Thin Layers of SrTiO3-TiO2 with Eutectic Composition for Photoelectrochemical Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Magnetron Sputtering
2.2. Material Characterization
2.3. Photoelectrochemical (PEC) Analysis
3. Results and Discussion
3.1. Layers of SrTiO3-TiO2-Eutectic Composition
3.2. Material Characterization
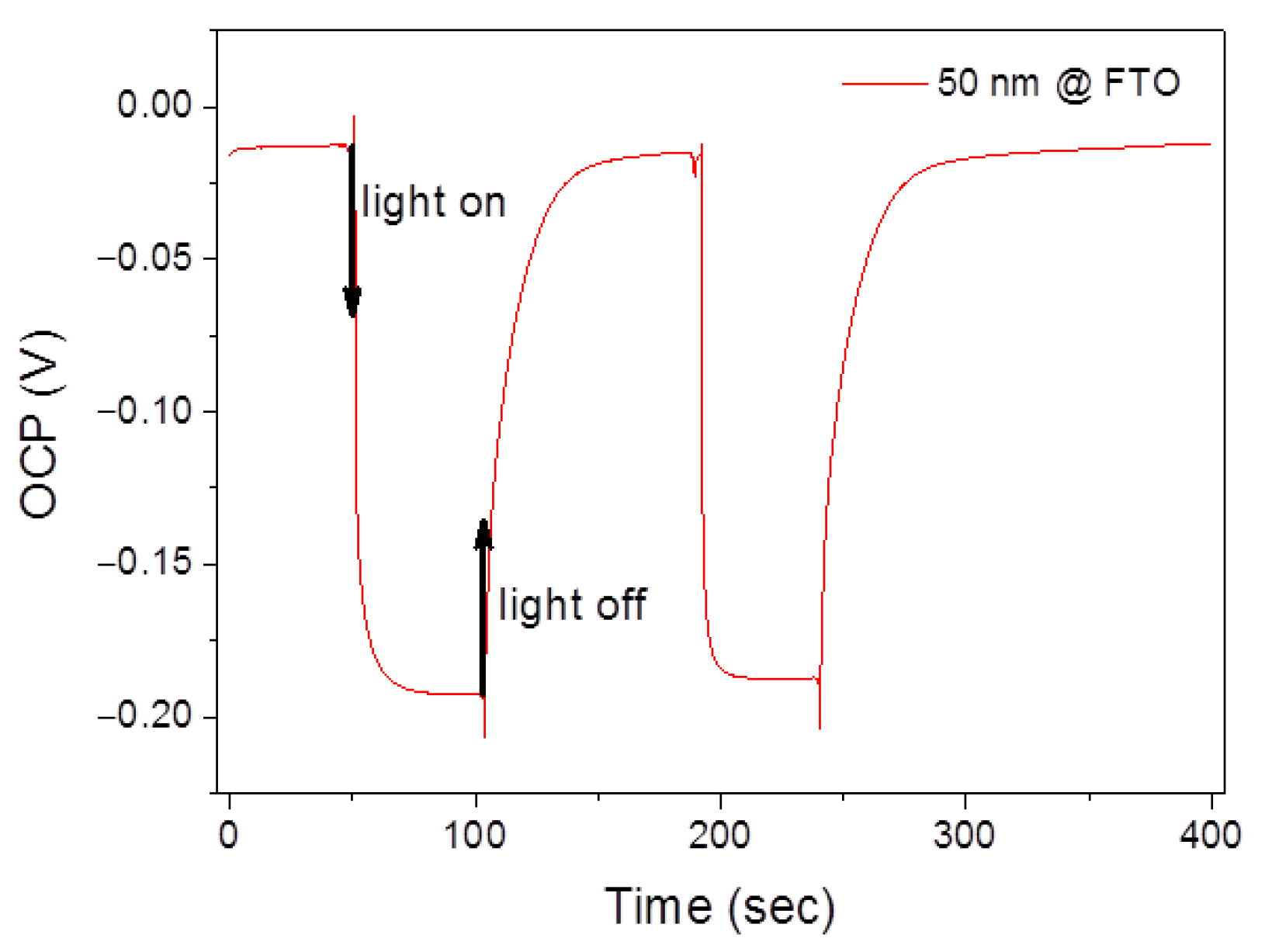
3.3. Photoelectrochemical (PEC) Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osterloh, F.E. Inorganic Materials as Catalysts for Photochemical Splitting of Water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Rajeshwar, K. Fundamentals of Semiconductor Electrochemistry and Photoelectrochemistry; Encyclopedia of Electrochemistry; Viley-VCH Verlag: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Currao, A. Photoelectrochemical Water Splitting. CHIMIA 2007, 61, 815–819. [Google Scholar] [CrossRef]
- Shao, J.; Wang, X.-T.; Xu, H.; Zhao, X.-D.; Niu, J.-M.; Zhang, Z.-D.; Huang, Y.-L.; Duan, J.-Z. Photoelectrochemical Performance of SnS2 Sensitized TiO2 Nanotube for Protection of 304 Stainless Steel. J. Electrochem. Soc. 2021, 168, 016511. [Google Scholar] [CrossRef]
- Llorca-Martínez, F.J.; Orera, V. Directionally solidified eutectic ceramic oxides. Prog. Mater. Sci. 2006, 51, 711–809. [Google Scholar] [CrossRef]
- Pawlak, D.A.; Kolodziejak, K.; Diduszko, R.; Rozniatowski, K.; Kaczkan, M.; Malinowski, M.; Kisielewski, J.; Lukasiewicz, T. The PrAlO3−Pr2O3 Eutectic, its Microstructure, Instability, and Luminescent Properties. Chem. Mater. 2007, 19, 2195–2202. [Google Scholar] [CrossRef]
- Pawlak, D.A.; Turczynski, S.; Gajc, M.; Kolodziejak, K.; Diduszko, R.; Rozniatowski, K.; Smalc, J.; Vendik, I. How Far Are We from Making Metamaterials by Self-Organization? The Microstructure of Highly Anisotropic Particles with an SRR-Like Geometry. Adv. Funct. Mater. 2010, 20, 1116–1124. [Google Scholar] [CrossRef]
- Gajc, M.; Surma, H.B.; Klos, A.; Sadecka, K.; Orlinski, K.; Nikolaenko, A.E.; Zdunek, K.; Pawlak, D.A. Nanoparticle Direct Doping: Novel Method for Manufacturing Three-Dimensional Bulk Plasmonic Nanocomposites. Adv. Funct. Mater. 2013, 23, 3443–3451. [Google Scholar] [CrossRef]
- Deska, R.; Sadecka, K.; Olesiak-Bańska, J.; Matczyszyn, K.; Pawlak, D.A.; Samoć, M. Nonlinear plasmonics in eutectic composites: Second harmonic generation and two-photon luminescence in a volumetric Bi2O3-Ag metamaterial. Appl. Phys. Lett. 2017, 110, 031102. [Google Scholar] [CrossRef]
- Myroshnychenko, V.; Stefanski, A.; Manjavacas, A.; Kafesaki, M.; Merino, R.I.; Orera, V.M.; Pawlak, D.A.; De Abajo, F.J.G. Interacting plasmon and phonon polaritons in aligned nano- and microwires. Opt. Express 2012, 20, 10879–10887. [Google Scholar] [CrossRef]
- Wysmulek, K.; Sar, J.; Osewski, P.; Orlinski, K.; Kolodziejak, K.; Trenczek-Zajac, A.; Radecka, M.; Pawlak, D.A. A SrTiO3-TiO2 eutectic composite as a stable photoanode material for photoelectrochemical hydrogen production. Appl. Catal. B Environ. 2017, 206, 538–546. [Google Scholar] [CrossRef]
- Kolodziejak, K.; Sar, J.; Wysmulek, K.; Osewski, P.; Warczak, M.; Sadkowski, A.; Radecka, M.; Pawlak, D.A. When eutectic composites meet photoelectrochemistry—Highly stable and efficient UV–visible hybrid photoanodes. J. Catal. 2017, 352, 93–101. [Google Scholar] [CrossRef]
- Bienkowski, K.; Turczynski, S.; Diduszko, R.; Gajc, M.; Gorecka, E.; Pawlak, D.A. Growth of a Plate-Shaped SrTiO3–TiO2 Eutectic. Cryst. Growth Des. 2011, 11, 3935–3940. [Google Scholar] [CrossRef]
- Feng, J.; Luo, W.; Fang, T.; Lv, H.; Wang, Z.; Gao, J.; Liu, W.; Yu, T.; Li, Z.; Zou, Z. Highly Photo-Responsive LaTiO2N Photoanodes by Improvement of Charge Carrier Transport among Film Particles. Adv. Funct. Mater. 2014, 24, 3535–3542. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Vesborg, P.C.K.; Jaramillo, T.F. Effect of Film Morphology and Thickness on Charge Transport in Ta3N5/Ta Photoanodes for Solar Water Splitting. J. Phys. Chem. C 2012, 116, 15918–15924. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, G.; Xian, H.; Zhang, H. Synthesis of SrO(SrTiO3)n (n = 1, 2, ∞) compounds and electronic structure analysis. Mater. Res. Bull. 2008, 43, 2554–2562. [Google Scholar] [CrossRef]
- Zhang, J.; Bang, J.H.; Tang, C.; Kamat, P.V. Tailored TiO2−SrTiO3 Heterostructure Nanotube Arrays for Improved Photoelectrochemical Performance. ACS Nano 2010, 4, 387–395. [Google Scholar] [CrossRef]
- Baek, J.-Y.; Duy, L.T.; Lee, S.Y.; Seo, H. Aluminum doping for optimization of ultrathin and high-k dielectric layer based on SrTiO3. J. Mater. Sci. Technol. 2020, 42, 28–37. [Google Scholar] [CrossRef]
- Gillispie, M.A.; van Hest, M.F.A.M.; Dabney, M.S.; Perkins, J.D.; Ginley, D.S. rf magnetron sputter deposition of transparent conducting Nb-doped TiO2 films on SrTiO3. J. Appl. Phys. 2007, 101, 033125. [Google Scholar] [CrossRef]
- Jabbar, S.; Ahmad, R.; Chu, P.K. Morphological and electrical properties of SrTiO3/TiO2/SrTiO3 sandwich structures prepared by plasma sputtering. Chin. Phys. B 2017, 26, 10702. [Google Scholar] [CrossRef]
- Shang, Q.; Yu, T.; Tan, X.; Zhang, Z.; Zou, Y.; Zhang, L.; Zhang, Y.; Wang, S. Enhanced photoelectrochemical performance of {001}TiO2/{001}SrTiO3 epitaxial heterostructures. J. Solid State Electrochem. 2016, 20, 123–132. [Google Scholar] [CrossRef]
- Rahman, M.Y.A.; Samsuri, S.A.M.; Umar, A.A. TiO2–SrTiO3 composite photoanode: Effect of strontium precursor concentration on the performance of dye-sensitized solar cells. Appl. Phys. A 2019, 125, 59. [Google Scholar] [CrossRef]
- Ruzimuradov, O.; Sharipov, K.; Yarbekov, A.; Saidov, K.; Hojamberdiev, M.; Prasad, R.M.; Cherkashinin, G.; Riedel, R. A facile preparation of dual-phase nitrogen-doped TiO2–SrTiO3 macroporous monolithic photocatalyst for organic dye photodegradation under visible light. J. Eur. Ceram. Soc. 2015, 35, 1815–1821. [Google Scholar] [CrossRef]
- Zhu, X.; Gu, P.; Wu, H.; Yang, D.; Sun, H.; Wangyang, P.; Li, J.; Tian, H. Influence of substrate on structural, morphological and optical properties of TiO2thin films deposited by reaction magnetron sputtering. AIP Adv. 2017, 7, 125326. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Flores, A.M.; Usiobo, O.J.; Audinot, J.-N.; Heyberger, R.; Choquet, P.; Boscher, N.D. Low Temperature Open-Air Plasma Deposition of SrTiO3 Films for Solar Energy Harvesting: Impact of Precursors on the Properties and Performances. ACS Appl. Mater. Interfaces 2022, 14, 8527–8536. [Google Scholar] [CrossRef]
- Roy, R.; (Pennsylvania State University, University Park: College Township, PA, USA). Private communication, 1957.
- The International Centre for Diffraction Data USA. 2012. Available online: www.icdd.com (accessed on 9 September 2022).
- Ramírez, M.O.; Molina, P.; Mateos, L.; Turczyński, S.; Kaczkan, M.; Malinowski, M.; Pawlak, D.A.; E Bausa, L. Pr3+-Based Fluorescent TiO2 Split Ring Resonator-like Crystalline Microstructures. Sci. Adv. Mater. 2013, 5, 921–926. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Factors Affecting the Performance and Applicability of SrTiO3Photoelectrodes for Photoinduced Cathodic Protection. J. Electrochem. Soc. 2017, 164, C1067–C1075. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Yu, H.; Jiang, X.; Sun, D. A heterogeneous TiO2/SrTiO3 coating on titanium alloy with excellent photocatalytic antibacterial, osteogenesis and tribocorrosion properties. Surf. Coat. Technol. 2022, 431, 128008. [Google Scholar] [CrossRef]
- Kwong, W.L.; Savvides, N.; Sorrell, C. Electrodeposited nanostructured WO3 thin films for photoelectrochemical applications. Electrochim. Acta 2012, 75, 371–380. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Shang, D.S.; Shi, L.; Sun, J.R.; Shen, B.G.; Zhuge, F.; Li, R.W.; Zhao, Y.G. Improvement of reproducible resistance switching in polycrystalline tungsten oxide films by in situ oxygen annealing. Appl. Phys. Lett. 2010, 96, 072103. [Google Scholar] [CrossRef] [Green Version]
- Helander, M.G.; Greiner, M.T.; Wang, Z.B.; Tang, W.M.; Lu, Z.H. Work function of fluorine doped tin oxide. J. Vac. Sci. Technol. A 2011, 29, 011019. [Google Scholar] [CrossRef]
- Maeda, K.; Terashima, H.; Kase, K.; Higashi, M.; Tabata, M.; Domen, K. Surface Modification of TaON with Monoclinic ZrO2 to Produce a Composite Photocatalyst with Enhanced Hydrogen Evolution Activity under Visible Light. Bull. Chem. Soc. Jpn. 2008, 81, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Deutsch, T.G.; Dinh, H.N.; Domen, K.; Emery, K.; Forman, A.J.; Gaillard, N.; Garland, R.; Heske, C.; Jaramillo, T.F. Experimental Considerations. In Photoelectrochemical Water Splitting; Springer: Berlin/Heidelberg, Germany, 2013; pp. 17–44. [Google Scholar]
- Lopes, T.; Andrade, L.; Le Formal, F.; Gratzel, M.; Sivula, K.; Mendes, A. Hematite photoelectrodes for water splitting: Evaluation of the role of film thickness by impedance spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 16515–16523. [Google Scholar] [CrossRef] [PubMed]
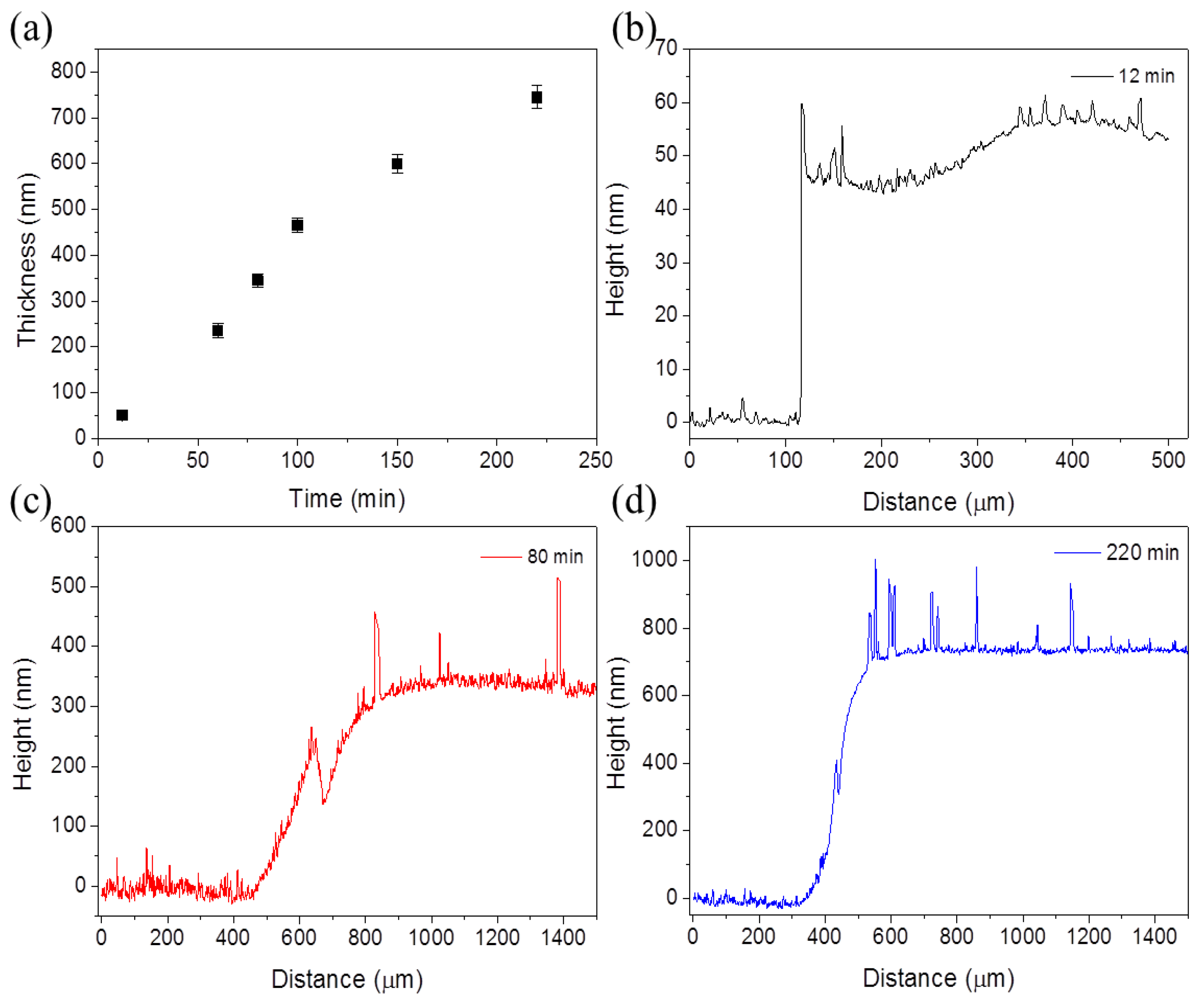
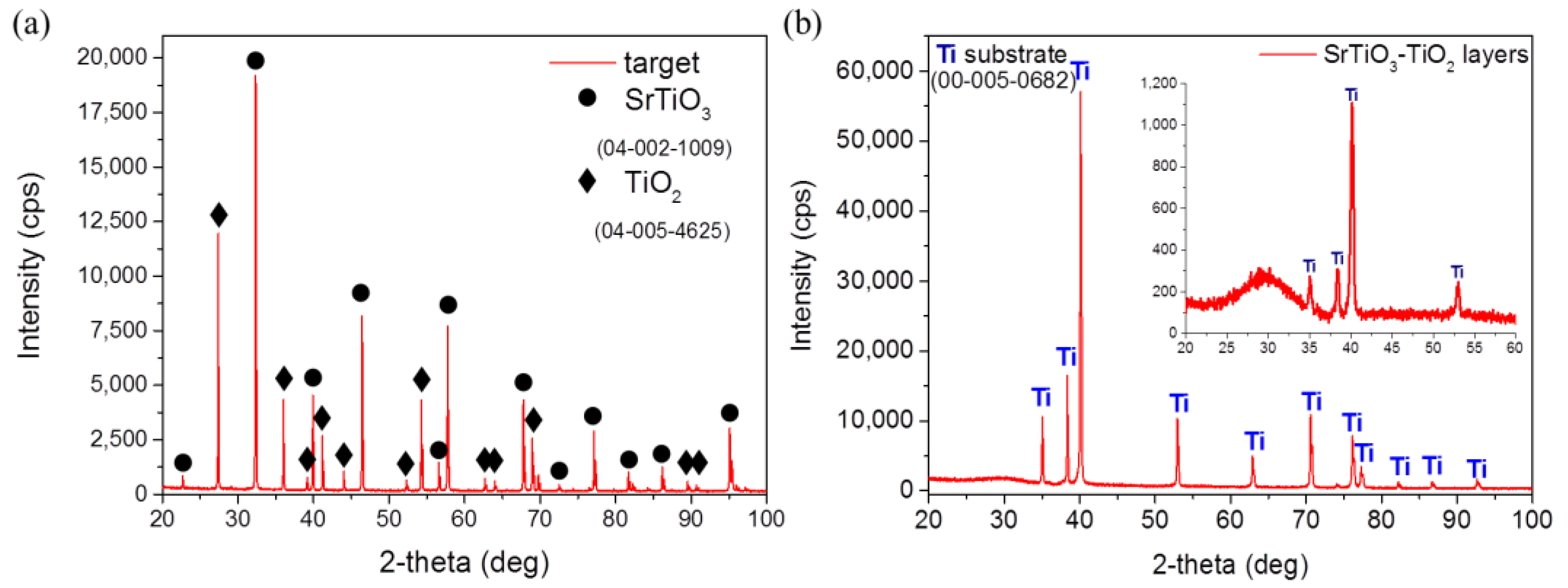
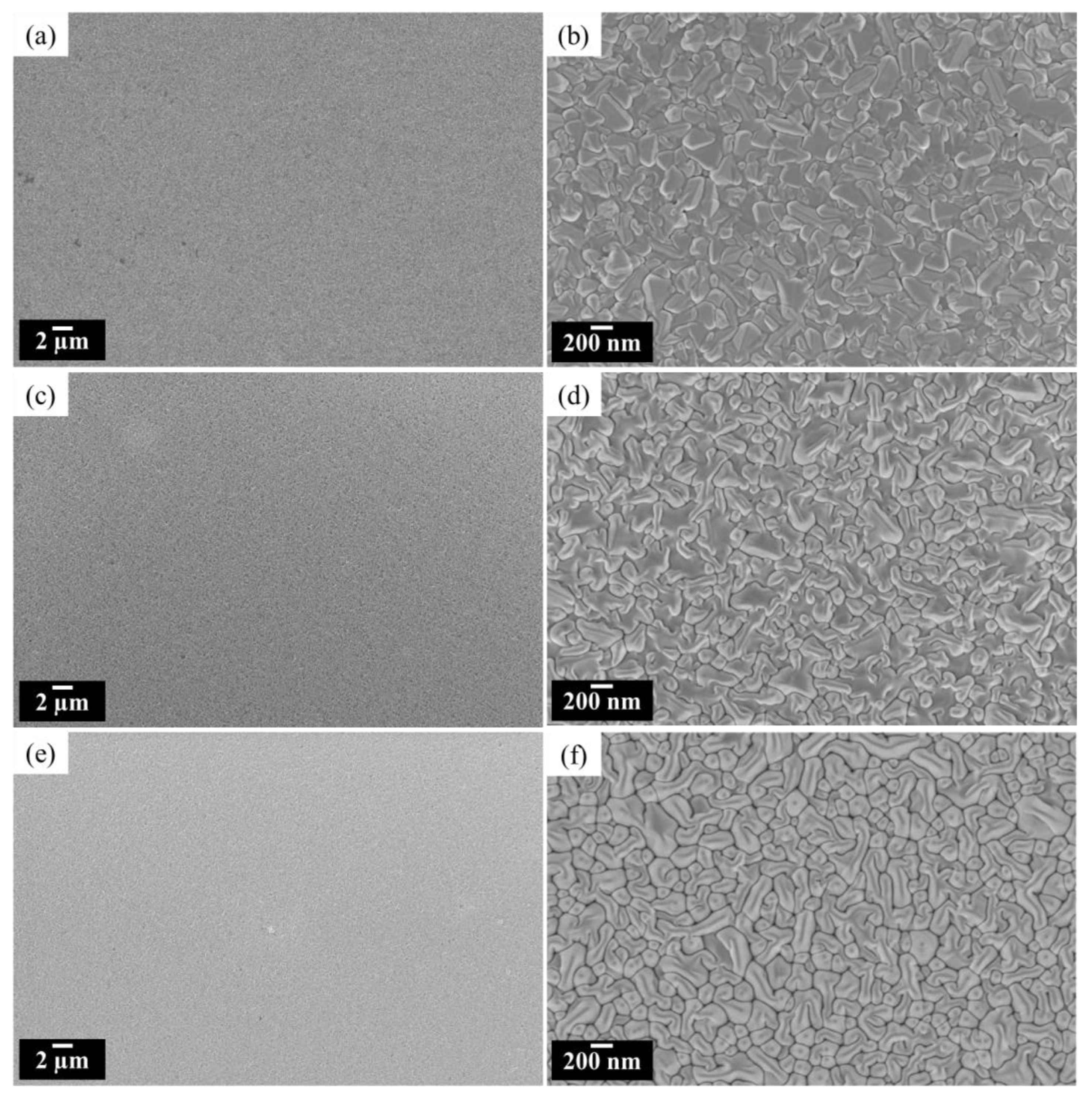

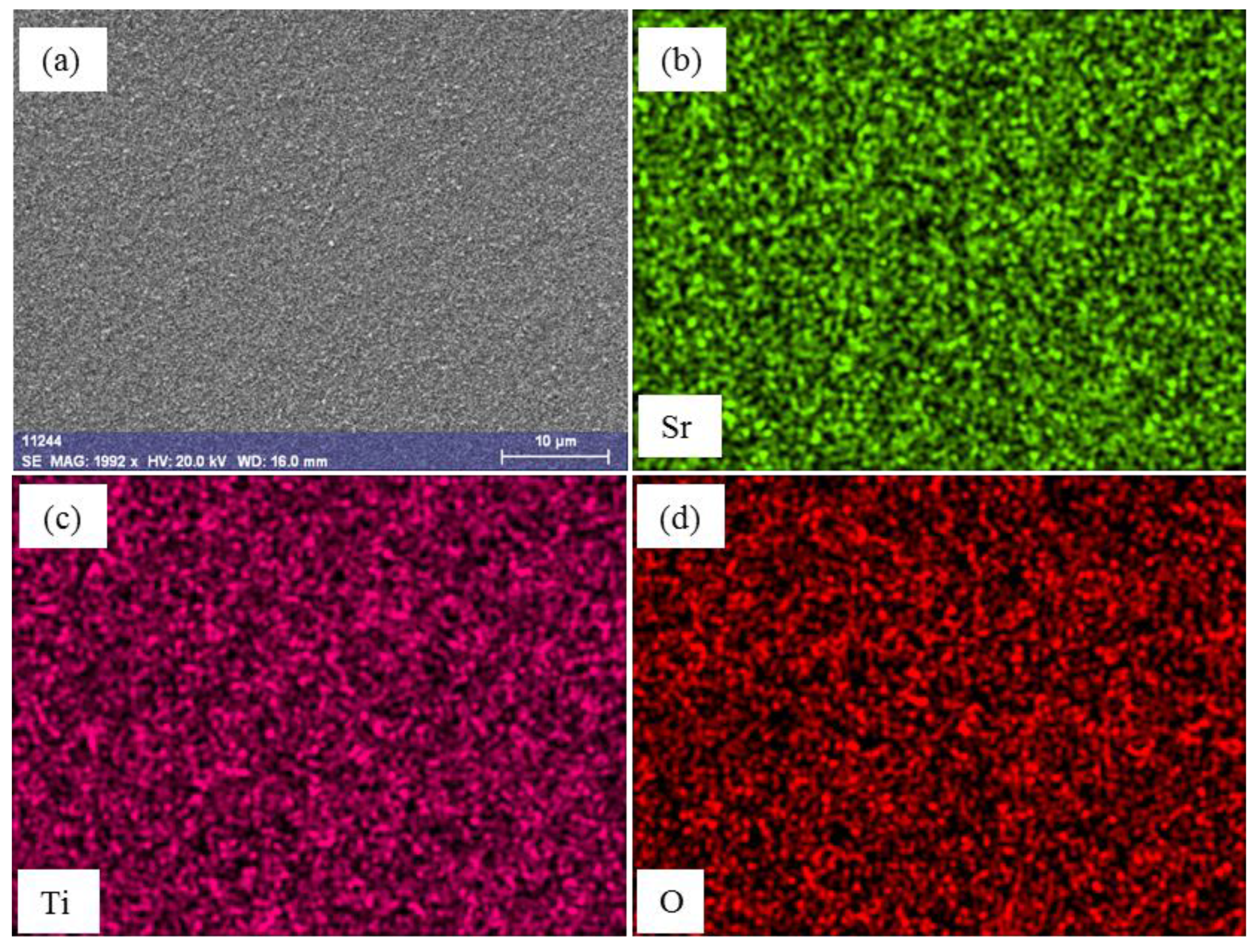

| PAr (hPa) | Power (W) | Target to Substrate Distance (mm) | Sputtering Time (min) | Thickness (nm) |
|---|---|---|---|---|
| 2 × 10−2 | 400 | 43 | 12 | 40–60 |
| 2 × 10−2 | 400 | 43 | 60 | 220–250 |
| 2 × 10−2 | 400 | 43 | 80 | 330–360 |
| 2 × 10−2 | 400 | 43 | 100 | 450–480 |
| 2 × 10−2 | 400 | 43 | 150 | 580–620 |
| 2 × 10−2 | 400 | 43 | 220 | 720–770 |
| SrTiO3-TiO2 @ FTO | |||||||
|---|---|---|---|---|---|---|---|
| Thickness (nm) | Rs (Ω) | Rsc (Ω) | Csc (F) | Rct (MΩ) | Qdl (F s(n − 1)) | n | |
| 750 | Dark | 30.0 | 75.3 | 3.51 × 10−9 | 23.2 | 1.21 × 10−8 | 0.95 |
| 750 | Light | 29.8 | 74.4 | 3.57 × 10−9 | 10.5 | 1.23 × 10−8 | 0.95 |
| 350 | Dark | 23.5 | 84.5 | 3.88 × 10−9 | 53.1 | 1.94 × 10−8 | 0.98 |
| 350 | Light | 23.0 | 82.9 | 3.85 × 10−9 | 4.29 | 2.05 × 10−8 | 0.98 |
| 50 | Dark | 28.9 | 99.8 | 3.48 × 10−9 | 11.0 | 2.87 × 10−7 | 0.96 |
| 50 | Light | 22.0 | 102.0 | 3.01 × 10−9 | 1.60 | 4.71 × 10−7 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sar, J.; Kołodziejak, K.; Orliński, K.; Gajewski, M.; Teodorczyk, M.; Pawlak, D.A. Thin Layers of SrTiO3-TiO2 with Eutectic Composition for Photoelectrochemical Water Splitting. Coatings 2022, 12, 1876. https://doi.org/10.3390/coatings12121876
Sar J, Kołodziejak K, Orliński K, Gajewski M, Teodorczyk M, Pawlak DA. Thin Layers of SrTiO3-TiO2 with Eutectic Composition for Photoelectrochemical Water Splitting. Coatings. 2022; 12(12):1876. https://doi.org/10.3390/coatings12121876
Chicago/Turabian StyleSar, Jaroslaw, Katarzyna Kołodziejak, Krzysztof Orliński, Michal Gajewski, Marian Teodorczyk, and Dorota Anna Pawlak. 2022. "Thin Layers of SrTiO3-TiO2 with Eutectic Composition for Photoelectrochemical Water Splitting" Coatings 12, no. 12: 1876. https://doi.org/10.3390/coatings12121876
APA StyleSar, J., Kołodziejak, K., Orliński, K., Gajewski, M., Teodorczyk, M., & Pawlak, D. A. (2022). Thin Layers of SrTiO3-TiO2 with Eutectic Composition for Photoelectrochemical Water Splitting. Coatings, 12(12), 1876. https://doi.org/10.3390/coatings12121876







