Silver and Silver Nanoparticles for the Potential Treatment of COVID-19: A Review
Abstract
Highlights
- Colloidal form of silver is biologically more reactive than the ionic form.
- Silver nanoparticles (AgNPs) can strongly bind to the spikes of the SARS-CoV-2 virus and prevent host cell attachment.
- Anti-viral activity of the AgNPs mainly depends on their size and structural features.
- AgNPs could be used to tackle the variants of SARS-CoV-2 virus effectively.
- Increased toxicity of AgNPs at the higher concentrations is a point of concern to be addressed.
Abstract
1. Introduction
2. Role of Silver (Ag) in Medicine
3. Variants of Coronaviruses
3.1. Treatment of COVID-19 Using Silver and Silver Nanoparticles (AgNPs)
3.2. Application of Silver Nanoparticles for Controlling COVID-19
3.3. Future Scope
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murarkar, S.; Gothankar, J.; Doke, P.; Dhumale, G.; Pore, P.D.; Lalwani, S.; Quraishi, S.; Patil, R.S.; Waghachavare, V.; Dhobale, R. Prevalence of the acute respiratory infections and associated factors in the rural areas and urban slum areas of western Maharashtra, India: A community-based cross-sectional study. Front. Public Health 2021, 9, 723807. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Simões, E.A.F.; Rudan, I.; Gessner, B.D.; Azziz-Baumgartner, E.; Zhang, J.S.F.; Feikin, D.R.; Mackenzie, G.A.; Moiïsi, J.C.; Roca, A. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet 2013, 381, 1380. [Google Scholar] [CrossRef]
- Acter, T.; Uddin, N.; Das, J.; Akhter, A.; Choudhury, T.R.; Kim, S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020, 730, 138996. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Website. Available online: https://covid19.who.int/ (accessed on 25 September 2021).
- AlSamman, M.; Caggiula, A.; Ganguli, S.; Misak, M.; Pourmand, A. Non-respiratory presentations of COVID-19, a clinical review. Am. J. Emerg. Med. 2020, 38, 2444. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO-China Study Team. WHO-convened global study of origins of SARS-CoV-2: China part (text extract). Infect. Dis. Immun. 2021, 1, 125. [Google Scholar]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Burke, R.M.; Midgley, C.M.; Dratch, A.; Fenstersheib, M.; Haupt, T.; Holshue, M.; Ghinai, I.; Jarashow, M.C.; Lo, J.; McPherson, T.D. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January–February 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 245. [Google Scholar] [CrossRef]
- Guo, Y.; Korteweg, C.; McNutt, M.A.; Gu, J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008, 133, 4. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020, 23, 1843. [Google Scholar] [CrossRef]
- Chang, L.; Zhao, L.; Gong, H.; Wang, L.; Wang, L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg. Infect. Dis. 2020, 26, 1631. [Google Scholar] [CrossRef]
- Chang, L.; Yan, Y.; Wang, L. Coronavirus disease 2019: Coronaviruses and blood safety. Transfus. Med. Rev. 2020, 34, 75. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Teng, T.; Abdalla, A.E.; Zhu, W.; Xie, L.; Wang, Y.; Guo, X. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses 2020, 12, 244. [Google Scholar] [CrossRef]
- Thippareddi, H.; Balamurugan, S.; Patel, J.; Singh, M.; Brassard, J. Coronaviruses–Potential human threat from foodborne transmission? Lebensm Wiss Technol. 2020, 134, 110147. [Google Scholar] [CrossRef]
- Kwok, Y.L.A.; Gralton, J.; McLaws, M.L. Face touching: Un hábito frecuente que tiene implicaciones para la higiene de las manos. Am. J. Infectar. 2015, 43, 112. [Google Scholar]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. A review and analysis Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010, 76, 2712. [Google Scholar] [CrossRef]
- Sizun, J.; Yu, M.W.N.; Talbot, P.J. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: A possible source ofhospital-acquired infections. J. Hosp. Infect. 2000, 46, 55. [Google Scholar] [CrossRef]
- Duan, S.-M.; Zhao, X.-S.; Wen, R.-F.; Huang, J.J.; Pi, G.-H.; Zhang, S.-X.; Sheng, J.H.; Bi, L.; Ruan, L.; Dong, X.-P.; et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003, 16, 246. [Google Scholar]
- Sportelli, C.N. Materials Science Help the Fight against SARS-CoV-2. Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.R.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral properties of copper and its alloys to inactivate covid-19 virus: A review. Biometals 2021, 34, 1217. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.; Mantilaka, M.; Ruparathna, K.A.A.; Rajapakshe, R.; Sameera, S.A.L.; Thilakarathna, M. Filler matrix interfaces of inorganic/biopolymer composites and their applications. Interfaces Part. Fibre Reinf. Compos. 2020, 1, 95–112. [Google Scholar]
- Longano, D.; Ditaranto, N.; Sabbatini, L.; Torsi, L.; Cioffi, N. Synthesis and antimicrobial activity of copper nanomaterials. In Nano-Antimicrobials; Springer: Berlin/Heidelberg, Germany, 2012; p. 85. [Google Scholar]
- Bogdanovic, U.; Vodnik, V.; Mitric, M.; Dimitrijevic, S.; Skapin, S.D.; Zunic, V.; Budimir, M.; Stoiljkovic, M. Nanomaterial with High Antimicrobial Efficacy Copper/Polyaniline Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 1955. [Google Scholar] [CrossRef] [PubMed]
- Galúcio, J.M.P.; de Souza, S.G.B.; Vasconcelos, A.A.; Lima, A.K.O.; da Costa, K.S.; de Campos Braga, H.; Taube, P.S. Synthesis, characterization, applications, and toxicity of green synthesized nanoparticles. Curr. Pharm. Biotechnol. 2022, 23, 420. [Google Scholar] [CrossRef]
- Chiriac, V.; Stratulat, D.N.; Calin, G.; Nichitus, S.; Burlui, V.; Stadoleanu, C.; Popa, M.; Popa, I.M. Antimicrobial property of zinc based nanoparticles. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; p. 12055. [Google Scholar]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Ranska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941. [Google Scholar] [CrossRef]
- Balraj, B.; Senthilkumar, N.; Potheher, I.V.; Arulmozhi, M. Characterization, antibacterial, anti-arthritic and in-vitro cytotoxic potentials of biosynthesized magnesium oxide nanomaterial. Mater. Sci. Eng. B 2018, 231, 121. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver in health care: Antimicrobial effects and safety in use. Biofunctional Text. Ski. 2006, 33, 17. [Google Scholar]
- Melayie, A.; Youngs, J.W. Silver and its application on antimicrobial agents. Expert. Opin. Ther. Pat. 2005, 15, 125. [Google Scholar] [CrossRef]
- Walker, M.; Parsons, D. The biological fate of silver ions following the use of silver-containing wound care products–a review. Int. Wound J. 2014, 11, 496. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Mashrur, F.R.; Chhoan, A.P.; Shahriar, S.M.; Haidere, M.F.; Runa, N.J.; Kim, S.; Kweon, D.-H.; Hosseinzadeh, H.; Cho, J.Y. Silver nanoparticles as potential antiviral agents. Pharmaceutics 2021, 13, 2034. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894. [Google Scholar] [CrossRef]
- Holden, N.E. History of the Origin of the Chemical Elements and Their Discoverers; Brookhaven National Lab. (BNL): Upton, NY, USA, 2019. [Google Scholar]
- Barillo, D.J.; Marx, D.E. Silver in medicine: A brief history BC 335 to present. Burns 2014, 40, S3. [Google Scholar] [CrossRef]
- Leone, G.; Pepi, S.; Consumi, M.; Mahdizadeh, F.F.; Lamponi, S.; Magnani, A. Phosphorylated xanthan gum-Ag (I) complex as antibacterial viscosity enhancer for eye drops formulation. Carbohydr. Polym. 2021, 267, 118196. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996, 116, 71. [Google Scholar] [CrossRef]
- Ludwigson, D.C.; Mears, D.C. Today’s prosthetic metals. J. Miner. Met. Mater. Soc. 1964, 16, 226. [Google Scholar] [CrossRef]
- Konop, M.; Damps, T.; Misicka, A.; Rudnicka, L. Certain aspects of silver and silver nanoparticles in wound care: A minireview. J. Nanomater. 2016, 2016, 7614753. [Google Scholar] [CrossRef]
- Pietro Reverberi, A.; Vocciante, M.; Salerno, M.; Ferretti, M.; Fabiano, B. Green synthesis of silver nanoparticles by low-energy wet bead milling of metal spheres. Materials 2020, 13, 63. [Google Scholar] [CrossRef]
- Zakaria, M.A.; Menazea, A.A.; Mostafa, A.M.; Al-Ashkar, E.A. Ultra-thin silver nanoparticles film prepared via pulsed laser deposition: Synthesis, characterization, and its catalytic activity on reduction of 4-nitrophenol. Surf. Interfaces 2020, 19, 100438. [Google Scholar] [CrossRef]
- Semenova, A.A.; Ivanov, V.K.; Savilov, S.V.; Goodilin, E.A. Unusual silver nanostructures prepared by aerosol spray pyrolysis. CrystEngComm 2013, 15, 7863. [Google Scholar] [CrossRef]
- Colson, P.; Henrist, C.; Cloots, R. Nanosphere lithography: A powerful method for the controlled manufacturing of nanomaterials. J. Nanomater. 2013, 2013, 948510. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Zamarreño, C.R.; Arregui, F.J.; Matías, I.R. An antibacterial coating based on a polymer/sol-gel hybrid matrix loaded with silver nanoparticles. Nanoscale Res. Lett. 2011, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Nasretdinova, G.R.; Fazleeva, R.R.; Mukhitova, R.K.; Nizameev, I.R.; Kadirov, M.K.; Ziganshina, A.Y.; Yanilkin, V.V. Electrochemical mediated synthesis of silver nanoparticles in solution. Russ. J. Electrochem. 2015, 51, 1029. [Google Scholar] [CrossRef]
- Raza, S. Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Borase, H.P.; Salunke, B.K.; Salunkhe, R.B.; Patil, C.D.; Hallsworth, J.E.; Kim, B.S.; Patil, S.V. Plant extract: A promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol. 2014, 173, 1. [Google Scholar] [CrossRef]
- Maya, F.; Muhl, S.; Peña, O.; Miki-Yoshida, M. Synthesis and characterization of silver–carbon nanoparticles produced by high-current pulsed arc. Thin Solid Films 2009, 518, 1484. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. Int. J. 1999, 18, 89. [Google Scholar] [CrossRef]
- Hollinger, M.A. Toxicological aspects of topical silver pharmaceuticals. Crit. Rev. Toxicol. 1996, 26, 255.2. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In AIP Conference Proceedings; AIP Publishing LLC: Bristol, UK, 2016; p. 20048. [Google Scholar]
- Hayat, M.; Uzair, M.; Ali Syed, R.; Arshad, M.; Bashir, S. Status of COVID-19 vaccination around South Asia. Hum. Vaccin. Immunother. 2022, 18, 2016010. [Google Scholar] [CrossRef]
- Marshall, J.P.; Schneider, R.P. Systemic argyria secondary to topical silver nitrate. Arch. Dermatol. 1977, 113, 1077. [Google Scholar] [CrossRef]
- Fung, M.C.; Bowen, D.L. Silver products for medical indications: Risk-benefit assessment. J. Toxicol. Clin. Toxicol. 1996, 34, 119. [Google Scholar] [CrossRef]
- Tashi, T.; Gupta, N.V.; Mbuya, V.B. Silver nanoparticles: Synthesis, mechanism of antimicrobial action, characterization, medical applications, and toxicity effects. J. Chem. Pharm. Res. 2016, 8, 526. [Google Scholar]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. Int. J. 2008, 27, 1972. [Google Scholar] [CrossRef]
- Rezvani, E.; Rafferty, A.; McGuinness, C.; Kennedy, J. Adverse effects of nanosilver on human health and the environment. Acta Biomater. 2019, 94, 145. [Google Scholar] [CrossRef]
- Ayrault, S.; Priadi, C.R.; Evrard, O.; Lefèvre, I.; Bonté, P. Silver and thallium historical trends in the Seine River basin. J. Environ. Monit. 2010, 12, 2177. [Google Scholar] [CrossRef]
- Rai, M.; Agarkar, G. Plant–fungal interactions: What triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 2016, 42, 428. [Google Scholar] [CrossRef]
- Furno, F.; Morley, K.S.; Wong, B.; Sharp, B.L.; Arnold, P.L.; Howdle, S.M.; Bayston, R.; Brown, P.D.; Winship, P.D.; Reid, H.J. Silver nanoparticles and polymeric medical devices: A new approach to prevention of infection? J. Antimicrob. Chemother. 2004, 54, 1019. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Marx, D.E.; Barillo, D.J. Silver in medicine: The basic science. Burns 2014, 40 (Suppl. S1), S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Damm, C.; Münstedt, H.; Rösch, A. The antimicrobial efficacy of polyamide 6/silver-nano-and microcomposites. Mater. Chem. Phys. 2008, 108, 61. [Google Scholar] [CrossRef]
- Anastasiadis, S.H.; Chrissopoulou, K.; Frick, B. Structure and dynamics in polymer/layered silicate nanocomposites. Mater. Sci. Eng. B 2008, 152, 33. [Google Scholar] [CrossRef]
- Chen, M.; Wang, M.-Y.; Han, J.-T.; Zhang, J.-Y.; Li, Z.-Y.; Qian, D.-J. Preparation and study of polyacryamide-stabilized silver nanoparticles through a one-pot process. J. Phys. Chem. B 2006, 110, 11224. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Chen, Y.; Chen, C.; Hu, L.; Wang, H.; Li, Y. Toward simultaneous compatibilization and nucleation of fully biodegradabe nanocomposites: Effect of nanorod-assisted interfacial stereocomplex crystals in immiscible polymer blends. Compos. Part B Eng. 2022, 234, 109708. [Google Scholar] [CrossRef]
- Bragg, P.D.; Rainnie, D.J. The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol. 1974, 20, 883. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef]
- Möhler, J.S.; Sim, W.; Blaskovich, M.A.T.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol. Adv. 2018, 36, 1391. [Google Scholar] [CrossRef]
- Lansdown, A.B.G.; Williams, A. How safe is silver in wound care? J. Wound Care 2004, 13, 131. [Google Scholar] [CrossRef]
- Lansdown, A.B.G.; Williams, A.; Chandler, S.; Benfield, S. Silver absorption and antibacterial efficacy of silver dressings. J. Wound Care 2005, 14, 155–160. [Google Scholar] [CrossRef]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in nanotechnology towards development of silver nanoparticle-based wound-healing agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. A review of the use of silver in wound care: Facts and fallacies. Br. J. Nurs. 2004, 13, S6. [Google Scholar] [CrossRef]
- Zhao, G.J.; Stevens, S.E. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals 1998, 11, 27–32. [Google Scholar] [CrossRef]
- Spacciapoli, P.; Buxton, D.; Rothstein, D.; Friden, P. Antimicrobial activity of silver nitrate against periodontal pathogens. J. Periodontal Res. 2001, 36, 108. [Google Scholar] [CrossRef]
- Teirumnieks, E.; Balchev, I.; Ghalot, R.S.; Lazov, L. Antibacterial and anti-viral effects of silver nanoparticles in medicine against COVID-19—A review. Laser Phys. 2020, 31, 13001. [Google Scholar] [CrossRef]
- Nazeruddin, G.M.; Prasad, N.R.; Prasad, S.R.; Shaikh, Y.I.; Waghmare, S.R.; Adhyapak, P. Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind. Crops Prod. 2014, 60, 212. [Google Scholar] [CrossRef]
- Mpenyana-Monyatsi, L.; Mthombeni, N.H.; Onyango, M.S.; Momba, M.N.B. Cost-effective filter materials coated with silver nanoparticles for the removal of pathogenic bacteria in groundwater. Int. J. Environ. Res. Public Health 2012, 9, 244. [Google Scholar] [CrossRef]
- Ahmadi, S. The importance of silver nanoparticles in human life. Adv. Appl. NanoBio-Technol. 2020, 1, 5. [Google Scholar]
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103. [Google Scholar]
- Siadati, S.A.; Afzali, M.; Sayyadi, M. Could silver nano-particles control the 2019-nCoV virus?; An urgent glance to the past. Chem. Rev. Lett. 2020, 3, 9. [Google Scholar]
- Behbudi, G. Effect of silver nanoparticles disinfectant on covid-19. Adv. Appl. NanoBio-Technol. 2021, 2, 63. [Google Scholar]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli, Nanomedicine Nanotechnology. Biol. Med. 2007, 3, 168. [Google Scholar]
- Fisman, D.N.; Tuite, A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: A retrospective cohort study in Ontario, Canada. Cmaj 2021, 193, E1619. [Google Scholar] [CrossRef] [PubMed]
- Sokhansanj, B.A.; Zhao, Z.; Rosen, G.L. Interpretable and predictive deep modeling of the SARS-CoV-2 spike protein sequence. medRxiv 2021. [Google Scholar] [CrossRef]
- Caputo, V.; Calvino, G.; Strafella, C.; Termine, A.; Fabrizio, C.; Trastulli, G.; Ingrascì, A.; Peconi, C.; Bardini, S.; Rossini, A. Tracking the Initial Diffusion of SARS-CoV-2 Omicron Variant in Italy by RT-PCR and Comparison with Alpha and Delta Variants Spreading. Diagnostics 2022, 12, 467. [Google Scholar] [CrossRef]
- Boshier, F.A.T.; Venturini, C.; Stirrup, O.; Guerra-Assunção, J.A.; Alcolea-Medina, A.; Becket, A.H.; Byott, M.; Charalampous, T.; da Silva Filipe, A.; Frampton, D. The Alpha variant was not associated with excess nosocomial SARS-CoV-2 infection in a multi-centre UK hospital study. J. Infect. 2021, 83, 693. [Google Scholar] [CrossRef]
- Brejová, B.; Hodorová, V.; Boršová, K.; Čabanová, V.; Szemes, T.; Mišík, M.; Klempa, B.; Nosek, J.; Vinař, T. Sequencing SARS-CoV-2 in Slovakia: An Unofficial Genomic Surveillance Report. medRxiv 2021. [Google Scholar] [CrossRef]
- Salleh, M.Z.; Derrick, J.P.; Deris, Z.Z. Structural evaluation of the spike glycoprotein variants on SARS-CoV-2 transmission and immune evasion. Int. J. Mol. Sci. 2021, 22, 7425. [Google Scholar] [CrossRef]
- Islam, S.; Islam, T.; Islam, M.R. New coronavirus variants are creating more challenges to global healthcare system: A brief report on the current knowledge. Clin. Pathol. 2022, 15, 2632010X221075584. [Google Scholar] [CrossRef]
- Nangunoori, S.; Appavu, R.; Kethar, J. The Connection between COVID-19 Variants (Gamma and Delta Variant) and Demographics Using Python. J. Stud. Res. 2021, 10, 1. [Google Scholar] [CrossRef]
- CDC COVID-19 Vaccine Breakthrough Case Investigations Team; Birhane, M.; Bressler, S.; Chang, G.; Clark, T.; Dorough, L.; Fischer, M.; Watkins, L.F.; Goldstein, J.M.; Kugeler, K.; et al. COVID-19 vaccine breakthrough infections reported to CDC—United States, January 1–April 30, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 792. [Google Scholar]
- Johnsen, U.; Sutter, J.; Schulz, A.; Tästensen, J.; Schönheit, P. XacR—A novel transcriptional regulator of D-xylose and L-arabinose catabolism in the haloarchaeon H aloferax volcanii. Environ. Microbiol. 2015, 17, 1663. [Google Scholar] [CrossRef]
- Maggi, F.; Novazzi, F.; Genoni, A.; Baj, A.; Spezia, P.G.; Focosi, D.; Zago, C.; Colombo, A.; Cassani, G.; Pasciuta, R. Imported SARS-CoV-2 variant P. 1 in traveler returning from Brazil to Italy. Emerg. Infect. Dis. 2021, 27, 1249. [Google Scholar] [CrossRef]
- Carroll, T.; Fox, D.; van Doremalen, N.; Ball, E.; Morris, M.K.; Sotomayor-Gonzalez, A.; Servellita, V.; Rustagi, A.; Yinda, C.K.; Fritts, L. The B. 1.427/1.429 (epsilon) SARS-CoV-2 variants are more virulent than ancestral B. 1 (614G) in Syrian hamsters. PLoS Pathog. 2022, 18, e1009914. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Murrone, A.; De Luca, L.; Roncon, L.; Di Lenarda, A.; Valente, S.; Caldarola, P.; Riccio, C.; Oliva, F.; Gulizia, M.M. COVID-19, Vaccines, and Thrombotic Events: A Narrative Review. J. Clin. Med. 2022, 11, 948. [Google Scholar] [CrossRef]
- Vaughan, A. Omicron emerges. New Sci. 2021, 252, 7. [Google Scholar] [CrossRef]
- Thakur, S.; Sasi, S.; Pillai, S.G.; Nag, A.; Shukla, D.; Singhal, R.; Phalke, S.; Velu, G.S.K. SARS-CoV-2 Mutations and Their Impact on Diagnostics, Therapeutics and Vaccines. Front. Med. 2022, 9, 815389. [Google Scholar] [CrossRef]
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 variants of concern. Yonsei Med. J. 2021, 62, 961. [Google Scholar] [CrossRef]
- Torjesen, I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ 2021, 29, n2943. [Google Scholar] [CrossRef]
- Khandia, R.; Singhal, S.; Alqahtani, T.; Kamal, M.A.; Nahed, A.; Nainu, F.; Desingu, P.A.; Dhama, K. Emergence of SARS-CoV-2 Omicron (B. 1.1. 529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ. Res. 2022, 209, 112816. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.R.; Spratt, A.N.; Sharma, K.; Chand, H.S.; Byrareddy, S.N.; Singh, K. Omicron SARS-CoV-2 variant: Unique features and their impact on pre-existing antibodies. J. Autoimmun 2022, 126, 102779. [Google Scholar] [CrossRef] [PubMed]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B. 1.617. 2) compared with alpha (B. 1.1. 7) variants of concern: A cohort study. Lancet Infect. Dis. 2022, 22, 35. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, S.; Ansari, S.; Paweska, J.T.; Maurya, V.K.; Tripathi, A.K.; Abdel-Moneim, A.S. Characterization of the novel SARS-CoV-2 Omicron (B. 1.1. 529) variant of concern and its global perspective. J. Med. Virol. 2022, 94, 1738. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.P. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. 2021, 12, 08971900211009650. [Google Scholar] [CrossRef]
- Oliver, S.E.; Wallace, M.; See, I.; Mbaeyi, S.; Godfrey, M.; Hadler, S.C.; Jatlaoui, T.C.; Twentyman, E.; Hughes, M.M.; Rao, A.K. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices—United States, December 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 90. [Google Scholar]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99. [Google Scholar] [CrossRef]
- Baraniuk, C. What do we know about China’s covid-19 vaccines? BMJ 2021, 373, n912. [Google Scholar] [CrossRef]
- Lawton, G. Sputnik V vaccine goes global. New Sci. 2021, 250, 10. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72. [Google Scholar] [CrossRef]
- Das, C.; Paul, S.S.; Saha, A.; Singh, T.; Saha, A.; Im, J.; Biswas, G. Silver-based nanomaterials as therapeutic agents against coronaviruses: A review. Int. J. Nanomed. 2020, 15, 9301. [Google Scholar] [CrossRef]
- Bahrami, A.; Arabestani, M.R.; Taheri, M.; Farmany, A.; Norozzadeh, F.; Hosseini, S.M.; Nozari, H.; Nouri, F. Exploring the role of heavy metals and their derivatives on the pathophysiology of COVID-19. Biol. Trace Elem. Res. 2022, 200, 2639. [Google Scholar] [CrossRef]
- Crane, M.J.; Devine, S.; Jamieson, A.M. Graphene oxide/silver nanoparticle ink formulations rapidly inhibit influenza A virus and OC43 coronavirus infection in vitro. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zachar, O. Nanomedicine formulations for respiratory infections by inhalation delivery: COVID-19 and beyond. Med. Hypotheses 2022, 159, 110753. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Pereira, A.E.S.; De Oliveira, J.L.; Carvalho, L.B.; Guilger-Casagrande, M.; De Lima, R.; Fraceto, L.F. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnol. 2020, 18, 1. [Google Scholar] [CrossRef]
- Bui, T.Q.; Loan, H.T.P.; My, T.T.A.; Quang, D.T.; Thuy, B.T.P.; Nhan, V.D.; Quy, P.T.; Van Tat, P.; Dao, N.D.Q.; Trung, T. A density functional theory study on silver and bis-silver complexes with lighter tetrylene: Are silver and bis-silver carbenes candidates for SARS-CoV-2 inhibition? Insight from molecular docking simulation. RSC Adv. 2020, 10, 30961. [Google Scholar] [CrossRef]
- Rezaee, P.; Akbari, M.; Morad, R.; Koochaki, A.; Maaz, M.; Jamshidi, Z. First principle simulation of coated hydroxychloroquine on Ag, Au and Pt nanoparticle as a potential candidate for treatment of SARS-CoV-2 (COVID-19). arXiv 2020, arXiv:2006.02343. [Google Scholar]
- Ghaffari, M.; Mollazadeh-Bajestani, M.; Moztarzadeh, F.; Uludağ, H.; Hardy, J.G.; Mozafari, M. An overview of the use of biomaterials, nanotechnology, and stem cells for detection and treatment of COVID-19: Towards a framework to address future global pandemics. Emergent Mater. 2021, 4, 19. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G. A proposed insight into the anti-viral potential of metallic nanoparticles against novel coronavirus disease-19 (COVID-19). Bull. Natl. Res. Cent. 2021, 45, 36. [Google Scholar] [CrossRef]
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Al-Sanea, M.M.; Abelyan, N.; Abdelgawad, M.A.; Musa, A.; Ghoneim, M.M.; Al-Warhi, T.; Aljaeed, N.; Alotaibi, O.J.; Alnusaire, T.S.; Abdelwahab, S.F. Strawberry and ginger silver nanoparticles as potential inhibitors for SARS-CoV-2 assisted by in silico modeling and metabolic profiling. Antibioticse 2021, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Allawadhi, P.; Singh, V.; Khurana, A.; Khurana, I.; Allwadhi, S.; Kumar, P.; Banothu, A.K.; Thalugula, S.; Barani, P.J.; Naik, R.R. Silver nanoparticle based multifunctional approach for combating COVID-19. Sens. Int. 2021, 2, 100101. [Google Scholar] [CrossRef] [PubMed]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D. Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Morey, J.; Torres, M.; Seqqat, R.; de las Nieves Pina, M. Silver nanoparticles as a potential treatment against SARS-CoV-2: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1707. [Google Scholar] [CrossRef]
- Zachar, O. Formulations for COVID-19 earlystage treatment via silver nanoparticles inhalation delivery at home and hospital. Sci. Prepr. 2020. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef]
- Campbell, S.; Rein, A. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J. Virol. 1999, 73, 2270. [Google Scholar] [CrossRef]
- Swann, H.; Sharma, A.; Preece, B.; Peterson, C.A.; Eldredge, A.; Belnap, D.M.; Vershinin, M.; Saffarian, S. Minimal system for assembly of SARS-CoV-2 virus like particles. Sci. Rep. 2021, 11, 21877. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Gad, S.F.; Muhammad, K.; Batiha, G.E.; Hetta, H.F. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine 2020, 15, 2085. [Google Scholar] [CrossRef]
- Vishnuvarthanan, M.; Rajeswari, N. Food packaging: Pectin–laponite–Ag nanoparticle bionanocomposite coated on polypropylene shows low O2 transmission, low Ag migration and high antimicrobial activity. Environ. Chem. Lett. 2019, 17, 439–445. [Google Scholar] [CrossRef]
- Kumar, A.; Nath, K.; Parekh, Y.; Enayathullah, M.G.; Bokara, K.K.; Sinhamahapatra, A. Antimicrobial silver nanoparticle-photodeposited fabrics for SARS-CoV-2 destruction. Colloid Interface Sci. Commun. 2021, 45, 100542. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull. 2021, 79, 5747–5771. [Google Scholar] [CrossRef]
- Hewawaduge, C.; Senevirathne, A.; Jawalagatti, V.; Kim, J.W.; Lee, J.H. Copper-impregnated three-layer mask efficiently inactivates SARS-CoV2. Environ. Res. 2021, 196, 110947. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Almanza-Reyes, H.; Moreno, S.; Plascencia-López, I.; Alvarado-Vera, M.; Patrón-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A. Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: In vitro and in vivo. PLoS ONE 2021, 16, 0256401. [Google Scholar] [CrossRef]
- Tabrez, S.; Jabir, N.R.; Adhami, V.M.; Khan, M.I.; Moulay, M.; Kamal, M.A.; Mukhtar, H. Nano-encapsulated dietary polyphenols for cancer prevention and treatment: Successes and challenges. Nanomedicine 2020, 15, 1147. [Google Scholar]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713. [Google Scholar] [CrossRef]

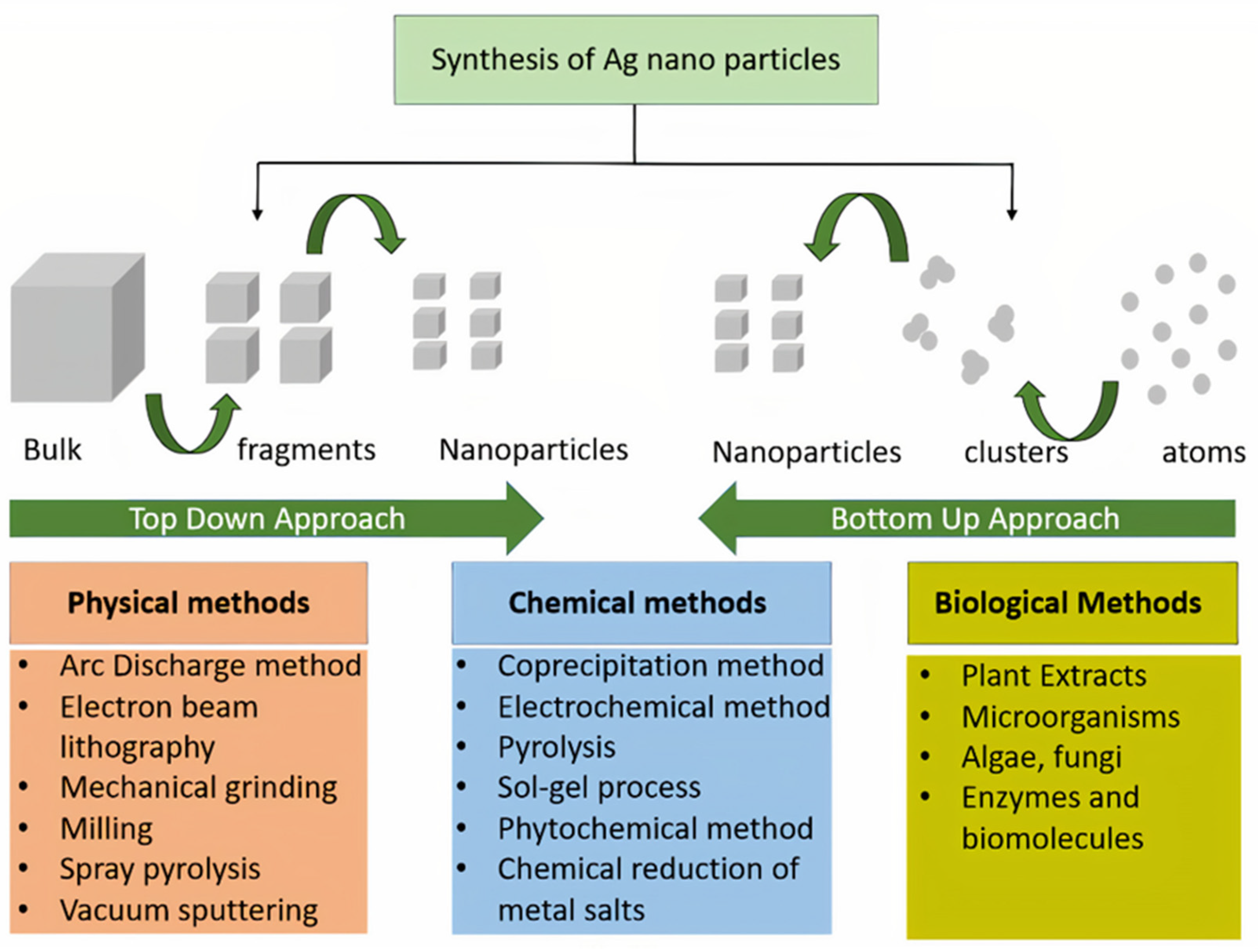

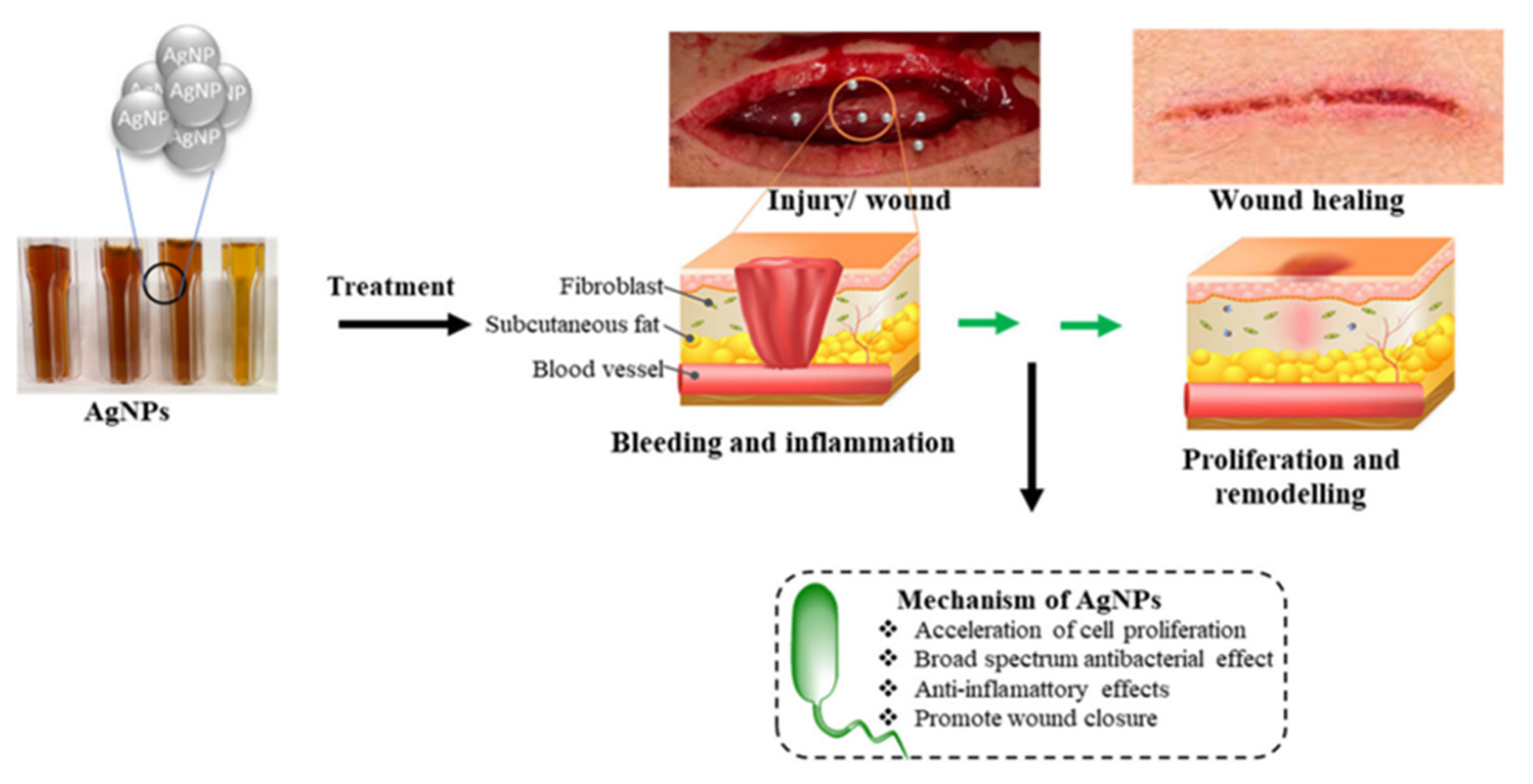


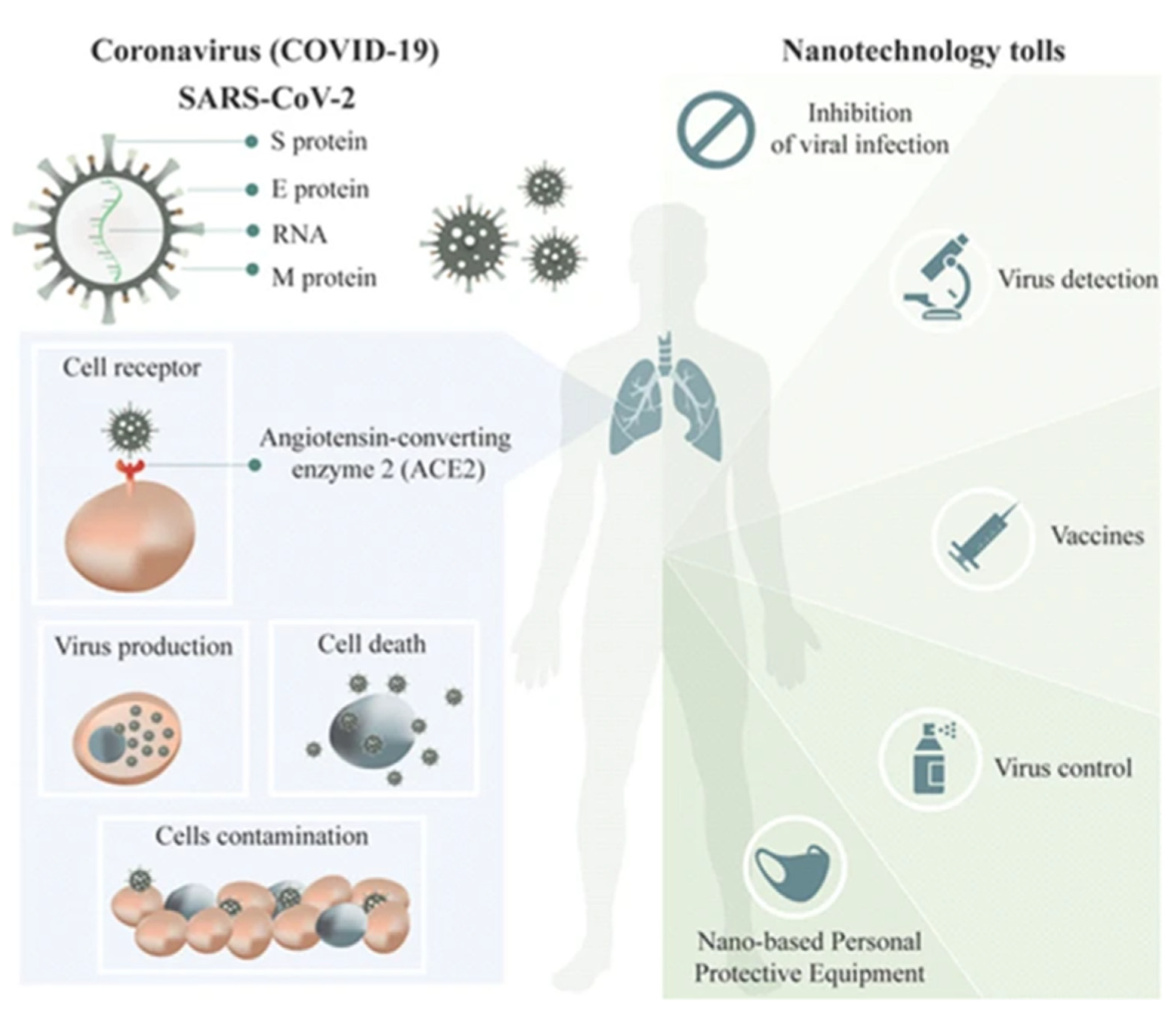
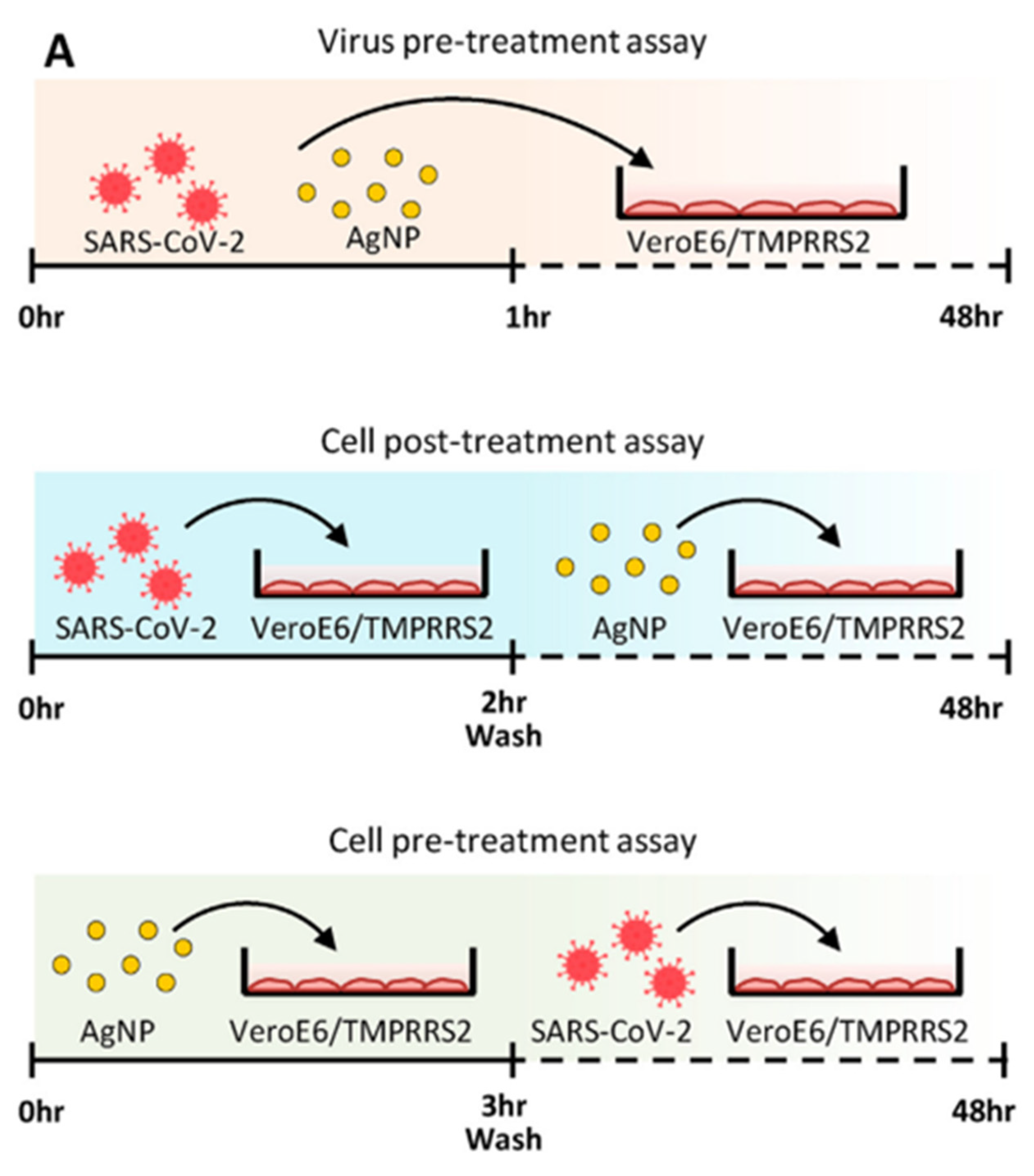
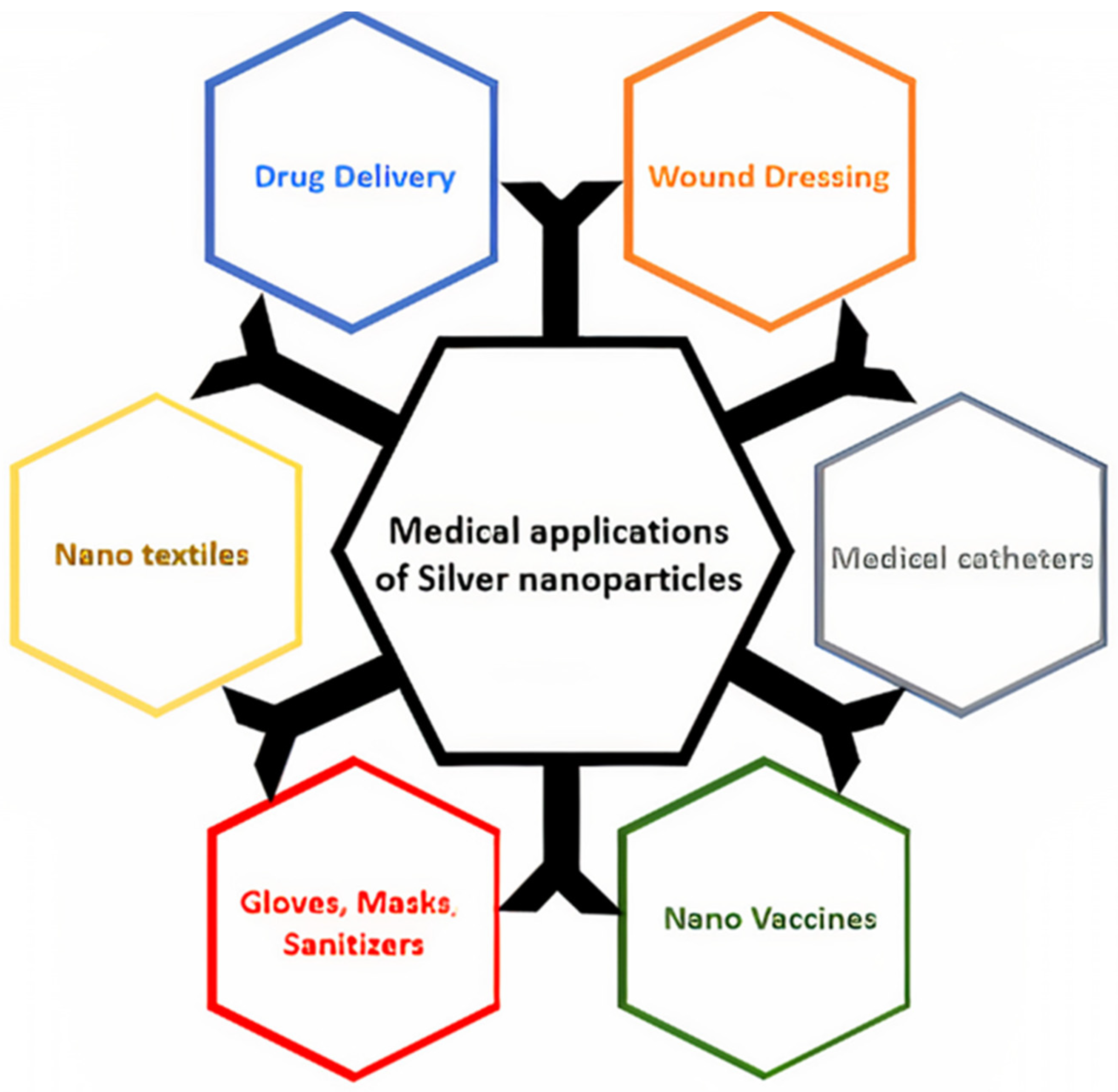
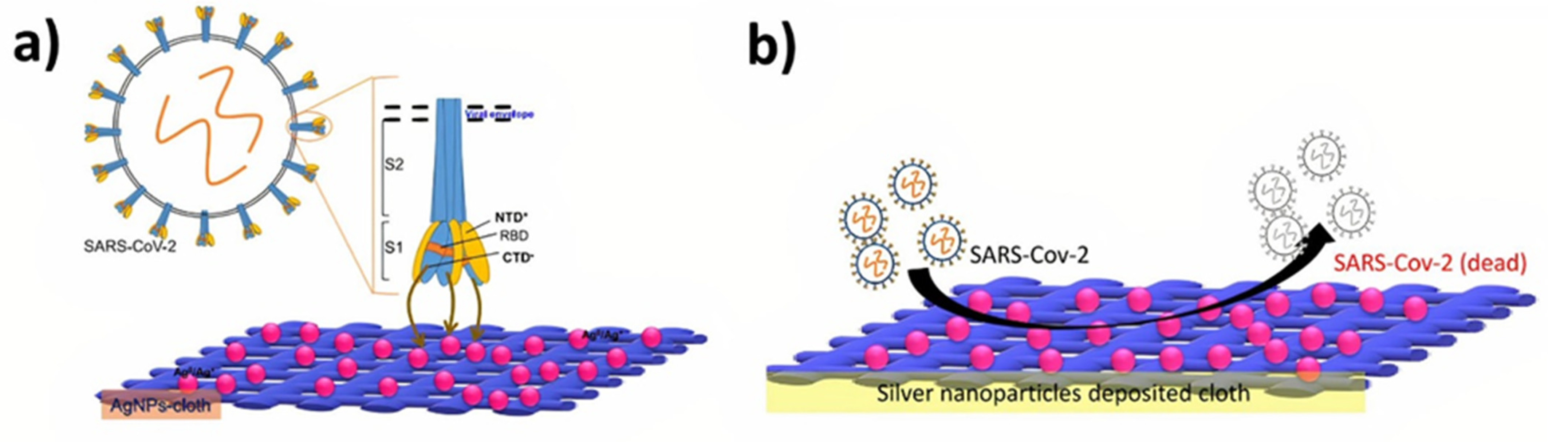
| Sl No. | Month and Year | Type of Variant | Country of Origin | Reference |
|---|---|---|---|---|
| 01 | December 2019 | SARS-CoV-2 | China | [98] |
| 02 | March 2020 | Epsilon | California | [100] |
| 03 | May 2020 | Beta | South Africa | [92] |
| 04 | September 2020 | Alpha | United Kingdom | [98] |
| 05 | October 2020 | Kappa | India | [5] |
| 06 | October 2020 | Delta | India | [91] |
| 07 | November 2020 | Gamma | Brazil | [96] |
| 08 | November 2020 | Iota | USA | [5] |
| 09 | December 2020 | Lambda | Peru | [101] |
| 10 | December 2020 | Eta | Multiple Countries | [5] |
| 11 | January 2021 | Theta | Philippines | [98] |
| 12 | January 2021 | Mu | Columbia | [5] |
| 13 | November 2021 | Omicron | South Africa | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arjun, P.N.J.; Sankar, B.; Shankar, K.V.; Kulkarni, N.V.; Sivasankaran, S.; Shankar, B. Silver and Silver Nanoparticles for the Potential Treatment of COVID-19: A Review. Coatings 2022, 12, 1679. https://doi.org/10.3390/coatings12111679
Arjun PNJ, Sankar B, Shankar KV, Kulkarni NV, Sivasankaran S, Shankar B. Silver and Silver Nanoparticles for the Potential Treatment of COVID-19: A Review. Coatings. 2022; 12(11):1679. https://doi.org/10.3390/coatings12111679
Chicago/Turabian StyleArjun, Phalalochanan Nair Jayapal, Bipin Sankar, Karthik V. Shankar, Naveen V. Kulkarni, Subbarayan Sivasankaran, and Balakrishnan Shankar. 2022. "Silver and Silver Nanoparticles for the Potential Treatment of COVID-19: A Review" Coatings 12, no. 11: 1679. https://doi.org/10.3390/coatings12111679
APA StyleArjun, P. N. J., Sankar, B., Shankar, K. V., Kulkarni, N. V., Sivasankaran, S., & Shankar, B. (2022). Silver and Silver Nanoparticles for the Potential Treatment of COVID-19: A Review. Coatings, 12(11), 1679. https://doi.org/10.3390/coatings12111679









