Abstract
As electrolytic plasma polishing (EPP) offers the advantages of strong shape adaptability, high efficiency, and environmental friendliness, it has great application prospects in biomedical material processing. However, the effect of EPP on the biological performance of the treated surfaces remains unclear. In the present study, the effects of EPP on the surface roughness, micro-morphology, corrosion behavior, and cell response of 316LVM were investigated. The results revealed that the surface roughness (Ra) was reduced from 0.3108 to 0.0454 µm upon EPP, and the sharp peaks and protrusions produced as a result of mechanical grinding were removed. The corrosion current density decreased from 1.129 to 0.164 µA/cm2, while the charge transfer resistance increased from 513.3 to 17,430 kΩ·cm2, which implied that EPP treatment could significantly improve the corrosion resistance of 316LVM. Furthermore, affected by the sharp ridges on both sides of the groove, the outward spreading of osteoblasts (MC3T3-E1) on the untreated samples was inhibited, and the edges were curled. The cells grew along the direction of the mechanical processing texture on the untreated samples, while they grew randomly in all directions on the surface treated using EPP, which adversely affected the growth, spreading, and migration of the cells.
1. Introduction
Austenitic stainless steels, such as AISI 316LVM, exhibit excellent corrosion resistance, commendable comprehensive mechanical properties, excellent processing and formability, good biocompatibility, and low cost. Therefore, these steels are used widely in medical devices and implants, such as knee prosthesis, hip prosthesis, fracture internal fixation devices, dental orthopedic devices, cardiovascular stents, artificial heart valves, and coronary artery stents [1,2,3,4]. The surface characteristics of metal implants, such as roughness, morphology, and chemical elemental composition, exert significant impacts on the biocompatibility and corrosion resistance of the implants, thereby influencing the performance and service life of the implants [5,6].
The corrosion resistance of the implants is an important factor that determines the success or failure of the operation. When metal implants corrode inside the biological environment, they release metal ions that may cause inflammation, allergies, and other adverse effects [7]. Long-term corrosion may lead to mechanical loosening of the prosthesis [8]. In addition, the surface characteristics of the implant will affect the cell adhesion, proliferation, differentiation, and other life activities, and is also an important factor affecting the performance of the implants [9]. Currently, researchers have developed a variety of techniques to improve the surface properties of implants, thereby increasing their corrosion resistance and biocompatibility. These include mechanical grinding [10], electrochemical polishing [11], plasma alloying technique [12], laser processing [13,14], shot peening [15], cold spraying [16], and so on.
Electrolytic plasma polishing (EPP) is an advanced technology for polishing metal and alloy surfaces. The EPP device generally consists of a DC power supply, temperature control system, polishing tank, electrolyte, cathode, and anode (workpiece). The workpiece (surface to be polished) is immersed in the polishing solution at a certain temperature and then a high voltage is applied between the anode and the cathode. Under the action of electrochemistry and the thermal effect of the current, a vapor gas envelope (VGE) is formed on the anode surface [17,18]. The VGE is ionized under the action of high voltage and plasma discharge medium is formed [19,20]. The anode surface is oxidized under the action of electrochemistry and plasma chemistry and the generated oxide is removed under complex physical and chemical actions, such as discharge bombardment, gas layer flow, and electrochemical dissolution [21,22,23,24]. When a dynamic balance is reached between the generation and removal of the oxide, the polishing of the anode surface is realized [25]. The EPP technology is applicable to the surface of products with complex geometric structures. The roughness of the product is greatly reduced upon treatment with EPP and the surface becomes free of burrs [26]. The processing requires a short duration for completion, which facilitates mass production [26,27]. Moreover, a low concentration salt is used as an electrolyte, rendering this process environmentally friendly [27,28]. EPP may be utilized to process biomedical metal materials, such as stainless steels, cobalt-chromium alloys, and titanium alloys [20,24]. In addition, the polishing of additive manufacturing products can be achieved through EPP technology [29,30]. EPP has a wide application potential in the field of biomedical product processing [30,31]. It can be used as the final processing process of the product and is also expected to be used in the pretreatment or post-treatment of coating, spraying, surface micro and nano structure [32,33], and other processing. Henning Zeidler conducted a preliminary study to reveal the effect of EPP-polished surface on biocompatibility and reported that the EPP-polished surface would not affect the survival of cells and could limit the growth of dental plaque bacteria [34]. However, the effects of EPP on biological corrosion behavior and cellular response remain to be elucidated so far.
The present study investigated the effects of EPP on biological corrosion resistance and osteoblast response using medical stainless steel 316LVM as the research object. The samples’ surface roughness, microscopic morphology, and chemical composition changes prior to and after treatment with the electrolytic plasma processing were characterized using scanning electron microscopy (SEM), ultra-depth three-dimensional microscope, surface profilometer, and X-ray photoelectron spectroscopy (XPS). Then, the corrosion resistance of the sample surface in simulated body fluid was examined. Electrochemical technology was adopted to analyze the EPP-treated surface’s polarization curve and impedance spectrum. The strengthening mechanism of corrosion resistance was analyzed in combination with the surface changes. Furthermore, osteoblasts (MC3T3-E1) were inoculated on the surfaces of the samples and the effect and the underlying mechanism of EPP treatment on the growth and morphology of these cells were studied.
2. Materials and Methods
2.1. Preparation of Samples
The EPP device is depicted in Figure 1. The samples of size 20 mm × 15 mm × 3 mm were cut from the cold-rolled plates of 316LVM austenitic stainless steel with the following chemical composition (in wt.%): 0.019 C, 0.47 Si, 1.46 Mn, 0.016 P, 0.002 S, 18.29 Cr, 14.77 Ni, 0.02 Cu, 2.68 Mo, 0.082 N, and 62.191 Fe. A 2 mm hole was drilled in each sample close to the edge of the short side to hang the samples. All samples were then placed on a polishing machine and polished using 120-mesh sandpaper to remove the oxide scale and render the roughness as consistent as possible. Then, the samples were divided into a control group and test group, and the samples of the control group were used as the initial samples before EPP treatment. The samples of the test group were processed by EPP in 3% (wt.%) ammonium sulfate solution at a temperature of 88–92 °C for 15 min and the processing voltage was 300 V.
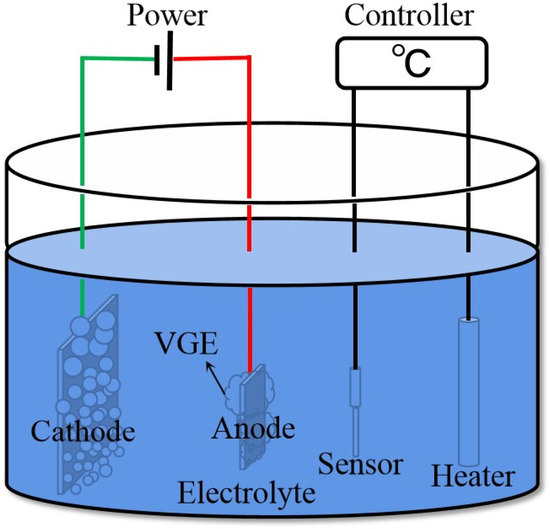
Figure 1.
Schematic illustration of the electrolytic plasma polishing apparatus.
2.2. Sample Characterization
The electron micrographs of the samples at 800× and 2000× were taken with a TESCAN VEGA scanning electron microscope. An ultra-depth of field microscope (Leica DM6M, Wetzlar, Germany) was employed to analyze the 3D morphology and microstructure of the samples. The values of surface roughness parameters (Ra, Rq, and Rz) were obtained with a Marh M400 surface profilometer and each sample was measured five times. A Thermo Electron Escalab 250xi multifunctional surface analysis system was employed to conduct the X-ray photoelectron spectroscopy (XPS) of the samples using a monochromatic Al Kα X-ray source (h = 1486.6 eV) and an X-ray spot size of 500 µm. The survey spectra were recorded at a step size of 1 eV and the high-resolution spectra of the Fe 2p and Cr 2p were recorded at a step size of 0.1 eV. Binding energies were calculated with respect to C 1s at 284.8 eV and were measured with a precision of ±0.05 eV. Curve fitting of the spectra was performed using the XPS PEAK software and 0-iterative Shirley-type background subtraction.
2.3. Electrochemical Measurement
Electrochemical measurements were conducted using a CHI660E electrochemical workstation. CHI version 18.04 was employed to control the instrument and for data collection and treatment. All measurements were conducted in a three-electrode system, in which the counter electrode (CE) was a platinum electrode, the reference electrode (RE) was a saturated calomel electrode, and the samples served as the working electrode (WE). The WE samples were placed in a specially constructed corrosion electrochemical cell, which exposed 1 cm2 of the sample to the solution. The corrosion tests were conducted in Hank’s balanced salt solution at 37.0 ± 0.5 °C under the control of a water bath. The composition of Hank’s balanced salt solution (per L, dissolved in distilled water) was as follows: 8.0 g NaCl, 0.4 g KCl, 0.14 g CaCl2, 0.35 g NaHCO3, 1.0 g C6H12O6, 0.1 g MgCl2·6H2O, 0.06 g MgSO4·7H2O, 0.06 g KH2PO4, and 0.06 g Na2HPO4·12H2O. A minimum of three samples were used in each corrosion test to ensure the accuracy and repeatability of the measured data. The order of measurements was as follows. Open circuit potential (OCP) was measured and recorded for 1800 s to ensure the stability of the alloy samples in Hank’s solution. After the OCP measurement, electrochemical impedance spectroscopy (EIS) was performed potentiostatically, in which peak-to-peak sinusoidal voltage signals of 5 mV were run over the frequency range of 10−2–105 Hz. The data obtained from EIS were processed using the software ZVIEW 3.1. Afterward, potentiodynamic polarization tests were conducted in a sweeping range of −0.5 to +0.5 V (vs. OCP) at a sweeping step of 1 mV/s.
2.4. Culture of Osteoblasts
The osteoblasts (MC3T3-E1; Shanghai Cell Bank, Chinese Academy of Sciences, Shanghai, China) were cultured in α-MEM medium inside a humidified, constant temperature incubator with a saturated humidity, a constant temperature of 37.0 °C, and a stable CO2 content of 5%. The culture medium comprised α-MEM dry powder 10.2 g/L, fetal bovine serum 10% (vol.%), penicillin 100 units/mL, streptomycin 100 µg/mL, and sodium bicarbonate 2.2 g/L. The samples were soaked in 75% alcohol for 30 min, followed by three rinses with sterile phosphate-buffered saline (PBS) and then incubation.
The cell toxicity of the samples was evaluated by subjecting osteoblasts to LIVE/DEAD cell staining. The stain comprised 5 µL Calcein AM, 20 µL ethidium homodimer-1(EthD-1), and 10 mL PBS. Calcein AM emits green fluorescence when it comes into contact with living cells. At the same time, EthD-1 passes through the damaged membrane structure of dead cells and binds to the nucleic acid to emit red fluorescence from inside the entering cells. The cells were cultured on the surfaces of the samples for 24 h. After the culture growth was terminated, the samples were rinsed gently with PBS three times, and then 50 µL of live/dead fluorescence stain was added dropwise to the surface of each sample. This was followed by incubation of the samples in the dark at a constant temperature inside an incubator for 1 h. After 1 h, the samples were rinsed gently with PBS and observed and photographed under a fluorescence microscope.
The cytoskeleton assembly ability of osteoblasts was evaluated using the mixed staining method involving fluorescein isothiocyanate (FITC) and 4, 6-diamidino-2-phenylindole (DAPI). FITC stains fibrins, such as microtubule filaments, in the cells, while DAPI stains the location of the nucleus. The cells were cultured on the surfaces of the samples for one day and three days, followed by rinsing with PBS, fixing in paraformaldehyde for 30 min, and rinsing gently with PBS solution again to remove the excess paraformaldehyde. Then, 50 µL of the FITC stain was added dropwise to the surface of each sample and staining was allowed to occur in the dark for 40 min. After gently rinsing with PBS, 50 µL DAPI stain was added to each sample’s surface and stained in the dark for 10 min. The samples were then rinsed and observed, and then photographed under a fluorescence microscope.
The cells were cultured for one day and then fixed in 2.5% glutaraldehyde for 30 min, followed by dehydration in graded ethanol and a subsequently sputtered Au thin layer. Cell morphology was examined using SEM. Cell morphology was observed with scanning electron microscope at 200×, 1000×, 10,000×, and 50,000× magnifications.
3. Results and Discussions
3.1. Surface Roughness and Morphology
The scanning electron microscopy images and the three-dimensional topographies of the samples before and after electrolytic plasma polishing are depicted in Figure 2. Deep valleys and sharp peaks were observed on the surface of the sample after mechanical grinding. In the EPP process, owing to the tip effect, the local field intensity and the charge density at the peaks on the sample surface are larger, which leads to an accelerated reaction rate and preferential removal of the peaks. After the peaks are completely removed, the surface of the sample would be uniformly removed, finally leaving the surface of the sample flat and smooth. In comparison with the original samples, the EPP-treated samples exhibited significant reductions in the surface roughness parameters Ra, Rq, and Rz, from 0.3108 to 0.0454 µm, 0.4419 to 0.0585 µm, and 3.2604 to 0.3678 µm, respectively (Figure 3).
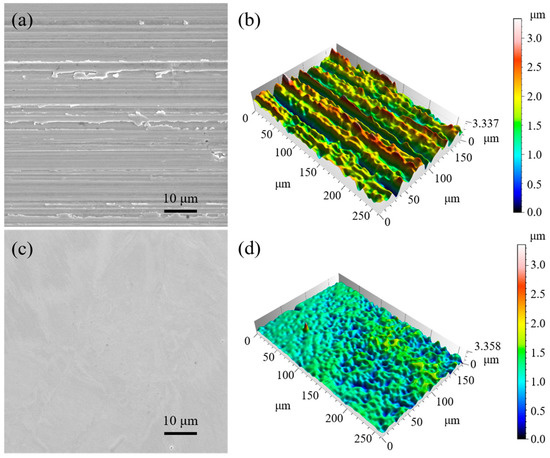
Figure 2.
SEM images and 3D topographies of the samples: (a,b) prior to EPP and (c,d) after EPP.

Figure 3.
Surface roughness parameters of the samples prior to and after EPP.
3.2. XPS Analysis
In the EPP process, the formation and removal of oxides occur on the metal surface, causing changes in the content and valence of the metal elements. Therefore, X-ray photoelectron spectroscopy (XPS) analysis of the untreated and EPP-treated surfaces was performed to investigate and compare the variations occurring at these surfaces. Figure 4 depicts the high-resolution XPS spectra and the deconvoluted results of the Cr 2p3/2 and Fe 2p3/2 of untreated and EPP-treated samples. The black lines indicate the test spectrum, while the red lines indicate the sum of the deconvoluted XPS spectrum. In the chromium (Cr 2p3/2) spectra of the samples, three main peaks were observed at 574.0, 576.4, and 577.6 eV, which corresponded to Cr, Cr2O3, and Cr(OH)3, respectively, attributed to the metallic states Cr(0) and Cr(III) [35,36]. Similarly, in the iron (Fe 2p3/2) spectra of the samples, four main peaks were observed at around 707.0, 708.0, 710.0, and 711.6 eV, which corresponded to Fe, Fe3O4, Fe2O3, and Fe(OH)3, respectively, attributed to the metallic states Fe(0), Fe(II), and Fe(III) [35,36]. A comparison of the areas of the deconvolution results indicated that the relative content of Fe(0) in the samples increased after EPP, which was attributed to the exposure of the new metal matrix after the removal of the oxides. Chromium mainly exists in the form of oxides. As chromium has a lower electrode potential compared with iron, it was oxidized again, and the relative content of Cr(0) exhibited no significant change.
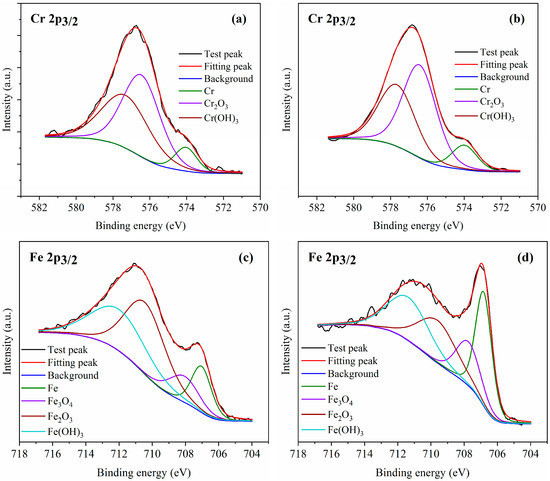
Figure 4.
The high-resolution spectra of Cr 2p3/2 and Fe 2p3/2 (a,c) prior to and (b,d) after EPP.
Chromium and iron are the most important elements that affect the corrosion resistance of stainless steel, and increasing the atomic concentration ratio of chromium to iron (Cr/Fe) is beneficial to improving the corrosion resistance of stainless steel [6]. In the present study, the element sensitivity factor method was adopted to calculate the Cr/Fe of the samples prior to and after EPP, and the results are presented in Table 1. In comparison with the untreated samples, Cr/Fe was significantly increased after EPP, which was consistent with the change trend of 316 stainless steel after electrochemical polishing [6]. This suggests that iron is more easily removed than chromium during EPP, which may be attributed to the fact that electrochemical reaction is the main method for material removal [37,38] and the chemical properties of Cr oxides are more stable than those of Fe [39], with relatively less removal.

Table 1.
Atomic concentration ratio of Cr to Fe (Cr/Fe).
3.3. Biological Corrosion Resistance Analysis
The anodic polarization curves of the original and EPP-treated 316LVM stainless steel samples in Hank’s solution are depicted in Figure 5. The corrosion parameters of the samples derived from these polarization curves are presented in Table 2, where icorr denotes the corrosion current density, Ecorr denotes the corrosion potential versus saturated calomel electrode (SCE), βc denotes the cathode Tafel slope, and βa denotes the anode Tafel slope. The corrosion potential of the samples was observed to increase after EPP, from −0.276 to −0.261 V, while the corrosion current density was reduced from 1.129 to 0.164 µA/cm2. The corrosion potential and corrosion current density of CoCr Mo alloy and structural steel in 3.5% sodium chloride solution have the same trend before and after EPP polishing [30,40]. According to Faraday’s law, the corrosion rate of the metal is proportional to the corrosion current density [41], which confirms that the surface of 316LVM stainless steel samples polished using electrolytic plasma had a stronger corrosion resistance in Hank’s solution compared with the surface of the untreated sample.
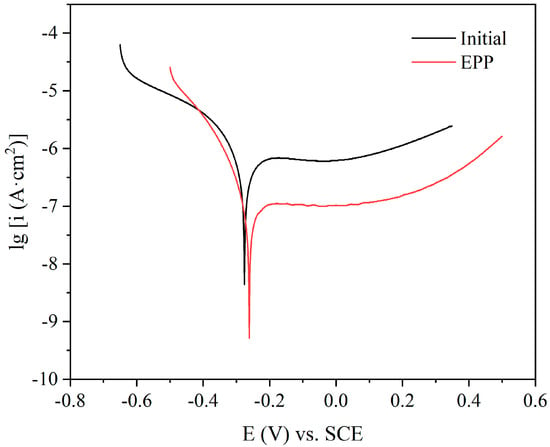
Figure 5.
Tafel polarization curves of samples in Hank’s solution prior to and after EPP.

Table 2.
Fitting results derived from the Tafel polarization curves.
Figure 6 illustrates the EIS data presented in the form of (a) Nyquist plots and (b) Bode plots for untreated and EPP-treated samples. The Nyquist plots characterize the relationships between the real and imaginary parts of the impedance. In these plots, only single semi-circular capacitance is shown for each sample, indicating the existence of one time constant [42]. In comparison with the smaller diameter of the untreated samples, a much larger loop diameter was observed in the EPP samples. It is known that the radius of the capacitor arc in the Nyquist diagram reflects the impedance of the passive film formed on the sample surface, implying that the capacitor arc with a larger radius generally specifies a higher impedance of passive film [2]. Therefore, it was inferred that the surfaces of the EPP samples had greater resistance to electrochemical dissolution compared with the untreated surface, and the corrosion resistance of the former was stronger, which is consistent with the results obtained regarding the lower corrosion current density of these samples.
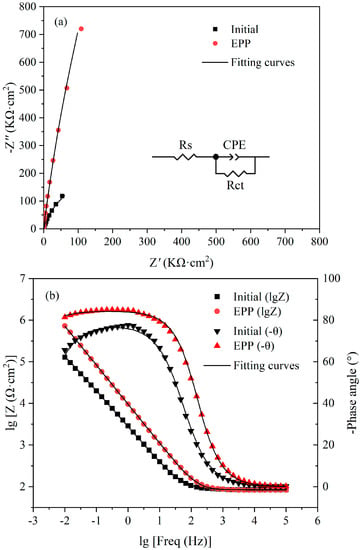
Figure 6.
The results of the electrochemical impedance spectroscopy analysis: (a) Nyquist plots and (b) Bode plots.
In the Bode impedance diagram, two distinct regions were observed. A flat portion of the curve was observed from the middle to high frequency (103–105 Hz) region, which was a response to the resistance of the electrolyte. In the frequency range of 10−2–102 Hz, the impedance increased with a decrease in the frequency, exhibiting a linear relationship. The impedance of the EPP samples was significantly higher than that of the untreated samples. In the Bode phase angle diagram, the phase angle approached 0° at a high frequency (104–105 Hz), which also indicated the constant resistance of electrolyte in the EIS measurements. In the frequency range of 10−2–104 Hz, the phase angles of the EPP samples were significantly larger than those of the untreated surface. These results demonstrated that electrolytic plasma polishing could effectively improve the corrosion resistance of the 316LVM stainless steel surface. In addition, the maximum phase angles of the untreated and EPP-treated samples were 77.6° and 84.2°, respectively. The modulus of the phase angle maxima is lower than 90°, suggesting that the non-ideal capacitor behavior of the passive films formed on the samples [43].
The equivalent electrical circuit (EEC) depicted in Figure 6a was used for the quantitative characterization of the electrochemical behavior at the interface between the passive film and the electrolyte medium. In the EEC, Rs denotes the solution resistance, Rct denotes the charge transfer resistance, and CPE is the constant phase element. The EIS results were fitted using the ZsimpWin software (v 3.60) and the fitted results are listed in Table 3. The χ2 value of less than 10−3 indicated a good quality fitting [41,44]. As all EIS tests were conducted in Hank’s solution under the same conditions, the solution resistance values were the same. In comparison with the untreated sample, the EPP-treated sample exhibited a great increase in the charge transfer resistance, from 531.3 to 17,430 kΩ·cm2. The larger Rct value of the EPP samples implied that their interfacial charge transfer was impeded, which suppressed the anodic dissolution process and decreased the corrosion rate. The EIS results were in good agreement with the anodic polarization curve.

Table 3.
Fitting results derived from the electrochemical impedance spectra.
The content of chromium element is a key factor affecting the corrosion resistance of stainless steel, because it can form a chromium trioxide passivation film with high stability and low ionic conductivity, which has a protective effect on the substrate [45]. Iron is the element with the highest content in stainless steel and it is also the main element for the corrosion reaction of stainless steel. Increasing the atomic concentration ratio of chromium to iron (Cr/Fe) is beneficial to improving the corrosion resistance of stainless steel. The difference in corrosion resistance of the samples before and after EPP is related to a change in the chromium/iron ratio. Compared with the untreated samples, the chromium/iron ratio of the samples after EPP treatment increased, resulting in an increase in chromium oxide content in the passive film and a decrease in the acceptor concentration in the corrosion reaction. Consequently, the charge transfer resistance increased and the corrosion current density decreased, improving the corrosion resistance. The CPE exponent value (n) defines the degree of deviation from pure capacitive behavior (n = 1). The increase in the n value after EPP treatment is related to the decrease in surface roughness, and it has been reported that the n value is inversely proportional to the interfacial roughness [46]. In addition, the surface roughness of the samples before EPP is larger, which led to a larger contact area with the corrosion medium, thereby increasing the area of corrosion reaction. The surface of a metal is composed of microscopic peaks and valleys. The larger roughness of a sample surface implies that it has higher peaks and deeper valleys. In corrosive media, these peaks are often unstable and more likely to induce the occurrence of corrosion reactions. After EPP, the higher peaks on the sample surface were removed, greatly reducing the number of sites prone to corrosion reactions. Therefore, the corrosion resistance of samples is improved after EPP, owing to the combined effect of the reduction of surface roughness and increased ratio of chromium to iron.
3.4. Osteoblast Response
The live/dead staining of osteoblasts (cultured for one day) is depicted in Figure 7a,b. Few dead cells (red) were observed in any of the groups, indicating a good level of viability of osteoblasts on the untreated samples and EPP-treated samples. Figure 7c–j shows the results of the cytoskeleton staining and nuclear staining of cells, where the green parts were the outline of the cells and the blue parts were the nucleus. The cells spread along the direction of the grinding marks on the surface of the untreated samples, as shown in Figure 7c,g. However, on the surface of the EPP-treated samples, the cells exhibited a trend of random spread in all directions, as shown in Figure 7e,i. It indicated that osteoblasts could recognize surfaces with different degrees of roughness and texture. The spread direction of the cells was seriously impacted by the topography of the material surface, exhibiting a topographic induction trend, which is related to the degree of roughness and the direction of surface texture. After three days of culture, the spreading area of cells on the surface of the samples treated with EPP was larger than that of the control group, indicating that a flat surface is more conducive to the growth and spreading of cells.
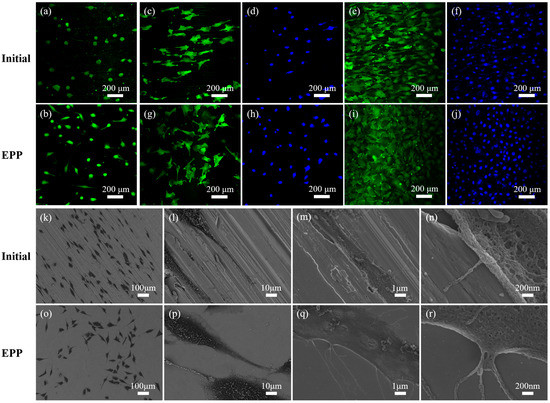
Figure 7.
Optical and SEM images of osteoblasts: live/dead staining (a,b), FITC and DAPI fluorescence staining (c–j), and osteoblasts morphologies (k–r).
Figure 7k–r shows the results of the scanning electron microscope observation of the growth morphology of osteoblasts on the samples. Comparing Figure 7k,o, it can be seen that cells grew along the texture on the surface of untreated samples and there was no fixed growth direction on the EPP-treated surface, which is consistent with the results of the cytoskeleton staining. At the same time, it was found that the pseudopodia of cells on the EPP-treated surface were longer than those on the untreated surface. As can be seen from Figure 7l,p, pseudopodia of cells on the EPP-treated surface were wider than those on the untreated surface, and the cells were flat on the untreated surface as if they had infiltrated into the material, while on EPP-treated samples, they were convex and adhered to the surface. On the untreated samples, cells were affected by the microscopic topography of the samples, sinking into grooves (Figure 7m) or hanging in mid-air by the support of peaks (Figure 7n), and the edges of the cells were curled, which adversely affected the growth, spreading, and migration of these cells. It was, therefore, concluded that the improvement in the microtopography of the materials using EPP is beneficial to the growth, spreading, and migration of osteoblasts.
4. Conclusions
To explore the effect of EPP treatment on the surface characteristics and properties of biomedical alloy, 316LVM medical stainless steel was taken as the research object. The changes in surface roughness, micromorphology, and chemical elements before and after EPP treatment were analyzed. The effects and mechanism of EPP treatment on biological corrosion resistance and osteoblast responses were studied. The results show that the EPP treatment effectively removes the microscopic protrusions on the surface of the material, making it flat and smooth, and greatly reducing its roughness (Ra was reduced from 0.3108 μm to 0.0454 μm). After EPP treatment, the charge transfer resistance of 316LVM in simulated body fluid increased from 513.3 to 17,430 kΩ·cm2 and the corrosion current density decreased from 1.129 to 0.164 µA/cm2, indicating that its corrosion resistance was improved, which is related to the improvement in surface morphology and the increase in the chromium/iron ratio. In addition, the improvement in the microtopography of the materials treated by EPP is beneficial to the growth, spreading, and migration of osteoblasts. The research results are of great significance in extending the application range of EPP and further provide a new idea for the polishing of medical alloys.
Author Contributions
Conceptualization, H.D. and H.S.; data curation, D.Y., S.L. and J.W.; formal analysis, H.D. and Y.X.; investigation, S.L., J.W. and Y.X.; methodology, H.D.; project administration, H.S.; software, G.J. and D.Y.; validation, H.D., H.S. and G.J.; visualization, H.D. and G.J.; writing—original draft, H.D.; writing—review and editing, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shanxi Provincial Key Research and Development Project, grant number 201903D121091.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maver, U.; Xhanari, K.; Žižek, M.; Gradišnik, L.; Repnik, K.; Potočnik, U.; Finšgar, M. Carboxymethyl cellulose/diclofenac bioactive coatings on AISI 316LVM for controlled drug delivery, and improved osteogenic potential. Carbohydr. Polym. 2020, 230, 115612. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, M.J.K.; Deen, K.M.; Greenlee-Wacker, M.C.; Haider, W. Additively manufactured 316L stainless steel with improved corrosion resistance and biological response for biomedical applications. Addit. Manuf. 2019, 27, 8–19. [Google Scholar] [CrossRef]
- Zivic, F.; Babic, M.; Grujovic, N.; Mitrovic, S.; Adamovic, D. Influence of loose PMMA bone cement particles on the corrosion assisted wear of the orthopedic AISI 316LVM stainless steel during reciprocating sliding. Wear 2013, 300, 65–77. [Google Scholar] [CrossRef]
- Galván, J.; Larrea, M.; Braceras, I.; Multigner, M.; González-Carrasco, J. In vitro corrosion behaviour of surgical 316LVM stainless steel modified by Si+ ion implantation—An electrochemical impedance spectroscopy study. J. Alloys Compd. 2016, 676, 414–427. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Jia, W.; Ma, D.; Huang, X.; Yu, S.; Wu, Y. Ta2O5-doped TiO2 nanotubes on Ti6Al4V alloy with improved cytocompatibility to endothelial cells. Vacuum 2022, 195, 110713. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Li, L.; Shum-Tim, D.; Davis, E.C.; Omanovic, S. Electrochemical polishing as a 316L stainless steel surface treatment method: Towards the improvement of biocompatibility. Corros. Sci. 2014, 87, 89–100. [Google Scholar] [CrossRef]
- Antunes, R.; Rodas, A.; Lima, N.; Higa, O.; Costa, I. Study of the corrosion resistance and in vitro biocompatibility of PVD TiCN-coated AISI 316L austenitic stainless steel for orthopedic applications. Surf. Coat. Technol. 2010, 205, 2074–2081. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Q.; Ren, Y.; Jin, D.; Liu, D.; Moradi, M.; Chen, X.; Li, H.; Xu, D.; Wang, F. Corrosion behavior of high nitrogen nickel-free austenitic stainless steel in the presence of artificial saliva and Streptococcus mutans. Bioelectrochemistry 2021, 142, 107940. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, J.; Huang, X.; Ma, Y.; Zhou, B.; Liu, Z.; Xue, Y.; Yu, S.; Wu, Y. Anodic growth of Ta-Ti-O nanotube arrays on Ta/Ti6Al4V alloy layer. Mater. Charact. 2021, 182, 111572. [Google Scholar] [CrossRef]
- Irving, M.; Murphy, M.F.; Lilley, F.; French, P.W.; Burton, D.R.; Dixon, S.; Sharp, M.C. The use of abrasive polishing and laser processing for developing polyurethane surfaces for controlling fibroblast cell behaviour. Mater. Sci. Eng. C 2017, 71, 690–697. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Deen, K.; Cano, L.; Haider, W. The effects of parametric changes in electropolishing process on surface properties of 316L stainless steel. Appl. Surf. Sci. 2017, 410, 432–444. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, B.; Gao, J.; Hei, H.; Ma, Y.; Huang, X.; Liu, Z.; Xue, Y.; Yu, S.; Wu, Y. A scalelike micro/nano-textured structure on Ti-based implants with enhanced cytocompatibility and osteogenic activities. Surf. Coat. Technol. 2021, 422, 127497. [Google Scholar] [CrossRef]
- Rafiee, K.; Naffakh-Moosavy, H.; Tamjid, E. The effect of laser frequency on roughness, microstructure, cell viability and attachment of Ti6Al4V alloy. Mater. Sci. Eng. C 2020, 109, 110637. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Olszta, M.; Edwards, D.; Engelhard, M.; Samanta, A.; Ding, H.; Murkute, P.; Isgor, O.B.; Rohatgi, A. Microstructural basis for improved corrosion resistance of laser surface processed AZ31 Mg alloy. Corros. Sci. 2021, 191, 109707. [Google Scholar] [CrossRef]
- Lu, F.-F.; Ma, K.; Li, C.-X.; Yasir, M.; Luo, X.-T.; Li, C.-J. Enhanced corrosion resistance of cold-sprayed and shot-peened aluminum coatings on LA43M magnesium alloy. Surf. Coat. Technol. 2020, 394, 125865. [Google Scholar] [CrossRef]
- Yao, H.-L.; Yi, Z.-H.; Yao, C.; Zhang, M.-X.; Wang, H.-T.; Li, S.-B.; Bai, X.-B.; Chen, Q.-Y.; Ji, G.-C. Improved corrosion resistance of AZ91D magnesium alloy coated by novel cold-sprayed Zn-HA/Zn double-layer coatings. Ceram. Int. 2020, 46, 7687–7693. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Parfenov, E.; Yerokhin, A.; Nevyantseva, R.; Gorbatkov, M.; Liang, C.-J.; Matthews, A. Towards smart electrolytic plasma technologies: An overview of methodological approaches to process modelling. Surf. Coat. Technol. 2015, 269, 2–22. [Google Scholar] [CrossRef]
- Ji, G.; Sun, H.; Duan, H.; Yang, D.; Sun, J. Effect of electrolytic plasma polishing on microstructural evolution and tensile properties of 316L stainless steel. Surf. Coat. Technol. 2021, 420, 127330. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, C.; Ding, F.; Yang, Y.; Zhang, T.; He, X.; Zheng, L.; Li, N. Principle, process, and application of metal plasma electrolytic polishing: A review. Int. J. Adv. Manuf. Technol. 2021, 114, 1893–1912. [Google Scholar] [CrossRef]
- Meletis, E.; Nie, X.; Wang, F.; Jiang, J. Electrolytic plasma processing for cleaning and metal-coating of steel surfaces. Surf. Coat. Technol. 2002, 150, 246–256. [Google Scholar] [CrossRef]
- Parfenov, E.; Farrakhov, R.; Mukaeva, V.; Gusarov, A.; Nevyantseva, R.; Yerokhin, A. Electric field effect on surface layer removal during electrolytic plasma polishing. Surf. Coat. Technol. 2016, 307, 1329–1340. [Google Scholar] [CrossRef]
- Yerokhin, A.; Mukaeva, V.R.; Parfenov, E.V.; Laugel, N.; Matthews, A. Charge transfer mechanisms underlying Contact Glow Discharge Electrolysis. Electrochimica Acta 2019, 312, 441–456. [Google Scholar] [CrossRef]
- Belkin, P.; Kusmanov, S.; Parfenov, E. Mechanism and technological opportunity of plasma electrolytic polishing of metals and alloys surfaces. Appl. Surf. Sci. Adv. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Nevyantseva, R.; Gorbatkov, S.; Parfenov, E.; Bybin, A. The influence of vapor–gaseous envelope behavior on plasma electrolytic coating removal. Surf. Coat. Technol. 2001, 148, 30–37. [Google Scholar] [CrossRef]
- Spica, A.; Roche, J.; Arurault, L.; Horville, M.; Rolet, J. Evolution of model roughness on quasi-pure aluminum during plasma electrolytic polishing. Surf. Coat. Technol. 2021, 428, 127839. [Google Scholar] [CrossRef]
- An, S.; Foest, R.; Fricke, K.; Riemer, H.; Fröhlich, M.; Quade, A.; Schäfer, J.; Weltmann, K.-D.; Kersten, H. Pretreatment of cutting tools by plasma electrolytic polishing (PEP) for enhanced adhesion of hard coatings. Surf. Coat. Technol. 2021, 405, 126504. [Google Scholar] [CrossRef]
- Gupta, P.; Tenhundfeld, G.; Daigle, E.; Ryabkov, D. Electrolytic plasma technology: Science and engineering—An overview. Surf. Coat. Technol. 2007, 201, 8746–8760. [Google Scholar] [CrossRef]
- Yang, L.; Laugel, N.; Housden, J.; Espitalier, L.; Matthews, A.; Yerokhin, A. Plasma additive layer manufacture smoothing (PALMS) technology—An industrial prototype machine development and a comparative study on both additive manufactured and conventional machined AISI 316 stainless steel. Addit. Manuf. 2020, 34, 101204. [Google Scholar] [CrossRef]
- Seo, B.; Park, H.-K.; Kim, H.G.; Kim, W.R.; Park, K. Corrosion behavior of additive manufactured CoCr parts polished with plasma electrolytic polishing. Surf. Coat. Technol. 2021, 406, 126640. [Google Scholar] [CrossRef]
- Nestler, K.; Böttger-Hiller, F.; Adamitzki, W.; Glowa, G.; Zeidler, H.; Schubert, A. Plasma electrolytic polishing—An overview of applied technologies and current challenges to extend the polishable material range. Procedia CIRP 2016, 42, 503–507. [Google Scholar] [CrossRef]
- Qi, X.; Bi, J. Plasmonic sensors relying on nanoparticle arrays created by a template-directed dewetting process. Opt. Commun. 2019, 453, 124328. [Google Scholar] [CrossRef]
- Blanco-Loimil, M.; Pardo, A.; Villar-Alvarez, E.; Martínez-González, R.; Topete, A.; Barbosa, S.; Taboada, P.; Mosquera, V. Development of ordered metal nanoparticle arrangements on solid supports by combining a green nanoparticle synthetic method and polymer templating for sensing applications. RSC Adv. 2016, 6, 60502. [Google Scholar] [CrossRef]
- Zeidler, H.; Boettger-Hiller, F.; Edelmann, J.; Schubert, A. Surface finish machining of medical parts using plasma electrolytic polishing. Procedia CIRP 2016, 49, 83–87. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K. Analysis of XPS results of AISI 316L SS electropolished and magnetoelectropolished at varying conditions. Surf. Coat. Technol. 2010, 204, 2583–2592. [Google Scholar] [CrossRef]
- Hermas, A. XPS analysis of the passive film formed on austenitic stainless steel coated with conductive polymer. Corros. Sci. 2008, 50, 2498–2505. [Google Scholar] [CrossRef]
- Zhou, C.; Su, H.; Qian, N.; Zhang, Z.; Xu, J. Characteristics and function of vapour gaseous envelope fluctuation in plasma electrolytic polishing. Int. J. Adv. Manuf. Technol. 2022, 119, 7815–7825. [Google Scholar] [CrossRef]
- Zhou, C.; Qian, N.; Su, H.; Zhang, Z.; Ding, W.; Xu, J. Effect of energy distribution on the machining efficiency and surface morphology of Inconel 718 nickel-based superalloy using plasma electrolytic polishing. Surf. Coat. Technol. 2022, 441, 128506. [Google Scholar] [CrossRef]
- Abouelata, A.; Awad, M.; Attia, A.; Youssef, G.I. Electrochemical polishing versus mechanical polishing of AISI 304: Surface and electrochemical study. J. Solid State Electrochem. 2022, 26, 121–129. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Tambovskiy, I.V.; Korableva, S.S.; Dyakov, I.G.; Burov, S.V.; Belkin, P.N. Enhancement of wear and corrosion resistance in medium carbon steel by plasma electrolytic nitriding and polishing. J. Mater. Eng. Perform. 2019, 28, 5425–5432. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Zhang, H.-Y.; Zheng, C.; Yang, H.-Y.; Qin, P.; Zhao, C.; Lu, S.; Liang, S.-X.; Chai, L.; Zhang, L.-C. Corrosion behavior and characteristics of passive films of laser powder bed fusion produced Ti–6Al–4V in dynamic Hank’s solution. Mater. Des. 2021, 208, 109907. [Google Scholar] [CrossRef]
- Ran, M.; Zhang, C.; Wen, L.; Zhou, H.; Zheng, W. Effect of surface mechanical attrition treatment on stainless steel corrosion. Surf. Eng. 2021, 37, 739–748. [Google Scholar] [CrossRef]
- Jinlong, L.; Meng, Y.; Miura, H.; Tongxiang, L. The effect of surface enriched chromium and grain refinement by ball milling on corrosion resistance of 316L stainless steel. Mater. Res. Bull. 2017, 91, 91–97. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Liu, L.; Xi, T.; Yang, C.; Li, Q.; Yang, K. Passivation potential regulating corrosion resistance and antibacterial property of 316L-Cu stainless steel in different simulated body fluids. Mater. Technol. 2020, 36, 118–130. [Google Scholar] [CrossRef]
- Yin, X.; Zhai, Q.; Zhang, Q.; Wang, K.; Meng, L.; Ma, Z.; Chen, G.; Wang, S.; Wang, L. Effect of tungsten particles on microstructure and properties of 316 L stainless steel manufactured by selective laser melting. J. Manuf. Process. 2021, 68, 210–221. [Google Scholar] [CrossRef]
- Williamson, R.; Disegi, J.; Janorkar, A.; Griggs, J.; Roach, M. Effect of duty cycle on the crystallinity, pore size, surface roughness and corrosion resistance of the anodized surface on titanium. Surf. Coat. Technol. 2015, 277, 278–288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).