Effect of Deposition Parameters on Morphological and Compositional Characteristics of Electrodeposited CuFeO2 Film
Abstract
:1. Introduction
2. Materials and Methods
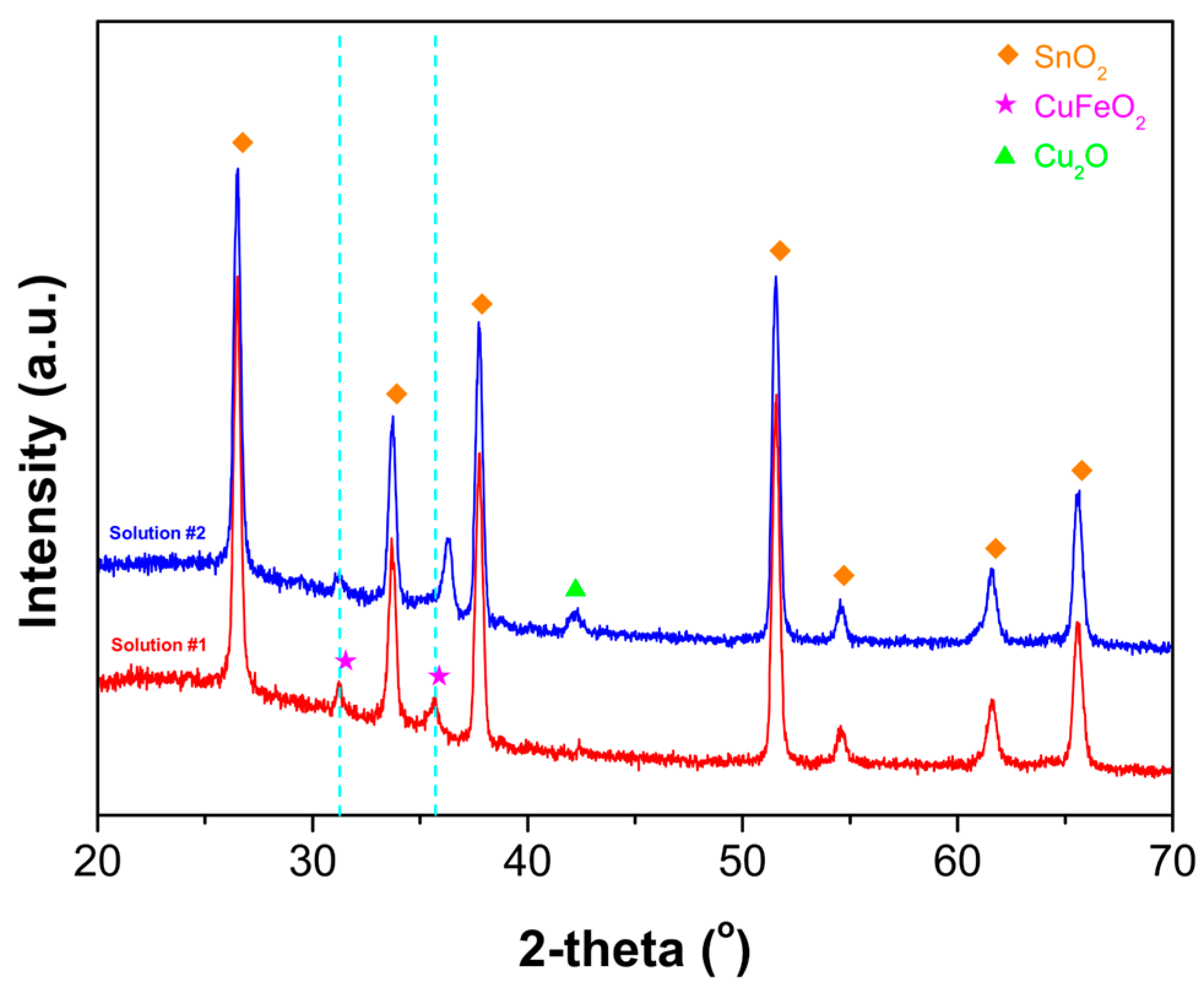
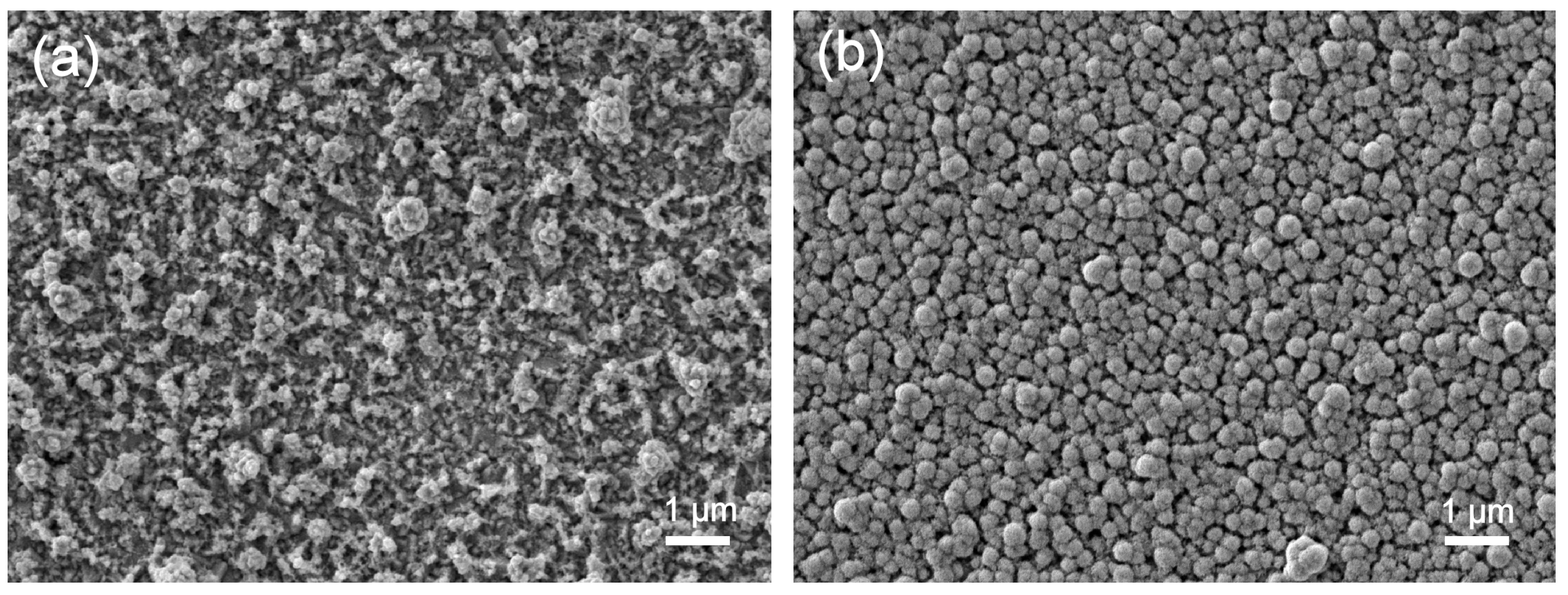
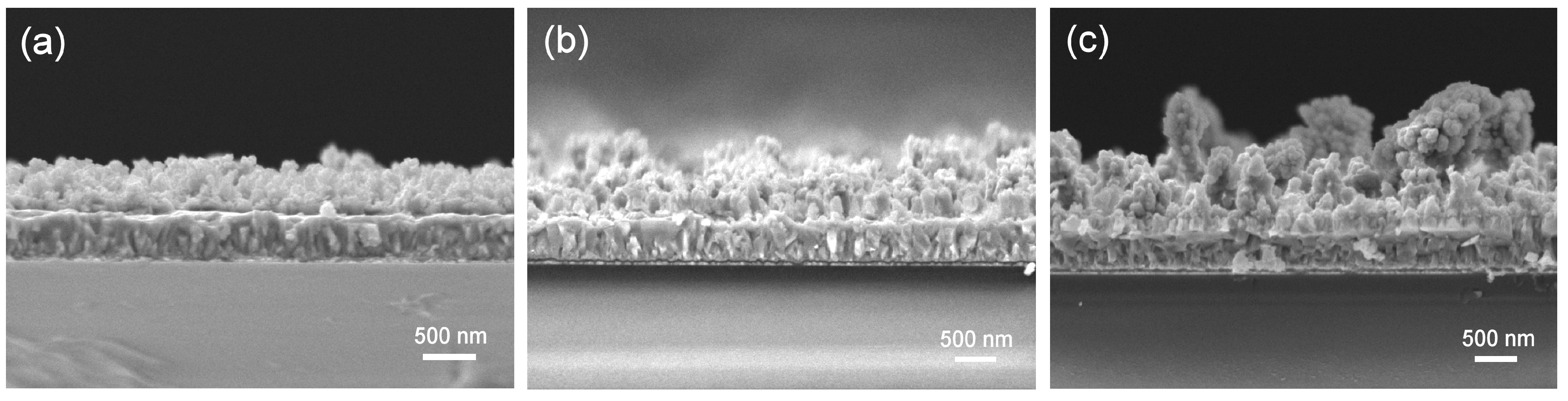
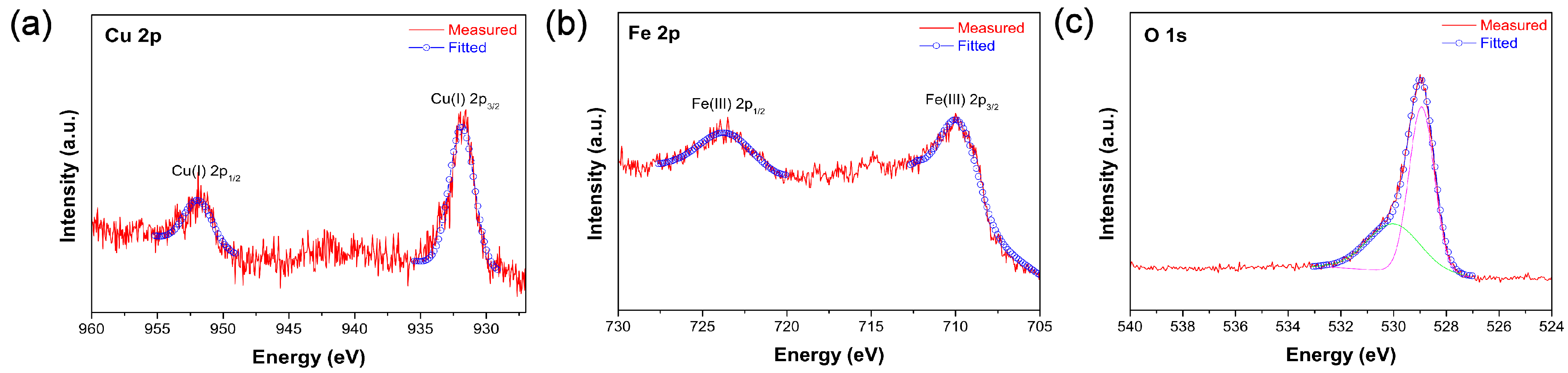
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.-Y.; Wu, J.-H. Transparent conductive CuFeO2 thin films prepared by sol-gel processing. Appl. Surf. Sci. 2012, 258, 4844–4847. [Google Scholar] [CrossRef]
- Deng, Z.; Fang, X.; Wang, X.; Wu, S.; Dong, W.; Shao, J.; Tao, R. Characterization of amorphous p-type transparent CuFeO2 thin films prepared by radio frequency magnetron sputtering method at room temperature. Thin Solid Film. 2015, 589, 17–21. [Google Scholar] [CrossRef]
- Mohamed, H.; Chikoidze, E.; Ratep, A.; Elsoud, A.M.A.; Boshta, M.; Osman, M.B.S. Synthesis of conducting single-phase CuFeO2 thin films by spray pyrolysis technique. Mater. Sci. Semicond. Process 2020, 107, 104831. [Google Scholar] [CrossRef]
- Elgazzar, E.; Tataroglu, A.; Al-Ghamdi, A.A.; Al-Tuki, Y.; Farooq, W.A.; El-Tantawy, F.; Yakuphanoglu, F. Thermal sensors based on delafossite film/p-silicon diode for low-temperature measurements. Appl. Phys. A 2016, 122, 617. [Google Scholar] [CrossRef]
- Sinnarasa, I.; Thimont, Y.; Barnabe, A.; Beaudhuin, M.; Moll, A.; Schorne-Pinto, J.; Tailhades, P.; Presmanes, L. Microstructural and transport properties of Mg-doped CuFeO2 thin films: A promising material for high accuracy miniaturized temperature sensors based on the Seebeck effect. J. Alloys Compd. 2020, 827, 154199. [Google Scholar] [CrossRef]
- Xia, N.; Shi, L.; Xia, Z.; Chen, B.; Jin, Z.; Wang, Y.; Ouyang, Z.; Zuo, H.; Shen, Y. Dynamic behavior of magnetoelectric coupling of CuFeO2 induced by a high magnetic field. J. Appl. Phys. 2014, 115, 114107. [Google Scholar] [CrossRef]
- Dai, H.; Ye, F.; Li, T.; Chen, Z.; Cao, X.; Wang, B. Impact of Li doping on the microstructure, defects, and physical properties of CuFeO2 multiferroic ceramics. Ceram. Int. 2019, 45, 24570–24577. [Google Scholar] [CrossRef]
- Crespo, C.T. Potentiality of CuFeO2-delafossite as a solar energy converter. Sol. Energy 2018, 163, 162–166. [Google Scholar] [CrossRef]
- Read, C.G.; Park, Y.; Choi, K.-S. Electrochemical synthesis of p-type CuFeO2 electrodes for use in a photoelectrochemical cell. J. Phys. Chem. Lett. 2012, 3, 1872–1876. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, Z.; Fang, X.; Huo, Z.; Wang, S.; Dong, W.; Shao, J.; Tao, R.; Song, C.; Wang, L. High photovoltages of CuFeO2 based p-type dye-sensitized solar cells. J. Alloys Compd. 2016, 685, 836–840. [Google Scholar] [CrossRef]
- Jin, Y.; Chumanov, G. Solution synthesis of pure 2H CuFeO2 at low temperature. RSC Adv. 2016, 6, 26392–26397. [Google Scholar] [CrossRef]
- Prevot, M.S.; Guijarro, N.; Sivula, K. Enhancing the performance of a robust sol-gel processed-type delafossite CuFeO2 photocathode for solar water reduction. ChemSusChem 2015, 8, 1359–1367. [Google Scholar] [CrossRef]
- Jang, Y.J.; Park, Y.B.; Kim, H.E.; Choi, Y.H.; Choi, S.H.; Lee, J.S. Oxygen-intercalated CuFeO2 photocathode fabricated by hybrid microwave annealing for efficient solar hydrogen production. Chem. Mater. 2016, 28, 6054–6061. [Google Scholar] [CrossRef]
- Oh, Y.; Yang, W.; Kim, J.; Jeong, S.; Moon, J. Enhanced photocurrent of transparent CuFeO2 photocathodes by self-light-harvesting architecture. ACS Appl. Mater. Interfaces 2017, 9, 14078–14087. [Google Scholar] [CrossRef]
- Boudoire, F.; Liu, Y.; Formal, F.L.; Guijarro, N.; Lhermitte, C.R.; Sivula, K. Spray synthesis of CuFeO2 photocathodes and in-operando assessment of charge carrier recombination. J. Phys. Chem. C 2021, 125, 10883–10890. [Google Scholar] [CrossRef]
- Prevot, M.S.; Jeanbourquin, X.A.; Bouree, W.S.; Abdi, F.; Friedrich, D.; Krol, R.; Guijarro, N.; Formal, F.L.; Sivula, K. Evaluating charge carrier transport and surface states in CuFeO2 photocathodes. Chem. Mater. 2017, 29, 4952–4962. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Zhao, Z.-Y.; Zhao, R.-D.; Yi, J.-H. Fundamental properties of delafossite CuFeO2 as photocatalyst for solar energy conversion. J. Alloys Compd. 2020, 819, 153032. [Google Scholar] [CrossRef]
- Preethi, S.; Vivek, S.; Priya, R.; Balakumar, S.; Babu, K.S. Enhanced photocatalytic performance of CuFeO2-ZnO heterostructures for methylene blue degradation under sunlight. J. Mater. Sci. Mater. Electron. 2021, 32, 22256–22269. [Google Scholar] [CrossRef]
- Tu, L.-W.; Chang, K.-S. Hydrothermal fabrication and photocatalytic study of delafossite (CuFeO2) thin films on fluorine-doped tin oxide substrate. Mater. Chem. Phys. 2021, 267, 124620. [Google Scholar] [CrossRef]
- Barnabe, A.; Mugnier, E.; Presmanes, L.; Thailhades, P. Preparation of delafossite CuFeO2 thin films by rf-sputtering on conventional glass substrate. Mater. Lett. 2006, 60, 3468–3470. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, Z.; Fang, X.; Dong, W.; Shao, J.; Tao, R.; Wang, S. Room temperature deposition of amorphous p-type CuFeO2 and fabrication of CuFeO2/n-Si heterojunction by RF sputtering method. Bull. Mater. Sci. 2016, 39, 883–887. [Google Scholar] [CrossRef] [Green Version]
- Ziani, N.; Aubry, E.; Martin, N.; Hirsinger, L.; Billard, A.; Briois, P.; Belkaid, M.S.; Yazdi, M.A.P. Influence of substrate temperature on delafossite CuFeO2 films synthesized by reactive magnetron sputtering. J. Alloys Compd. 2021, 876, 160169. [Google Scholar] [CrossRef]
- Li, S.Z.; Liu, J.; Wang, X.Z.; Yan, B.W.; Li, H.; Liu, J.-M. Epitaxial growth of delafossite CuFeO2 thin films by pulse laser deposition. Phys. B Condens. Mater. 2012, 407, 2412–2415. [Google Scholar] [CrossRef]
- Joshi, T.; Senty, T.R.; Trappen, R.; Zhou, J.; Chen, S.; Ferrari, P.; Borisov, P.; Song, X.; Holcomb, M.B.; Bristow, A.D.; et al. Structural and magnetic properties of epitaxial delafossite CuFeO2 thin films grown by pulsed laser deposition. J. Appl. Phys. 2015, 117, 013908. [Google Scholar] [CrossRef]
- Luo, S.; Fluri, A.; Zhang, S.; Liu, X.; Döbeli, M.; Harrington, G.F.; Tu, R.; Pergolesi, D.; Ishihara, T.; Lippert, T. Thickness-dependent microstructural properties of heteroepitaxial (00.1) CuFeO2 thin films on (00.1) sapphire by pulsed laser deposition. J. Appl. Phys. 2020, 127, 065301. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Huang, K.; Tang, Z.; Liu, G.; Li, Y. Chemical solution deposition and transport properties of epitaxial CuFeO2 thin films. Mater. Lett. 2011, 65, 3289–3291. [Google Scholar] [CrossRef]
- Gupta, R.K.; Cavas, M.; Al-Ghamdi, A.A.; Gafer, Z.H.; El-Tantawy, F.; Yakuphanoglu, F. Electrical and photoresponse properties of Al/p-CuFeO2/p-Si/Al MTCOS photodiode. Sol. Energy 2013, 92, 1–6. [Google Scholar] [CrossRef]
- Ito, M.; Izawa, C.; Watanabe, T. Direct fabrication of a CuFeO2/Fe photocathode for solar hydrogen production by hydrothermal method. Chem. Lett. 2017, 46, 814–816. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, L.; Hao, C. Lithium-doped CuFeO2 thin film electrodes for photoelectrochemical reduction of carbon dioxide to methanol. J. Electrochem. Soc. 2019, 166, H718. [Google Scholar] [CrossRef]
- Aqaei, F.; Zare, M.; Shafiekhani, A. Role of plasmonic Au nanoparticles embedded in the diamond-like carbon overlayer in the performance of CuFeO2 solar photocathodes. J. Solid State Electrochem. 2021, 25, 1139–1150. [Google Scholar] [CrossRef]
- Yin, G.; Liu, C.; Shi, T.; Ji, D.; Yao, Y.; Chen, Z. Porous BiVO4 coupled with CuFeO2 and NiFe layered double hydroxide as highly-efficient photoanode toward boosted photoelectrochemical water oxidation. J. Photochem. Photobiol. A Chem. 2022, 426, 113742. [Google Scholar] [CrossRef]
- Alkhayatt, A.H.O.; Thahab, S.M.; Zgair, I.A. Structure, surface morphology and optical properties of post-annealed delafossite CuFeO2 thin films. Optik 2016, 127, 3745–3749. [Google Scholar] [CrossRef]
- Garcia-Torregrosa, I.; Geertzema, Y.G.; Ismail, A.S.M.; Lee, T.-L.; de Grioot, F.M.F.; Weckhyusen, B.M. Facile two-step synthesis of delafossite CuFeO2 photocathodes by ultrasonic spray pyrolysis and hybrid microwave annealing. ChemPhotoChem 2019, 3, 1238–1245. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Haigh, J. Strengths and advantages of electrodeposition as a semiconductor growth technique for applications in microelectronic devices. J. Electrochem. Soc. 2006, 153, G47–G52. [Google Scholar] [CrossRef]
- Xiao, F.; Hangarter, C.; Yoo, B.; Rheem, Y.; Lee, K.-H.; Myung, N.V. Recent progress in electrodeposition of thermoelectric thin films and nanostructures. Electrochim. Acta 2008, 53, 8103–8117. [Google Scholar] [CrossRef]
- Riveros, G.; Garin, C.; Ramirez, D.; Dalchiele, E.A.; Marotti, R.E.; Pereyra, C.J.; Spera, E.; Gomez, H.; Grez, P.; Martin, F.; et al. Delafossite CuFeO2 thin films electrochemically grown from a DMSO based solution. Electrochim. Acta 2015, 164, 297–306. [Google Scholar] [CrossRef]
- Kang, U.; Park, H. A facile synthesis of CuFeO2 and CuO composite photocatalyst films for the production of liquid formate from CO2 and water over a month. J. Mater. Chem. A 2017, 5, 2123–2131. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, H.; Hao, Z.; Yu, M.; Chen, X.; Chen, J. Electrodeposition of (hydro)oxides for an oxygen evolution electrode. Chem. Sci. 2020, 11, 10614–10625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.; Yang, W.; Tan, J.; Lee, H.; Park, J.; Moon, J. Photoelectrodes based on 2D opals assembled from Cu-delafossite double-shelled microspheres for an enhanced photoelectrochemical response. Nanoscale 2018, 10, 3720–3729. [Google Scholar] [CrossRef]
- Oh, Y.; Yang, W.; Tan, J.; Lee, H.; Park, J.; Moon, J. Boosting visible light harvesting in p-type ternary oxides for solar-to-hydrogen conversion using inverse opal structure. Adv. Funct. Mater. 2019, 29, 1900194. [Google Scholar] [CrossRef]
- Wang, M.; Liu, C.; Shi, H.; Long, T.; Zhang, C.; Liu, B. Facile synthesis of chitosan-derived maillard reaction productions coated CuFeO2 with abundant oxygen vacancies for higher Fenton-like catalytic performance. Chemosphere 2021, 283, 131191. [Google Scholar] [CrossRef]
- Choudhary, S.; Sarma, J.V.N.; Pande, S.; Girad, S.A.; Turban, P.; Lepine, B.; Gangopadhyay, S. Oxidation mechanism of thin Cu films: A gateway towards the formation of single oxide phase. AIP Adv. 2018, 8, 055114. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Mamyrbekova, A.K.; Mamitova, A.D.; Turebekova, G.; Gul, K.; Mamyrbekova, A.K. Kinetics and mechanism of cathodic processes at electrolysis of Cu(NO3)2∙3H2O solution in dimethyl sulfoxide. Asian J. Chem. 2016, 28, 525–528. [Google Scholar] [CrossRef]
- De, M.; Gupta, S.P.S. Lattice imperfection studies in polycrystalline materials by x-ray diffraction line-profile analysis. Pramana 1984, 23, 721–744. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Fu, J.-R. Delafossite-CuFeO2 thin films prepared by atmospheric pressure plasma annealing. Mater. Lett. 2014, 120, 47–49. [Google Scholar] [CrossRef]
- Xiong, D.; Qi, Y.; Li, X.; Tao, H.; Chen, W.; Zhao, X. Hydrothermal synthesis of delafossite CuFeO2 crystals at 100 °C. RSC Adv. 2015, 5, 49280–49286. [Google Scholar] [CrossRef]
- Li, Z.; Wu, W.; Wang, M.; Wang, Y.; Ma, X.; Luo, L.; Chen, Y.; Kan, K.; Pan, Y.; Li, H.; et al. Ambient-pressure hydrogenation of CO2 into long-chain olefins. Nat. Commun. 2022, 13, 2396. [Google Scholar] [CrossRef] [PubMed]
- Son, M.-K.; Seo, H.; Watanabe, M.; Shiratani, M.; Ishihara, T. Characteristics of crystalline sputtered LaFeO3 thin films as photoelectrochemical water splitting photocathodes. Nanoscale 2020, 12, 9653–9660. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, M.-K. Effect of Deposition Parameters on Morphological and Compositional Characteristics of Electrodeposited CuFeO2 Film. Coatings 2022, 12, 1820. https://doi.org/10.3390/coatings12121820
Son M-K. Effect of Deposition Parameters on Morphological and Compositional Characteristics of Electrodeposited CuFeO2 Film. Coatings. 2022; 12(12):1820. https://doi.org/10.3390/coatings12121820
Chicago/Turabian StyleSon, Min-Kyu. 2022. "Effect of Deposition Parameters on Morphological and Compositional Characteristics of Electrodeposited CuFeO2 Film" Coatings 12, no. 12: 1820. https://doi.org/10.3390/coatings12121820
APA StyleSon, M.-K. (2022). Effect of Deposition Parameters on Morphological and Compositional Characteristics of Electrodeposited CuFeO2 Film. Coatings, 12(12), 1820. https://doi.org/10.3390/coatings12121820





