Antifungal Properties of Zinc Oxide Nanoparticles on Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. ZnO NPs Suspension Preparation
2.2. Characterization of ZnO NPs
2.3. Determination of Growth Pattern
2.4. Exposure to ZnO NPs
2.5. Growth Inhibition Test
2.5.1. Turbidity Determination
2.5.2. Colony Count
2.5.3. INT Assay
2.6. Surface Interaction Analysis of ZnO NPs on the Yeast Cell Wall
2.7. Scanning Electron Microscope and Energy Dispersive X-ray Analysis
2.8. Statistical Analysis
3. Results
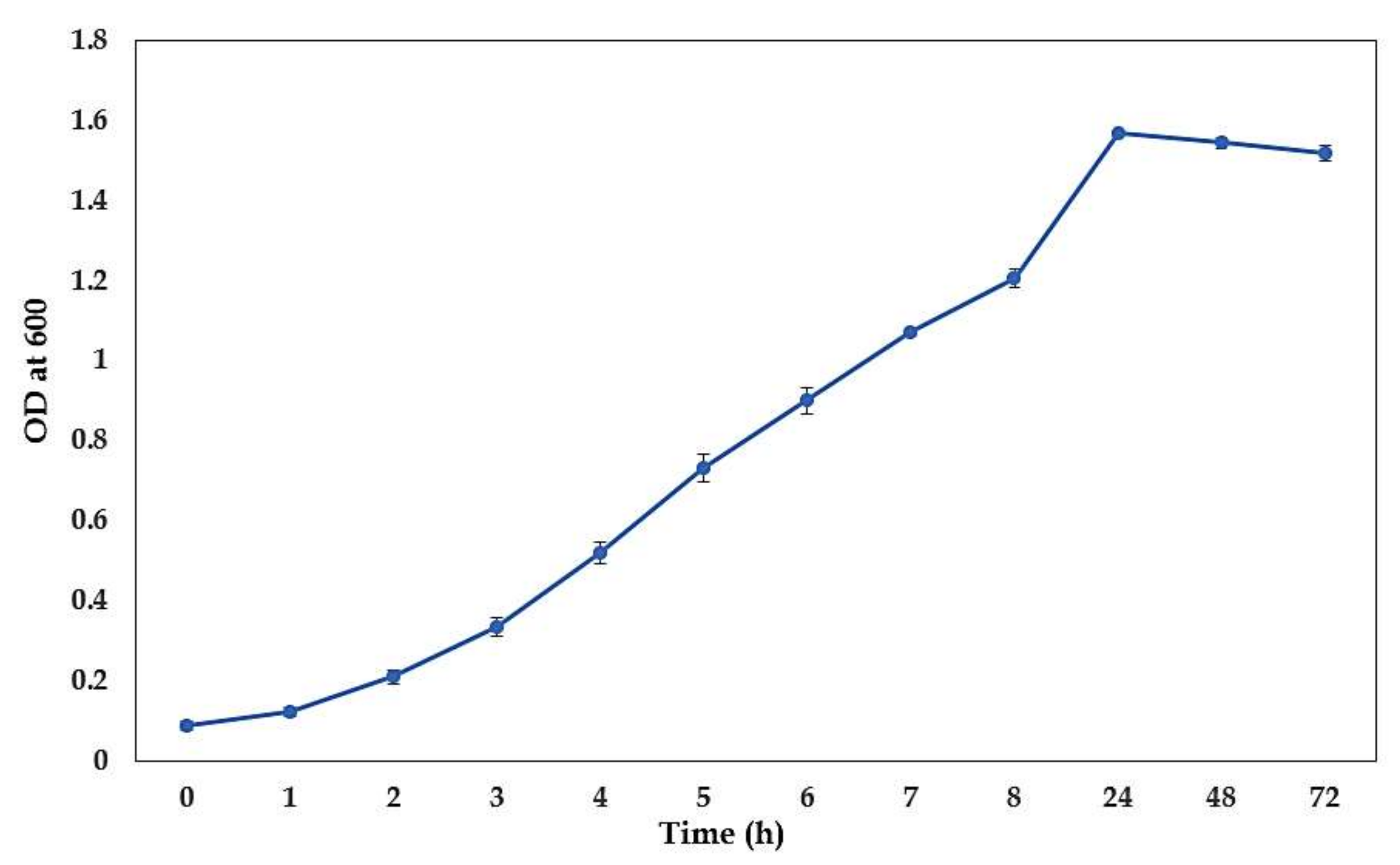
3.1. The growth Curve of C. albicans
3.2. Growth Inhibition Test
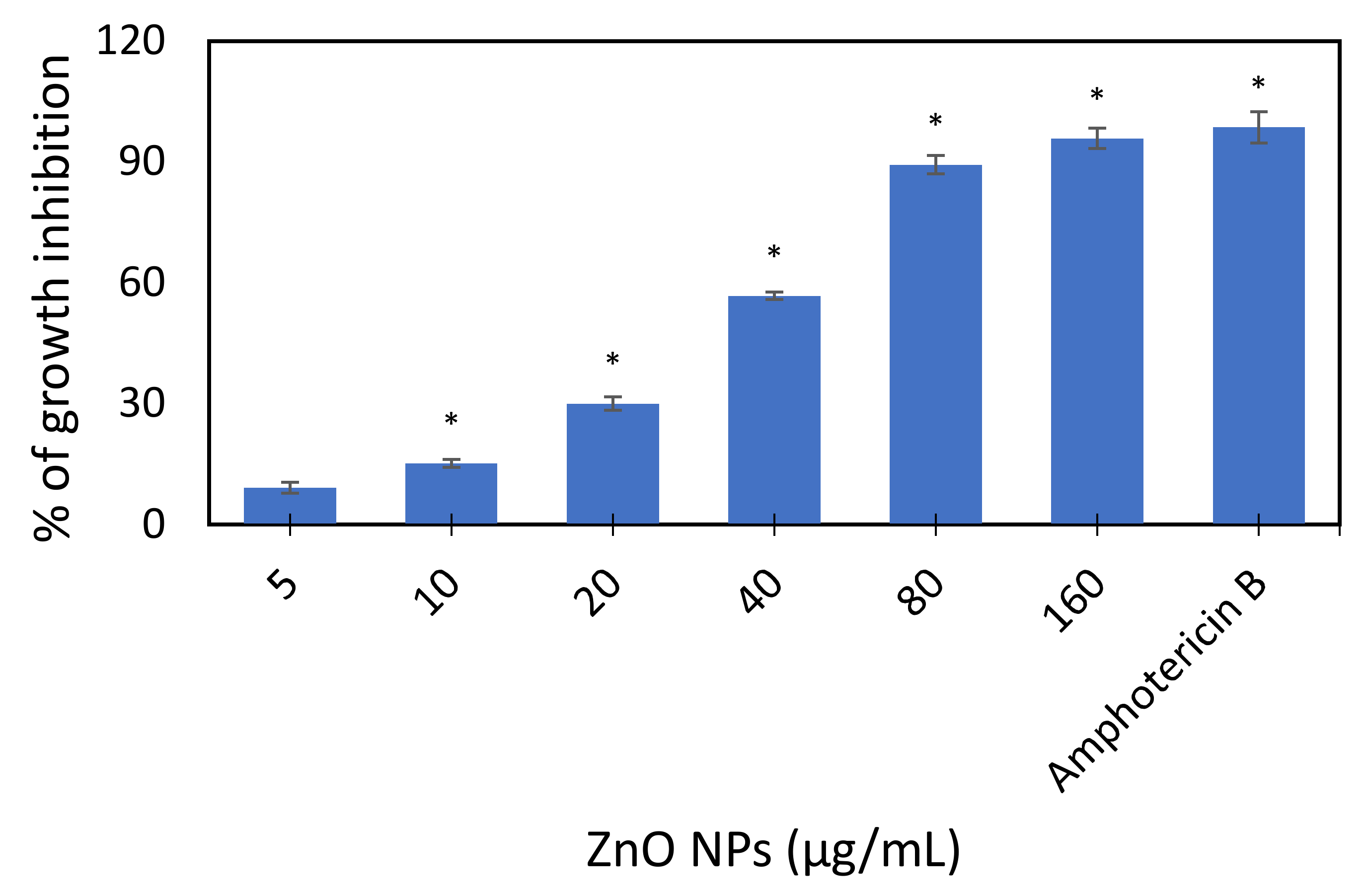
3.2.1. Turbidity Method
3.2.2. Colony Count Method
3.2.3. INT Assay
3.3. Surface Interaction and Cellular Accumulation of ZnO NPs on the Yeast Cell Wall
3.3.1. Fourier Transform Infrared (FTIR) Analysis
3.3.2. Energy Dispersive X-ray Analysis
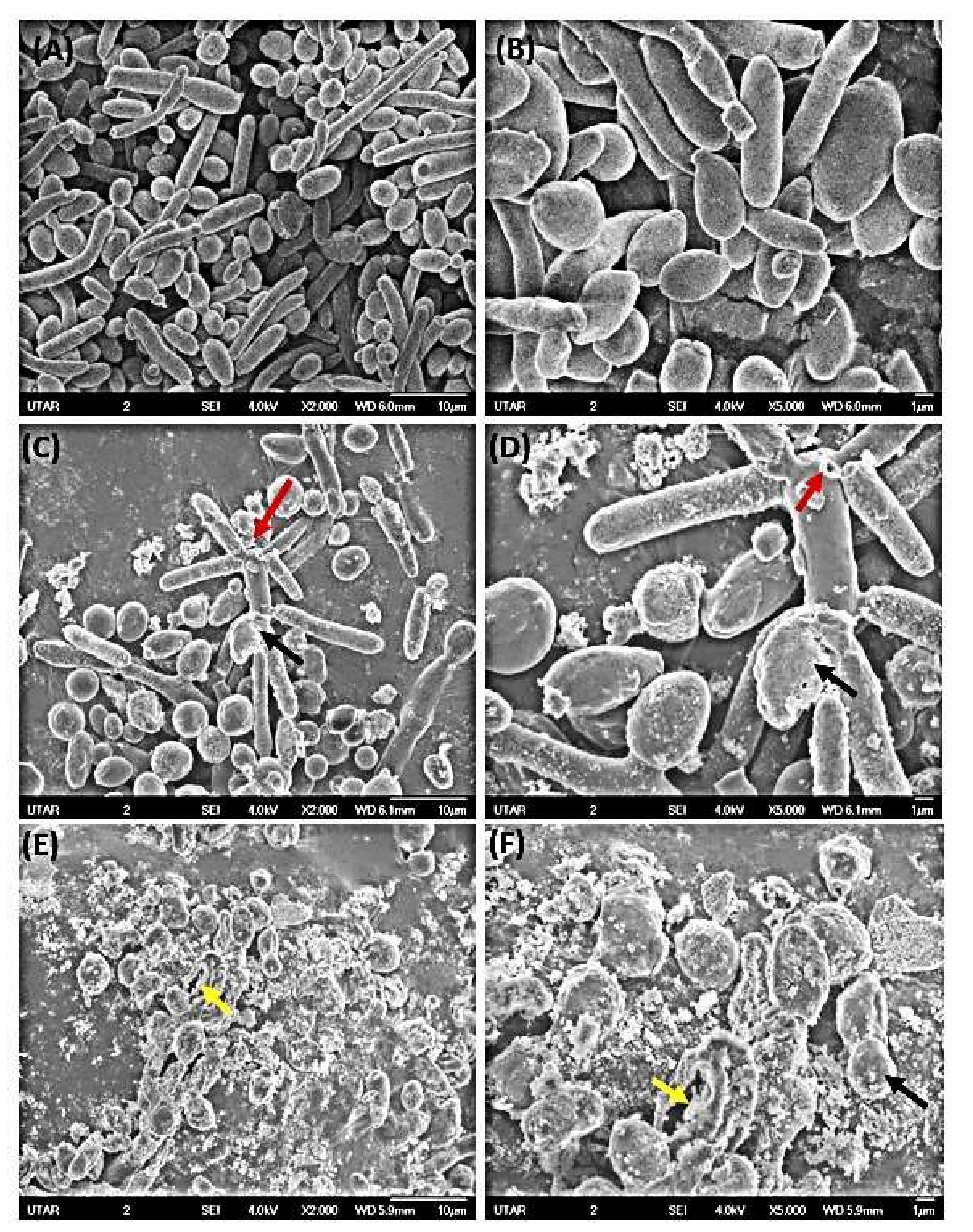
3.3.3. SEM Micrographs
4. Discussion
4.1. Growth Inhibitory Effect of ZnO NPs
4.2. Surface Interaction and Cellular Accumulation of ZnO NPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, G.K.K.; Padmavathi, A.R.; Nancharaiah, Y.V. Fungal infections: Pathogenesis, antifungals and alternate treatment approaches. Curr. Res. Microb. Sci. 2022, 3, 100137. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.R.; Robbins, N.; Cowen, L.E. The role of Candida albicans stress response pathways in antifungal tolerance and resistance. iScience 2022, 25, 103953. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.A.; Hussain, M.A.; Ahmad, Z. Candida albicans: A model organism for studying fungal pathogens. ISRN Microbiol. 2012, 2012, 538694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef] [PubMed]
- Graybill, J.R.; Burgess, D.S.; Hardin, T.C. Key issues concerning fungistatic versus fungicidal drugs. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Armengol, E.S.; Harmanci, M.; Laffleur, F. Current strategies to determine antifungal and antimicrobial activity of natural compounds. Microbiol. Res. 2021, 252, 126867. [Google Scholar] [CrossRef] [PubMed]
- Bhavyasree, P.G.; Xavier, T.S. Green synthesised copper and copper oxide-based nanomaterials using plant extracts and their application in antimicrobial activity. Curr. Res. Green Sustain. Chem. 2022, 5, 100249. [Google Scholar]
- Djearamane, S.; Ravintharan, T.; Liang, S.X.; Wong, L.S. Sensitivity of Proteus vulgaris to Zinc Oxide Nanoparticles. Sains Malays. 2022, 51, 1353–1362. [Google Scholar] [CrossRef]
- Owaid, M.N.; Rabeea, M.A.; Aziz, A.A.; Jameel, M.S.; Dheyab, M.A. Mycogenic fabrication of silver nanoparticles using Picoa, Pezizales, characterization and their antifungal activity. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100612. [Google Scholar] [CrossRef]
- Nidhin, M.; Saneha, D.; Hans, S.; Varghese, A.; Fatima, Z.; Hameed, S. Studies on the antifungal activity of biotemplated gold nanoparticles over Candida albicans. Mater. Res. Bull. 2019, 119, 110563. [Google Scholar]
- Hermida-Montero, L.A.; Pariona, N.; Mtz-Enriquez, A.I.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Aqueous-phase synthesis of nanoparticles of copper/copper oxides and their antifungal effect against Fusarium oxysporum. J. Hazard. Mater. 2019, 380, 120850. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathogen. 2018, 115, 2087–2292. [Google Scholar] [CrossRef]
- Dhanasegaran, K.; Djearamane, S.; Liang, S.X.; Wong, L.S.; Kasivelu, G.; Lee, P.F.; Lim, Y.M. Antibacterial properties of zinc oxide nanoparticles on Pseudomonas aeruginosa (ATCC 27853). Sci. Iran. 2021, 28, 3806–3815. [Google Scholar]
- Vijayakumar, S.; Vidhya, E.; Nilavukkarasi, M.; Punitha, V.N.; Praseetha, P.K. Potential eco-friendly Zinc Oxide nanomaterials through Phyco-nanotechnology—A review. Biocatal. Agric. Biotechnol. 2021, 35, 102050. [Google Scholar] [CrossRef]
- Abhishek, T.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar]
- Dutta, G.; Sugumaran, A. Bioengineered zinc oxide nanoparticles: Chemical, green, biological fabrication methods and its potential biomedical applications. J. Drug Deliv. Sci. Technol. 2021, 66, 102853. [Google Scholar]
- Karthikeyan, C.; Tharmalingam, N.; Varaprasad, K.; Mylonakis, E.; Yallapu, M.M. Biocidal and biocompatible hybrid nanomaterials from biomolecule chitosan, alginate and ZnO. Carbohydr. Polym. 2021, 274, 118646. [Google Scholar] [CrossRef] [PubMed]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- El-Diasty, E.M.; Ahmed, M.A.; Okasha, N.; Mansour, S.F.; El-Dek, S.I.; El-Khalek, H.M.; Youssif, M.H. Antifungal activity of zinc oxide nanoparticles against dermatophytic lesions of cattle. Rom. J. Biophys. 2013, 23, 191–202. [Google Scholar]
- Singh, P.; Nanda, P.A. Antimicrobial and antifungal potential of zinc oxide nanoparticles in comparison to conventional zinc oxide particles. J. Chem. Pharm. Res. 2013, 5, 457–463. [Google Scholar]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morliere, C.; Bolzinger, M.A. Antimicrobial activity of zinc oxide particles on five micro-organisms of the Challenge Tests related to their physicochemical properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Karimiyan, A.; Najafzadeh, H.; Ghorbanpour, M.; Hekmati-Moghaddam, S.H. Antifungal effect of magnesium oxide, zinc oxide, silicon oxide and copper oxide nanoparticles against Candida albicans. Zahedan J. Res. Med. Sci. 2015, 17, e2179. [Google Scholar] [CrossRef] [Green Version]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanely, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Djearamane, S.; Wong, L.S.; Lim, Y.M.; Lee, P.F. Oxidative stress effects of zinc oxide nanoparticles on freshwater microalga Haematococcus pluvialis. Ecol. Environ. Conserv. 2020, 26, 663–668. [Google Scholar]
- Djearamane, S.; Loh, Z.C.; Lee, J.J.; Wong, L.S.; Ranjithkumar, R.; Luque, P.A.; Gupta, P.K.; Liang, S.X.T. Remedial Aspect of Zinc Oxide Nanoparticles Against Serratia Marcescens and Enterococcus Faecalis. Front. Pharmacol. 2022, 13, 891304. [Google Scholar] [CrossRef] [PubMed]
- Sukri, S.N.A.M.; Shameli, K.; Wong, M.M.-T.; Teow, S.-Y.; Chew, J.; Ismail, N.A. Cytotoxicity and antibacterial activities of plant-mediated synthesized zinc oxide (ZnO) nanoparticles using Punica granatum (pomegranate) fruit peels extract. J. Mol. Struct. 2019, 1189, 57–65. [Google Scholar] [CrossRef]
- Kumar, H.; Rani, R. Structural and Optical Characterization of ZnO Nanoparticles Synthesized by Microemulsion Route. Int. Lett. Chem. Phys. Astron. 2013, 14, 26. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G.J.S.A.P.A.M. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Abomuti, M.A.; Danish, E.Y.; Firoz, A.; Hasan, N.; Malik, M.A. Green Synthesis of Zinc Oxide Nanoparticles Using Salvia officinalis Leaf Extract and Their Photocatalytic and Antifungal Activities. Biology 2021, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Ghose, R. Synthesis of zinc oxide nanoparticles by homogeneous precipitation method and its application in antifungal activity against Candida albicans. Ceram. Int. 2015, 41, 967–975. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, N.; Mishra, A.; Alam, H.; Manzoor, N.; Sardar, M. Biosynthesis, characterization, and antifungal activity of the silver nanoparticles against pathogenic Candida species. BioNanoScience 2015, 5, 65–74. [Google Scholar] [CrossRef]
- Melin, A.M.; Perromat, A.; Déléris, G. Pharmacologic application of Fourier transform IR spectroscopy: In vivo toxicity of carbon tetrachloride on rat liver. Biopolym. Orig. Res. Biomol. 2000, 57, 160–168. [Google Scholar] [CrossRef]
- Naumann, D. FT-infrared and FT-Raman spectroscopy in biomedical research. Appl. Spectrosc. Rev. 2001, 36, 239–298. [Google Scholar] [CrossRef]
- Banyay, M.; Sarkar, M.M.; Gräslund, A. A library of IR bands of nucleic acids in solution. Biophys. Chem. 2003, 104, 477–488. [Google Scholar] [CrossRef]
- Kačuráková, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Galichet, A.; Sockalingum, G.D.; Belarbi, A.; Manfait, M. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: Study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol. Lett. 2001, 197, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plata, M.R.; Koch, C.; Wechselberger, P.; Herwig, C.; Lendl, B.B. Determination of carbohydrates present in Saccharomyces cerevisiae using mid-infrared spectroscopy and partial least squares regression. Anal. Bioanal. Chem. 2013, 405, 8241–8250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lianga, S.X.; Wong, L.S.; Lim, Y.M.; Djearamane, S.; Lee, P.F. Effects of Zinc Oxide nanoparticles on Streptococcus pyogenes. S. Afr. J. Chem. Eng. 2020, 34, 63–71. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 3. [Google Scholar]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The Fugal Kingdom; ASM Press: Washington, DC, USA, 2017. [Google Scholar]
- Feofilova, E.P. The fungal cell wall: Modern concepts of its composition and biological function. Microbiology 2010, 79, 711–720. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The role of interparticle and external forces in nanoparticle assembly. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef]
- Anselm, O.; Chorover, J. Spectroscopic study of extracellular polymeric substances from Bacillus subtilis: Aqueous chemistry and adsorption effects. Biomacromolecules 2004, 5, 1219–1230. [Google Scholar]
- Parikh Sanjai, J.; Chorover, J. ATR-FTIR spectroscopy reveals bond formation during bacterial adhesion to iron oxide. Langmuir 2006, 22, 8492–8500. [Google Scholar] [CrossRef]
- Wei, J.; Yang, K.; Vachet, R.W.; Xing, B. Interaction between oxide nanoparticles and biomolecules of the bacterial cell envelope as examined by infrared spectroscopy. Langmuir 2010, 26, 18071–18077. [Google Scholar]
- Shoeb, M.; Singh, B.R.; Khan, J.A.; Khan, W.; Singh, B.N.; Singh, H.B.; Naqvi, A.H. ROS-dependent anticandidal activity of zinc oxide nanoparticles synthesized by using egg albumen as a biotemplate. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035015. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Mudiam, M.K.R.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.N.; Oliveira, R.C.; Nadres, E.T.; Paparella, A.; Rodrigues, D.F. A morphological, enzymatic and metabolic approach to elucidate apoptotic-like cell death in fungi exposed to h- and α-molybdenum trioxide nanoparticles. Nanoscale 2018, 10, 20702–20716. [Google Scholar]
- Lamsal, K.; Kim, S.W.; Jung, J.H.; Kim, Y.S.; Kim, K.S.; Lee, Y.S. Application of silver nanoparticles for the control of colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology 2011, 39, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. JBIC J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef]
- Ghorbani, F.; Gorji, P.; Mobarakeh, M.S.; Mozaffari, H.R.; Masaeli, R.; Safaei, M. Optimized Synthesis of Xanthan gum/ZnO/TiO2Nanocomposite with High Antifungal Activity against Pathogenic Candida albicans. J. Nanomater. 2022, 2022, 7255181. [Google Scholar] [CrossRef]
- Gharpure, S.; Yadwade, R.; Ankamwar, B. Non-antimicrobial and Non-anticancer Properties of ZnO Nanoparticles Biosynthesized Using Different Plant Parts of Bixa orellana. ACS Omega 2022, 7, 1914–1933. [Google Scholar] [CrossRef] [PubMed]






| ZnO NPs (μg/mL) | Number of Colonies |
|---|---|
| Mean Standard Deviation | |
| 0 | 197 7 |
| 5 | 200 6 |
| 10 | 120 29 |
| 20 | 52 9 |
| 40 | 10 6 |
| 80 | 2 2 |
| 160 | 2 1 |
| Amphotericin B | 0 0 |
| Absorption (cm−1) | Molecular Motion | Functional Group | Biomolecules |
|---|---|---|---|
| 34363447 29302923 | O–H and N–H stretching CH2 stretching | Alcohol, amide A Methyl group | Proteins, polysaccharides, chitin Lipids |
| 20782067 | CC stretching | Alkynes group | Hydrocarbon |
| 10761048 | C–O mainly by vibrations and absorptions of polysaccharides and phosphate groups | Phosphate group | Polysaccharides, mainly glucans and mannans, phospholipids |
| 16411638 | C=O stretching, Zn–O stretching | Amide I and amide II bands, respectively due to the C=O stretching and the NH bending of the peptide bond | Polypeptide, protein backbone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djearamane, S.; Xiu, L.-J.; Wong, L.-S.; Rajamani, R.; Bharathi, D.; Kayarohanam, S.; De Cruz, A.E.; Tey, L.-H.; Janakiraman, A.K.; Aminuzzaman, M.; et al. Antifungal Properties of Zinc Oxide Nanoparticles on Candida albicans. Coatings 2022, 12, 1864. https://doi.org/10.3390/coatings12121864
Djearamane S, Xiu L-J, Wong L-S, Rajamani R, Bharathi D, Kayarohanam S, De Cruz AE, Tey L-H, Janakiraman AK, Aminuzzaman M, et al. Antifungal Properties of Zinc Oxide Nanoparticles on Candida albicans. Coatings. 2022; 12(12):1864. https://doi.org/10.3390/coatings12121864
Chicago/Turabian StyleDjearamane, Sinouvassane, Lin-Jia Xiu, Ling-Shing Wong, Ranjithkumar Rajamani, Devaraj Bharathi, Saminathan Kayarohanam, Alice Escalante De Cruz, Lai-Hock Tey, Ashok Kumar Janakiraman, Mohammod Aminuzzaman, and et al. 2022. "Antifungal Properties of Zinc Oxide Nanoparticles on Candida albicans" Coatings 12, no. 12: 1864. https://doi.org/10.3390/coatings12121864
APA StyleDjearamane, S., Xiu, L.-J., Wong, L.-S., Rajamani, R., Bharathi, D., Kayarohanam, S., De Cruz, A. E., Tey, L.-H., Janakiraman, A. K., Aminuzzaman, M., & Selvaraj, S. (2022). Antifungal Properties of Zinc Oxide Nanoparticles on Candida albicans. Coatings, 12(12), 1864. https://doi.org/10.3390/coatings12121864








