Effect of Deposition Temperature on the Surface, Structural, and Mechanical Properties of HfO2 Using Chemical Vapor Deposition (CVD)
Abstract
1. Introduction
2. Experiments and Methods
2.1. Experimental Materials
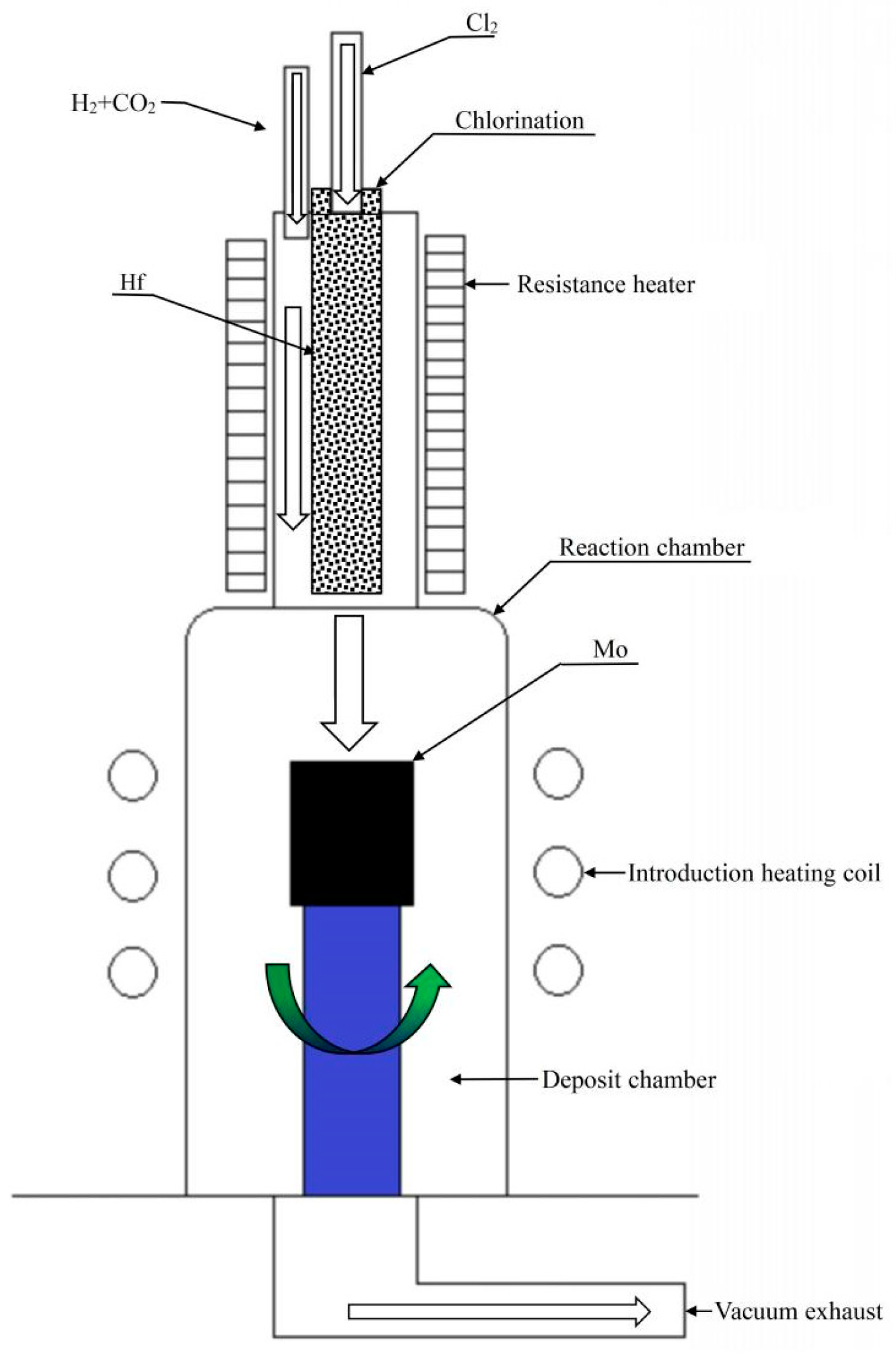
2.2. Experimental Methods
2.3. Measurement Methods
2.4. Thermodynamic Calculation of the Deposition Zone
3. Experimental Results and Analysis
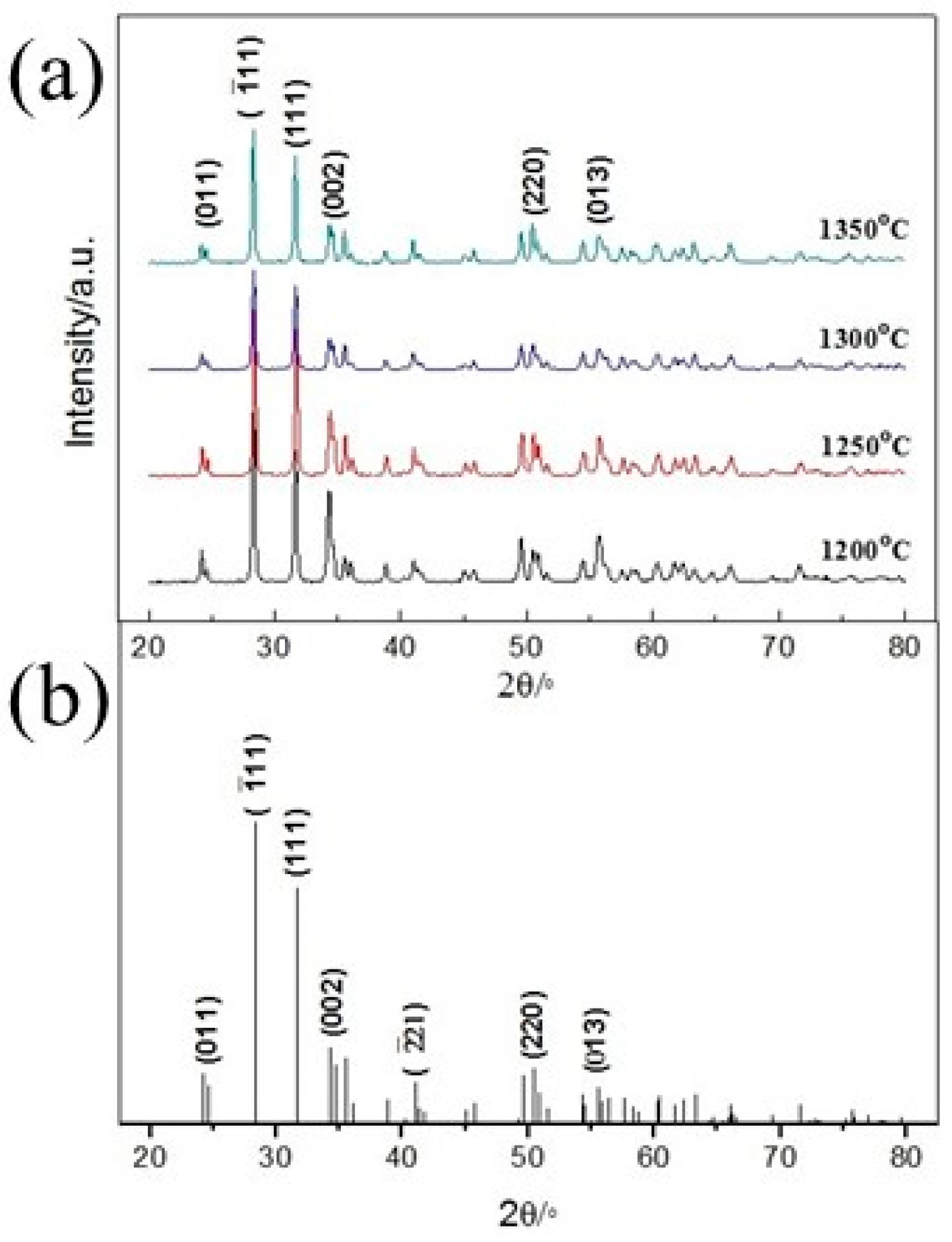
3.1. X-ray Diffraction Analysis
3.2. Surface Topography Analysis
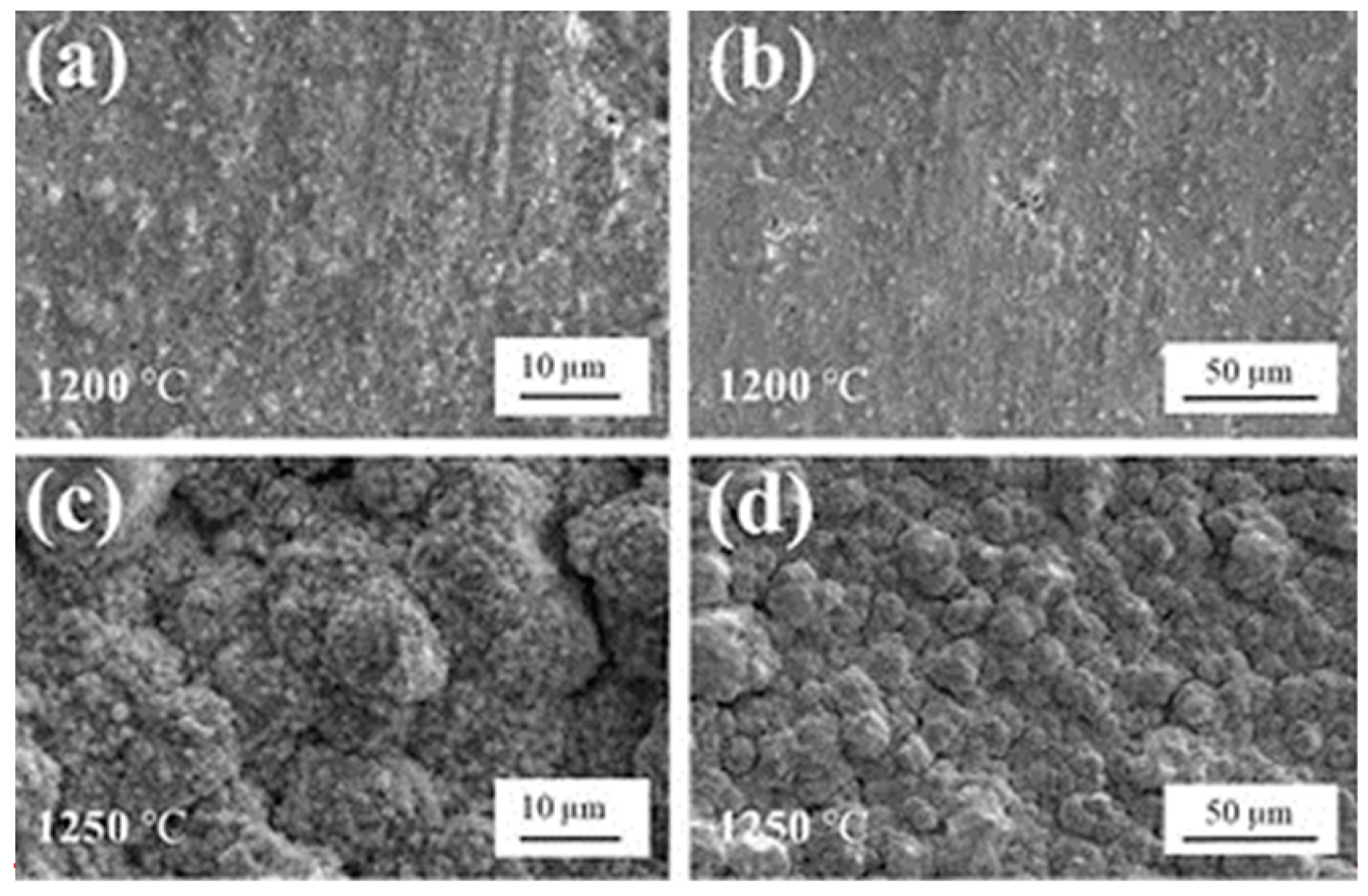
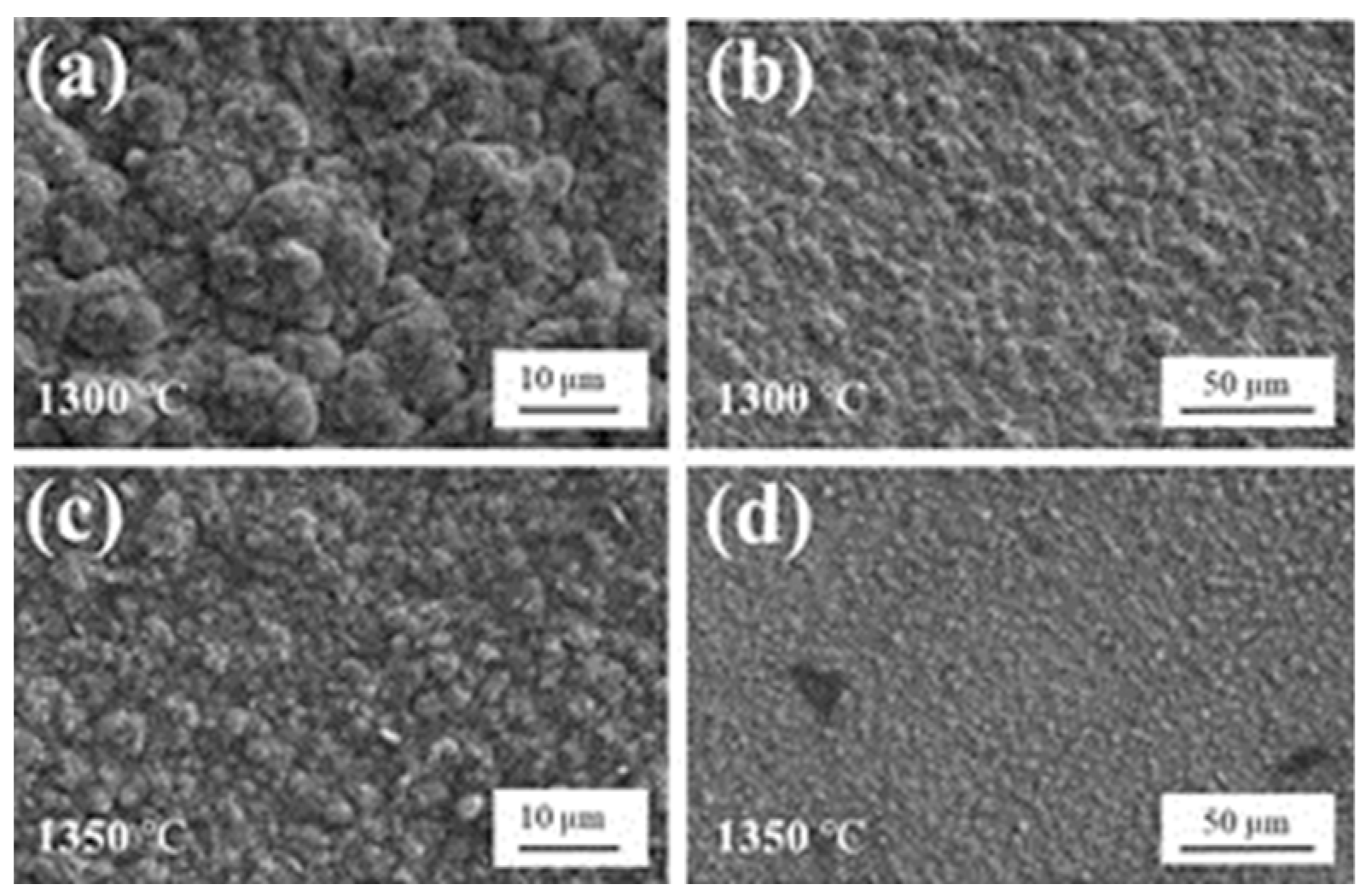
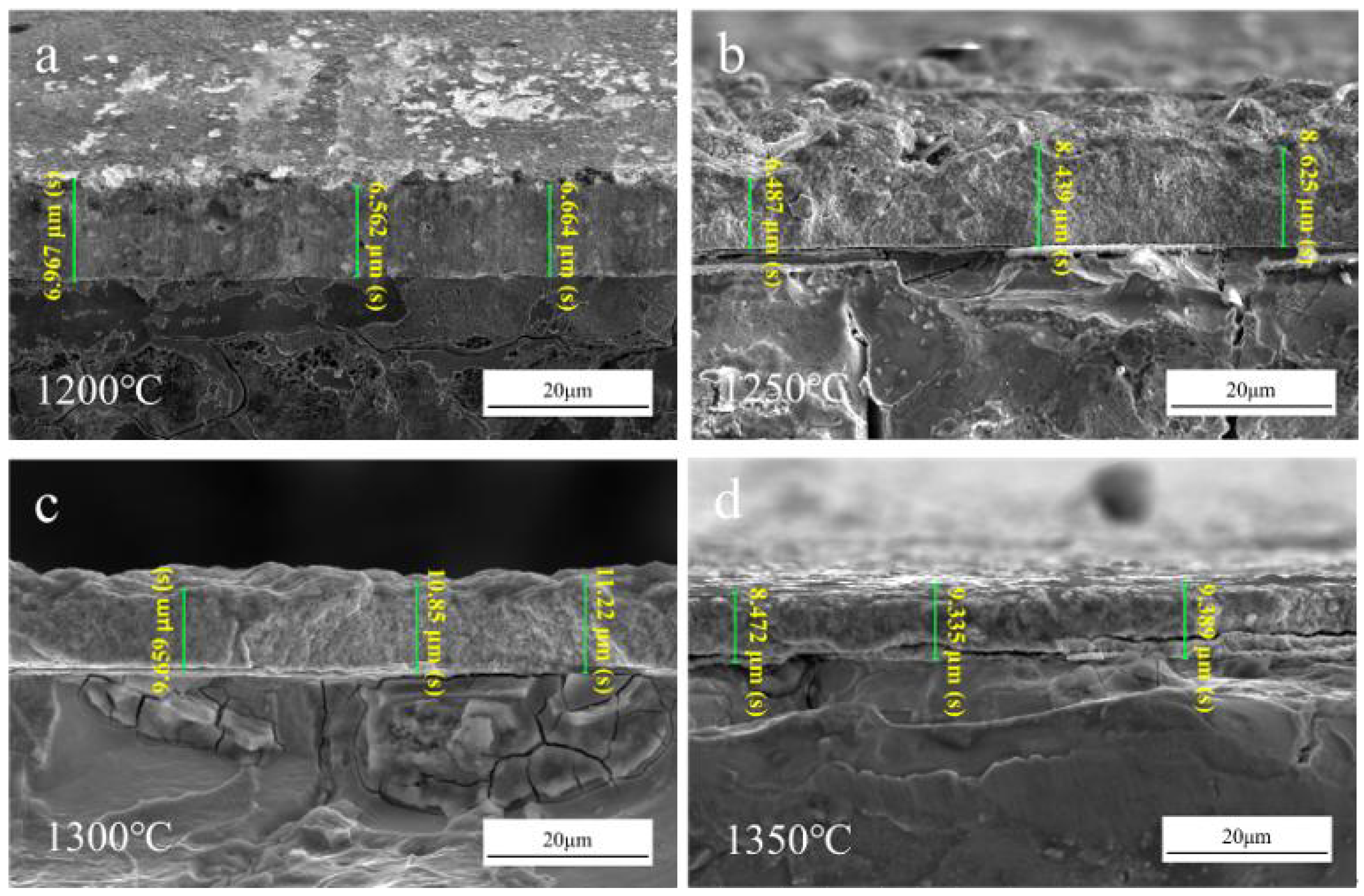
3.2.1. Surface Morphology and Thickness Characterization
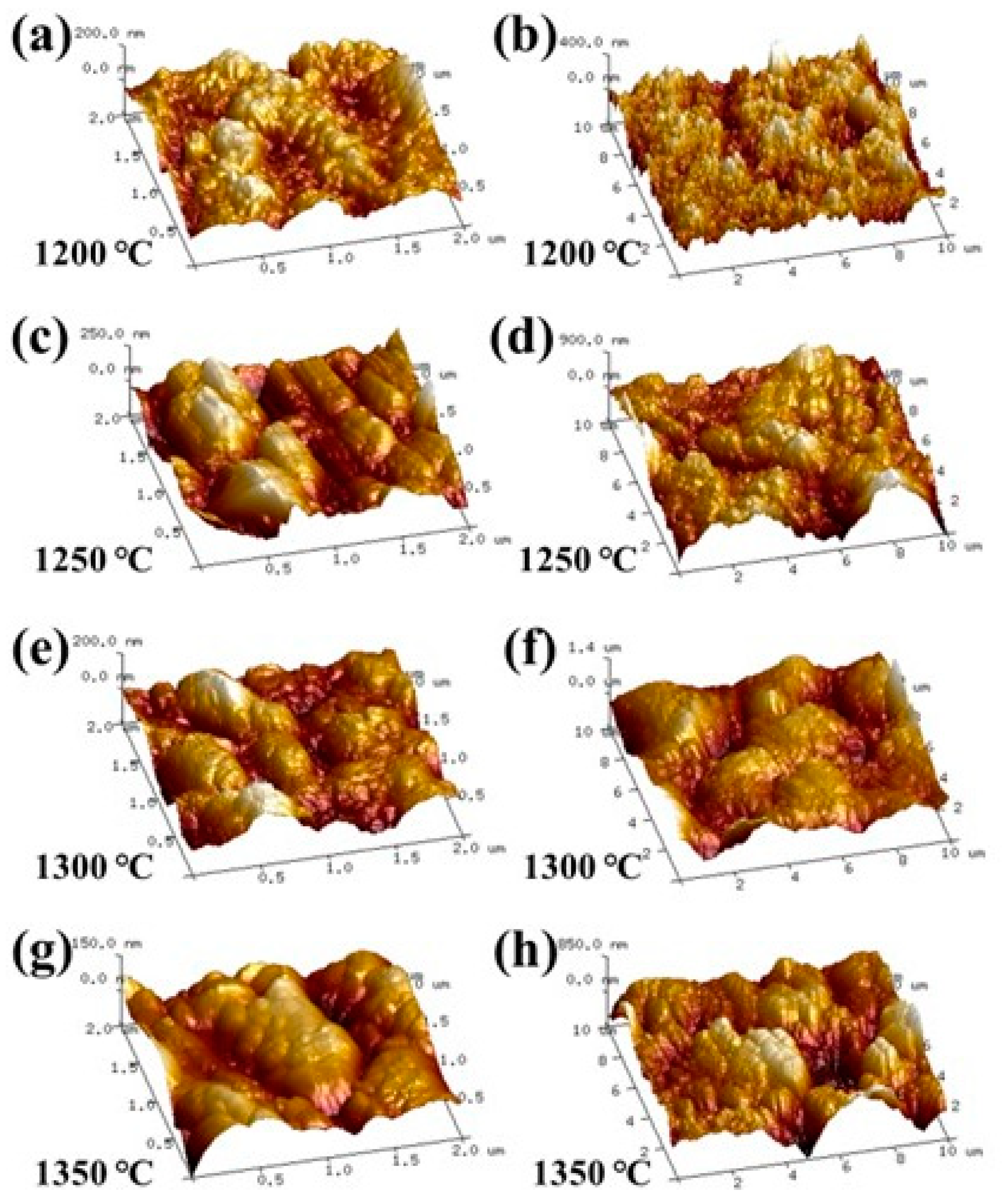
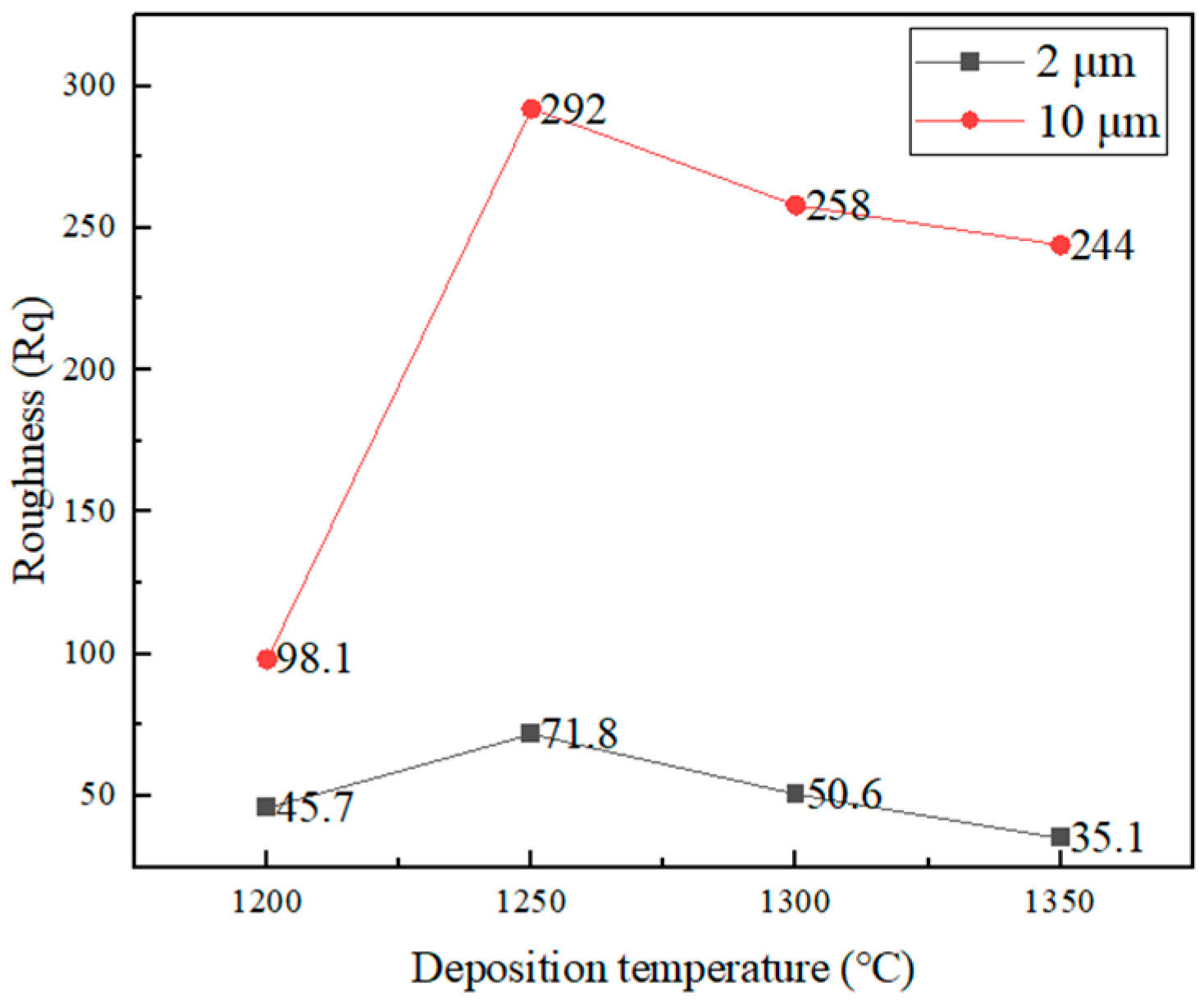
3.2.2. Surface Roughness Characterization
3.3. Density and Relative Density
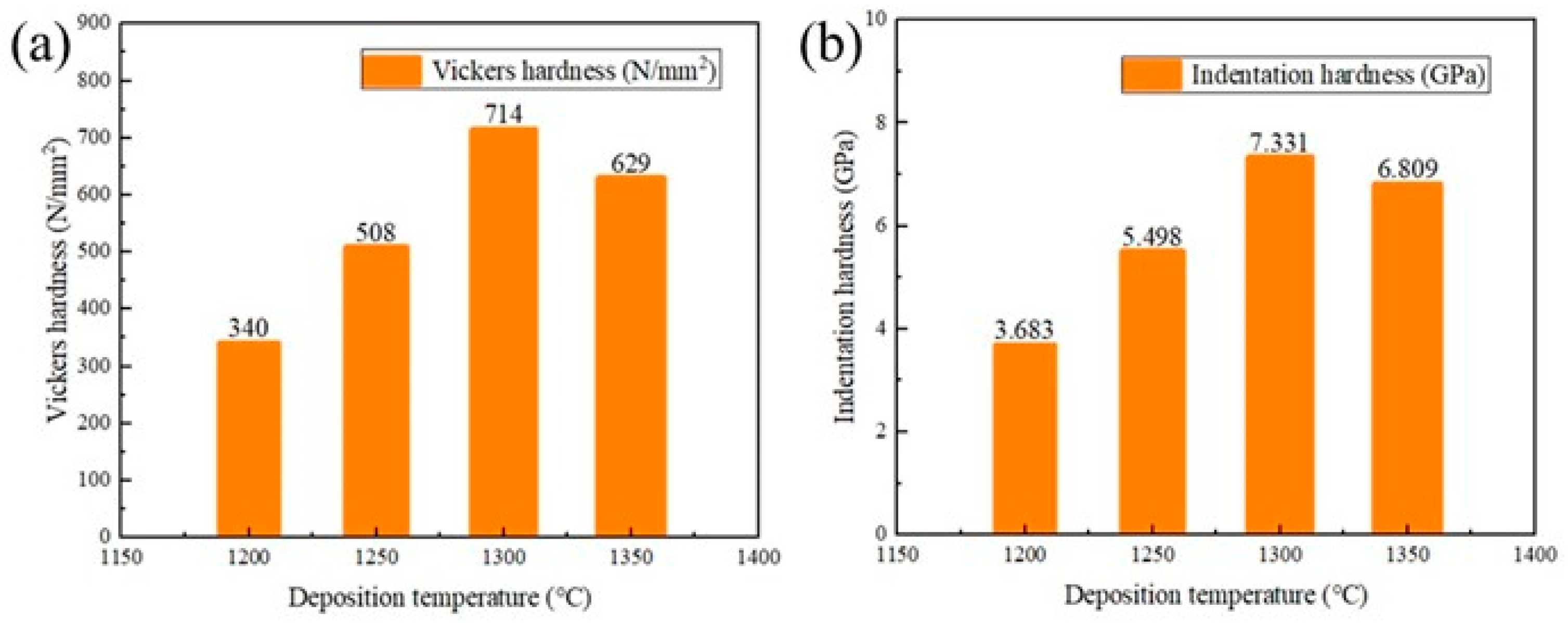
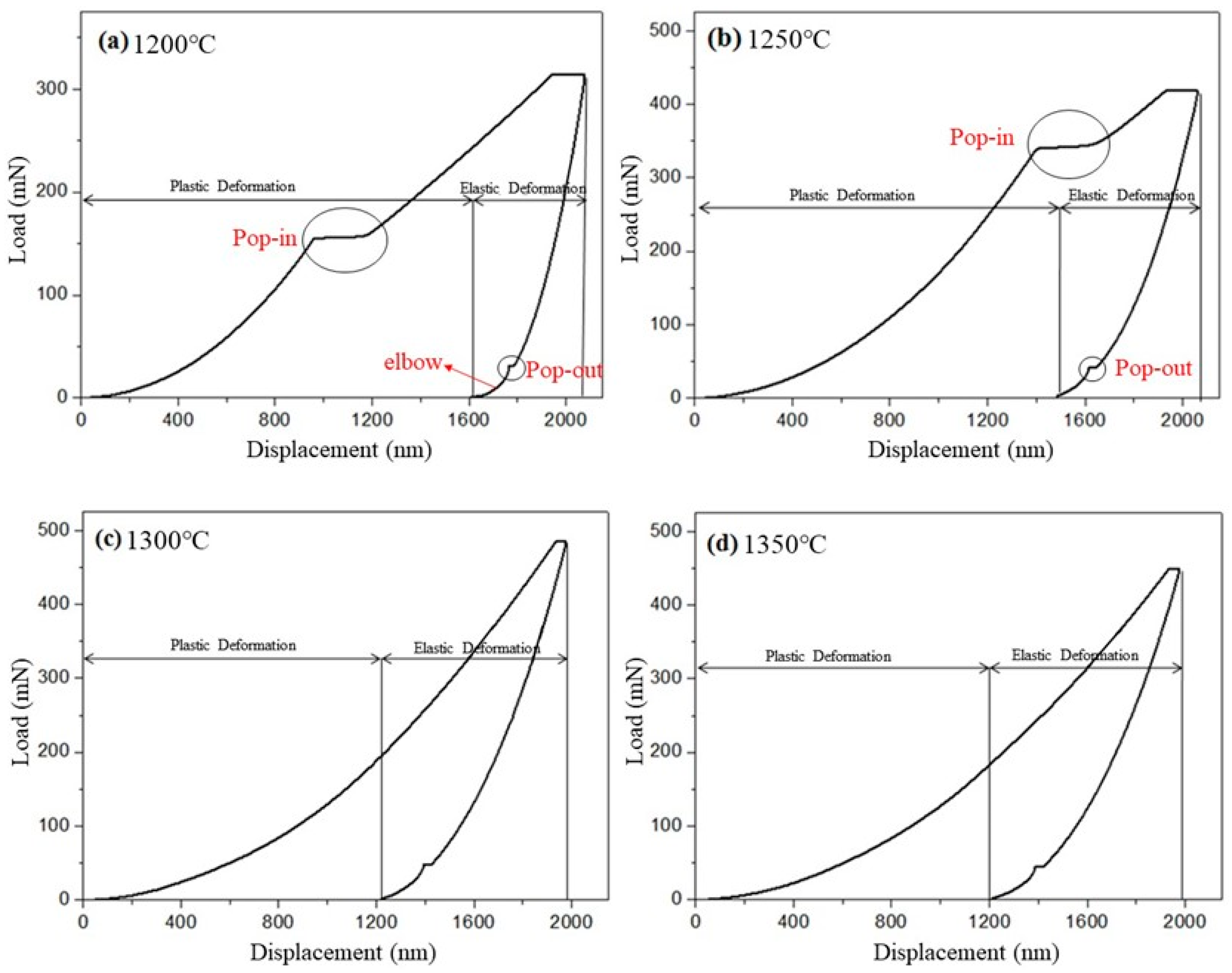
3.4. Hardness and Nanoindentation Load-Displacement Curves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, B.S.; He, R.J.; Hong, C.Q. Ablation behavior and mechanism of double-layer ZrB2-based ceramic coating for lightweight carbon-bonded carbonfiber composites under oxyacetylene torch at elevate temperature. J. Alloy Comp. 2018, 702, 551–561. [Google Scholar] [CrossRef]
- Ren, X.R.; Mo, H.S.; Wang, W.H. Ultrahigh temperature ceramic HfB2-SiC coating by liquid phase sintering method to protect carbon materials from oxidation. Mater. Chem. Phys. 2018, 217, 504–512. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, H.S.; Hong, B.G. Hafnium carbide protective layer coatings on carbon/carbon composites deposited with a vacuum plasma spray coating method. J. Eur. Ceram. Soc. 2016, 36, 1581–1587. [Google Scholar] [CrossRef]
- Shiryaeva, S.; Baranova, A.; Kiselev, O. Hafnium oxide as a nanoradiosensitizer under X-Ray irradiation of aqueous organic systems: A model study using the spin-trapping technique and Monte Carlo simulations. J. Phys. Chem. C. 2019, 123, 27375–27384. [Google Scholar] [CrossRef]
- Choi, H.H.; Jaesung, P.; Huh, O.S. Photoelectric memory effect in graphene heterostructure field-effect transistors based on dual dielectrics. ACS Photonics 2018, 5, 329–336. [Google Scholar] [CrossRef]
- Ramavenkateswari, K.; Venkatachalam, P. Proficiency of acceptor-donor-acceptor organic dye with Spiro-MeOTAD HTM on the photovoltaic performance of dye sensitized solar cell. Electron. Mater. Lett. 2016, 12, 628–637. [Google Scholar] [CrossRef]
- Jayaraman, V.; Sagadevan, S.; Sudhakar, R. Studies on optical and electrical properties of hafnium oxide nanoparticles. J. Electron. Mater. 2017, 46, 4392–4397. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhana, V.; Koratkar, N. Influence of high energy ion irradiation on structural, morphological and optical properties of high-K dielectric hafnium oxide (HfO2) thin films grown by atomic layer deposition. J. Alloy. Compd. 2020, 931, 154698. [Google Scholar] [CrossRef]
- Nigro, R.L.; Schilirò, E.; Mannino, G. Comparison between thermal and plasma enhanced atomic layer deposition processes for the growth of HfO2 dielectric layers. J. Cryst. Growth 2020, 539, 125624. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, W.L.; Zhu, R. Structural, optical, chemical and laser damage resistant properties of HfO2 films deposited by reactive electron beam evaporation. J. China Laser 2020, 47, 170–176. [Google Scholar]
- Zhang, W.Q.; Xiong, Y.H.; Wei, F. Preparation and properties of Nd2O3 doped HfO2 High K gate dielectric films by ALD. Rare Met. 2017, 7, 780–784. [Google Scholar]
- Wang, H.P.; Stevens, R. Hafnia and hafnia-toughened ceramics. J. Mater. 1992, 27, 5397–5430. [Google Scholar] [CrossRef]
- Rammula, R.; Aarik, J.; Mändar, H. Atomic layer de-position of HfO2: Effect of structure development on growth rate, morphology and optical properties of thinfilms. Appl. Surf. Sci. 2010, 257, 1043–1052. [Google Scholar] [CrossRef]
- Lukosius, M.; Walczyk, C.H.; Fraschke, M. High performance metal–insulator–metal capacitors with atomic vapor deposited HfO2 dielectrics. Thin Solid Film. 2010, 518, 4380–4384. [Google Scholar] [CrossRef]
- Lukosius, M.; Dabrowski, J.; Wolff, A. Direct growth of HfO2 on graphene by CVD. J. Vac. Sci. Technol. B. 2015, 1, A110. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, Q.; Wang, Z. Growth properties andoptical properties for HfO2 thinfilms deposited by atomic layer deposition. J. Alloy. Comp. 2018, 735, 1422–1426. [Google Scholar] [CrossRef]
- Gao, H.J.; Xiong, Y.Q.; Zhang, W.T. Principles of atomic layer deposition technology and its application in aerospace. J. J. Vac. Sci. Technol. 2022, 42, 237–243. [Google Scholar]
- Zhong, L.J.; Zhang, Z.H.; Stephen, A. Combinatorial CVD of ZrO2 or HfO2 compositional spreads with SiO2 for high K dielectrics. J. Mater. Chem. 2004, 14, 3203–3209. [Google Scholar] [CrossRef]
- Kang, B.R.; Kim, H.S. Characteristics of ZrC barrier coating on SiC-coated carbon/carbon composite developed by thermal spray process. Materials 2019, 12, 747. [Google Scholar] [CrossRef]
- Zhao, P. Preparation and Properties of Hafnium(IV) Oxide Films by Magnetron Sputtering; Dalian University of Technology: Dalian, China, 2019. [Google Scholar]
- Wan, Y.F.; Li, S.; Yang, P.Z. Progress in preparation and photoelectric properties of Hafnium oxide films. J. Yunnan Norm. Univ. 2021, 41, 12–21. [Google Scholar]
- Korotkov, R.Y.; Ricou, P.; Farran, J.E. Preferred orientations in polycrystalline SnO2 films grown by atmospheric pressure Chemical Vapor Deposition. J. Thin Solid Film. 2006, 502, 79–82. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, Z.H.; Xiong, X. Action mechanism of hydrogen gas on deposition of HfC coating using HfCl4-CH4-H2-Ar system. Appl. Surf. Sci. 2016, 390, 903–908. [Google Scholar] [CrossRef]

| Temperature (K) | ΦT (HfCl4) (J·mol−1·K−1) | ΦT (H2) (J·mol−1·K−1) | ΦT (CO2) (J·mol−1·K−1) | ΦT (HfO2) (J·mol−1·K−1) | ΦT (CO) (J·mol−1·K−1) | ΦT (HCl) (J·mol−1·K−1) | (J·mol−1·K−1) | ΔGT (J·mol·K −1) |
|---|---|---|---|---|---|---|---|---|
| 300 | 190.793 | 130.584 | 213.636 | 59.33 | 197.528 | 186.775 | 322.253 | −21,781 |
| 400 | 195.609 | 131.718 | 215.178 | 61.811 | 198.682 | 187.918 | 321.446 | −53,684 |
| 500 | 204.821 | 133.859 | 218.23 | 66.693 | 200.877 | 190.073 | 319.74 | −84,976 |
| 600 | 218.339 | 136.263 | 221.778 | 72.338 | 203.355 | 192.492 | 314.595 | −213,442 |
| 700 | 252.252 | 138.683 | 225.44 | 78.135 | 205.861 | 194.927 | 289.067 | −227,032 |
| 800 | 279.591 | 141.03 | 229.058 | 83.84 | 208.299 | 197.29 | 269.831 | −240,550 |
| 900 | 302.341 | 143.272 | 232.568 | 89.352 | 210.635 | 199.551 | 254.805 | −254,010 |
| 1000 | 321.731 | 145.403 | 235.946 | 94.638 | 212.86 | 201.703 | 242.741 | −267,426 |
| 1100 | 338.572 | 147.425 | 239.187 | 99.689 | 214.977 | 203.748 | 232.839 | −280,808 |
| 1200 | 353.42 | 149.344 | 242.292 | 104.512 | 216.99 | 205.692 | 224.568 | −294,167 |
| 1300 | 366.673 | 151.169 | 245.268 | 109.12 | 218.907 | 207.544 | 217.563 | −307,517 |
| 1400 | 378.624 | 152.906 | 248.124 | 113.526 | 220.735 | 209.31 | 211.552 | −320,858 |
| 1500 | 389.495 | 154.564 | 250.867 | 117.745 | 222.483 | 210.998 | 206.346 | −334,204 |
| 1600 | 399.456 | 156.149 | 253.507 | 121.792 | 224.156 | 212.614 | 201.792 | −347,552 |
| 1700 | 408.642 | 157.667 | 256.05 | 125.679 | 225.76 | 214.165 | 197.783 | −360,916 |
| 1800 | 417.16 | 159.123 | 258.504 | 129.419 | 227.302 | 215.657 | 194.237 | −374,312 |
| 1900 | 425.097 | 160.524 | 260.876 | 133.022 | 228.787 | 217.093 | 191.071 | −387,720 |
| 2000 | 432.525 | 161.872 | 263.171 | 136.572 | 230.218 | 218.478 | 188.309 | −390,843 |
| (−111) | (111) | (002) | (020) | (200) | (022) | (220) | (013) | |
|---|---|---|---|---|---|---|---|---|
| 1200 °C | 0.700 | 0.696 | 1.569 | 0.826 | 0.535 | 1.189 | 0.778 | 1.707 |
| 1250 °C | 0.707 | 0.797 | 1.125 | 0.883 | 0.823 | 1.180 | 1.019 | 1.469 |
| 1300 °C | 0.739 | 0.803 | 0.950 | 0.976 | 0.915 | 1.180 | 1.055 | 1.383 |
| 1350 °C | 0.750 | 0.781 | 0.919 | 0.964 | 0.868 | 1.121 | 1.256 | 1.340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, M.; Zhu, J.; Luo, Y.; Cai, H.; Li, X.; Wang, X.; Wei, Y.; Wang, X.; Hu, C.; Hu, J.; et al. Effect of Deposition Temperature on the Surface, Structural, and Mechanical Properties of HfO2 Using Chemical Vapor Deposition (CVD). Coatings 2022, 12, 1731. https://doi.org/10.3390/coatings12111731
Bi M, Zhu J, Luo Y, Cai H, Li X, Wang X, Wei Y, Wang X, Hu C, Hu J, et al. Effect of Deposition Temperature on the Surface, Structural, and Mechanical Properties of HfO2 Using Chemical Vapor Deposition (CVD). Coatings. 2022; 12(11):1731. https://doi.org/10.3390/coatings12111731
Chicago/Turabian StyleBi, Mengran, Junyu Zhu, Yuan Luo, Hongzhong Cai, Xuming Li, Xian Wang, Yan Wei, Xiao Wang, Changyi Hu, Jinquan Hu, and et al. 2022. "Effect of Deposition Temperature on the Surface, Structural, and Mechanical Properties of HfO2 Using Chemical Vapor Deposition (CVD)" Coatings 12, no. 11: 1731. https://doi.org/10.3390/coatings12111731
APA StyleBi, M., Zhu, J., Luo, Y., Cai, H., Li, X., Wang, X., Wei, Y., Wang, X., Hu, C., Hu, J., Zhang, G., Wang, X., & Zhang, X. (2022). Effect of Deposition Temperature on the Surface, Structural, and Mechanical Properties of HfO2 Using Chemical Vapor Deposition (CVD). Coatings, 12(11), 1731. https://doi.org/10.3390/coatings12111731





