Fluids 2019, 31, 027104. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.F.; Ma, X.J.; Fan, B.F.; Wang, G. Stability and process parameter optimization for a vertical rotating ZnO-MOCVD reaction chamber. Vacuum 2018, 149, 60–68. [Google Scholar] [CrossRef]
- Venu Gopal, V.R.; Kamila, S. Effect of temperature on the morphology of ZnO nanoparticles: A comparative study. Appl. Nanosci. 2017, 7, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.F.; Kuo, H.H.; Leu, I.C.; Hon, M.H. The effects of thickness and operation temperature on ZnO: Al thin film CO gas sensor. Sens. Actuators B Chem. 2002, 84, 258–264. [Google Scholar] [CrossRef]
- Dauelsberg, M.; Talalaev, R. Progress in modeling of III-nitride MOVPE. Prog. Cryst. Growth Charact. Mater. 2020, 66, 100486. [Google Scholar] [CrossRef]
- Mehmood, S.; Rehman, M.A.; Ismail, H.; Mirza, B.; Bhatti, A.S. Significance of postgrowth processing of ZnO nanostructures on antibacterial activity against gram-positive and gram-negative bacteria. Int. J. Nanomed. 2015, 10, 4521. [Google Scholar] [CrossRef]




| General | Boundary Condition | Parameters |
|---|---|---|
| Operating temperature | - | 300 K |
| Operating pressure | - | 975 mbar |
| Inlet | Velocity inlet | 4 slm; II/VI = 0.33 |
| Outlet | Pressure outlet | p = 0 (gauge) |
| Susceptor | No-slip–stationary wall with chemical reaction | 593 to 713 K |
| Walls | No-slip | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wu, R.; Zhou, X.; Wang, H.; Hu, Y.; Nie, D.; Bao, D. Numerical Modelling on the Effect of Temperature on MOCVD Growth of ZnO Using Diethylzinc and Tertiarybutanol. Coatings 2022, 12, 1991. https://doi.org/10.3390/coatings12121991
Wu Y, Wu R, Zhou X, Wang H, Hu Y, Nie D, Bao D. Numerical Modelling on the Effect of Temperature on MOCVD Growth of ZnO Using Diethylzinc and Tertiarybutanol. Coatings. 2022; 12(12):1991. https://doi.org/10.3390/coatings12121991
Chicago/Turabian StyleWu, Yiyi, Rui Wu, Xiaosong Zhou, Hongli Wang, Yang Hu, Dengpan Nie, and Dongmei Bao. 2022. "Numerical Modelling on the Effect of Temperature on MOCVD Growth of ZnO Using Diethylzinc and Tertiarybutanol" Coatings 12, no. 12: 1991. https://doi.org/10.3390/coatings12121991
APA StyleWu, Y., Wu, R., Zhou, X., Wang, H., Hu, Y., Nie, D., & Bao, D. (2022). Numerical Modelling on the Effect of Temperature on MOCVD Growth of ZnO Using Diethylzinc and Tertiarybutanol. Coatings, 12(12), 1991. https://doi.org/10.3390/coatings12121991





