Recycling of Glass Waste by Deposition of TiO2 for the Intensification of the Photocatalytic Effect in the Purification of Wastewater
Abstract
1. Introduction
2. Materials and Methods
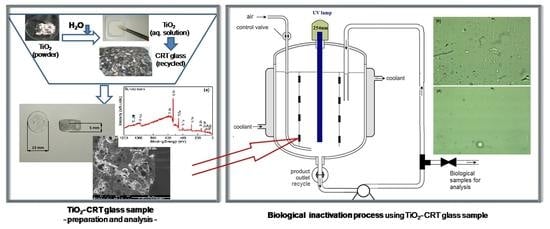
2.1. TiO2 Covered CRT Glass Samples
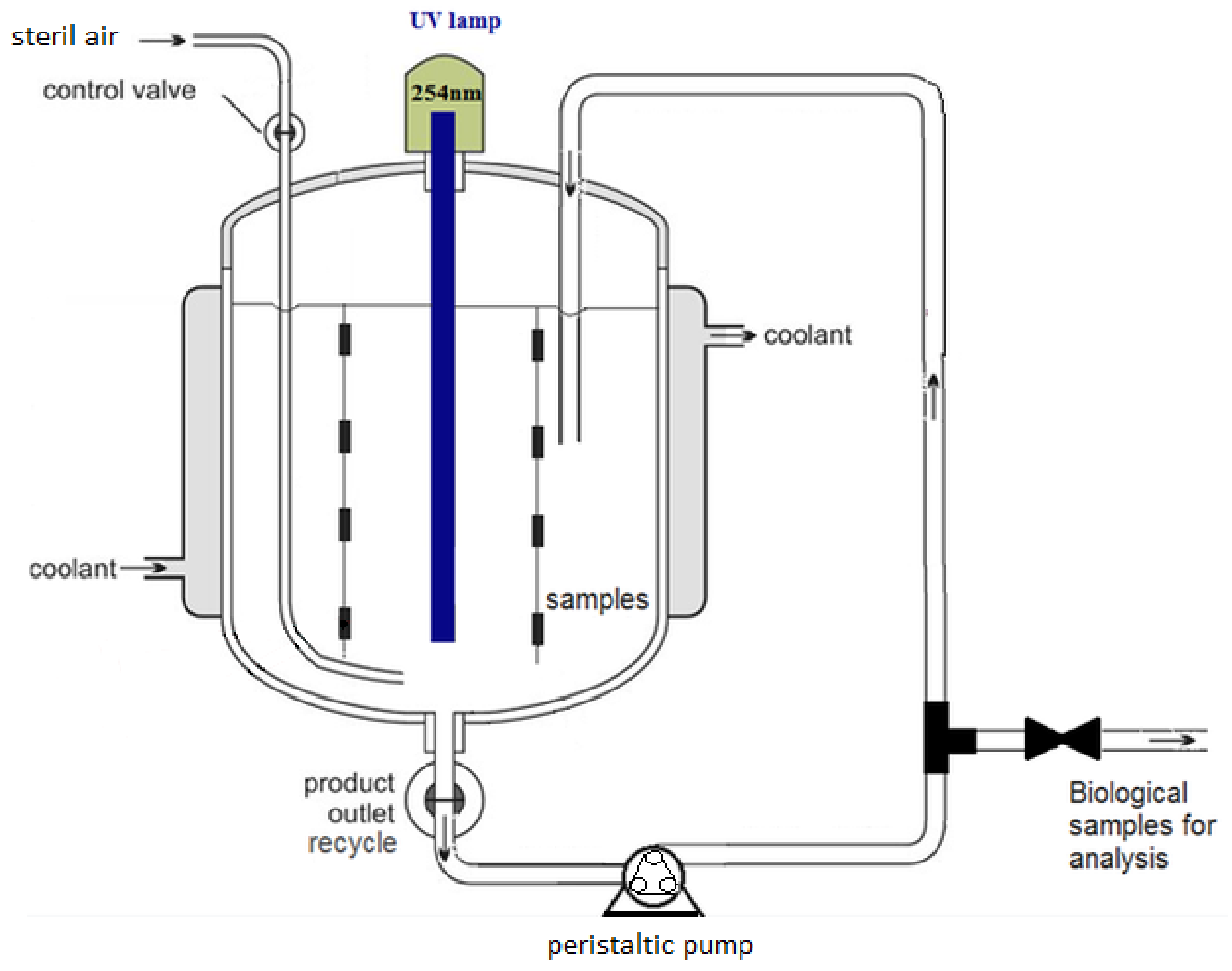
2.2. The Experimental Set-Up
| Control sample | Cultivation of the bacterial strain in the photoreactor (under the conditions described above) under UV and without photocatalytic material |
| Experiment 1 | Cultivation of the bacterial strain under UV and in the presence of photocatalytic material (3 wt. % TiO2) |
| Experiment 2 | Cultivation of the bacterial strain under UV and in the presence of photocatalytic material (1 wt. % TiO2) |
2.3. Instrumental Analysis
3. Results and Discussions
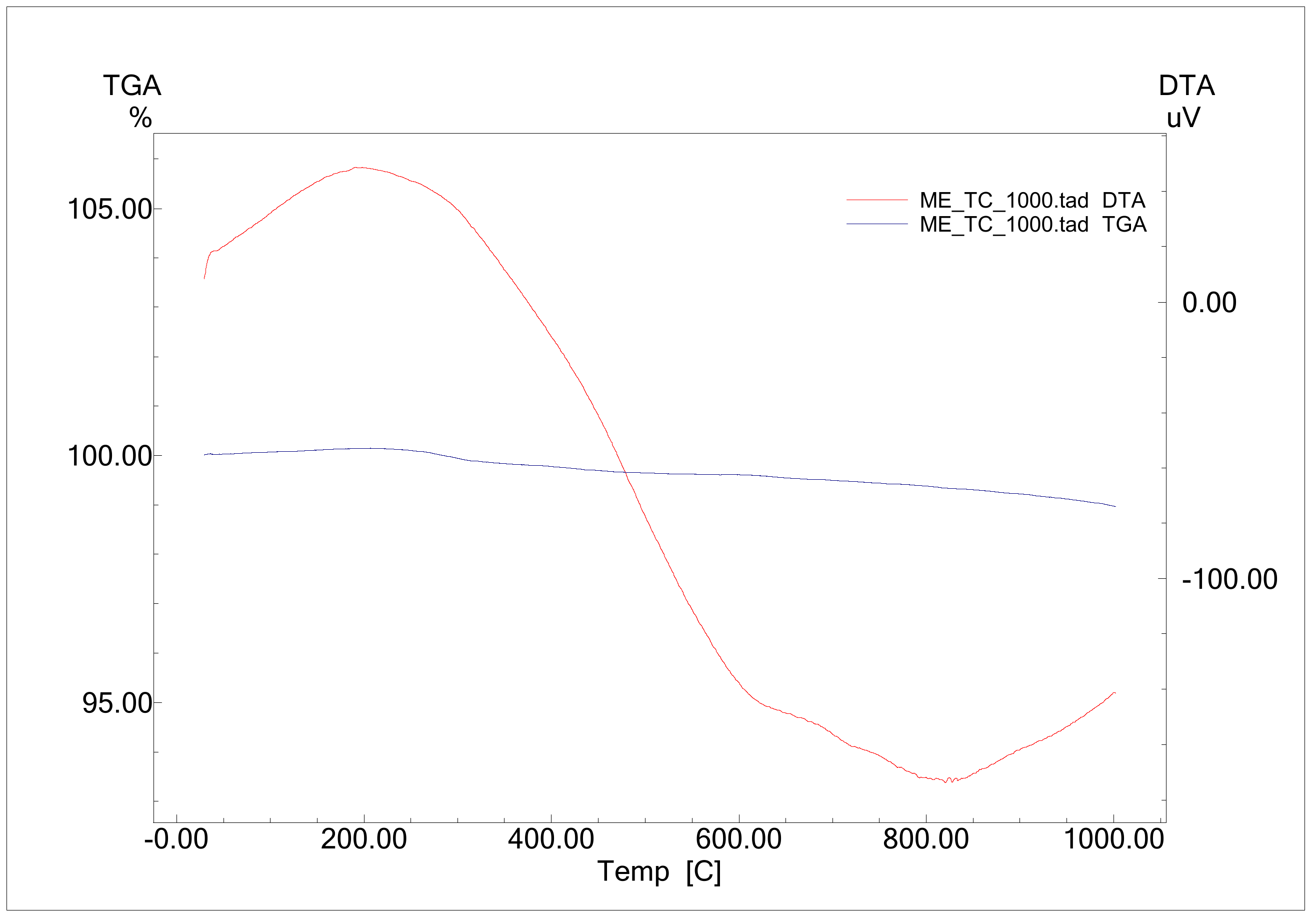
3.1. Thermal and Microstructural Characterization of CRT Waste
3.2. NAP-XPS Analysis
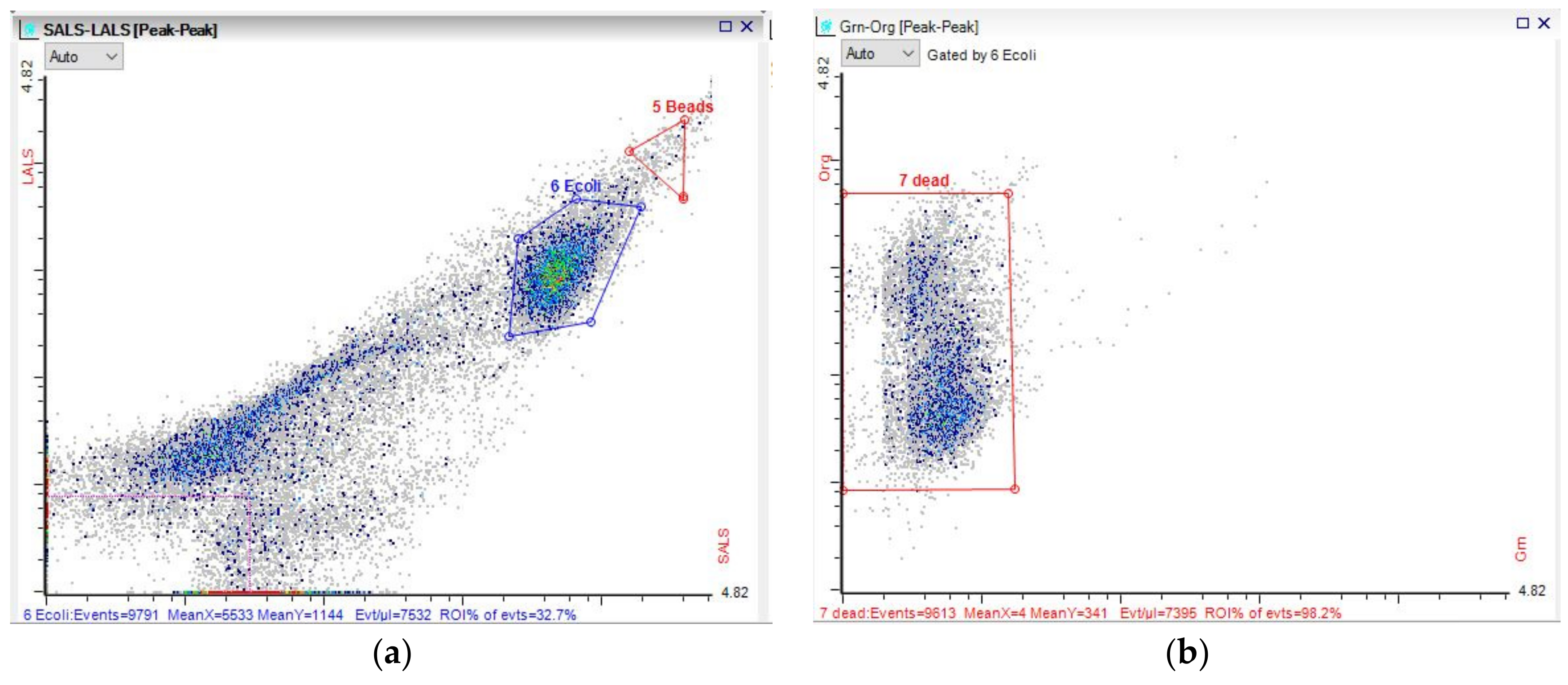
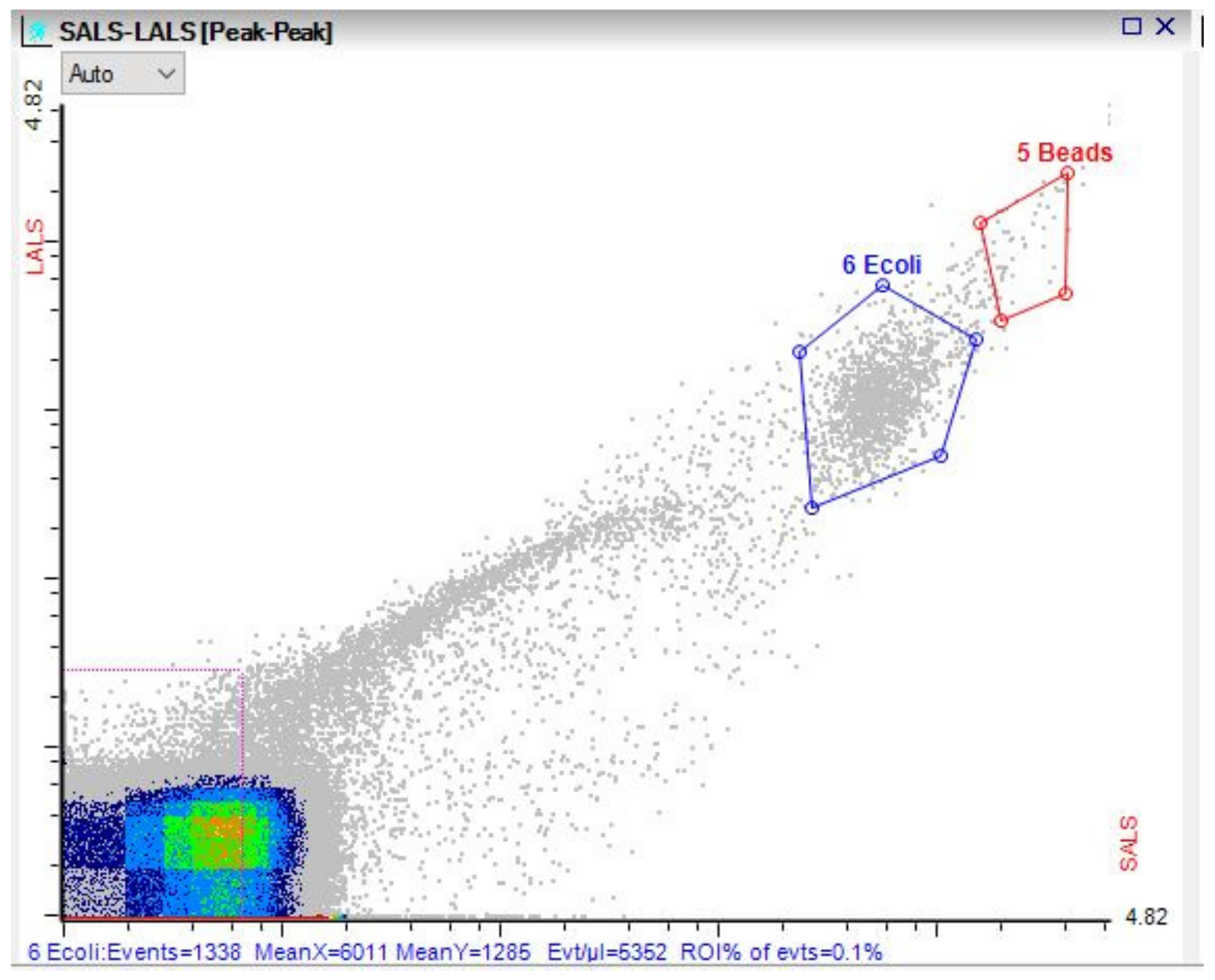
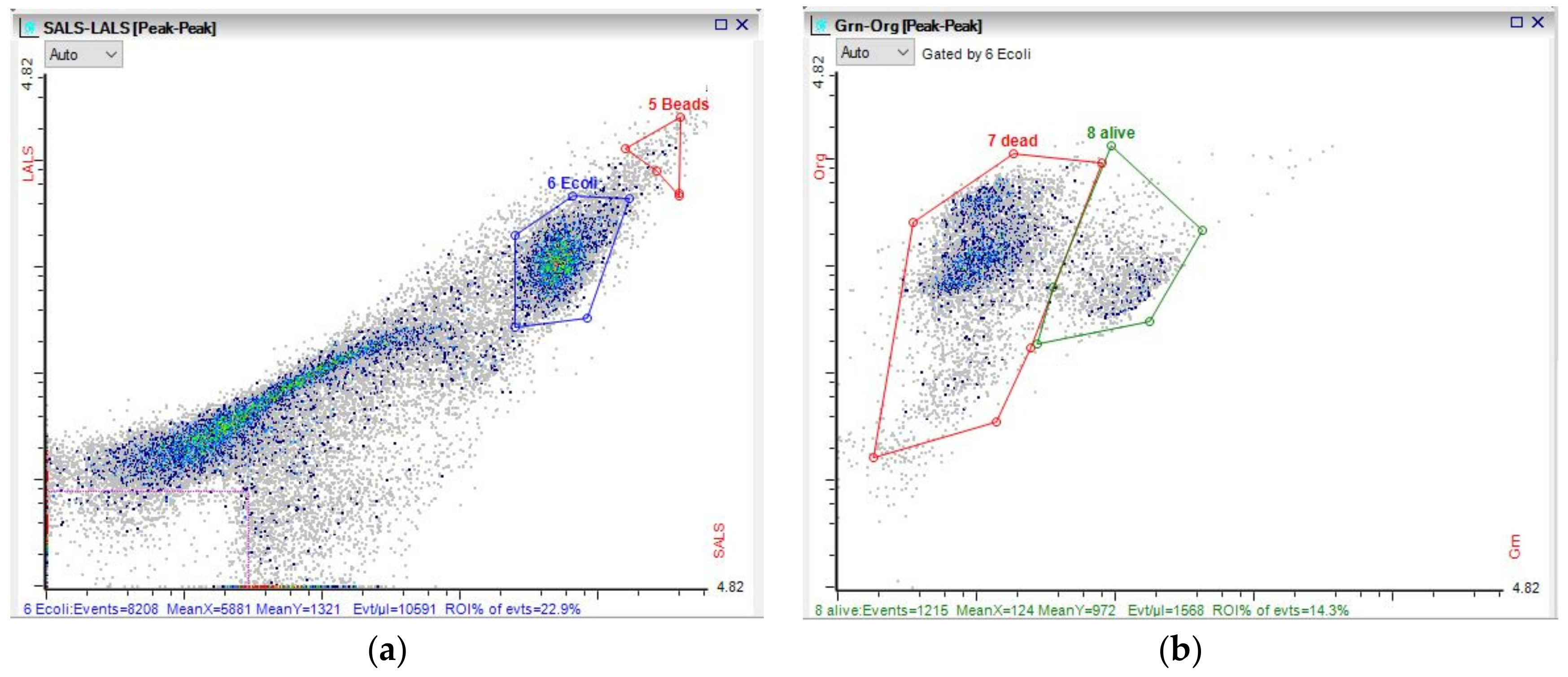
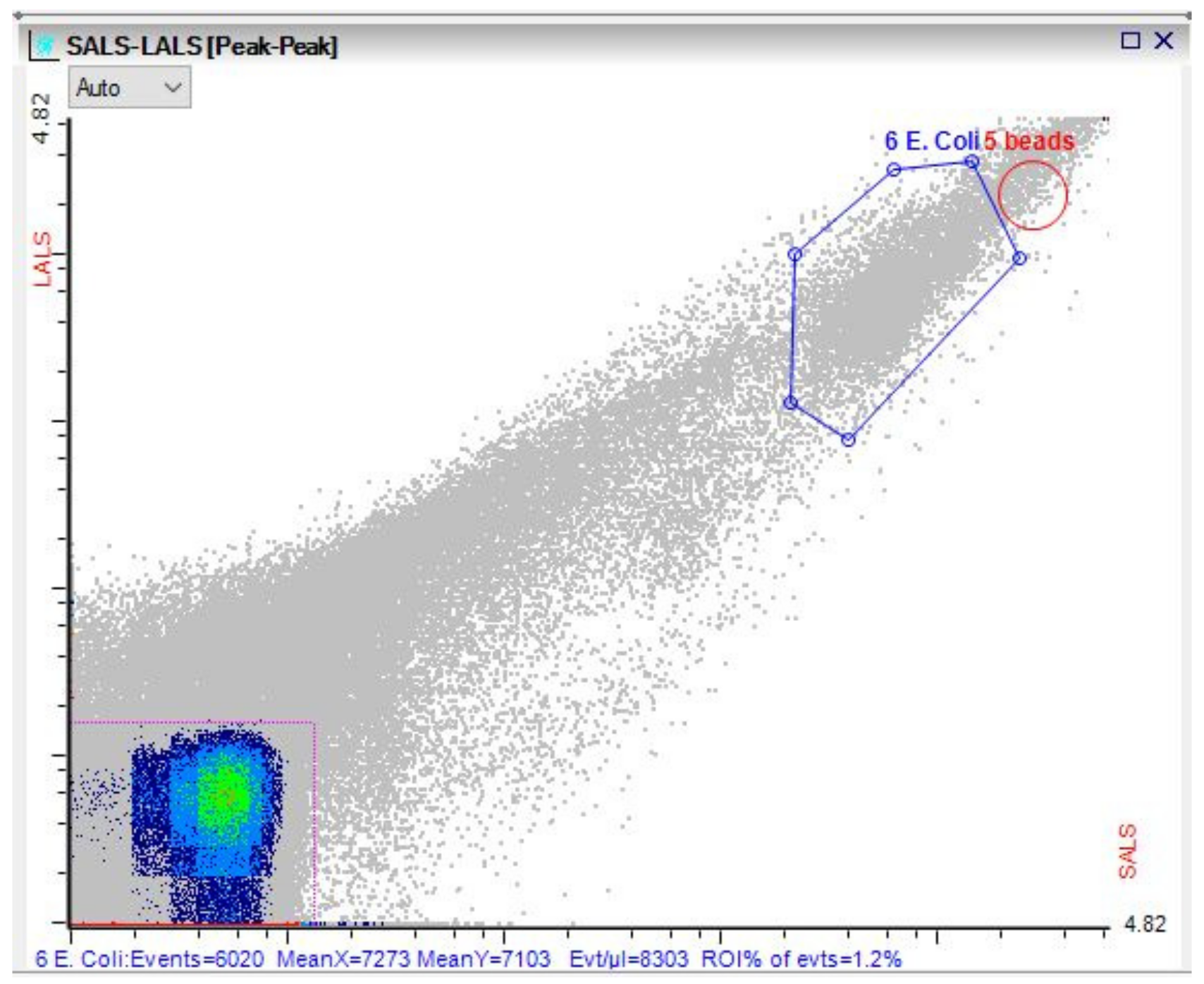
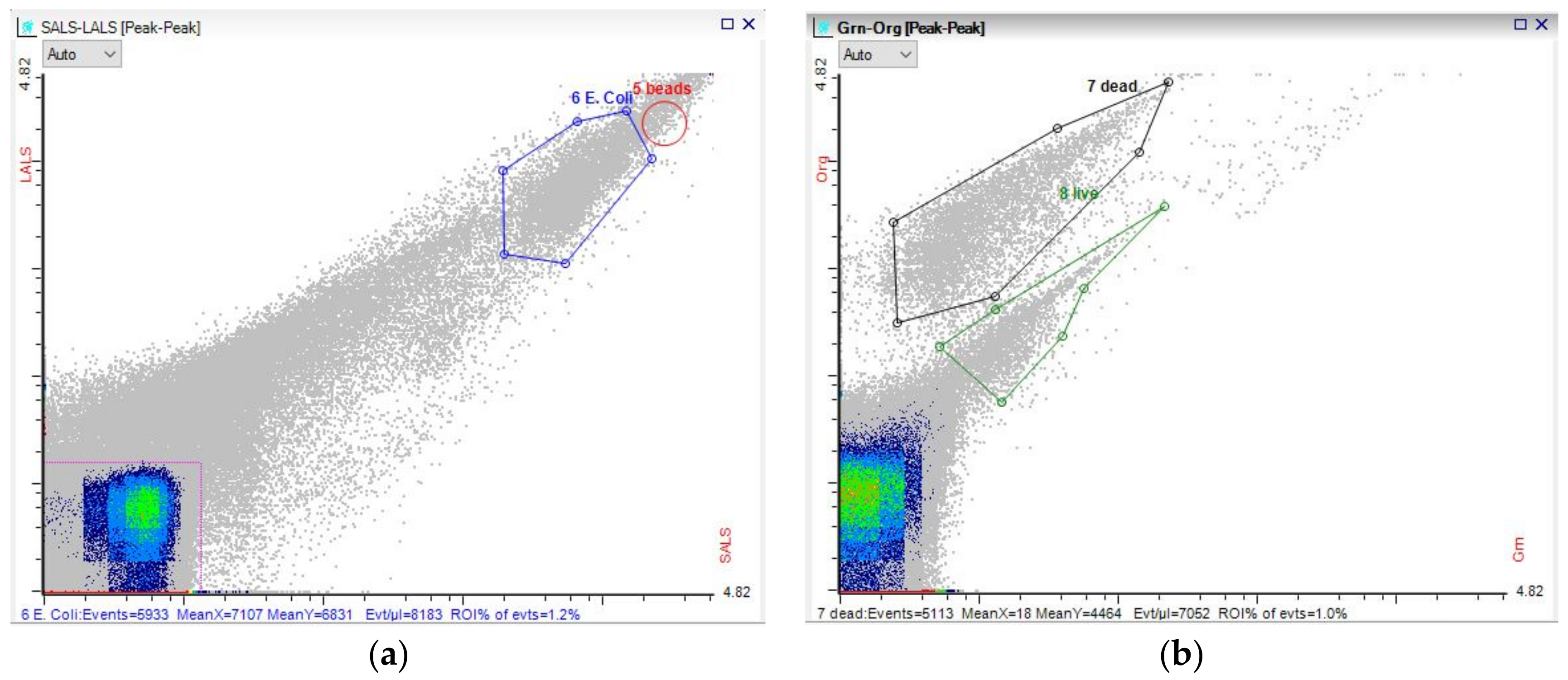
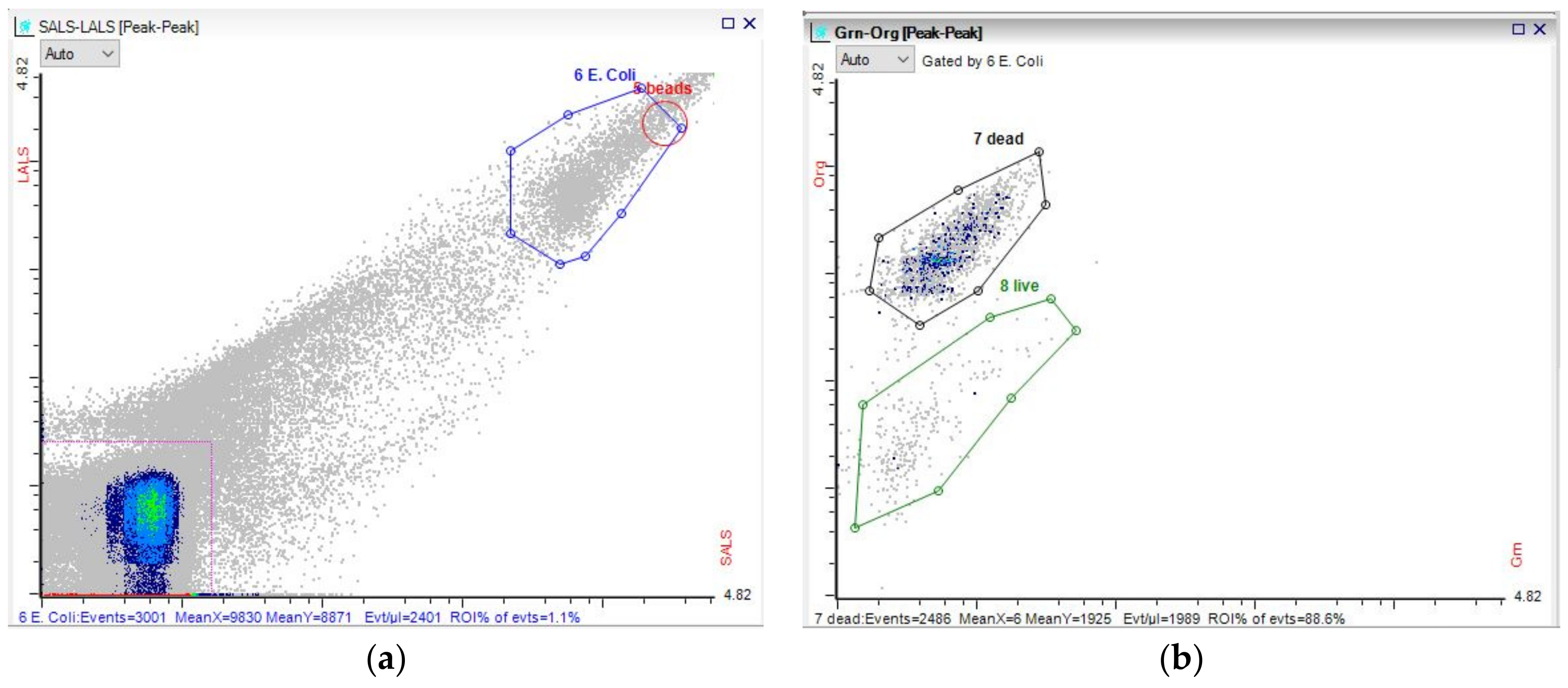
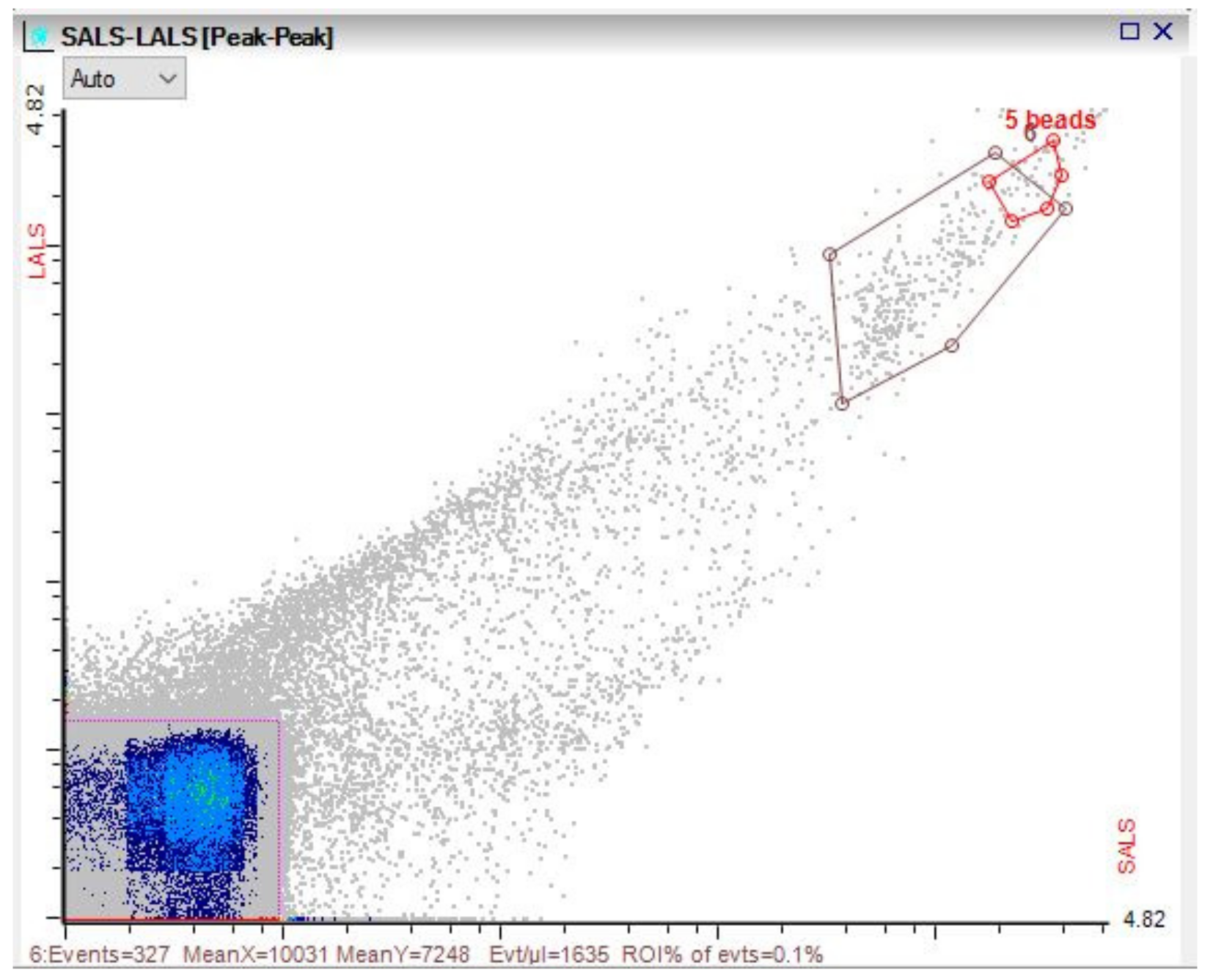
3.3. Microbial Dynamics
3.4. Mathematical Calculus
3.5. Kinetics of Inactivation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


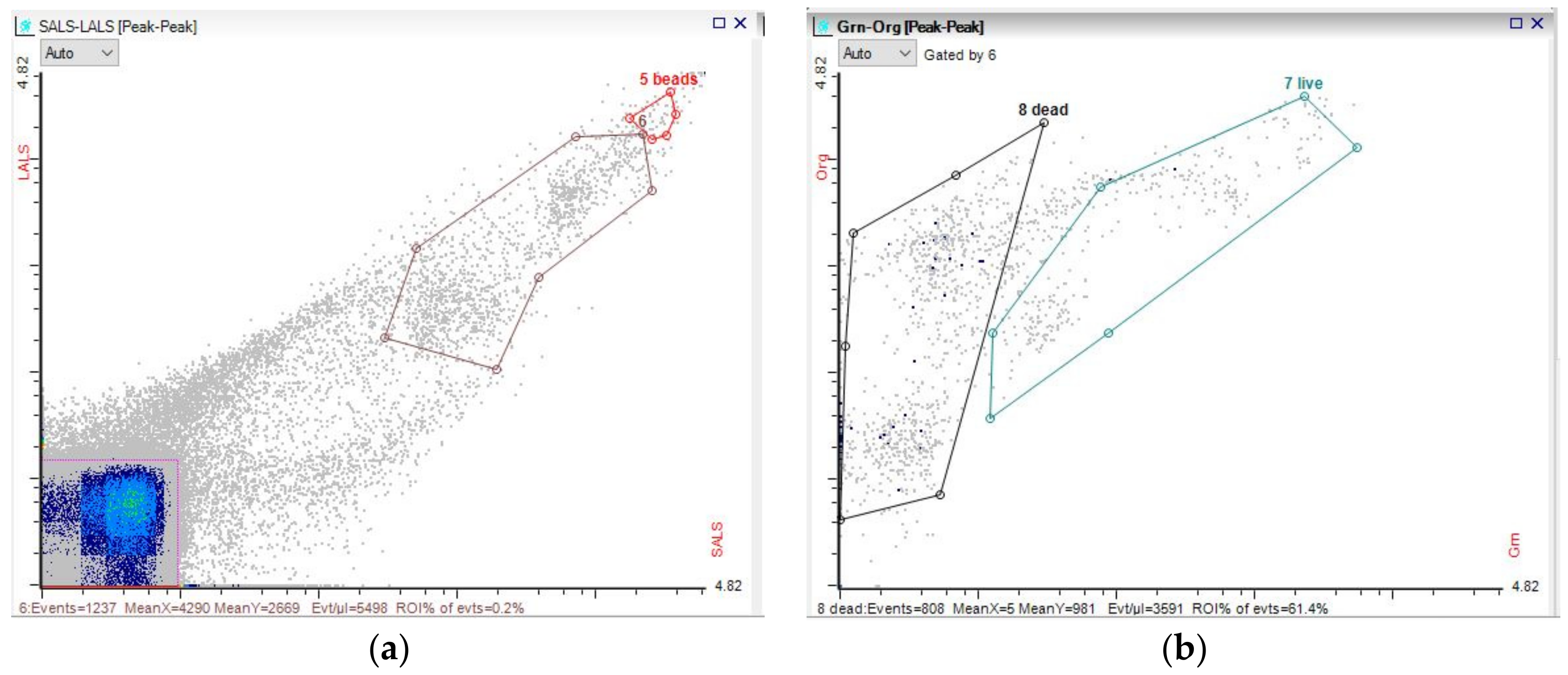
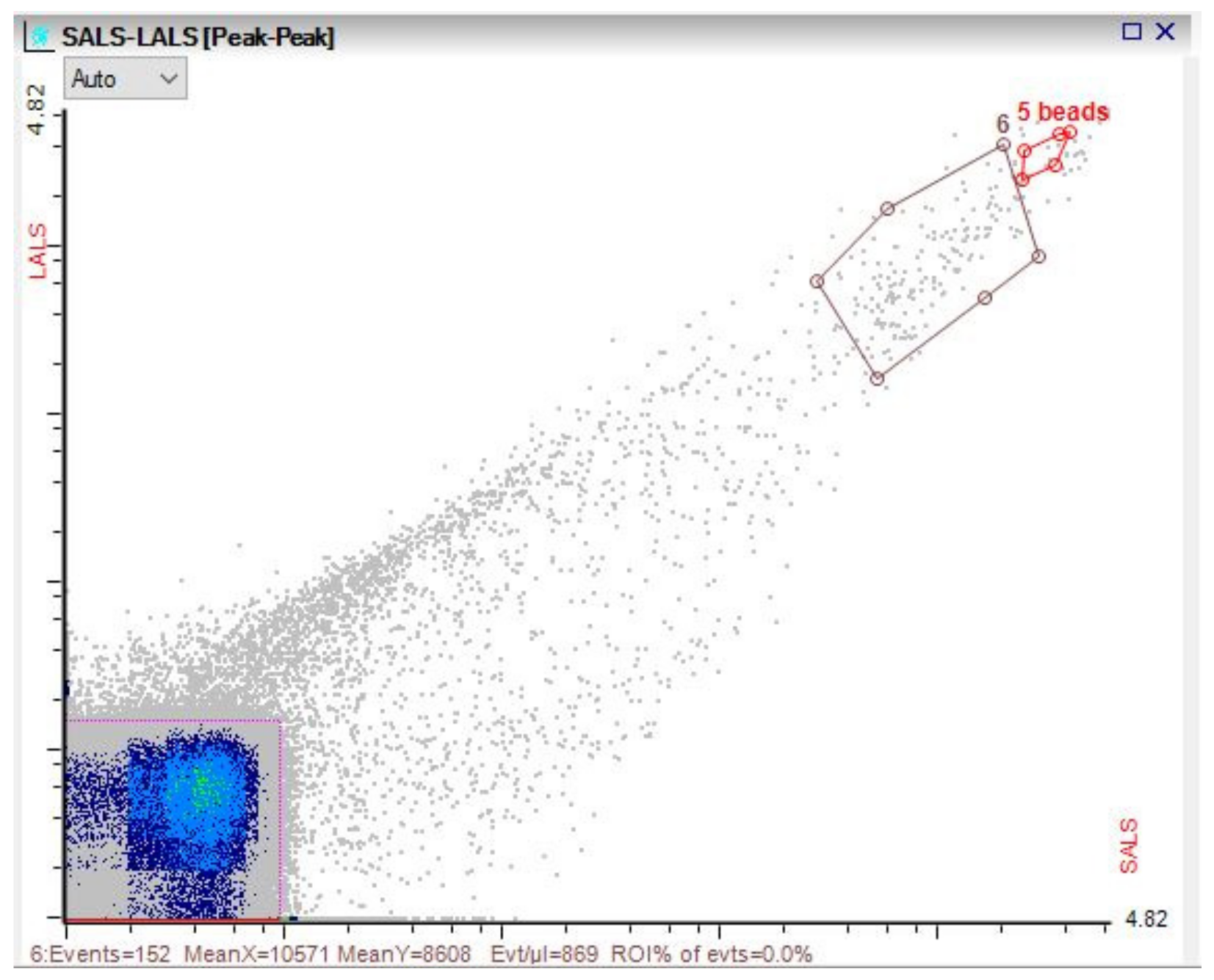

References
- Skuras, D.; Tyllianakis, E. The perception of water related risks and the state of the water environment in the European Union. Water Res. 2018, 143, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Deng, X.; Wan, Q.; Xu, X.; Huang, T. Photoreactivation of fungal spores in water following UV disinfection and their control using UV-based advanced oxidation processes. Water Res. 2019, 148, 1–9. [Google Scholar] [CrossRef]
- Templeton, M.R.; Oddy, F.; Leung, W.; Rogers, M. Chlorine and UV disinfection of ampicillin-resistant and trimethoprim-resistant Escherichia coli. Can. J. Civ. Eng. 2009, 36, 889–894. [Google Scholar] [CrossRef]
- Santos, A.L.; Oliveira, V.; Baptista, I.; Henriques, I.; Gomes, N.C.M.; Almeida, A.; Correia, A.; Cunha, Â. Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch. Microbiol. 2013, 195, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Gemici, B.T.; Karel, F.B.; Karaer, F.; Koparal, A.S. Water disinfection with advanced methods: Successive and hybrid application of antibacterial column with silver, ultrasound and UV radiation. Appl. Ecol. Environ. Res. 2018, 16, 4667–4680. [Google Scholar] [CrossRef]
- Quang, D.V.; Sarawade, P.B.; Jeon, S.J.; Kim, S.H.; Kim, J.-K.; Chai, Y.G.; Kim, H.T. Effective water disinfection using silver nanoparticle containing silica beads. Appl. Surf. Sci. 2013, 266, 280–287. [Google Scholar] [CrossRef]
- Xu, N.; Cheng, X.; Wang, D.; Xu, X.; Huangfu, X.; Li, Z. Effects of Escherichia coli and phosphate on the transport of titanium dioxide nanoparticles in heterogeneous porous media. Water Res. 2018, 146, 264–274. [Google Scholar] [CrossRef]
- Oh, J.; Salcedo, D.E.; Medriano, C.A.; Kim, S. Comparison of different disinfection processes in the effective removal of antibiotic-resistant bacteria and genes. J. Environ. Sci. 2014, 26, 1238–1242. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Yousefi, M.; Kor, Y.; Roohafzaee, M. Inactivation of Escherichia coli in Water by Combined Process of Silver Nanoparticle and Ultraviolet Radiation. Health Scope 2016, 6, e39102. [Google Scholar] [CrossRef]
- Alrousan, D.M.A.; Dunlop, P.S.M.; McMurray, T.A.; Byrne, J.A. Photocatalytic inactivation of E. coli in surface water using immobilised nanoparticle TiO2 films. Water Res. 2009, 43, 47–54. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Hamidinezhad, H.; Haddadi, H.; Mahmoudi, M. Double-doped TiO2 nanoparticles as an efficient visible-light-active photocatalyst and antibacterial agent under solar simulated light. Appl. Surf. Sci. 2014, 301, 338–345. [Google Scholar] [CrossRef]
- Venieri, D.; Fraggedaki, A.; Kostadima, M.; Chatzisymeon, E.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar light and metal-doped TiO2 to eliminate water-transmitted bacterial pathogens: Photocatalyst characterization and disinfection performance. Appl. Catal. B Environ. 2014, 154–155, 93–101. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Z.; Tay, S.L.; Gao, W. Photocatalytic TiO2 nanoparticles enhanced polymer antimicrobial coating. Appl. Surf. Sci. 2014, 290, 274–279. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.; Xie, X.; Chen, H.; Lin, J.; Dionysiou, D.D. Removing Escherichia coli from water using zinc oxide-coated zeolite. Water Res. 2018, 141, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Gerardin, F.; André, J.-C.; Pons, M.-N.; Zahraa, O. Study of photocatalytic damages induced on E. coli by different photocatalytic supports (various types and TiO2 configurations). J. Photochem. Photobiol. A Chem. 2011, 222, 323–329. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified titanium dioxide for photocatalytic applications. In Photocatalysts—Applications and Attributes; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Monteiro, R.A.R.; Miranda, S.M.; Vilar, V.J.P.; Pastrana-Martínez, L.M.; Tavares, P.B.; Boaventura, R.A.R.; Faria, J.L.; Pinto, E.; Silva, A.M.T. N-modified TiO2 photocatalytic activity towards diphenhydramine degradation and Escherichia coli inactivation in aqueous solutions. Appl. Catal. B Environ. 2015, 162, 66–74. [Google Scholar] [CrossRef]
- Rojviroon, T.; Sirivithayapakorn, S. E. coli Bacteriostatic Action Using TiO2 Photocatalytic Reactions. Int. J. Photoenergy 2018, 2018, 8474017. [Google Scholar] [CrossRef]
- Figoli, A.; Hoinkis, J.; Altinkaya, S.A.; Bundschuh, J. Application of Nanotechnology in Membranes for Water Treatment; CRC Press: London, UK, 2017. [Google Scholar] [CrossRef]
- Gayan, E.; Manas, P.; Alvarez, I.; Condon, S. Mechanism of the Synergistic Inactivation of Escherichia coli by UV-C Light at Mild Temperatures. Appl. Environ. Microbiol. 2013, 79, 4465–4473. [Google Scholar] [CrossRef]
- Leung, Y.H.; Xu, X.; Ma, A.P.Y.; Liu, F.; Ng, A.M.C.; Shen, Z.; Gethings, L.A.; Guo, M.Y.; Djurišić, A.B.; Lee, P.K.H.; et al. Toxicity of ZnO and TiO2 to Escherichia coli cells. Sci. Rep. 2016, 6, 35243. [Google Scholar] [CrossRef]
- Matafonova, G.G.; Batoev, V.B.; Linden, K.G. Photocatalytic-based inactivation of E. coli by UV 282 nm XeBr Excilamp. J. Environ. Sci. Health Part A 2013, 48, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Varnagiris, S.; Urbonavicius, M.; Sakalauskaite, S.; Daugelavicius, R.; Pranevicius, L.; Lelis, M.; Milcius, D. Floating TiO2 photocatalyst for efficient inactivation of E. coli and decomposition of methylene blue solution. Sci. Total Environ. 2020, 720, 137600. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, K. Fundamentals of medicinal application of titanium dioxide nanoparticles. In Nanoparticles Technology; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Jiang, X.; Lv, B.; Wang, Y.; Shen, Q.; Wang, X. Bactericidal mechanisms and effector targets of TiO2 and Ag-TiO2 against Staphylococcus aureus. J. Med. Microbiol. 2017, 66, 440–446. [Google Scholar] [CrossRef]
- Nyangaresi, P.O.; Qin, Y.; Chen, G.; Zhang, B.; Lu, Y.; Shen, L. Effects of single and combined UV-LEDs on inactivation and subsequent reactivation of E. coli in water disinfection. Water Res. 2018, 147, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Fendrich, M.; Quaranta, A.; Orlandi, M.; Bettonte, M.; Miotello, A. Solar Concentration for Wastewaters Remediation: A Review of Materials and Technologies. Appl. Sci. 2018, 9, 118. [Google Scholar] [CrossRef]
- Ferro, G.; Fiorentino, A.; Alferez, M.C.; Polo-López, M.I.; Rizzo, L.; Fernández-Ibáñez, P. Urban wastewater disinfection for agricultural reuse: Effect of solar driven AOPs in the inactivation of a multidrug resistant E. coli strain. Appl. Catal. B Environ. 2015, 178, 65–73. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Mansilla, H.D.; Mora, A.; Pincheira, C.; Mondaca, M.A.; Marcato, P.D.; Durán, N.; Freer, J. New photocatalytic reactor with TiO2 coating on sintered glass cylinders. Appl. Catal. B Environ. 2007, 76, 57–63. [Google Scholar] [CrossRef]
- Anpo, M.; Che, M.; Werner, O.; Haag, B.C.G.; Knoezinger, H. Advances in Catalysis; Academic Press: New York, NY, USA, 1999. [Google Scholar]
- Hidalgo, M.C.; Bahnemann, D. Highly photoactive supported TiO2 prepared by thermal hydrolysis of TiOSO4: Optimisation of the method and comparison with other synthetic routes. Appl. Catal. B Environ. 2005, 61, 259–266. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Yu, M.; Liu, L.; Li, J. An overall Solution to Cathode-Ray Tube (CRT) Glass Recycling. Procedia Environ. Sci. 2016, 31, 887–896. [Google Scholar] [CrossRef]
- Choi, B.-C.; Xu, L.-H.; Kim, H.-T.; Bahnemann, D.W. Photocatalytic Characteristics on Sintered Glass and Micro Reactor. J. Ind. Eng. Chem. 2006, 12, 663–672. [Google Scholar]
- Dey, T. UV-reflecting sintered nano-TiO2 thin film on glass for anti-bird strike application. Surf. Eng. 2021, 37, 688–694. [Google Scholar] [CrossRef]
- Eftimie, M.; Melinescu, A. Glassceramics from CRT glass waste with TiO2 as nucleating agent. Rev. Rom. Mater. Rom. J. Mater. 2015, 45, 424–427. [Google Scholar]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Chi Fru, E.; Ofiţeru, I.D.; Lavric, V.; Graham, D.W. Non-linear population dynamics in chemostats associated with live–dead cell cycling in Escherichia coli strain K12-MG1655. Appl. Microbiol. Biotechnol. 2011, 89, 791–798. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Babalola, S.O.; Onwudiwe, D.C. Photocatalytic Inactivation as a Method of Elimination of E. coli from Drinking Water. Appl. Sci. 2021, 11, 1313. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli: Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Janus, M.; Kusiak-Nejman, E.; Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Zając, K.; Morawski, A.W. Bacterial Inactivation on Concrete Plates Loaded with Modified TiO2 Photocatalysts under Visible Light Irradiation. Molecules 2019, 24, 3026. [Google Scholar] [CrossRef]
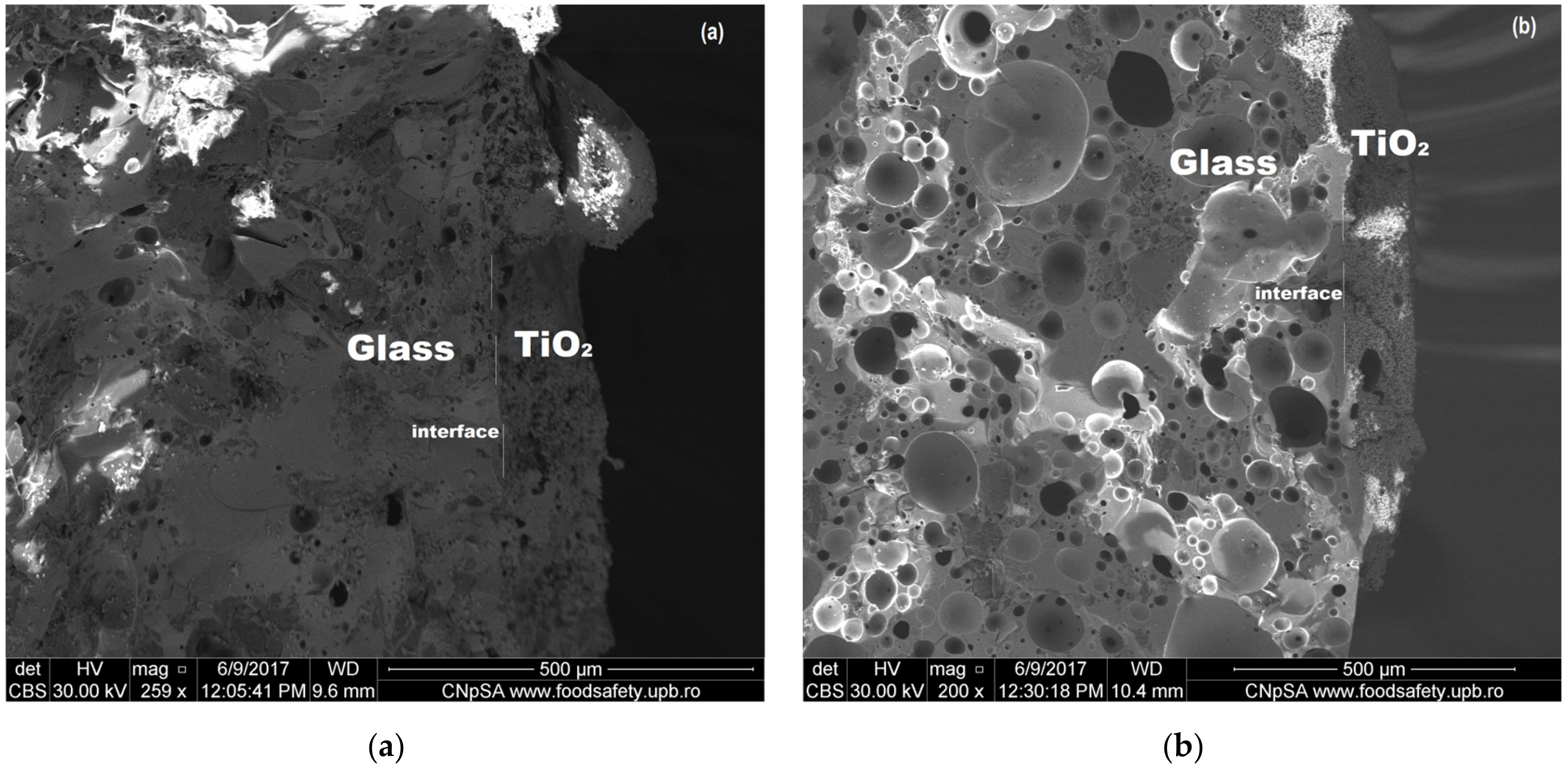

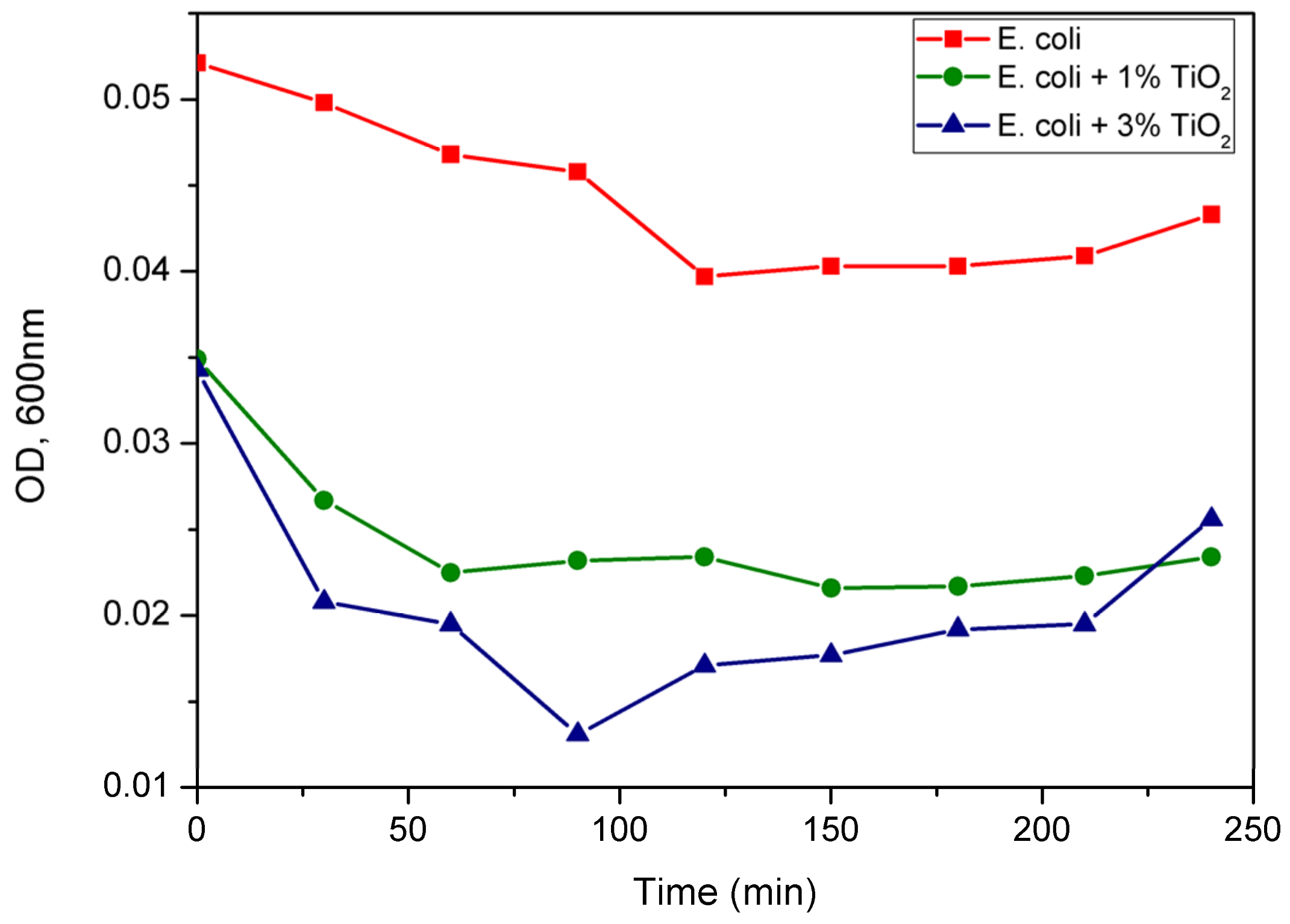


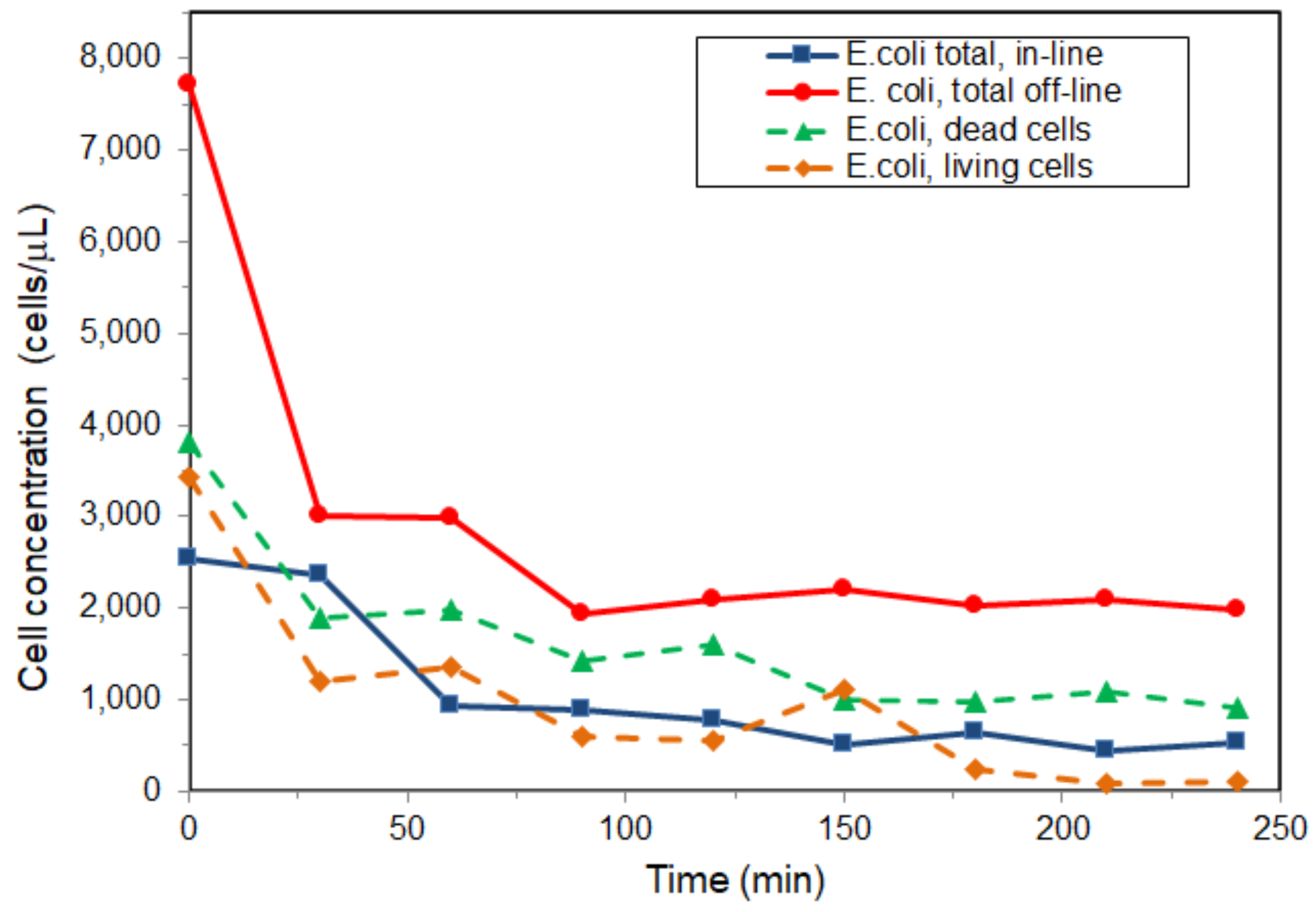
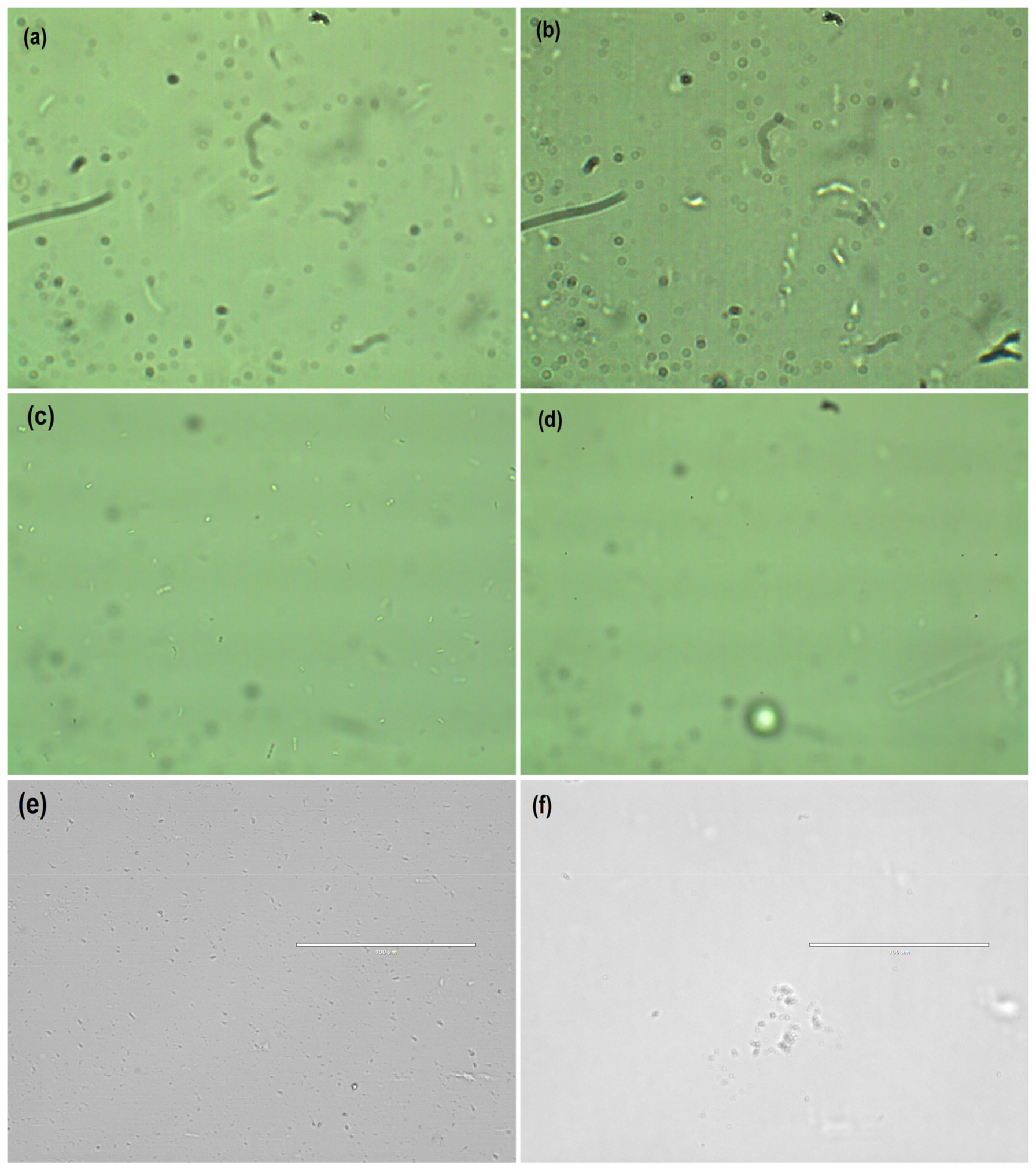
| Composition (wt %) | Frontal panel | Cone |
|---|---|---|
| SiO2 | 71.57 | 55.22 |
| Na2O | 18.05 | 13.78 |
| K2O | 3.57 | 3.14 |
| PbO | - | 18.83 |
| Al2O3 | 3.14 | 4.44 |
| BaO | 3.48 | - |
| MgO | - | 3.2 |
| CaO | - | 1.07 |
| Time, min | Efficiency, % | ||
|---|---|---|---|
| Control | Experiment 1 | Experiment 2 | |
| 0 | 95.0 ± 0.21 | 48.54 ± 0.33 | 55.59 ± 0.47 |
| 30 | 77.64 ± 0.11 | 75.83 ± 0.27 | 60.07 ± 0.36 |
| 60 | 65.53 ± 0.09 | 79.84 ± 0.24 | 54.59 ± 0.41 |
| 90 | 70.59 ± 0.10 | 81.18 ± 0.15 | 69.43 ± 0.29 |
| 120 | 73.61 ± 0.12 | 82.20 ± 0.19 | 73.92 ± 0.11 |
| 150 | 77.67 ± 0.11 | 91.30 ± 0.07 | 50.00 ± 0.43 |
| 180 | 79.91 ± 0.05 | 91.10 ± 0.09 | 88.21 ± 0.12 |
| 210 | 81.82 ± 0.06 | 90.64 ± 0.07 | 96.18 ± 0.09 |
| 240 | 88.81 ± 0.07 | 89.28 ± 0.23 | 94.70 ± 0.08 |
| Experiment | Inactivation Kinetic Constant, k, min-1 |
|---|---|
| Control | 5.094 |
| Experiment 1 | 4.120 |
| Experiment 2 | 3.547 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isopencu, G.; Eftimie, M.; Melinescu, A.; Dancila, A.M.; Mares, M. Recycling of Glass Waste by Deposition of TiO2 for the Intensification of the Photocatalytic Effect in the Purification of Wastewater. Coatings 2022, 12, 1794. https://doi.org/10.3390/coatings12111794
Isopencu G, Eftimie M, Melinescu A, Dancila AM, Mares M. Recycling of Glass Waste by Deposition of TiO2 for the Intensification of the Photocatalytic Effect in the Purification of Wastewater. Coatings. 2022; 12(11):1794. https://doi.org/10.3390/coatings12111794
Chicago/Turabian StyleIsopencu, Gabriela, Mihai Eftimie, Alina Melinescu, Annette Madelene Dancila, and Monica Mares. 2022. "Recycling of Glass Waste by Deposition of TiO2 for the Intensification of the Photocatalytic Effect in the Purification of Wastewater" Coatings 12, no. 11: 1794. https://doi.org/10.3390/coatings12111794
APA StyleIsopencu, G., Eftimie, M., Melinescu, A., Dancila, A. M., & Mares, M. (2022). Recycling of Glass Waste by Deposition of TiO2 for the Intensification of the Photocatalytic Effect in the Purification of Wastewater. Coatings, 12(11), 1794. https://doi.org/10.3390/coatings12111794