Metallic Materials for Hydrogen Storage—A Brief Overview
Abstract
:1. Introduction
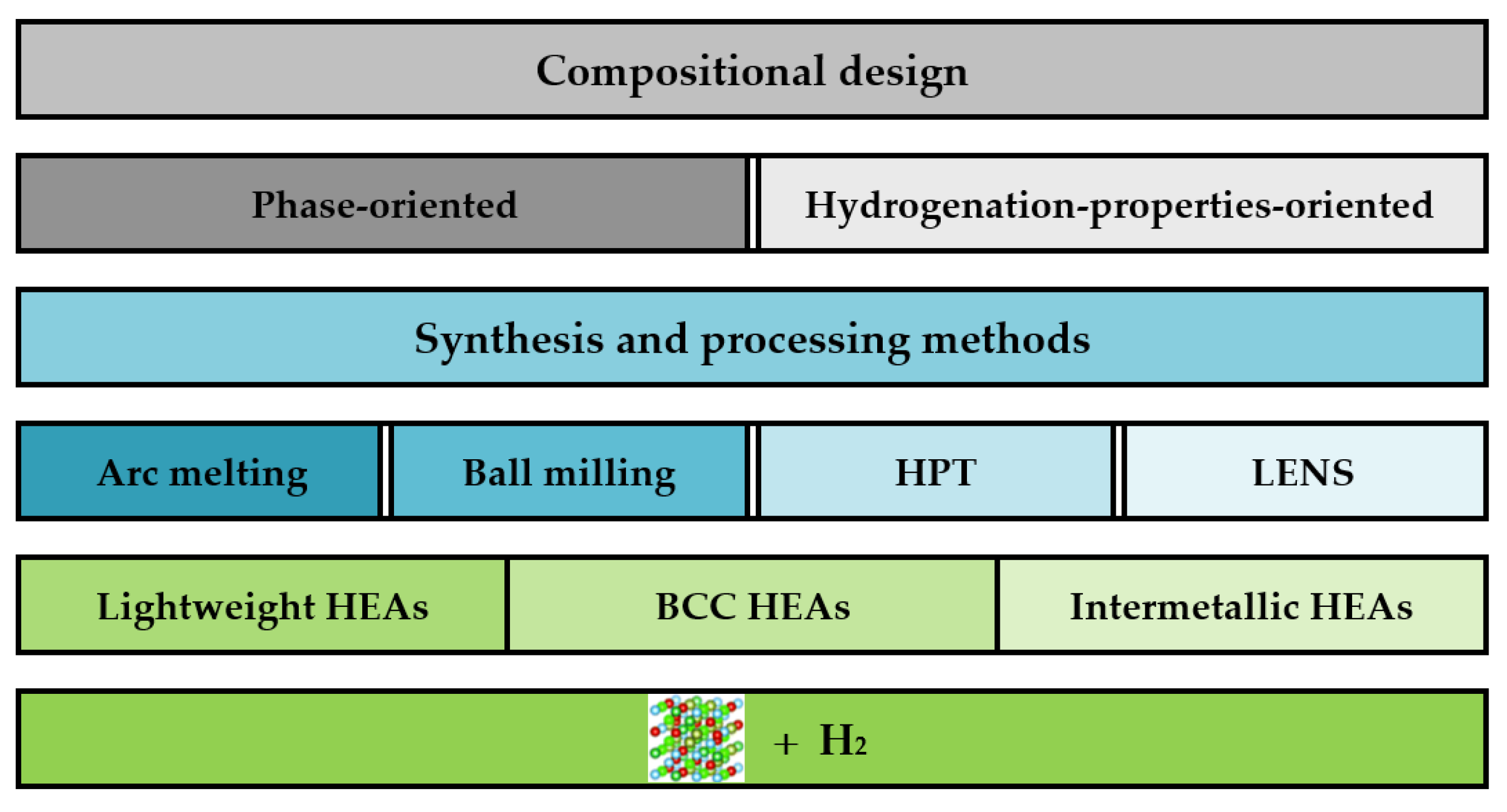
2. Methods for Designing the Composition of Alloys
2.1. Empirical Approach
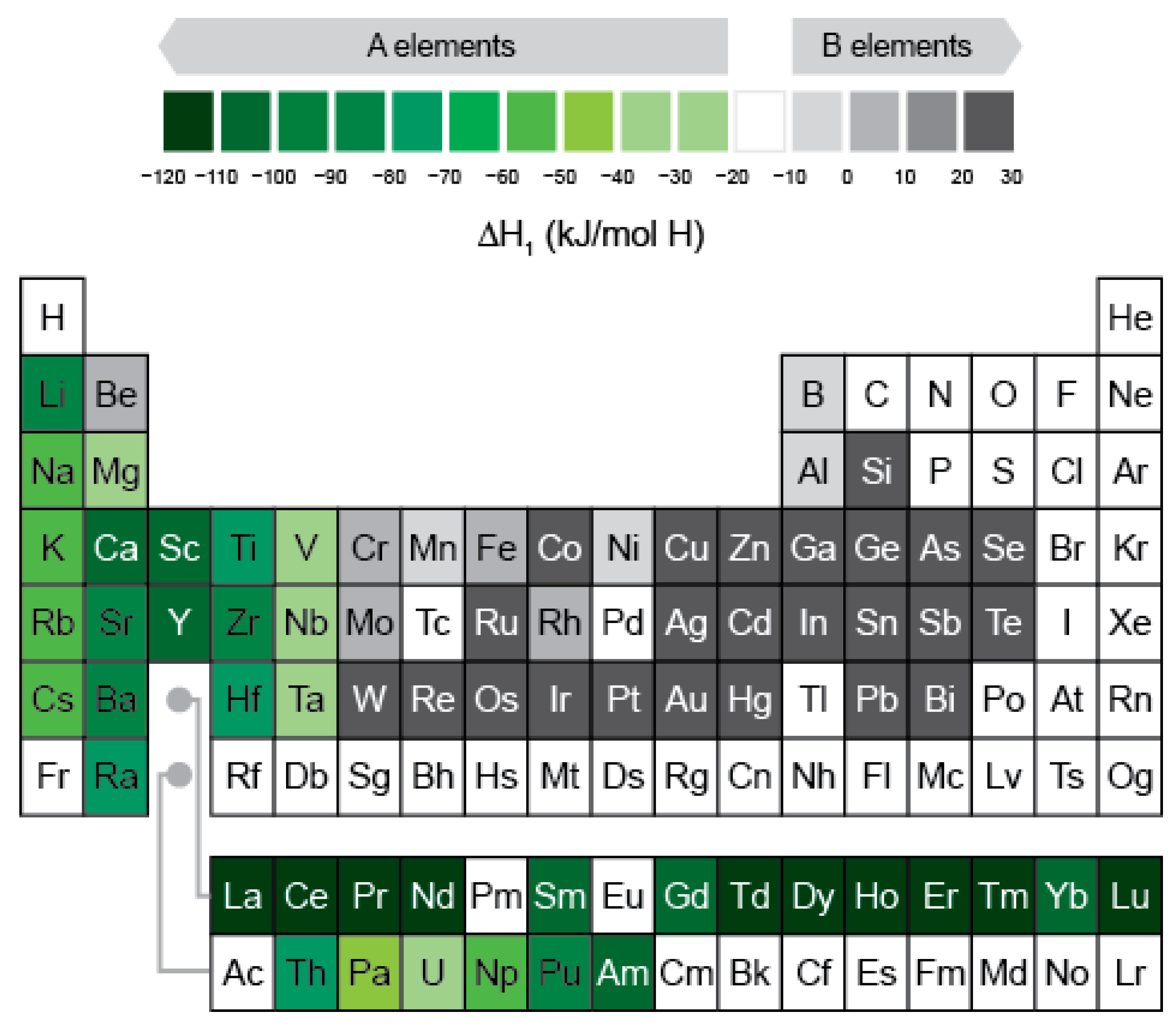
- Selection of the AB2 or AB system, where A represents hydride-forming elements such as Mg, Ti, Zr, V, Nb, etc. and B represents elements with low chemical affinity for hydrogen such as Cr, Mn, Fe, Co, Ni, etc.;
- Valence electron concentration (VEC) 6.4–6.5;
- The stability of the Laves phase, which should be investigated by thermodynamic calculations using the CALPHAD method (phase diagram calculation).
2.2. Semiempirical Approach
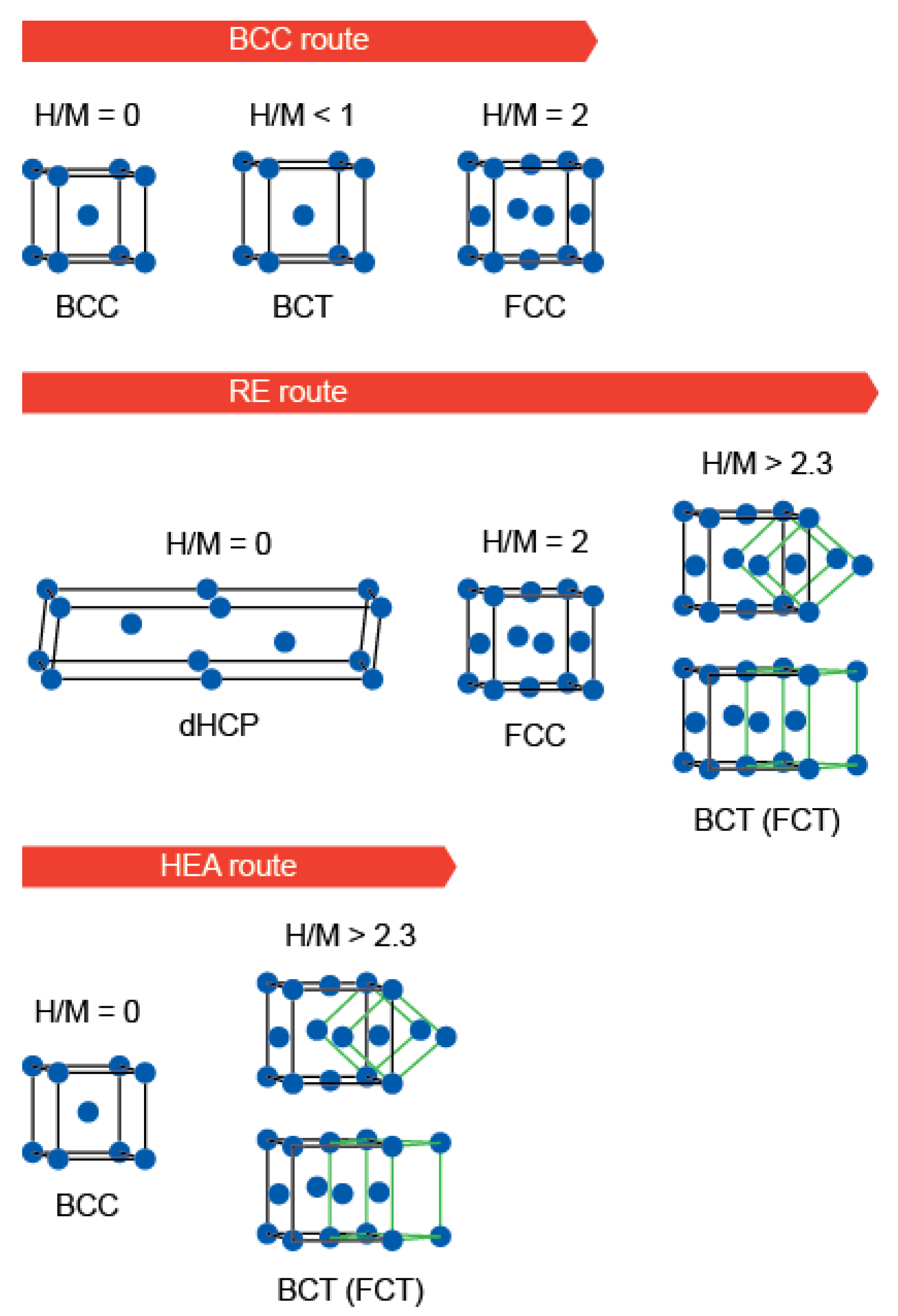
3. Absorption Properties of Selected HEAs
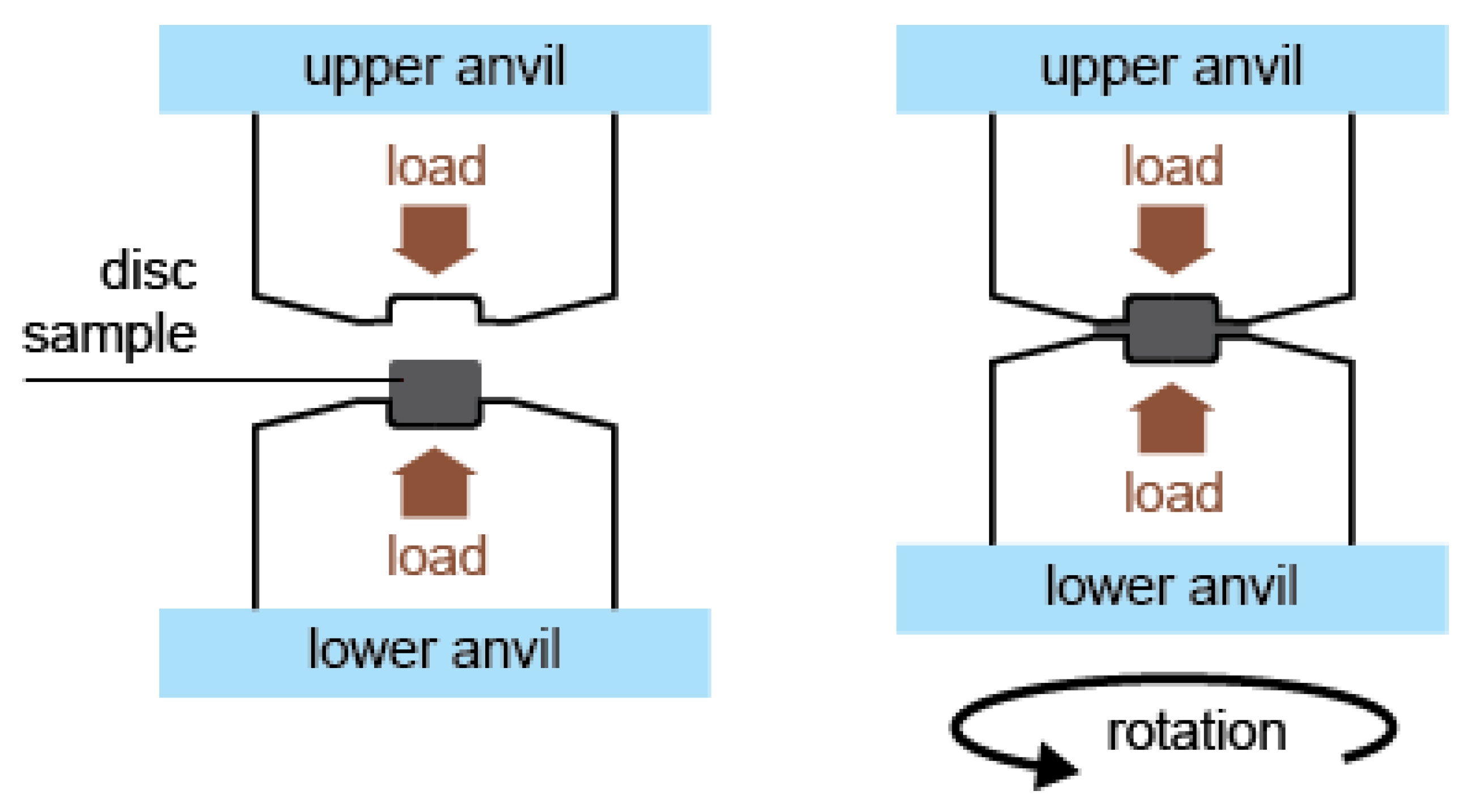
4. Manufacturing Processes (Methods) for Increasing Absorption Capacity
5. Summary
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Normalized Chemical Composition Ordered by Atomic Number | Synthesis and Processing | Alloy Phase | Hydride Phase | Structural Transf. upon Hydrogenation | H2 Absorp. Capacity (wt%) | H/M | H2 Absorp. Kinetics | Hydride Decompos. Onset/Peak Temperatures (K) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ti0.2Zr0.2Nb0.4Hf0.2 | Arc melting | BCC | FCC | 1 step | 1.12 | — | — | 656/— | [2] |
| Ti0.2Zr0.2Nb0.3Mo0.1Hf0.2 | Arc melting | BCC | FCC | 1 step | 1.54 | — | — | 605/— | [2] |
| Ti0.2Zr0.2Nb0.2Mo0.2Hf0.2 | Arc melting | BCC | FCC | 1 step | 1.18 | — | — | 575/— | [2] |
| Ti0.2Zr0.2Nb0.1Mo0.3Hf0.2 | Arc melting | BCC | BCT | 1 step | 1.40 | — | — | 437/— | [2] |
| Ti0.2Zr0.2Mo0.4Hf0.2 | Arc melting | BCC | BCT | 1 step | 0.92 | — | — | 441/— | [2] |
| Ti0.2V0.2Zr0.2Nb0.2Hf0.2 | Arc melting | BCC | BCT | — | 2.1 (573 K) 2.2 (473 K) | 1.94 (573 K) 2 (473 K) | 1.7 wt% in 300 s (573 K, 2 MPa | 623/— | [11] |
| Ti0.25V0.25Zr0.25Nb0.25 | Arc melting | BCC | FCC | 1 step | — | 1.98 (293 K) | — | ~573/— | [60] |
| Ti0.22V0.22Zr0.22Nb0.11Hf0.22 | Arc melting | BCC | BCT | — | — | 1.82 (293 K) | — | ~573/— | [60] |
| Ti0.22V0.22Zr0.11Nb0.22Hf0.22 | Arc melting | BCC | FCC | — | — | 1.99 (293 K) | — | ~593/— | [60] |
| Ti0.22V0.22Zr0.22Nb0.22Hf0.11 | Arc melting | BCC | FCC | — | — | 2.00 (293 K) | — | ~593/— | [60] |
| Ti0.22V0.11Zr0.22Nb0.22Hf0.22 | Arc melting | BCC | FCC | 1 step | — | 1.96 (293 K) | — | ~573/— | [60] |
| Ti0.11V0.22Zr0.22Nb0.22Hf0.22 | Arc melting | BCC | FCC | — | — | 1.97 (273 K) | — | ~573/— | [60] |
| Ti0.2V0.2Zr0.2Nb0.2Hf0.2 | Arc melting | BCC | FCC | 1 step | — | 1.99 (293 K) | ~593/— | [60] | |
| Ti0.25V0.25Zr0.25Hf0.25 | Arc melting | BCC | Phase separation | — | — | — | — | — | [60] |
| Ti0.25V0.25Nb0.25Hf0.25 | Arc melting | BCC | FCC | — | — | 1.99 (293 K) | — | ~593/— | [60] |
| Ti0.25V0.25Nb0.25Hf0.25 | Arc melting | BCC | BCT | — | — | 1.98 (293 K) | — | ~623/— | [60] |
| V0.25Zr0.25Nb0.25Hf0.25 | Arc melting | BCC (major) Unknown (minor) | Phase separation | — | — | — | — | — | [60] |
| Ti0.2V0.2Zr0.2Nb0.2Hf0.2 | Arc melting | BCC | BCT | 1 step | 2.7 (573 K) | 2.5 (573 K) | — | ~473/~673 | [70] |
| Ti0.2V0.2Zr0.2Nb0.2Hf0.2 | Arc melting (followed by ball milling) | BCC | FCC (293 K) BCT (723 K) | 1 step | 1.8 | 1.9 (562 K) | — | — | [58] |
| Ti0.2Zr0.2Nb0.2Hf0.2Ta0.2 | Arc melting (homogenized by induction heating) | BCC | FCC | 2 step | — | ~2.0 (573 K) | — | ~593/~648 | [59] |
| * Ti0.2V0.2Zr0.2Nb0.2Mo0.2 | LENS—300 W | BCC (major) NbTi4 (minor) | FCC (TiHx) BCC (NbH0.4) | — | 2.3 (323 K) 1.78 (673 K) after activation | — | 2.3 wt% in 1380 s (303 K, 8.5 MPa H2) | — | [30] |
| ** Ti0.2V0.2Zr0.2Nb0.2Mo0.2 | LENS—1000 W (3×) | BCC (major) Zr-rich (Ppt) | BCC (major) Zr-rich (Ppt) | — | 0.59 (323 K) 0.61 (673 K) after activation | — | 0.59 wt% in 1380 s (303 K, 8.5 MPa H2) | — | [30] |
| Ti0.25V0.25Zr0.25Nb0.25 | Arc melting | BCC | FCC | 1 step (phase separation upon 1 cycle) | — | ~1.9 | — | — | [65] |
| Ti0.24V0.24Zr0.28Nb0.24 | Arc melting | BCC | FCC | 1 step (phase separation upon 1 cycle) | — | ~1.9 | — | — | [65] |
| Ti0.22V0.22Zr0.33Nb0.22 | Arc melting | BCC | FCC | 1 step (phase separation upon 1 cycle) | — | ~1.9 | — | — | [65] |
| Ti0.21V0.21Zr0.37Nb0.21 | Arc melting | BCC | FCC | 1 step (phase separation upon 1 cycle) | — | ~1.9 | — | — | [65] |
| Ti0.2V0.2Zr0.4Nb0.2 | Arc melting | BCC | FCC | 1 step (phase separation upon 1 cycle) | — | ~1.9 | — | — | [65] |
| Ti0.25V0.25Zr0.04Nb0.25Ta0.21 | Arc melting | BCC | FCC (major) BCT (minor) | — | — | ~1.9 | — | — | [65] |
| Ti0.25V0.25Zr0.125Nb0.25Ta0.125 | Arc melting | BCC | FCC (major) BCC (minor) | 1 step (phase separation upon 1 cycle) | — | ~1.9 | — | — | [65] |
| Ti0.25V0.25Zr0.19Nb0.25Ta0.06 | Arc melting | BCC | FCC (major) BCC (minor) | 1 step (phase separation upon 1 cycle) | — | ~1.9 | — | — | [65] |
| Ti0.25V0.25Nb0.25Ta0.25 | Arc melting | BCC | FCC (major) BCT (minor) | — | — | 1.9 | — | ~498/— | [65] |
| * (VFe)60 (TiCrCo)40- xZrx | Arc melting | BCC (major) Laves phase C14 (minor) CeO2 (minor) FCC (minor) | — | 2 steps | 3.5 (298 K) | [64] | |||
| Ti0.25V0.25Cr0.25Mo0.25 | Arc melting | BCC | BCC | 1 step | — | ∼0.75 | — | ∼523/— | [42] |
| Ti0.2V0.2Cr0.2Nb0.2Ta0.2 | Arc melting | BCC | FCC (major) BCC (minor) | 1 step | — | ∼1.9 | — | 473 (1.Max) ~556 (2.Max)/— | [42] |
| Ti0.2V0.2Zr0.2Nb0.2Hf0.2 | Arc melting | BCC | FCC | 1 step | — | ∼1.9 | 553 (1.Max) ~666 (2.Max)/— | [42] | |
| Ti0.25V0.25Nb0.25Hf0.25 | Arc melting | BCC | FCC | 1 step | — | ∼2 | — | 553 (1.Max) ~648 (2.Max)/— | [42] |
| ** Ti0.25V0.25Nb0.25Ta0.25 | Arc melting | BCC | FCC (major) BCC (minor) | 1 step | — | ∼1.9 | — | ~503 (1.Max) ~602 (2.Max)/— | [42] |
| *** Ti0.25V0.25Cr0.25Nb0.25Mo 0.25 | Arc melting | BCC | FCC (major) BCC (minor) | 1 step | — | ∼2 | — | 473 (1. Max) ~556 (2.Max)/— | [42] |
| Ti0.25Zr0.25Nb0.25Hf0.25 | Arc melting | BCC | BCT | 1 step | — | ∼2 | — | 553 (1.Max) ~694 (2.Max)/— | [42] |
| Normalized Chemical Composition Ordered by Atomic Number | Chemical Composition | Synthesis Method | Alloy Phase | Maximum H2 Storage Capacity (wt% H2) | H2 Absorption Kinetics | H/M | Hydride Decomposition Temperature (K) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ti0.20Fe0.40Ni0.15Zr0.20Nb0.05 | Arc melting | Laves phases C14 (major) BCC (minor) | 1.38 | — | 0.95 | 305 ** | [33] | |
| Ti0.20Fe0.20Ni0.20Zr0.20Nb0.20 | Arc melting | Laves phases C14 (major) BCC (minor) | 1.64 | — | 1.17 | 305 ** | [33] | |
| Ti0.17V0.17Cr0.17Fe0.17Ni0.17Zr0.17 | LENS | Laves phases C14 (major) α-Ti solid solution (minor) | 1.81 | — | — | 323 * | [30] | |
| TixVyMnFeCoZrz | 0.5 ≤ x ≤ 2.5 0.4 ≤ y ≤ 3.0 0.4 ≤ z ≤ 3.0 | Arc melting | Laves phases C14 | 0.03–1.80 | 18 ≤ t0.9 (s) ≤ 1250 (298 K, 0.97 MPa H2) | 0.02–1.17 | 298 * | [54] |
| Ti0.17Cr0.17Mn0.17Fe0.17Ni0.17Zr0.17 | Arc melting + HPT | C14 Laves (major) | 1.7 | 1.6 wt% H2 in 60 s (303 K, 3.9 MPa H2) | 1 | 305 * | [43] |
| Normalized Chemical Composition Ordered by Atomic Number | Synthesis and Processing | Phases | H2 Storage Capacity (wt%) | H2 Absorp. Kinetics | H/M | Hydride Decompos. Onset/Peak Temperatures (K) | Enthalpy of Hydrogen Solution (kJ mol−1 H) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mg0.20Ti0.20V0.20Cr0.20Fe0.20 | Reactive milling
| BCC (major) MgH2 (minor) Amorphous phase (minor) | 0.3 (623 K) | 0.2 wt% in 3600 s (303 K, 2 MPa) | — | 483/520 (1.Max) 633 (2.Max) | — | [15] |
| Mg0.20Al0.20V0.20Cr0.20Ni0.20 | Mechanical alloying
| BCC | — | — | — | — | — | [38] |
| Mg0.20Al0.20V0.20Cr0.20Ni0.20 | Reactive milling
| BCC | 0.3 | — | 0.09–0.14 | 589/650 | +12.2 | [38] |
| Mg0.28Al0.19V0.28Cr0.19Ni0.06 | Reactive milling
| BCC (major) BCC (minor) MgH2 (minor) | 0.28–0.41 (calc, based on XRD data) | — | 0.11–0.16 | — | +9.84 | [38] |
| Mg0.26Al0.31V0.31Cr0.06Ni0.06 | Reactive milling
| BCC (major) BCC (minor) MgH2 (minor) | — | — | — | — | +8.36 | [38] |
| Mg0.22Ti0.22Cr0.11Mn0.11Ni0.11Nb0.22 | Reactive milling
| BCC (major) Cr (minor) Mn (minor) | — | — | 0.8 | — | — | [50] |
| Mg (minor) | [50] | ||||||
| Mg0.22Ti0.22Cr0.11Mn0.11Ni0.11Nb0.22 | Reactive milling
| FCC (major) Cr (minor) Mn (minor) Mn (minor) Mn2NiH4 (minor) | 1.6 | — | — | 493/576 (1. Max) 653 (2. Max) | — | [50] |
| Mg0.22Ti0.22Fe0.11Co0.11Ni0.11Zr0.22 | Reactive milling
| BCC | 1.2 (623 K) | 1.0 wt.% in1800 s (623 K, 2 MPa H2) | 0.67 | — | −14.4 | [51] |
| Mg0.22Ti0.22Fe0.11Co0.11Ni0.11Zr0.22 | Reactive milling
| FCC | — | — | — | 503/573 (1. Max) 648 (2. Max) | — | [51] |
| Al0.17Cr0.17Mn0.17Fe0.17Ni0.17W0.17 | Mechanical alloying
| BCC (major) FCC (minor) | 0.62 (293 K) | — | — | 358/— | +11 | [52] |
| Mg0.10Ti0.30V0.25Zr0.10Nb0.25 | Mechanical alloying
| BCC | 2.7 (298 K) | 2.7 wt% in 60 s (298 K, 2.5 MPa) | 1.72 | 523/563 | — | [48] |
| Mg0.10Ti0.30V0.25Zr0.10Nb0.25 | Reactive milling
| FCC | — | — | 1.65 | — | — | [48] |
References
- Hu, J.; Zhang, J.; Xiao, H.; Xie, L.; Sun, G.; Shen, H.; Li, P.; Zhang, J.; Zu, X. A first-principles study of hydrogen storage of high entropy alloy TiZrVMoNb. Int. J. Hydrog. Energy 2021, 46, 21050–21058. [Google Scholar] [CrossRef]
- Shen, H.; Hu, J.; Li, P.; Huang, G.; Zhang, J.; Zhang, J.; Mao, Y.; Xiao, H.; Zhou, X.; Zu, X.; et al. Compositional dependence of hydrogenation performance of Ti-Zr-Hf-Mo-Nb high-entropy alloys for hydrogen/tritium storage. J. Mater. Sci. Technol. 2020, 55, 116–125. [Google Scholar] [CrossRef]
- Rabiee, A.; Mohseni-Bonab, M.B. Maximizing hosting capacity of renewable energy sources in distribution networks: A multi-objective and scenario-based approach. Energy 2017, 120, 417–430. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Zhang, Y.; Zhang, Y.; Wu, Z.; Zhang, Z.; Zhao, F.; Huot, J.; Novaković, J.G.; Novaković, N. Recent progress on the development of high entropy alloys (HEAs) for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2022, 47, 11236–11249. [Google Scholar] [CrossRef]
- Beccali, M.; Brunone, S.; Finocchiaro, P.; Galletto, J.M. Method for size optimisation of large wind–hydrogen systems with high penetration on power grids. Appl. Energy 2013, 102, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Hydrogen Storage: Hydrogen and Fuel Cell Technologies Office. Energy.gov [online]. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 2 October 2022).
- Boateng, E.; Chen, A. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022. [Google Scholar] [CrossRef]
- Broom, D.P.; Webb, C.J.; Fanourgakis, G.S.; Froudakis, G.E.; Trikalitis, P.N.; Hirscher, M. Concepts for improving hydrogen storage in nanoporous materials. Int. J. Hydrog. Energy 2019, 44, 7768–7779. [Google Scholar] [CrossRef]
- Zacharia, R.; Rather, S.U. Review of Solid State Hydrogen Storage Methods Adopting Different Kinds of Novel Materials. J. Nanomater. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrog. Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Desantis, D.; Mason, J.A.; James, B.D.; Houchins, C.; Long, J.R.; Veenstra, M. Techno-economic Analysis of Metal-Organic Frameworks for Hydrogen and Natural Gas Storage. Energy Fuels 2017, 31, 2024–2032. [Google Scholar] [CrossRef]
- Kurko, S.; Milanović, I.; Grbović Novaković, J.; Ivanović, N.; Novaković, N. Investigation of surface and near-surface effects on hydrogen desorption kinetics of MgH2. Int. J. Hydrog. Energy 2014, 39, 862–867. [Google Scholar] [CrossRef]
- Scheemann, A.J.; White, L.; Kangs, S.Y.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef] [PubMed]
- Losertová, M. Vodíkové Hospodářství; VŠB-TUO: Ostrava, The Czech Republic, 2010. [Google Scholar]
- de Marco, M.O.; Li, Y.; Li, H.-W.; Edalati, K.; Floriano, R. Mechanical Synthesis and Hydrogen Storage Characterization of MgVCr and MgVTiCrFe High-Entropy Alloy. Adv. Eng. Mater. 2020, 22, 1901079. [Google Scholar] [CrossRef]
- Edalati, K.; Li, H.-W.; Kilmametov, A.; Floriano, R.; Borchers, C. High-Pressure Torsion for Synthesis of High-Entropy Alloys. Metals 2021, 11, 1263. [Google Scholar] [CrossRef]
- Marques, F.; Balcerzak, M.; Winkelmann, F.; Zepon, G.; Felderhoff, M. Review and outlook on high-entropy alloys for hydrogen storage. Energy Environ. Sci. 2021, 14, 5191–5227. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Berti, N.; Cuevas, F.; Latroche, M.; Baricco, M. Substitutional effects in TiFe for hydrogen storage: A comprehensive review. Mater. Adv. 2021, 2, 2524–2560. [Google Scholar] [CrossRef]
- Lys, A.; Fadonougbo, J.O.; Faisal, M.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Park, J.; Cho, Y.-W. Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review. Hydrogen 2020, 1, 38–63. [Google Scholar] [CrossRef]
- Grbović Novaković, J.; Novaković, N.; Kurko, S.; Govedarović, S.M.; Pantić, T.; Mamula, B.P.; Batalović, K.; Radaković, J.; Rmuš, J.; Shelyapina, M.; et al. Influence of Defects on the Stability and Hydrogen-Sorption Behavior of Mg-Based Hydrides. Chemphyschem 2019, 20, 1216–1247. [Google Scholar] [CrossRef]
- Milanović, I.; Milošević, S.; Rašković-Lovre, Ž.; Novaković, N.; Vujasin, R.; Matović, L.; Fernández, J.F.; Sánchez, C.; Novaković, J.G. Microstructure and hydrogen storage properties of MgH2–TiB2–SiC composites. Ceram. Int. 2013, 39, 4399–4405. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yao, H.; Qiao, J.-W.; Gao, M.; Hawk, J.; Ma, S.-G.; Zhou, H. MoNbTaV Medium-Entropy Alloy. Entropy 2016, 18, 189. [Google Scholar] [CrossRef] [Green Version]
- Floriano, R.; Zepon, G.; Edalati, K.; Fontana GL, B.G.; Mohammadi, A.; Ma ZLi, H.-W.; Contieri, R.J. Hydrogen storage in TiZrNbFeNi high entropy alloys, designed by thermodynamic calculations. Int. J. Hydrog. Energy 2020, 45, 33759–33770. [Google Scholar] [CrossRef]
- Batalović, K.; Radaković, J.; Paskaš Mamula, B.; Kuzmanović, B.; Medić Illić, M. Predicting the Heat of Hydride Formation by Graph Neural Network—Exploring the Structure–Property Relation for Metal Hydrides. Adv. Theory Simul. 2022, 5, 2200293. [Google Scholar] [CrossRef]
- Edalati, K.; Shao, H.; Emami, H.; Iwaoka, H.; Akiba, E.; Horita, Z. Activation of titanium-vanadium alloy for hydrogen storage by introduction of nanograins and edge dislocations using high-pressure torsion. Int. J. Hydrog. Energy 2016, 41, 8917–8924. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. A review on high-pressure torsion (HPT) from 1935 to 1988. Mater. Sci. Eng. A 2016, 652, 325–352. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using Laser Engineered Net Shaping (LENS). Int. J. Hydrog. Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Thermodynamics-Interaction Studies-Solids, Liquids and Gases; Moreno-Pirajan, J.C. (Ed.) InTechOpen: London, UK, 2011; ISBN 978-953-307-563-1. [Google Scholar]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Floriano, R.; Zepon, G.; Edalati, K.; Fontana GL, B.G.; Mohammadi, A.; Ma, Z.; Li, H.-W.; Contieri, R.J. Hydrogen storage properties of new A3B2-type TiZrNbCrFe high-entropy alloy. Int. J. Hydrog. Energy 2021, 46, 23757–23766. [Google Scholar] [CrossRef]
- Shao, H.; Asano, H.; Enoki, H.; Akiba, E. Preparation and hydrogen storage properties of nanostructured Mg-Ni BCC alloys. J. Alloy. Compd. 2009, 477, 301–306. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Manivasagam, T.; Kiraz, K.; Notten, P. Electrochemical and Optical Properties of Magnesium-Alloy Hydrides Reviewed. Crystals 2012, 2, 1410–1433. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, J.H.; Fleischer, R.L. (Eds.) Intermetallic Compounds; Wiley-VCH: Hoboken, NJ, USA, 2002; Volume 3, p. 1086. ISBN 0-471-49315-5. [Google Scholar]
- Strozi, R.B.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Synthesis and hydrogen storage behavior of Mg-V-Al-Cr-Ni high entropy alloys. Int. J. Hydrog. Energy 2021, 46, 2351–2361. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sørby, M.H.; Sahlberg, M.; Hauback, B.C. Counting electrons—A new approach to tailor the hydrogen sorption properties of high-entropy alloys. Acta Mater. 2019, 175, 121–129. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Mohammadi, A.; Li, Y.; Zepon, G.; Li, H.-W.; Edalati, K. Reversible room temperature hydrogen storage in high-entropy alloy TiZrCrMnFeNi. Scr. Mater. 2020, 178, 387–390. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Ågren, J. Calculation of phase diagrams: Calphad. Curr. Opin. Solid State Mater. Sci. 1996, 1, 355–360. [Google Scholar] [CrossRef]
- Spencer, P.J. A brief history of CALPHAD. Calphad 2008, 32, 1–8. [Google Scholar] [CrossRef]
- Witman, M.; Ek, G.; Ling, S.; Chames, J.; Agarwal, S.; Wong, J.; Allendorf, M.D.; Sahlberg, M.; Stavila, V. Data-Driven Discovery and Synthesis of High Entropy Alloy Hydrides with Targeted Thermodynamic Stability. Chem. Mater. 2021, 33, 4067–4076. [Google Scholar] [CrossRef]
- Montero, J.; Ek, G.; Sahlberg, M.; Zlotea, C. Improving the hydrogen cycling properties by Mg addition in Ti-V-Zr-Nb refractory high entropy alloy. Scr. Mater. 2021, 194, 113699. [Google Scholar] [CrossRef]
- Coudert, F.-X. Materials Databases: The Need for Open, Interoperable Databases with Standardized Data and Rich Metadata. Adv. Theory Simul. 2019, 2, 1900131. [Google Scholar] [CrossRef]
- Marques, F.; Pinto, H.C.; Figueroa SJ, A.; Winkelmann, F.; Felderhoff, M.; Botta, W.J.; Zepon, G. Mg-containing multi-principal element alloys for hydrogen storage: A study of the MgTiNbCr0.5Mn0.5Ni0.5 and Mg0.68TiNbNi0.55 compositions. Int. J. Hydrog. Energy 2020, 45, 19539–19552. [Google Scholar] [CrossRef]
- Zepon, G.; Leiva, D.R.; Strozi, R.B.; Bedoch, A.; Figueroa SJ, A.; Ishikawa, T.T.; Botta, W.J. Hydrogen-induced phase transition of MgZrTiFe0.5Co0.5Ni0.5 high entropy alloy. Int. J. Hydrog. Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Sharma, V.K.; Sahu, P.; Kumar, V. Synthesis and characterization of hydrogenated novel AlCrFeMnNiW high entropy alloy. Int. J. Hydrog. Energy 2020, 45, 16984–16991. [Google Scholar] [CrossRef]
- Hu, J.; Shen, H.; Jiang, M.; Gong, H.; Xiao, H.; Liu, Z.; Sun, G.; Zu, X. A DFT Study of Hydrogen Storage in High-Entropy Alloy TiZrHfScMo. Nanomaterials 2019, 9, 461. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.-F.; Chen, S.-K.; Sheu, J.-H.; Lin, W.-E.; Yeh, J.-W.; Lin, S.-J.; Liou, T.-H.; Wang, C.-W. Hydrogen storage properties of multi-principal-component CoFeMnTixVyZrz alloys. Int. J. Hydrog. Energy 2010, 35, 9046–9059. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, J.; Ek, G.; Laversenne, L.; Nassif, V.; Zepon, G.; Sahlberg, M.; Zlotea, C. Hydrogen storage properties of the refractory Ti–V–Zr–Nb–Ta multi-principal element alloy. J. Alloys Compd. 2020, 835, 155376. [Google Scholar] [CrossRef]
- Montero, J.; Zlotea, C.; Ek, G.; Crivello, J.C.; Laversenne, L.; Sahlberg, M. TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties. Molecules 2019, 24, 2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, D.; Ek, G.; Cedervall, J.; Zlotea, C.; Møller, K.T.; Hansen, T.C.; Bednarčík, J.; Paskevicius, M.; Sørby, M.H.; Jensen, T.R.; et al. Structure and Hydrogenation Properties of a HfNbTiVZr High-Entropy Alloy. Inorg. Chem. 2018, 57, 2103–2110. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.-P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Ek, G.; Nygård, M.M.; Pavan, A.F.; Montero, J.; Henry, P.F.; Sørby, M.H.; Witman, M.; Stavila, V.; Zlotea, C.; Hauback, B.C.; et al. Elucidating the Effects of the Composition on Hydrogen Sorption in TiVZrNbHf-Based High-Entropy Alloys. Inorg. Chem. 2021, 60, 1124–1132. [Google Scholar] [CrossRef]
- Westlake, D.G. Site occupancies and stoichiometries in hydrides of intermetallic compounds: Geometric considerations. J. Less Common Met. 1983, 90, 251–273, ISSN 00225088. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Wu, C.; Chen, Y.; Mao, Y.; Luo, L. Hydrogen storage and cyclic properties of (VFe)60(TiCrCo)40-xZrx (0 ≤ x ≤ 2) alloys. J. Alloys Compd. 2016, 663, 460–465. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sahlberg, M.; Sørby, M.H.; Hauback, B.C. Hydrogen storage in high-entropy alloys with varying degree of local lattice strain. Int. J. Hydrog. Energy 2019, 44, 29140–29149. [Google Scholar] [CrossRef] [Green Version]
- Sleiman, S.; Huot, J. Effect of particle size, pressure and temperature on the activation process of hydrogen absorption in TiVZrHfNb high entropy alloy. J. Alloys Compd. 2021, 861, 158615. [Google Scholar] [CrossRef]
- Montero, J.; Ek, G.; Laversenne, L.; Nassif, V.; Sahlberg, M.; Zlotea, C. How 10 at% Al Addition in the Ti-V-Zr-Nb High-Entropy Alloy Changes Hydrogen Sorption Properties. Molecules 2021, 26, 2470. [Google Scholar] [CrossRef] [PubMed]
- Edalati, K.; Akiba, E.; Horita, Z. High-pressure torsion for new hydrogen storage materials. Sci. Technol. Adv. Mater. 2018, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Huang, H.; Luo, A.A. Phase formations in low density high entropy alloys. Calphad 2017, 56, 19–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Lee, D.-H.; Lee, J.A.; Kim, W.-J.; Han, H.-N.; Ramamurty, U.; Suh, J.-Y.; Jang, J.-I. Hydrogen-induced nanohardness variations in a CoCrFeMnNi high-entropy alloy. Int. J. Hydrog. Energy 2017, 42, 12015–12021. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hájková, P.; Horník, J.; Čižmárová, E.; Kalianko, F. Metallic Materials for Hydrogen Storage—A Brief Overview. Coatings 2022, 12, 1813. https://doi.org/10.3390/coatings12121813
Hájková P, Horník J, Čižmárová E, Kalianko F. Metallic Materials for Hydrogen Storage—A Brief Overview. Coatings. 2022; 12(12):1813. https://doi.org/10.3390/coatings12121813
Chicago/Turabian StyleHájková, Pavlína, Jakub Horník, Elena Čižmárová, and František Kalianko. 2022. "Metallic Materials for Hydrogen Storage—A Brief Overview" Coatings 12, no. 12: 1813. https://doi.org/10.3390/coatings12121813
APA StyleHájková, P., Horník, J., Čižmárová, E., & Kalianko, F. (2022). Metallic Materials for Hydrogen Storage—A Brief Overview. Coatings, 12(12), 1813. https://doi.org/10.3390/coatings12121813







