A Stable Aqueous SnO2 Nanoparticle Dispersion for Roll-to-Roll Fabrication of Flexible Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental
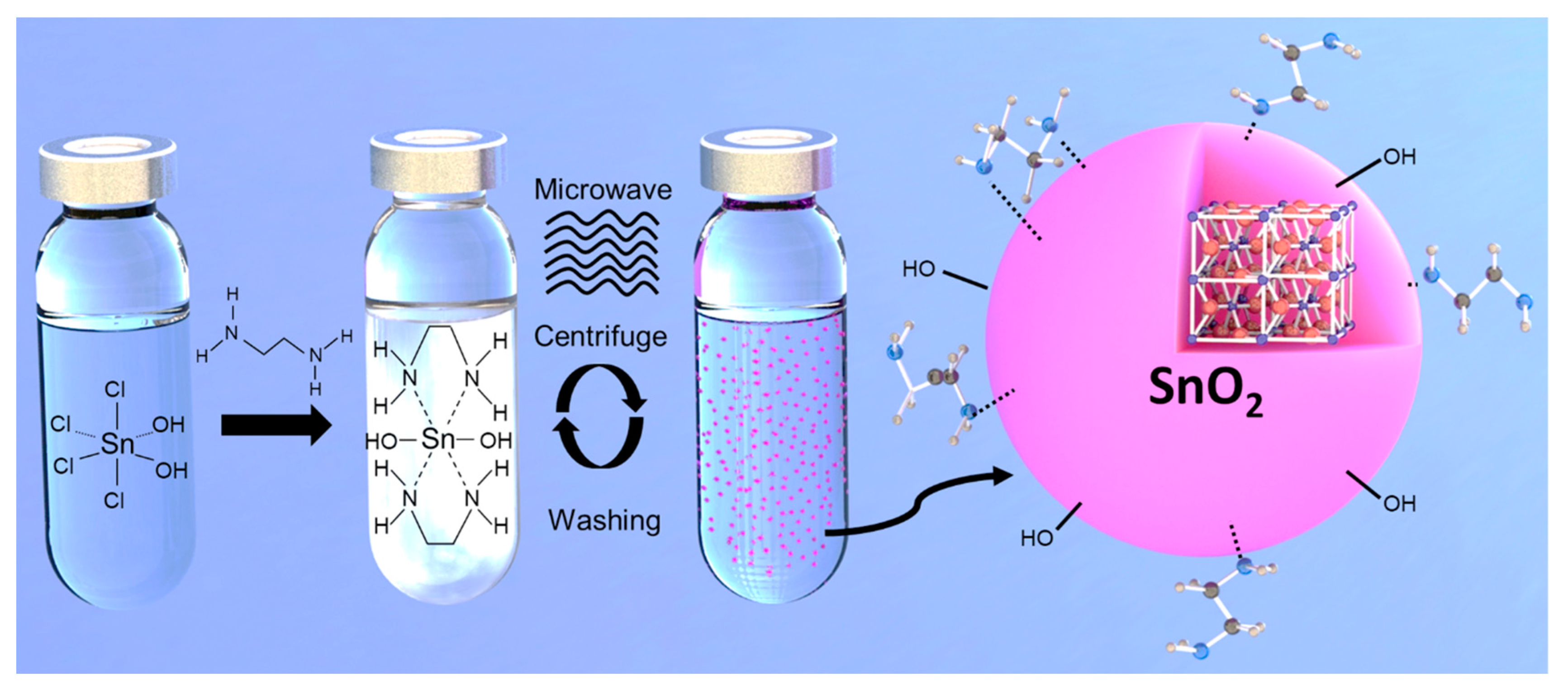
2.1. SnO2 Nanoparticle Dispersion
2.2. Perovskite Solar Cell Fabrication
2.2.1. Glass Device Fabrication
2.2.2. Flexible Device Fabrication
3. Characterization
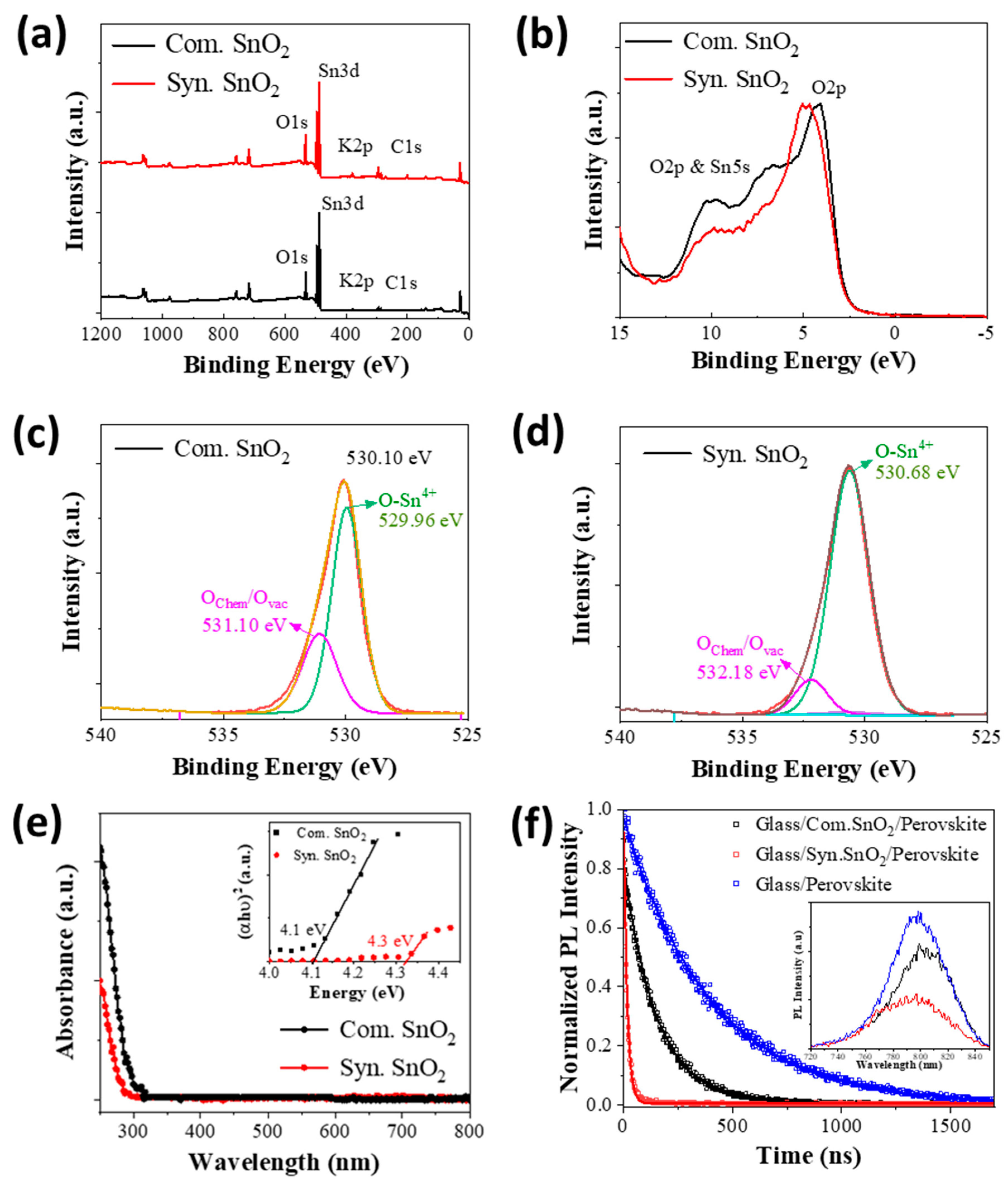
3.1. Physical Characterization
3.2. Optical Spectroscopy
3.3. Electronic Properties
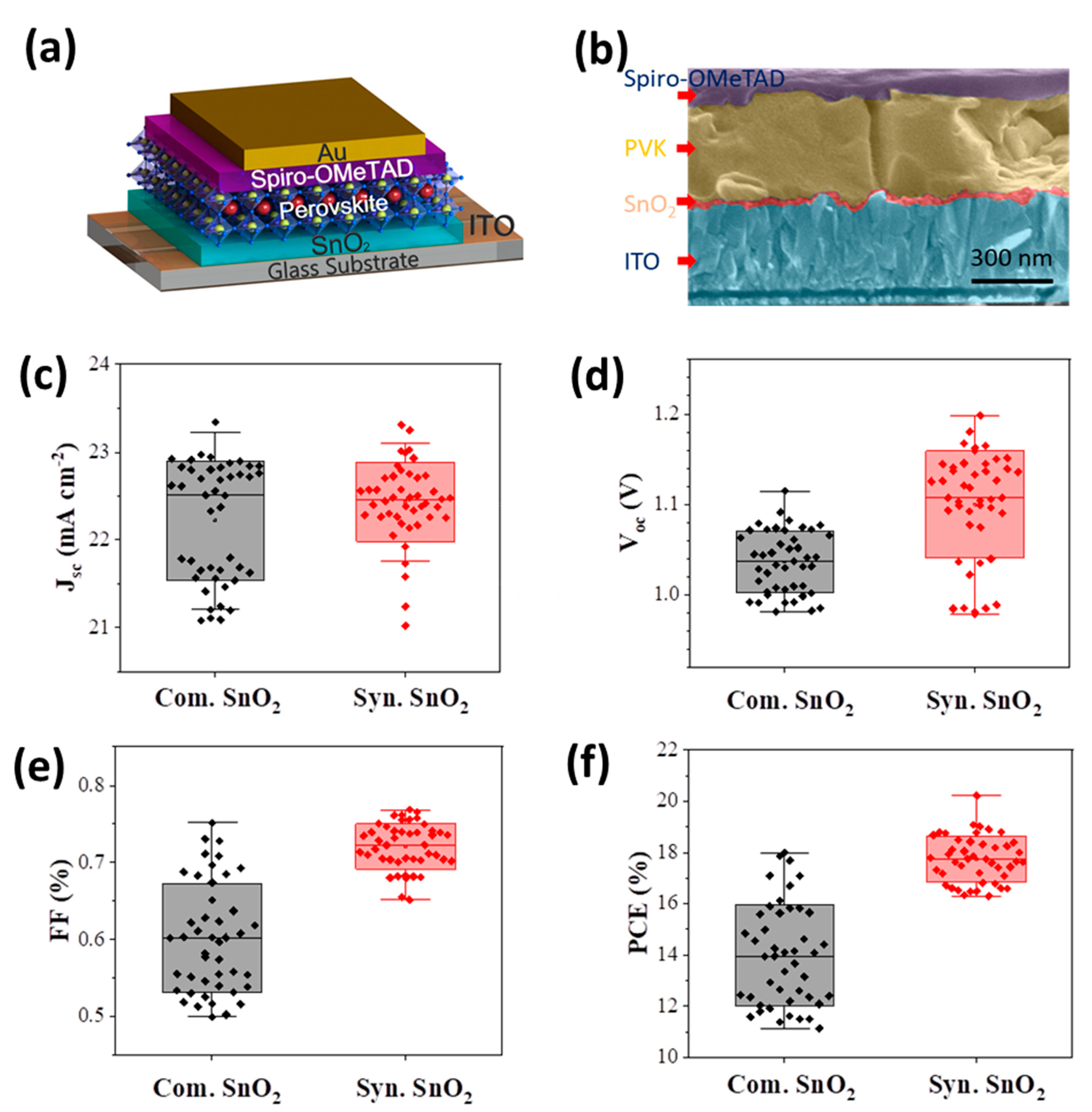
4. Results and Discussion
Flexible Devices Comprising a R2R-Deposited SnO2 ETL
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| PSCs | perovskite solar cells |
| R2R | roll-to-roll |
| PCE | power conversion efficiency |
| PV | photovoltaic |
| ETL | electron transporting layers |
| PET | polyethylene terephthalate |
| TiO2 | titanium dioxide |
| SnO2 | tin oxide |
| EDA | ethylene diamine |
| rpm | rotations per minute |
| PET | polyethylene terephthalate |
| ITO | tin doped indium oxide |
| TCO | transparent conducting oxide |
| HTL | hole transporting layer |
| TGA | thermogravimetric analysis |
| TEM | transmission electron microscopic |
| SEM | scanning electron microscopy |
| XRD | X-ray diffraction |
| AFM | atomic force microscope |
| XPS | X-ray photoelectron spectroscopy |
| UV-Vis | ultraviolet-Visible |
| FTIR | fourier-transform infrared |
| J-V | current density-voltage |
| IPCE | incident photon-to-electron conversion efficiency |
| PL | photoluminescence |
| SAED | selected area electron diffraction |
| VOC | open circuit voltage |
| FF | fill-factor |
| Jsc | short-circuit current density |
| MPP | maximum power point |
| RG | reverse-gravure |
References
- Extance, A. The Reality behind Solar Power’s next Star Material. Nature 2019, 570, 429–432. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, S.A.; Ramakrishna, S.; Aberle, A.G. Recent Progress in Flexible-Wearable Solar Cells for Self-Powered Electronic Devices. Energy Environ. Sci. 2020, 13, 685–743. [Google Scholar] [CrossRef]
- Tang, G.; Yan, F. Recent Progress of Flexible Perovskite Solar Cells. Nano Today 2021, 39, 101155. [Google Scholar] [CrossRef]
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J.; et al. The Main Progress of Perovskite Solar Cells in 2020–2021. Nano-Micro Lett. 2021, 13, 152. [Google Scholar] [CrossRef]
- Sutherland, L.J.; Weerasinghe, H.C.; Simon, G.P. A Review on Emerging Barrier Materials and Encapsulation Strategies for Flexible Perovskite and Organic Photovoltaics. Adv. Energy Mater. 2021, 11, 2101383. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Liu, J.; Cai, H. Review of Interface Passivation of Perovskite Layer. Nanomaterials 2021, 11, 775. [Google Scholar] [CrossRef]
- Le Corre, V.M.; Stolterfoht, M.; Perdigón Toro, L.; Feuerstein, M.; Wolff, C.; Gil-Escrig, L.; Bolink, H.J.; Neher, D.; Koster, L.J.A. Charge Transport Layers Limiting the Efficiency of Perovskite Solar Cells: How to Optimize Conductivity, Doping, and Thickness. ACS Appl. Energy Mater. 2019, 2, 6280–6287. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Chu, C.W.; Ng, A. Perspective on Predominant Metal Oxide Charge Transporting Materials for High-Performance Perovskite Solar Cells. Front. Mater. 2021, 8, 655207. [Google Scholar] [CrossRef]
- Jana, S. Advances in Nanoscale Alloys and Intermetallics: Low Temperature Solution Chemistry Synthesis and Application in Catalysis. Dalt. Trans. 2015, 44, 18692–18717. [Google Scholar] [CrossRef]
- Temple, T.L.; Bagnall, D.M. Optical Properties of Gold and Aluminium Nanoparticles for Silicon Solar Cell Applications. J. Appl. Phys. 2011, 109, 084343. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Atapattu, H.R.; Zhao, Q.; Gao, Y.; Finkenauer, B.P.; Wang, K.; Chen, K.; Park, S.M.; Coffey, A.H.; Zhu, C.; et al. Multifunctional Conjugated Ligand Engineering for Stable and Efficient Perovskite Solar Cells. Adv. Mater. 2021, 33, 2100791. [Google Scholar] [CrossRef]
- Tahir, A.A.; Peiris, T.A.N.; Wijayantha, K.G.U. Enhancement of Photoelectrochemical Performance of AACVD-Produced TiO2 Electrodes by Microwave Irradiation While Preserving the Nanostructure. Chem. Vap. Depos. 2012, 18, 107–111. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Sakuma, T.; Kaneko, T.; Tomita, K.; Isomura, M.; Taima, T.; Umezu, S.; Iwamori, S. Oblique Electrostatic Inkjet-Deposited TiO2 Electron Transport Layers for Efficient Planar Perovskite Solar Cells. Sci. Rep. 2019, 9, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, X.; Feng, X.; Zhao, X.; Fang, J. A Conjugated Ligand Interfacial Modifier for Enhancing Efficiency and Operational Stability of Planar Perovskite Solar Cells. Chem. Eng. J. 2021, 412, 128680. [Google Scholar] [CrossRef]
- Naz, S.; Javid, I.; Konwar, S.; Surana, K.; Singh, P.K.; Sahni, M.; Bhattacharya, B. A Simple Low Cost Method for Synthesis of SnO2 Nanoparticles and Its Characterization. SN Appl. Sci. 2020, 2, 975. [Google Scholar] [CrossRef]
- Aziz, M.; Saber Abbas, S.; Wan Baharom, W.R. Size-Controlled Synthesis of SnO2 Nanoparticles by Sol-Gel Method. Mater. Lett. 2013, 91, 31–34. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhou, X.; Gao, J.; Chen, W.; Wang, X.; Xu, B. Hydrothermally Treated SnO2 as the Electron Transport Layer in High-Efficiency Flexible Perovskite Solar Cells with a Certificated Efficiency of 17.3%. Adv. Funct. Mater. 2019, 29, 1807604. [Google Scholar] [CrossRef]
- Xie, H.; Yin, X.; Chen, P.; Liu, J.; Yang, C.; Que, W.; Wang, G. Solvothermal Synthesis of Highly Crystalline SnO2 Nanoparticles for Flexible Perovskite Solar Cells Application. Mater. Lett. 2019, 234, 311–314. [Google Scholar] [CrossRef]
- Eliwi, A.A.; Malekshahi Byranvand, M.; Fassl, P.; Khan, M.R.; Hossain, I.M.; Frericks, M.; Ternes, S.; Abzieher, T.; Schwenzer, J.A.; Mayer, T.; et al. Optimization of SnO2 Electron Transport Layer for Efficient Planar Perovskite Solar Cells with Very Low Hysteresis. Mater. Adv. 2022, 3, 456–466. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Lowerature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef]
- Song, K.; Zou, X.; Zhang, H.; Zhang, C.; Cheng, J.; Liu, B.; Yao, Y.; Wang, X.; Li, X.; Wang, Y.; et al. Effect of SnO2 Colloidal Dispersion Solution Concentration on the Quality of Perovskite Layer of Solar Cells. Coatings 2021, 11, 591. [Google Scholar] [CrossRef]
- Luan, Y.; Yi, X.; Mao, P.; Wei, Y.; Zhuang, J.; Chen, N.; Lin, T.; Li, C.; Wang, J. High-Performance Planar Perovskite Solar Cells with Negligible Hysteresis Using 2,2,2-Trifluoroethanol-Incorporated SnO2. iScience 2019, 16, 433–441. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Jayathilake, D.S.Y.; Peiris, T.A.N.; Sagu, J.S.; Potter, D.B.; Wijayantha, K.G.U.; Carmalt, C.J.; Southee, D.J. Microwave-Assisted Synthesis and Processing of Al-Doped, Ga-Doped, and Al, Ga Codoped ZnO for the Pursuit of Optimal Conductivity for Transparent Conducting Film Fabrication. ACS Sustain. Chem. Eng. 2017, 5, 4820–4829. [Google Scholar] [CrossRef] [Green Version]
- Ghanizadeh, S.; Peiris, T.A.N.; Jayathilake, D.S.Y.; Hutt, D.A.; Wijayantha, K.G.U.; Southee, D.J.; Conway, P.P.; Marchand, P.; Darr, J.A.; Parkin, I.P.; et al. Dispersion and Microwave Processing of Nano-Sized ITO Powder for the Fabrication of Transparent Conductive Oxides. Ceram. Int. 2016, 42, 18296–18302. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, I.A.; Nirmal Peiris, T.A.; Smith, T.D.; Upul Wijayantha, K.G. Hierarchical ZnO Nanorod Electrodes: Effect of Post Annealing on Structural and Photoelectrochemical Performance. Mater. Lett. 2013, 93, 333–336. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y. Microwave Solvothermal Synthesis of Flower-like SnS2 and SnO2 Nanostructures as High-Rate Anodes for Lithium Ion Batteries. Chem. Eng. J. 2013, 229, 183–189. [Google Scholar] [CrossRef]
- Krishna, M.; Komarneni, S. Conventional- vs Microwave-Hydrothermal Synthesis of Tin Oxide, SnO2 Nanoparticles. Ceram. Int. 2009, 35, 3375–3379. [Google Scholar] [CrossRef]
- Shi, S.; Hwang, J.-Y. Microwave-Assisted Wet Chemical Synthesis: Advantages, Significance, and Steps to Industrialization. J. Miner. Mater. Charact. Eng. 2003, 2, 101–110. [Google Scholar] [CrossRef]
- Peiris, T.A.N.; Weerasinghe, H.C.; Sharma, M.; Kim, J.-E.; Michalska, M.; Chandrasekaran, N.; Senevirathna, D.C.; Li, H.; Chesman, A.S.R.; Vak, D.; et al. Non-Aqueous One-Pot SnO2 Nanoparticle Inks and Their Use in Printable Perovskite Solar Cells. Chem. Mater. 2022, 34, 5535–5545. [Google Scholar] [CrossRef]
- Sutherland, L.J.; Vak, D.; Gao, M.; Peiris, T.A.N.; Jasieniak, J.; Simon, G.P.; Weerasinghe, H. Vacuum-Free and Solvent-Free Deposition of Electrodes for Roll-to-Roll Fabricated Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2202142. [Google Scholar] [CrossRef]
- Vak, D.; Weerasinghe, H.; Ramamurthy, J.; Subbiah, J.; Brown, M.; Jones, D.J. Reverse Gravure Coating for Roll-to-Roll Production of Organic Photovoltaics. Sol. Energy Mater. Sol. Cells 2016, 149, 154–161. [Google Scholar] [CrossRef]
- Vatanparast, M.; Taghizadeh, M.T. One-Step Hydrothermal Synthesis of Tin Dioxide Nanoparticles and Its Photocatalytic Degradation of Methylene Blue. J. Mater. Sci. Mater. Electron. 2016, 27, 54–63. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yang, X. Size-Controlled Synthesis and Characterization of Quantum-Size SnO2 Nanocrystallites by a Solvothermal Route. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 312, 219–225. [Google Scholar] [CrossRef]
- Rac, O.; Suchorska-Wozniak, P.; Fiedot, M.; Teterycz, H. Influence of Stabilising Agents and PH on the Size of SnO2 Nanoparticles. Beilstein J. Nanotechnol. 2014, 5, 2192–2201. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Wang, H.; Wang, Z.; Jiang, Y.; Cao, D.; Yang, G. Room-Temperature Synthesis of Colloidal SnO2 Quantum Dot Solution and Ex-Situ Deposition on Carbon Nanotubes as Anode Materials for Lithium Ion Batteries. J. Alloys Compd. 2016, 680, 109–115. [Google Scholar] [CrossRef] [Green Version]
- IR Spectroscopy Tutorial: Amines. Available online: https://www.orgchemboulder.com/Spectroscopy/irtutor/aminesir.shtml (accessed on 13 May 2022).
- Dontsova, T.A.; Nagirnyak, S.V.; Zhorov, V.V.; Yasiievych, Y.V. SnO2 Nanostructures: Effect of Processing Parameters on Their Structural and Functional Properties. Nanoscale Res. Lett. 2017, 12, 332. [Google Scholar] [CrossRef] [Green Version]
- Beedasy, V.; Smith, P.J. Printed Electronics as Prepared by Inkjet Printing. Materials 2020, 13, 704. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R.J. NIST Standard Reference Database 20- Version 3.4. Available online: https://srdata.nist.gov/xps/ (accessed on 25 January 2022).
- Thermo Scientific Thermo Scientific XPS: Knowledge Base. Available online: http://xpssimplified.com/elements/tin.php (accessed on 16 July 2022).
- Flak, D.; Braun, A.; Mun, B.S.; Park, J.B.; Parlinska-Wojtan, M.; Graule, T.; Rekas, M. Spectroscopic Assessment of the Role of Hydrogen in Surface Defects, in the Electronic Structure and Transport Properties of TiO2, ZnO and SnO2 Nanoparticles. Phys. Chem. Chem. Phys. 2013, 15, 1417–1430. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Passacantando, M.; Santucci, S.; Czempik, G.; Szuber, J. XPS Study of the Surface Chemistry of L-CVD SnO2 Thin Films after Oxidation. Thin Solid Films 2005, 490, 36–42. [Google Scholar] [CrossRef]
- Mullins, D.R.; Overbury, S.H.; Huntley, D.R. Electron Spectroscopy of Single Crystal and Polycrystalline Cerium Oxide Surfaces. Surf. Sci. 1998, 409, 307–319. [Google Scholar] [CrossRef]
- Cumpson, P.J. Angle-Resolved XPS and AES: Depth-Resolution Limits and a General Comparison of Properties of Depth-Profile Reconstruction Methods. J. Electron Spectrosc. Relat. Phenom. 1995, 73, 25–52. [Google Scholar] [CrossRef]
- Shah, L.R.; Ali, B.; Zhu, H.; Wang, W.G.; Song, Y.Q.; Zhang, H.W.; Shah, S.I.; Xiao, J.Q. Detailed Study on the Role of Oxygen Vacancies in Structural, Magnetic and Transport Behavior of Magnetic Insulator: Co-CeO2. J. Phys. Condens. Matter 2009, 21, 486004. [Google Scholar] [CrossRef] [Green Version]
- Naeem, M.; Hasanain, S.K.; Kobayashi, M.; Ishida, Y.; Fujimori, A.; Buzby, S.; Shah, S.I. Effect of Reducing Atmosphere on the Magnetism of Zn1-XCo XO (0 ≤ x ≤ 0.10) Nanoparticles. Nanotechnology 2006, 17, 2675–2680. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Cai, H.; Wu, Y.; Xing, Z.; Li, Z.; Xu, J.; Huang, L.; Ni, J.; Li, J.; Zhang, J. Enhanced Planar Perovskite Solar Cells with Efficiency Exceeding 16% via Reducing the Oxygen Vacancy Defect State in Titanium Oxide Electrode. Phys. Chem. Chem. Phys. 2017, 19, 13679–13686. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Liu, X.; Wang, S.; Yan, Z.; Chen, L.; Li, G.; Zhang, X.; Sun, W.; Lan, Z. High-Effective SnO2-Based Perovskite Solar Cells by Multifunctional Molecular Additive Engineering. J. Alloys Compd. 2021, 886, 161352. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A Comprehensive Review on Structures and Gas Sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Hamad, D.; Abdel-Latief, A.Y.; Abdel-Rahim, M.A. Preparation of Quantum Size of Tin Oxide: Structural and Physical Characterization. Prog. Nat. Sci. Mater. Int. 2016, 26, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Chen, S.; Wu, J.; Zhang, H.; Qin, M.; Lu, X.; Tu, Y.; Meng, Q.; Zhan, X. Fullerene Derivative Anchored SnO2 for High-Performance Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 3463–3471. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, M.; Yang, Z.; Qiu, H.; Kou, S.; Padhiar, M.A.; Bhatti, A.S.; Gaponenko, N.V. Pressure-Driven Transformation of CsPbBrI2Nanoparticles into Stable Nanosheets in Solution through Self-Assembly. J. Phys. Chem. Lett. 2020, 11, 9862–9868. [Google Scholar] [CrossRef]
- Xi, H.; Tang, S.; Ma, X.; Chang, J.; Chen, D.; Lin, Z.; Zhong, P.; Wang, H.; Zhang, C. Performance Enhancement of Planar Heterojunction Perovskite Solar Cells through Tuning the Doping Properties of Hole-Transporting Materials. ACS Omega 2017, 2, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Mohamad Noh, M.F.; Arzaee, N.A.; Safaei, J.; Mohamed, N.A.; Kim, H.P.; Mohd Yusoff, A.R.; Jang, J.; Mat Teridi, M.A. Eliminating Oxygen Vacancies in SnO2 Films via Aerosol-Assisted Chemical Vapour Deposition for Perovskite Solar Cells and Photoelectrochemical Cells. J. Alloys Compd. 2019, 773, 997–1008. [Google Scholar] [CrossRef]
- Ho, Y.C.; Hoque, M.N.F.; Stoneham, E.; Warzywoda, J.; Dallas, T.; Fan, Z. Reduction of Oxygen Vacancy Related Traps in TiO2 and the Impacts on Hybrid Perovskite Solar Cells. J. Phys. Chem. C 2017, 121, 23939–23946. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Caprioglio, P.; Wolff, C.M.; Márquez, J.A.; Nordmann, J.; Zhang, S.; Rothhardt, D.; Hörmann, U.; Amir, Y.; Redinger, A.; et al. The Impact of Energy Alignment and Interfacial Recombination on the Internal and External Open-Circuit Voltage of Perovskite Solar Cells. Energy Environ. Sci. 2019, 12, 2778–2788. [Google Scholar] [CrossRef] [Green Version]
- Keshtmand, R.; Zamani-Meymian, M.R.; Taghavinia, N. Improving the Performance of Planar Perovskite Solar Cell Using NH4Cl Treatment of SnO2 as Electron Transport Layer. Surf. Interfaces 2022, 28, 101596. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Wang, K.; Wu, C.; Zhu, X.; Feng, J.; Ren, X.; Fang, G.; Priya, S.; Liu, S. (Frank) High Efficiency Planar-Type Perovskite Solar Cells with Negligible Hysteresis Using EDTA-Complexed SnO2. Nat. Commun. 2018, 9, 3239. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Guo, F.; Wang, X.; Xu, K.; Lei, M.; Liang, Y.; Zhao, Y.; Xu, D. SnO2-in-Polymer Matrix for High-Efficiency Perovskite Solar Cells with Improved Reproducibility and Stability. Adv. Mater. 2018, 30, 1805153. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X.; Shen, J.; Wang, H.; Xue, J.; Su, C.; Guo, H.; Fu, G.; Kong, W.; Yang, S. Functionalized SnO2 Films by Using EDTA-2M for High Efficiency Perovskite Solar Cells with Efficiency over 23%. Chem. Eng. J. 2022, 430, 132683. [Google Scholar] [CrossRef]
- Song, P.; Shen, L.; Zheng, L.; Liu, K.; Tian, W.; Chen, J.; Luo, Y.; Tian, C.; Xie, L.; Wei, Z. Double-layered SnO2/NH4Cl-SnO2 for Efficient Planar Perovskite Solar Cells with Improved Operational Stability. Nano Sel. 2021, 2, 1779–1787. [Google Scholar] [CrossRef]
- Weerasinghe, H.C.; Rolston, N.; Vak, D.; Scully, A.D.; Dauskardt, R.H. A Stability Study of Roll-to-Roll Processed Organic Photovoltaic Modules Containing a Polymeric Electron-Selective Layer. Sol. Energy Mater. Sol. Cells 2016, 152, 133–140. [Google Scholar] [CrossRef]
- Jung, Y.S.; Hwang, K.; Heo, Y.J.; Kim, J.E.; Vak, D.; Kim, D.Y. Progress in Scalable Coating and Roll-to-Roll Compatible Printing Processes of Perovskite Solar Cells toward Realization of Commercialization. Adv. Opt. Mater. 2018, 6, 1701182. [Google Scholar] [CrossRef]

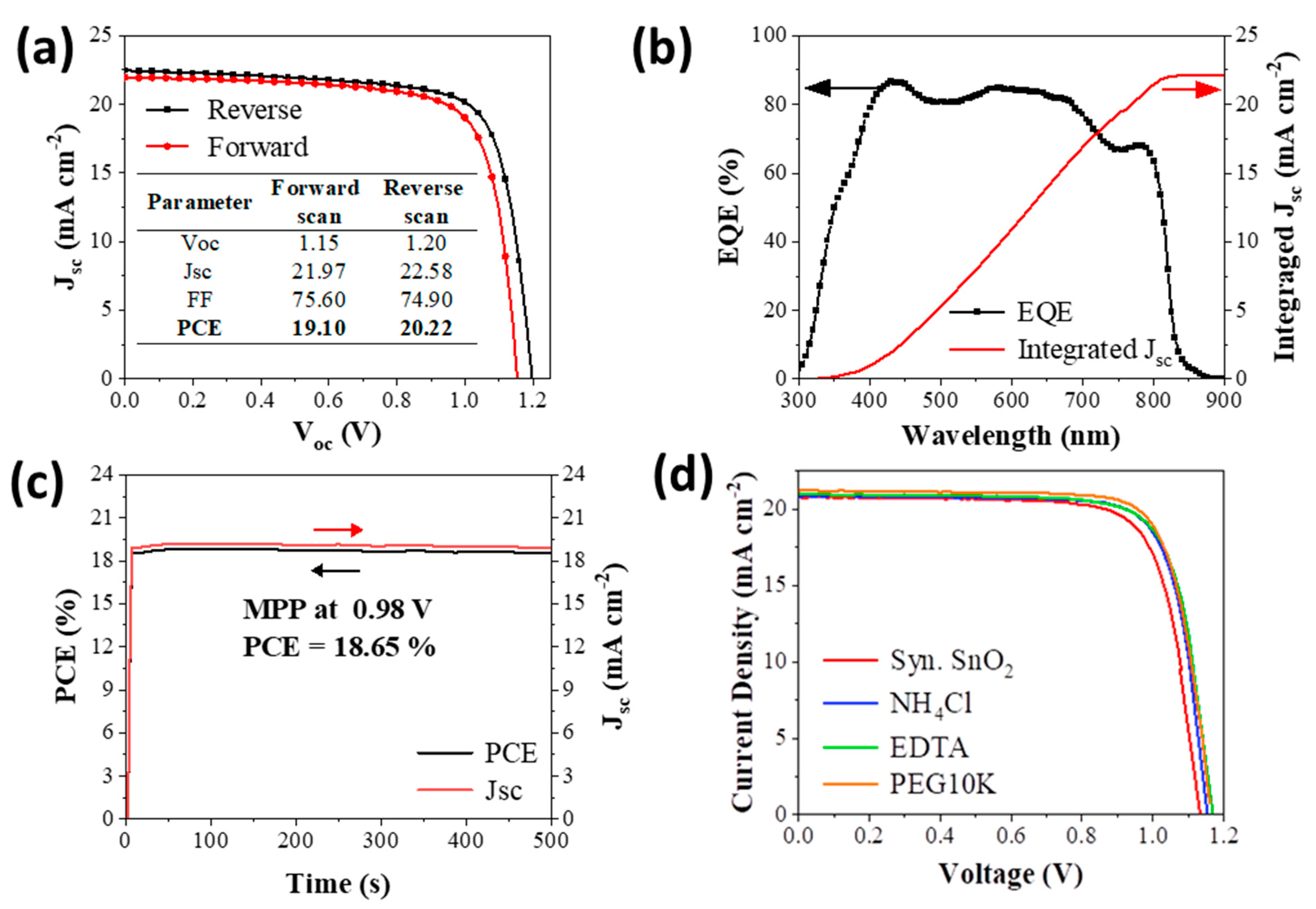

| Vo/V | Jsc/mA cm−2 | FF | PCE/% | ||
|---|---|---|---|---|---|
| SnO2 | FWD | 1.12 (1.12 ± 0.01) | 20.83 (20.78 ± 0.11) | 0.74 (0.74 ± 0.01) | 17.26 (17.22 ± 0.15) |
| REV | 1.13 (1.13 ± 0.01) | 20.88 (20.73 ± 0.17) | 0.76 (0.76 ± 0.01) | 17.93 (17.71 ± 0.14) | |
| NH4Cl- 1 ppm | FWD | 1.14 (1.14 ± 0) | 20.90 (20.82 ± 0.09) | 0.76 (0.75 ± 0.01) | 18.11 (17.92 ± 0.15) |
| REV | 1.15 (1.15 ± 0.01) | 20.85 (20.79 ± 0.06) | 0.78 (0.78 ± 0.01) | 18.70 (18.52 ± 0.17) | |
| EDTA- 0.1 ppm | FWD | 1.16 (1.16 ± 0) | 21.01 (20.86 ± 0.22) | 0.73 (0.73 ± 0.01) | 17.79 (17.69 ± 0.07) |
| REV | 1.16 (1.16 ± 0.01) | 21.02 (20.85 ± 0.15) | 0.77 (0.76 ± 0.01) | 18.78 (18.51 ± 0.19) | |
| PEG10K- 1 ppm | FWD | 1.15 (1.15 ± 0.01) | 21.21 (20.96 ± 0.24) | 0.77 (0.76 ± 0.01) | 18.79 (18.65 ± 0.14) |
| REV | 1.16 (1.16 ± 0) | 21.68 (21.50 ± 0.22) | 0.77 (0.77 ± 0.01) | 19.45 (19.18 ± 0.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peiris, T.A.N.; Benitez, J.; Sutherland, L.; Sharma, M.; Michalska, M.; Scully, A.D.; Vak, D.; Gao, M.; Weerasinghe, H.C.; Jasieniak, J. A Stable Aqueous SnO2 Nanoparticle Dispersion for Roll-to-Roll Fabrication of Flexible Perovskite Solar Cells. Coatings 2022, 12, 1948. https://doi.org/10.3390/coatings12121948
Peiris TAN, Benitez J, Sutherland L, Sharma M, Michalska M, Scully AD, Vak D, Gao M, Weerasinghe HC, Jasieniak J. A Stable Aqueous SnO2 Nanoparticle Dispersion for Roll-to-Roll Fabrication of Flexible Perovskite Solar Cells. Coatings. 2022; 12(12):1948. https://doi.org/10.3390/coatings12121948
Chicago/Turabian StylePeiris, T. A. Nirmal, Juan Benitez, Luke Sutherland, Manoj Sharma, Monika Michalska, Andrew D. Scully, Doojin Vak, Mei Gao, Hasitha C. Weerasinghe, and Jacek Jasieniak. 2022. "A Stable Aqueous SnO2 Nanoparticle Dispersion for Roll-to-Roll Fabrication of Flexible Perovskite Solar Cells" Coatings 12, no. 12: 1948. https://doi.org/10.3390/coatings12121948
APA StylePeiris, T. A. N., Benitez, J., Sutherland, L., Sharma, M., Michalska, M., Scully, A. D., Vak, D., Gao, M., Weerasinghe, H. C., & Jasieniak, J. (2022). A Stable Aqueous SnO2 Nanoparticle Dispersion for Roll-to-Roll Fabrication of Flexible Perovskite Solar Cells. Coatings, 12(12), 1948. https://doi.org/10.3390/coatings12121948








