Microstructure Investigation and Cyclic Oxidation Resistance of Ce-Si-Modified Aluminide Coating Deposited by Pack Cementation on Inconel 738LC
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Materials and Preparation
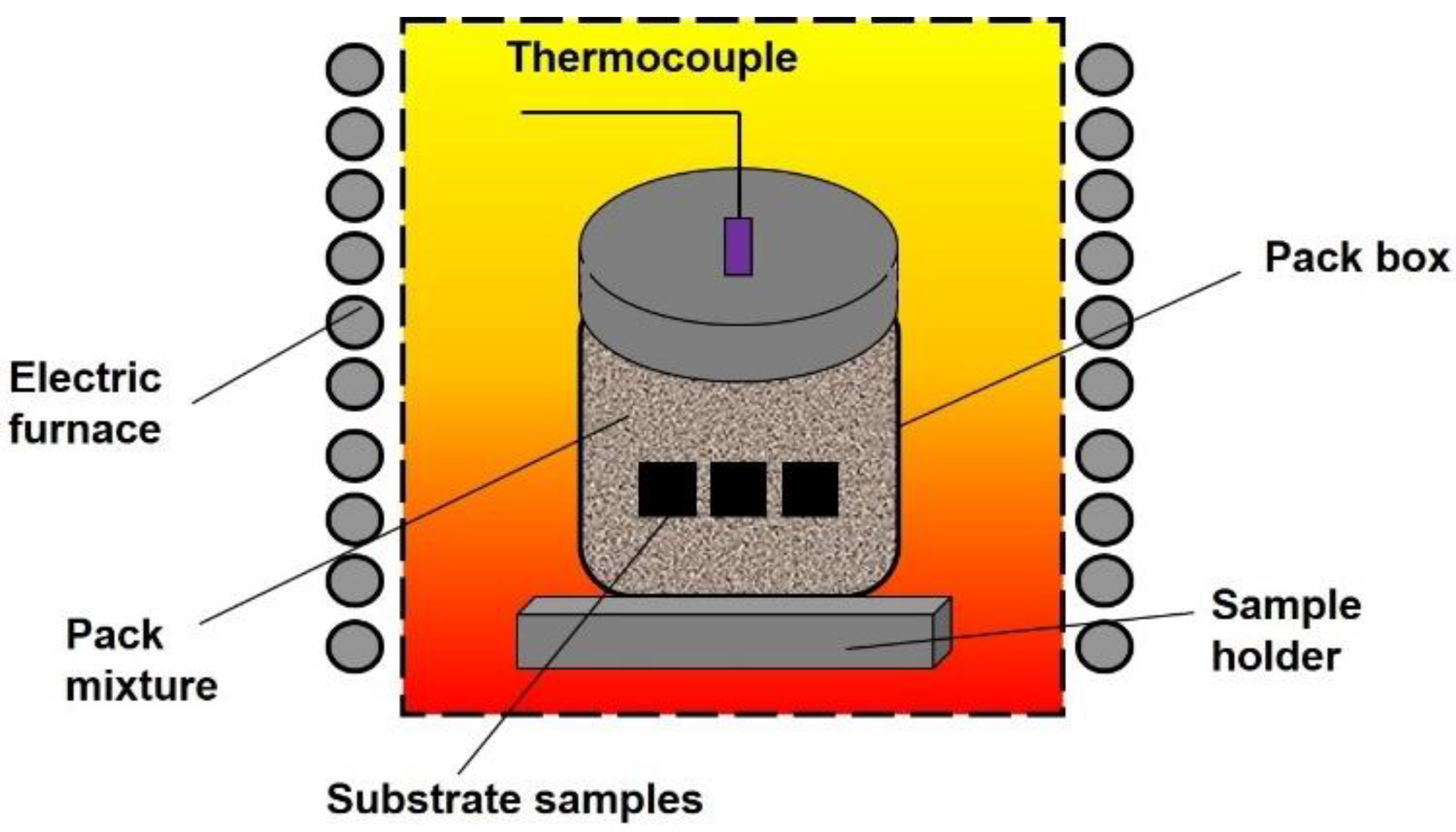
2.2. Coating Procedure
2.3. Microstructural Characterization
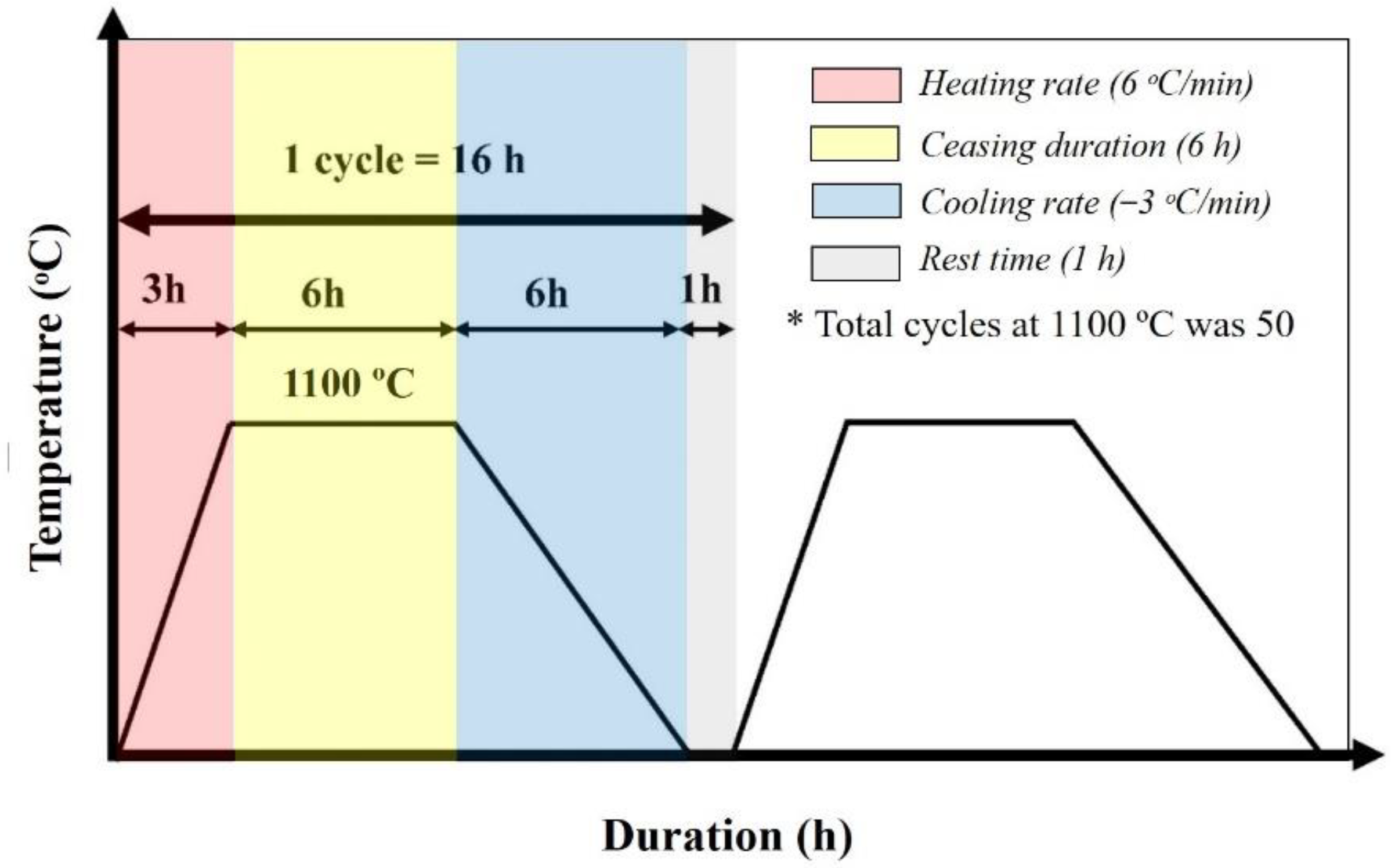
2.4. Cyclic Oxidation Tests
3. Results and Discussion
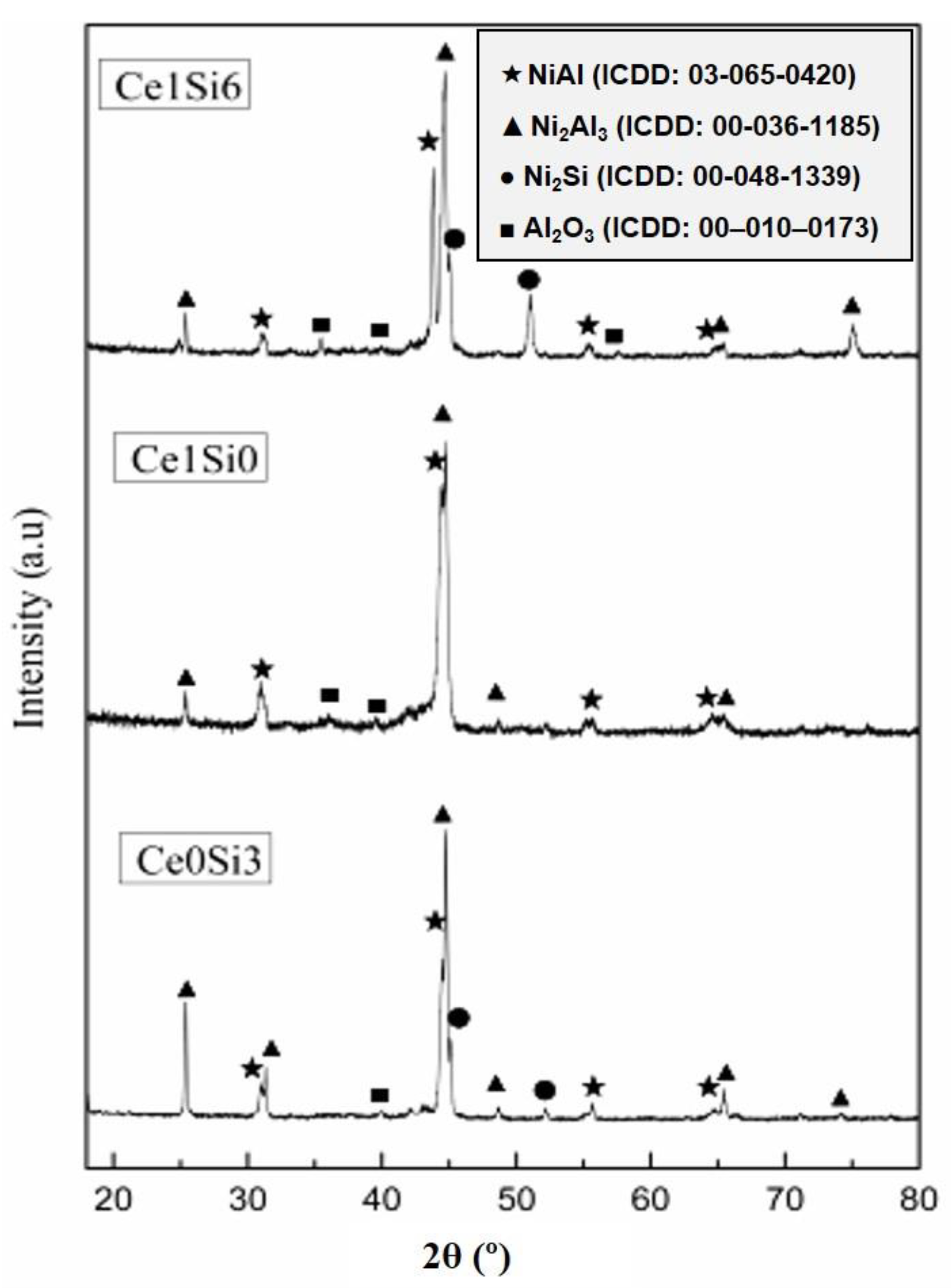
3.1. Microstructure of Al-Si-Ce Coatings
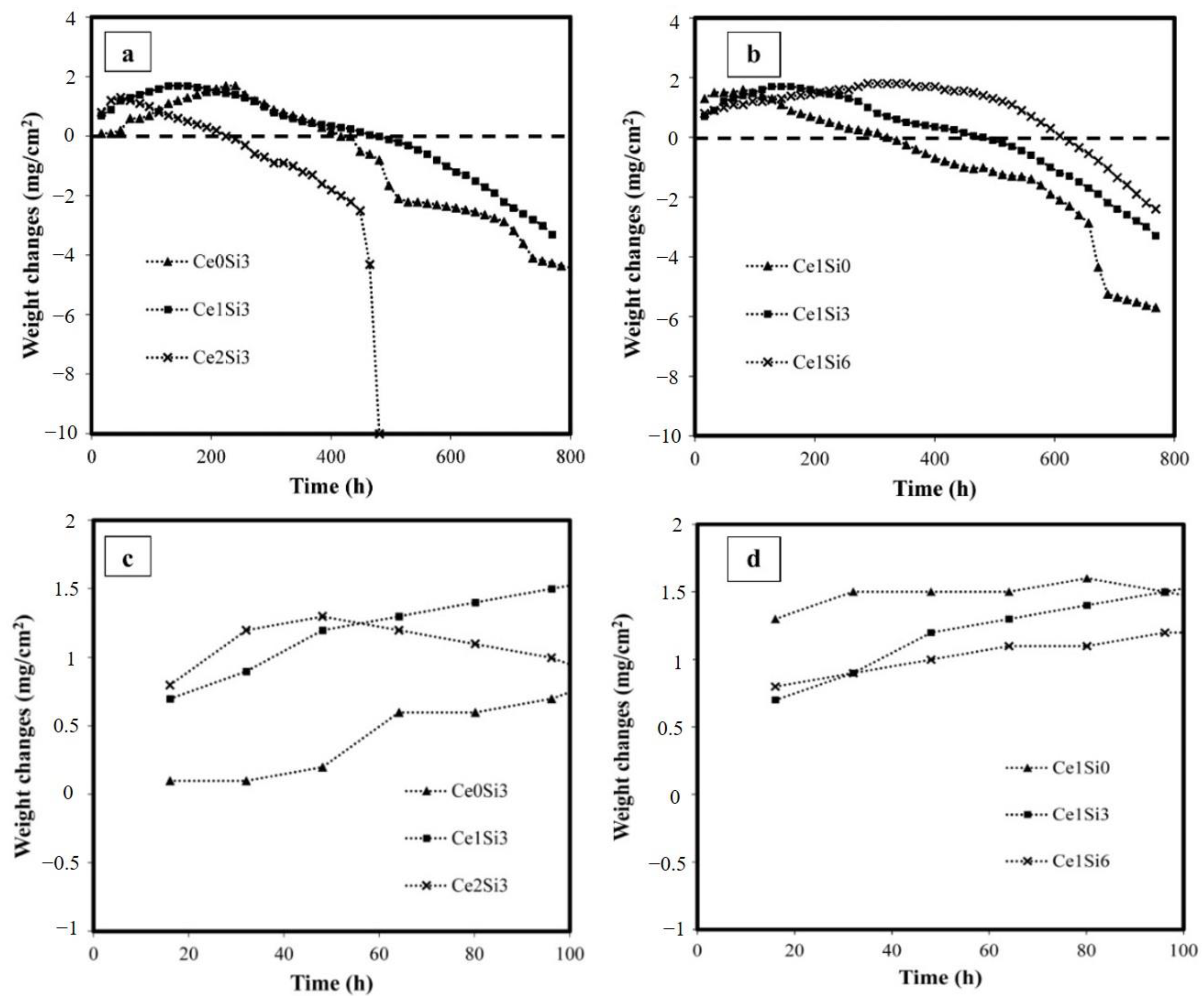
3.2. High-Temperature Oxidation
3.3. Characterization of Oxide Scale
4. Conclusions
- The cerium addition by 1% enhances the oxidation resistance, whereas the oxidation resistance is limited by increasing the cerium ratio to exceed 2%. This occurs through increasing the ratio of NiAl to Ni2Al3, inhibiting the outward/inward diffusion of intermetallic elements and oxygen, which is called the blocking effect, in the hot oxidation process, and promoting a finer-grained microstructure of the NiAl and Ni2Al3 phases in the coating.
- Increasing the cerium ratio to up to 2% strictly decreases the oxidation resistance, and a noticeable weight loss was observed after 450 h, which is mainly attributed to cerium placed into the solid solution increasing the energy of the system by the distortion and mismatch of the β-NiAl and Ni2Al3 lattice.
- Deposited silicon is mainly present in the form of NiAl1-nSin and Ni2Al3-nSin and δ-Ni2Si in the coatings. The reaction of δ-Ni2Si with oxygen forms active silicon atoms, and the silicon contents promote the transformation of γ′-Ni3Al into β-NiAl and Al, which improves the hot oxidation resistance of the coatings.
- The simultaneous addition of 1% cerium and 6% silicon, which is the Ce1Si6 sample, limits the oxygen diffusion and can synergistically promote the formation of the continuous Al2O3 layer, which can enhance the hot oxidation resistance of the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bose, S. Preface, in High Temperature Coatings; Bose, S., Ed.; Butterworth-Heinemann Burlington: Oxford, UK, 2007; pp. xi–xii. [Google Scholar]
- Xu, Z.; Wang, Z.; Niu, J.; He, L.; Mu, R.; Wang, K. Effects of deposition temperature on the kinetics growth and protective properties of aluminide coatings. J. Alloys Compd. 2015, 632, 238–245. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, J.; Niu, J.; He, L.; Mu, R.; Wang, Z. Isothermal oxidation and hot corrosion behaviors of diffusion aluminide coatings deposited by chemical vapor deposition. J. Alloys Compd. 2015, 637, 343–349. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.; Schütze, M. Low-activity aluminide coatings for superalloys using a slurry process free of halide activators and chromates. Surf. Coatings Technol. 2013, 222, 9–14. [Google Scholar] [CrossRef]
- Rajendran, R. Gas turbine coatings—An overview. Eng. Fail. Anal. 2012, 26, 355–369. [Google Scholar] [CrossRef]
- Goward, G.W. Progress in coatings for gas turbine airfoils. Surf. Coat. Technol. 1998, 108–109, 73–79. [Google Scholar] [CrossRef]
- Kourtidou, D.; Chaliampalias, D.; Vogiatzis, C.; Tarani, E.; Kamou, A.; Pavlidou, E.; Skolianos, S.; Chrissafis, K.; Vourlias, G. Deposition of Ni-Al coatings by pack cementation and corrosion resistance in high temperature and marine environments. Corros. Sci. 2018, 148, 12–23. [Google Scholar] [CrossRef]
- Kianicová, M.; Kafrík, J.; Trník, J. Degradation of Aluminide Coatings Deposited on Nickel Superalloys. Procedia Eng. 2018, 136, 346–352. [Google Scholar] [CrossRef]
- Pomeroy, M. Coatings for gas turbine materials and long term stability issues. Mater. Des. 2005, 26, 223–231. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Zhao, C.; Hao, W.; Wang, X.; Xiao, P. The oxidation performance for Zr-doped nickel aluminide coating by composite electrodepositing and pack cementation. Corros. Sci. 2017, 123, 103–115. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Xiao, P. Effect of microstructure on early oxidation of MCrAlY coatings. Acta Mater. 2018, 159, 150–162. [Google Scholar] [CrossRef]
- Bianco, R.; Rapp, R.A. Pack Cementation Aluminide Coatings on Superalloys: Codeposition of Cr and Reactive Elements. J. Electrochem. Soc. 1993, 140, 1181–1190. [Google Scholar] [CrossRef]
- Alam, Z.; Sarkar, S.B.; Das, D.K. Refurbishment of thermally degraded diffusion Pt-aluminide (PtAl) bond coat on a Ni-base superalloy. Surf. Coatings Technol. 2018, 354, 101–111. [Google Scholar] [CrossRef]
- Nicholls, J.R.; Long, K.A.; Simms, N.J. 4.05—Diffusion Coatings, in Shreir’s Corrosion; Stott, B., Ed.; Elsevier: Oxford, UK, 2010; pp. 2532–2555. [Google Scholar]
- Gleeson, B.; Cheung, W.H.; Costa, W.D.; Young, D.J. The hot-corrosion behavior of novel CO-deposited chromium-modified aluminide coatings. Oxid. Metals 1992, 38, 407–424. [Google Scholar] [CrossRef]
- He, H.; Liu, Z.; Wang, W.; Zhou, C. Microstructure and hot corrosion behavior of Co–Si modified aluminide coating on nickel based superalloys. Corros. Sci. 2015, 100, 466–473. [Google Scholar] [CrossRef]
- Fan, Q.; Peng, X.; Yu, H.; Jiang, S.; Gong, J.; Sun, C. The isothermal and cyclic oxidation behaviour of two Co modified aluminide coatings at high temperature. Corros. Sci. 2014, 84, 42–53. [Google Scholar] [CrossRef]
- Ahmadi, H.; Li, D. Mechanical and tribological properties of aluminide coating modified with yttrium. Surf. Coatings Technol. 2002, 161, 210–217. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H. Preparation and oxidation of an Al2O3-modified aluminide coating. Vacuum 2012, 86, 1353–1357. [Google Scholar] [CrossRef]
- Wang, Y.; Suneson, M. Oxidation behavior of Hf-modified aluminide coatings on Haynes-188 at 1050 °C. Surf. Coatings Technol. 2013, 215, 7–15. [Google Scholar] [CrossRef]
- Wang, Y.; Suneson, M.; Sayre, G. Synthesis of Hf-modified aluminide coatings on Ni-base superalloys. Surf. Coatings Technol. 2011, 206, 1218–1228. [Google Scholar] [CrossRef]
- Xiang, Z.; Datta, P. Codeposition of Al and Si on nickel base superalloys by pack cementation process. Mater. Sci. Eng. A 2003, 356, 136–144. [Google Scholar] [CrossRef]
- Streiff, R.; Boone, D.H. Corrosion resistant modified aluminide coatings. J. Mater. Eng. 1988, 10, 15–26. [Google Scholar] [CrossRef]
- Qian, L.; Xu, F.; Voisey, K.; Nekouie, V.; Zhou, Z.; Silberschmidt, V.V.; Hou, X. Incorporation and evolution of ZrO2 nano-particles in Pt-modified aluminide coating for high temperature applications. Surf. Coatings Technol. 2017, 311, 238–247. [Google Scholar] [CrossRef]
- Azarmehr, S.A.; Shirvani, K.; Schütze, M.; Galetz, M. Microstructural evolution of silicon-platinum modified aluminide coatings on superalloy GTD-111. Surf. Coatings Technol. 2017, 321, 455–463. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Datta, P.K. Formation of Hf- and W-modified aluminide coatings on nickel–base superalloys by the pack cemen-tation process. Mater. Sci. Eng. A 2003, 363, 185–192. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, S.; Li, H.; Wang, W.; Gong, J.; Sun, C. Microstructure and oxidation behaviour of an AlSiY/NiCrAlYSi composite coating at 1150°C. Corros. Sci. 2011, 53, 1417–1423. [Google Scholar] [CrossRef]
- Klöwer, J.; Brill, U.; Heubner, U. High temperature corrosion behaviour of nickel aluminides: Effects of chromium and zirco-nium. Intermetallics 1999, 7, 1183–1194. [Google Scholar] [CrossRef]
- Guo, H.; Li, D.; Zheng, L.; Gong, S.; Xu, H. Effect of co-doping of two reactive elements on alumina scale growth of β-NiAl at 1200 °C. Corros. Sci. 2014, 88, 197–208. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Guo, H.; Zhou, C. Cyclic oxidation resistance of Ce/Co modified aluminide coatings on nickel base superalloys. Corros. Sci. 2015, 94, 135–141. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Hu, H.; Meng, J.; Zhao, X. Effect of Y2O3 content in the pack mixtures on the cy-clic-oxidation of Y2O3-modified low temperature aluminide coatings on 309 stainless steel. Vaccum 2018, 158, 101–112. [Google Scholar] [CrossRef]
- Haijun, Z.; Jianfeng, S. Fabrication and Cyclic Oxidation of Y2O3/CeO2-Modified Low Temperature Aluminide Coatings. Rare Met. Mater. Eng. 2017, 46, 301–306. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Zhou, C. Improved hot corrosion resistance of Y–Ce–Co-modified aluminide coating on nickel base superalloys by pack cementation process. Corros. Sci. 2015, 92, 148–154. [Google Scholar] [CrossRef]
- Li, H.; Qiao, M.; Zhou, C. Formation and cyclic oxidation resistance of Hf–Co-modified aluminide coatings on nickel base superalloys. Mater. Chem. Phys. 2014, 143, 915–920. [Google Scholar] [CrossRef]
- Firouzi, A.; Shirvani, K. The structure and high temperature corrosion performance of medium-thickness aluminide coatings on nickel-based superalloy GTD-111. Corros. Sci. 2010, 52, 3579–3585. [Google Scholar] [CrossRef]
- Fu, C.; Kong, W.K.; Cao, G.H. Microstructure and oxidation behavior of Al + Si co-deposited coatings on nickel-based super-alloys. Surf. Coat. Technol. 2014, 258, 347–352. [Google Scholar] [CrossRef]
- Grünling, H.; Bauer, R. The role of silicon in corrosion-resistant high temperature coatings. Thin Solid Films 1982, 95, 3–20. [Google Scholar] [CrossRef]
- Kircher, T.; McMordie, B.; McCarter, A. Performance of a silicon-modified aluminide coating in high temperature hot corrosion test conditions. Surf. Coatings Technol. 1994, 68–69, 32–37. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Datta, P.K. Deposition of silicon modified aluminide coatings on nickel base superalloys by pack cementation process. Mater. Sci. Technol. 2003, 19, 935–942. [Google Scholar] [CrossRef]
- Meng, J.; Ji, Z. Effect of La2O3/CeO2 particle size on high-temperature oxidation resistance of electrodeposited Ni–La2O3/CeO2 composites. Trans. Nonferrous Met. Soc. China 2014, 24, 3571–3577. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, G.; Zhang, H.; Zhang, Y.; Qian, B. Effect of CeO2 on the microstructure and isothermal oxidation of Ni–Al alloy coatings transformed from electrodeposited Ni–Al films at 800°C. Vacuum 2009, 83, 1333–1339. [Google Scholar] [CrossRef]
- Tan, X.; Peng, X.; Wang, F. The mechanism for self-formation of a CeO2 diffusion barrier layer in an aluminide coating at high temperature. Surf. Coatings Technol. 2013, 224, 62–70. [Google Scholar] [CrossRef]
- Bouchaud, B.; Rannou, B.; Pedraza, F. Slurry aluminizing mechanisms of Ni-based superalloys incorporating an electrosynthe-sized ceria diffusion barrier. Mater. Chem. Phys. 2013, 143, 416–424. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, H.; Zhang, H. Oxidation behavior of aluminide coatings on carbon steel with and without electrodeposited Ni–CeO2 film by low-temperature pack cementation. Vacuum 2011, 86, 210–217. [Google Scholar] [CrossRef]
- Peng, X.; Guan, Y.; Dong, Z.; Xu, C.; Wang, F. A fundamental aspect of the growth process of alumina scale on a metal with dispersion of CeO2 nanoparticles. Corros. Sci. 2011, 53, 1954–1959. [Google Scholar] [CrossRef]
- Safari, M.; Shahriari, F.; Nogorani. Formation mechanism of high activity aluminide coating on Ni-CeO2 coated Rene 80 alloy. Surf. Coat. Technol. 2017, 329 (Suppl. C), 218–223. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Q.; Wang, H.; Cui, W.; Yuan, X. Micromechanism characteristics ofmodified Al-Si coating by lasermelt injection CeO2 nano-particles. Surf. Coat. Technol. 2017, 319, 88–94. [Google Scholar] [CrossRef]
- Saito, Y.; Önay, B. Improvements of Scale Adherence on Heat-Resisting Alloys and Coatings by Rare Earth Additions. Surf. Coat. Technol. 1990, 336–346. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, C. Hot Corrosion Behavior of Si–Y–Co-Modified Aluminide Coating Exposed to NaCl + Na2SO4 Salt at 1173 °K. Oxid. Met. 2016, 85, 205–217. [Google Scholar] [CrossRef]
- Fukumoto, M.; Yokobori, A.; Hara, M. Formation of the β-NiAl Containing Hf by the Simultaneous Electrodeposition of Al and Hf using a Molten-Salt and the Cyclic Oxidation Behavior. Oxid. Met. 2016, 85, 17–28. [Google Scholar] [CrossRef]
- Pint, B.; Haynes, J.; Besmann, T. Effect of Hf and Y alloy additions on aluminide coating performance. Surf. Coatings Technol. 2010, 204, 3287–3293. [Google Scholar] [CrossRef]
- Goward, G.W.; Cannon, L.W. Pack Cementation Coatings for Superalloys: A Review of History, Theory, and Practice. J. Eng. Gas Turbines Power 1988, 110, 150–154. [Google Scholar] [CrossRef]
- Pan, X.M.; Jin, Z.P.; Zhao, J.-C. Determination of the isothermal sections of the Al-Ni-Si ternary system at 750 °C and 850 °C. Met. Mater. Trans. A 2005, 36, 1757–1767. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhou, Y.-B.; Sun, J.-F. Preparation and oxidation behaviour of electrodeposited Ni–CeO2 nanocomposite coatings. Trans. Nonferrous Met. Soc. China 2013, 23, 2011–2020. [Google Scholar] [CrossRef]
- Romanowska, J.; Morgiel, J.; Zagula-Yavorska, M.; Sieniawski, J. Nanoparticles in hafnium-doped aluminide coatings. Mater. Lett. 2015, 145, 162–166. [Google Scholar] [CrossRef]
- Tan, X.; Peng, X.; Wang, F. The effect of grain refinement on the adhesion of an alumina scale on an aluminide coating. Corros. Sci. 2014, 85, 280–286. [Google Scholar] [CrossRef]


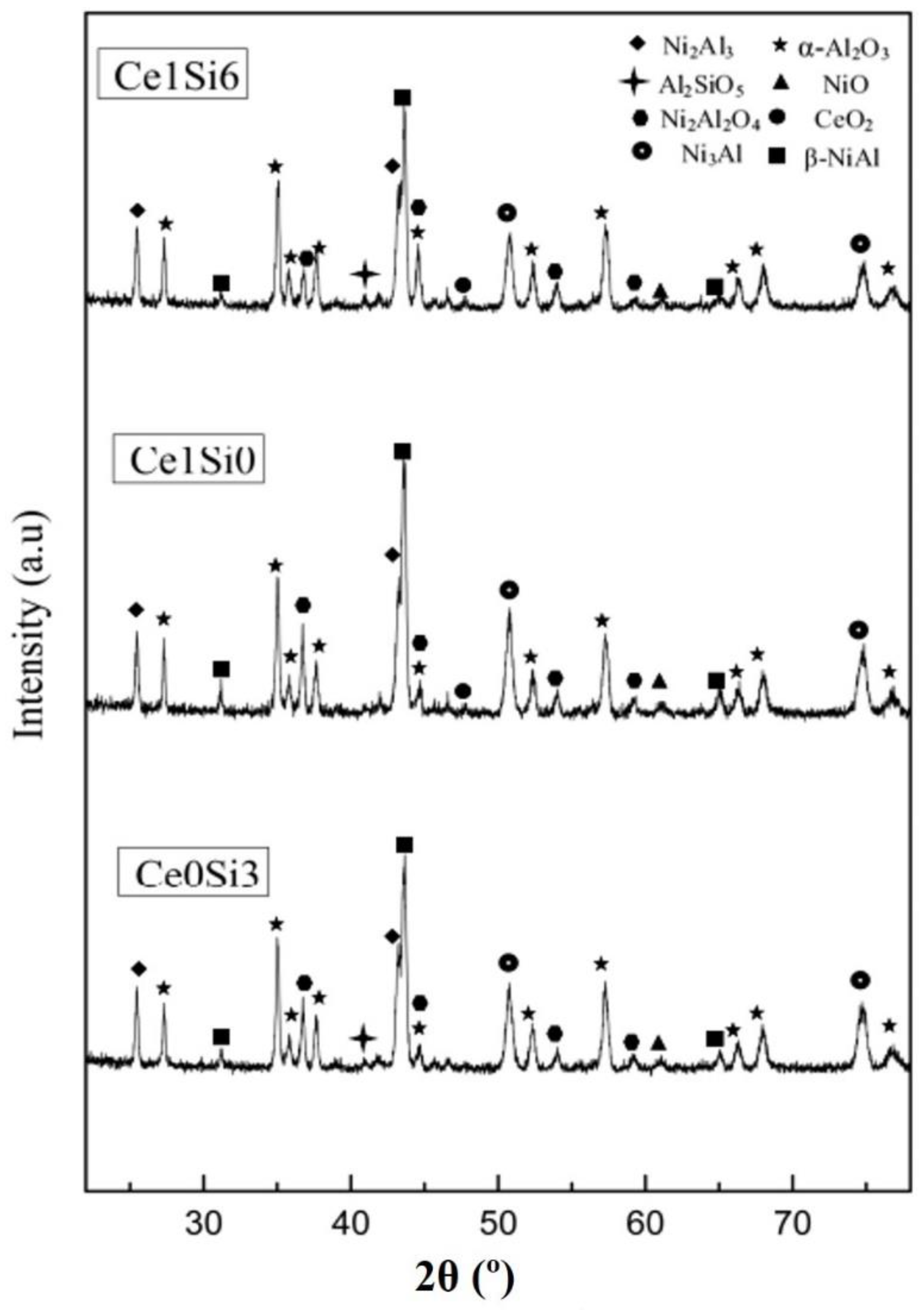
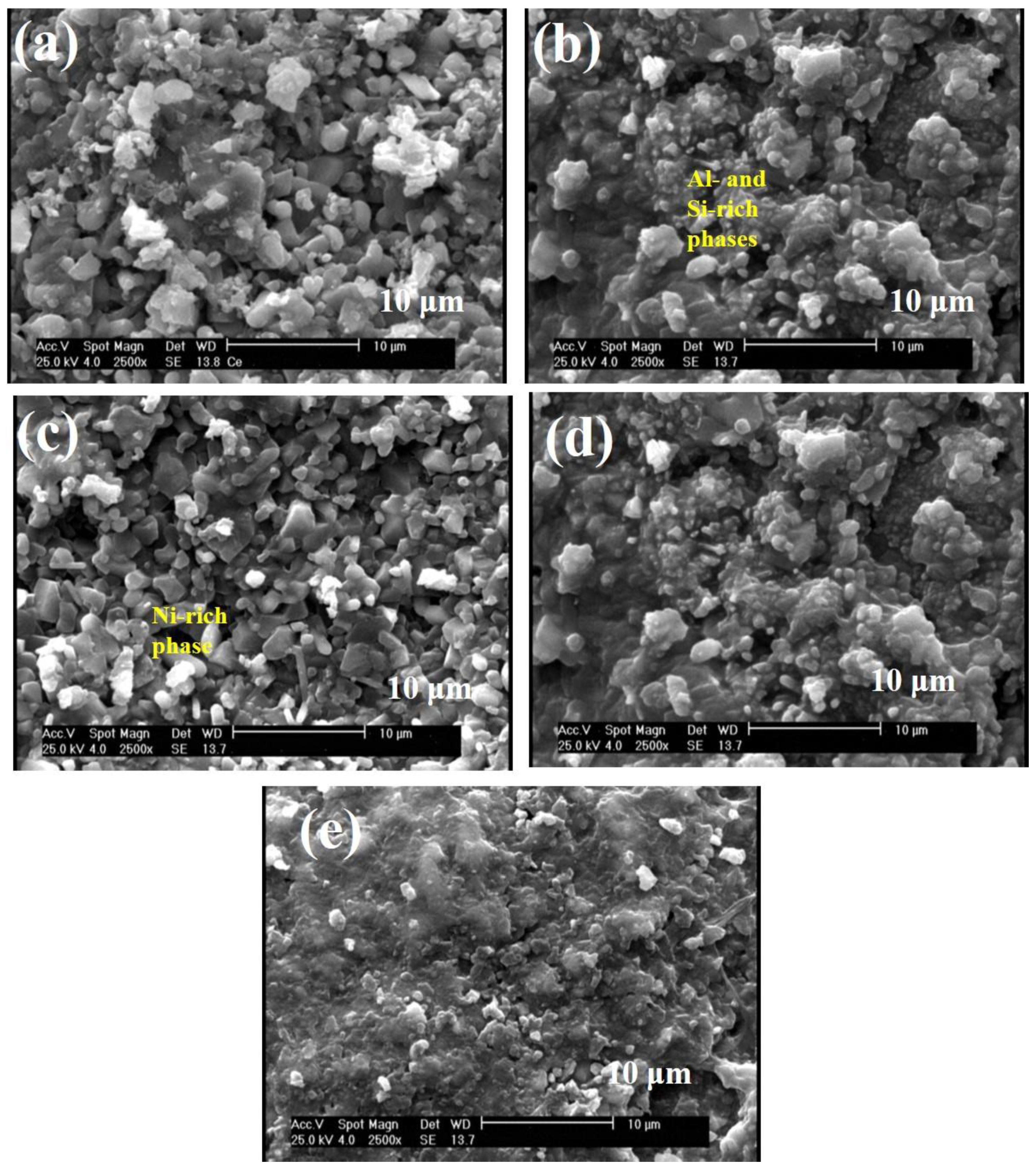
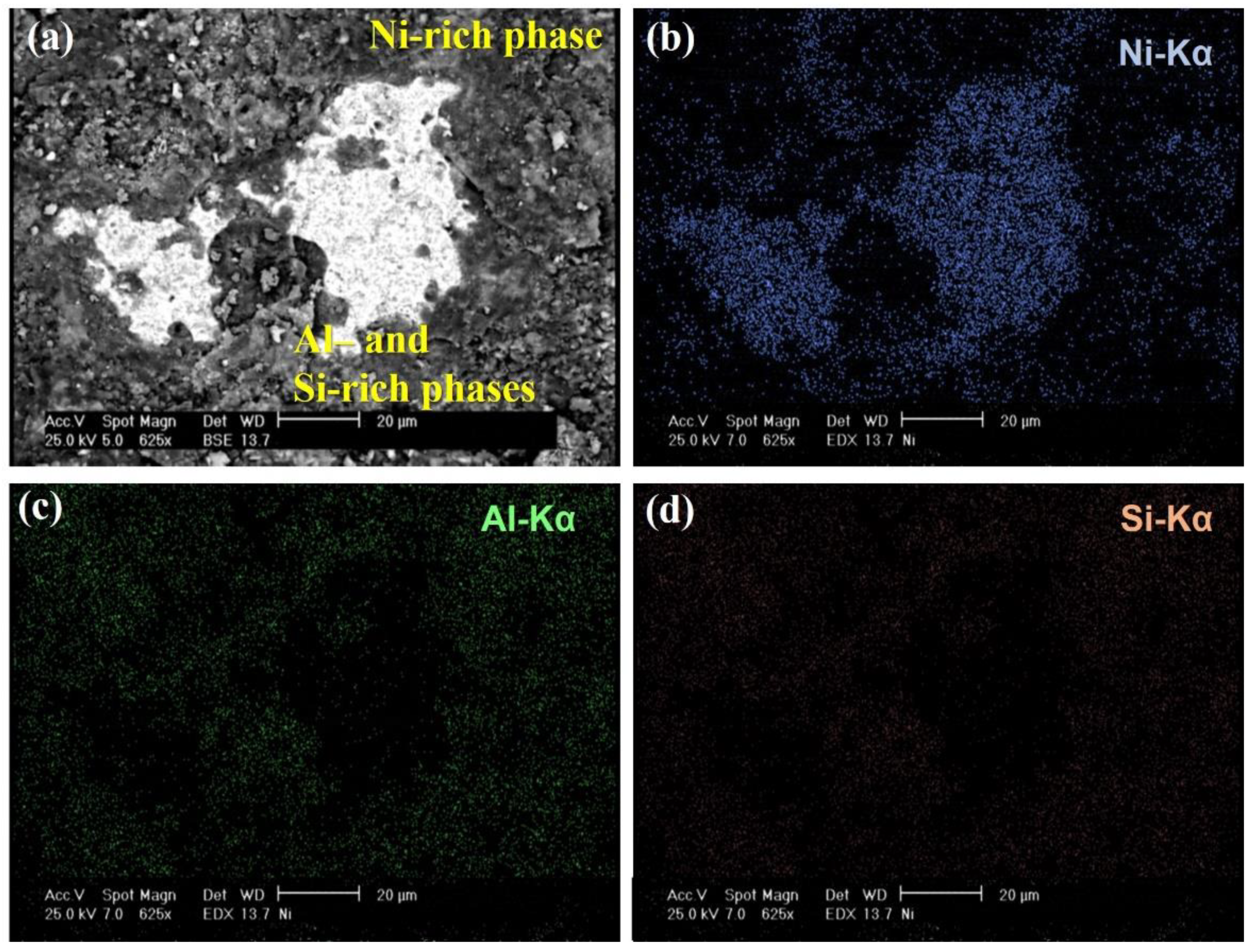
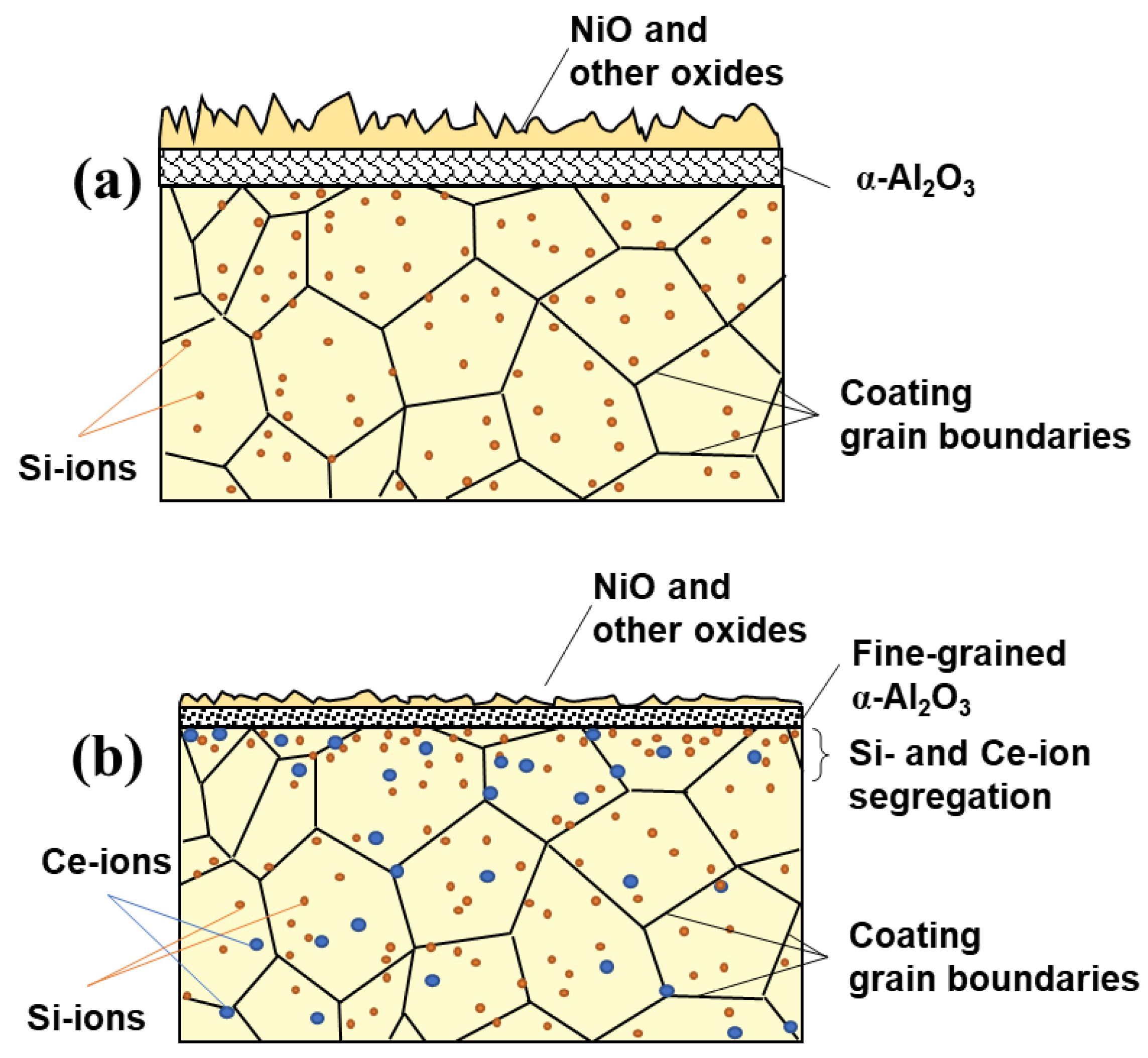
| Element | wt.% |
|---|---|
| Ni | Bal. |
| Cr | 16 |
| Co | 8.5 |
| Ti | 3.4 |
| W | 2.6 |
| Ta | 1.7 |
| Al | 3.4 |
| Mo | 0.9 |
| Sample | Code | Content of Composition (wt.%) | |||
|---|---|---|---|---|---|
| Al | Si | CeCl3 | NH4Br | ||
| No.1 | Ce0Si3 | 6 | 3% | 0 | 2.0 |
| No.2 | Ce1Si3 | 6 | 3% | 1% | 2.0 |
| No.3 | Ce2Si3 | 6 | 3% | 2% | 2.0 |
| No.2 | Ce1Si3 | 6 | 3% | 1% | 2.0 |
| No.4 | Ce1Si0 | 6 | 0% | 1% | 2.0 |
| No.5 | Ce1Si6 | 6 | 6% | 1% | 2.0 |
| Elements | Ce0Si3 | Ce1Si3 | Ce2Si3 | Ce1Si0 | Ce1Si6 |
|---|---|---|---|---|---|
| Ni | 34.80 | 41.67 | 42.66 | 54.49 | 41.86 |
| Cr | 9.86 | 11.06 | 10.61 | 1.34 | 11.82 |
| Co | 4.00 | 5.53 | 6.2 | 5.28 | 4.87 |
| Al | 46.90 | 38.63 | 33.67 | 32.25 | 37.51 |
| Si | 1.37 | 0.38 | 0.25 | 0.01 | 1.03 |
| Ce | 0.01 | 0.70 | 0.93 | 0.75 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nourpoor, P.; Javadian, S.; Sabour Rouh Aghdam, A.; Ghadami, F. Microstructure Investigation and Cyclic Oxidation Resistance of Ce-Si-Modified Aluminide Coating Deposited by Pack Cementation on Inconel 738LC. Coatings 2022, 12, 1491. https://doi.org/10.3390/coatings12101491
Nourpoor P, Javadian S, Sabour Rouh Aghdam A, Ghadami F. Microstructure Investigation and Cyclic Oxidation Resistance of Ce-Si-Modified Aluminide Coating Deposited by Pack Cementation on Inconel 738LC. Coatings. 2022; 12(10):1491. https://doi.org/10.3390/coatings12101491
Chicago/Turabian StyleNourpoor, Parviz, Soheila Javadian, Alireza Sabour Rouh Aghdam, and Farzin Ghadami. 2022. "Microstructure Investigation and Cyclic Oxidation Resistance of Ce-Si-Modified Aluminide Coating Deposited by Pack Cementation on Inconel 738LC" Coatings 12, no. 10: 1491. https://doi.org/10.3390/coatings12101491
APA StyleNourpoor, P., Javadian, S., Sabour Rouh Aghdam, A., & Ghadami, F. (2022). Microstructure Investigation and Cyclic Oxidation Resistance of Ce-Si-Modified Aluminide Coating Deposited by Pack Cementation on Inconel 738LC. Coatings, 12(10), 1491. https://doi.org/10.3390/coatings12101491






