Americium Sorption by Microplastics in Aqueous Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Analytical Methods
2.2. (De)Sorption Experiments
3. Results and Discussion
3.1. pH and Contact Time Effect on the Am-241 Sorption by PN6 and PE
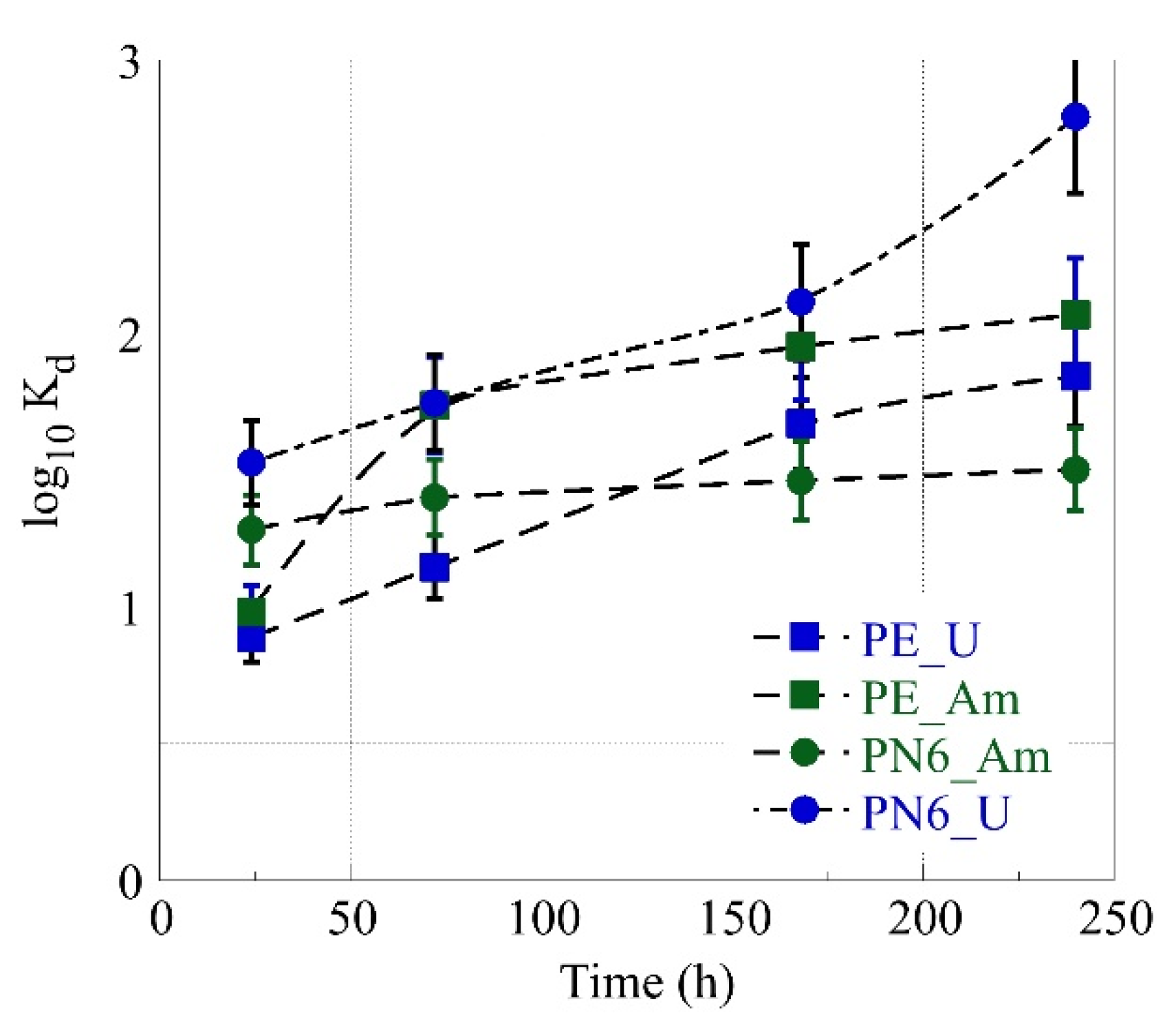
3.2. Simultaneous Am-241 and U-232 Sorption by PN6 and PE as Function of Time
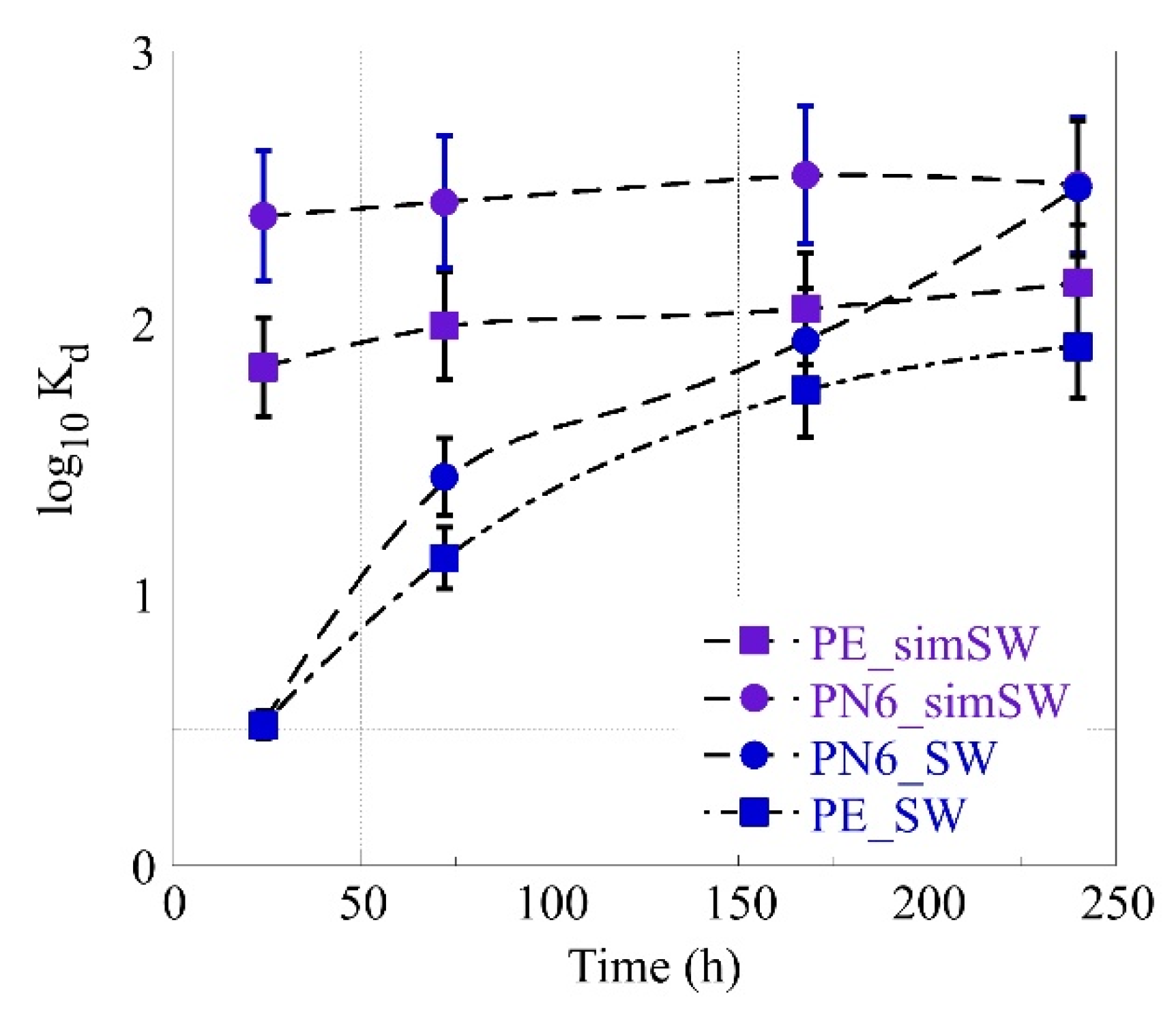
3.3. Am-241 Sorption by MP’s in Environmental Water Solutions
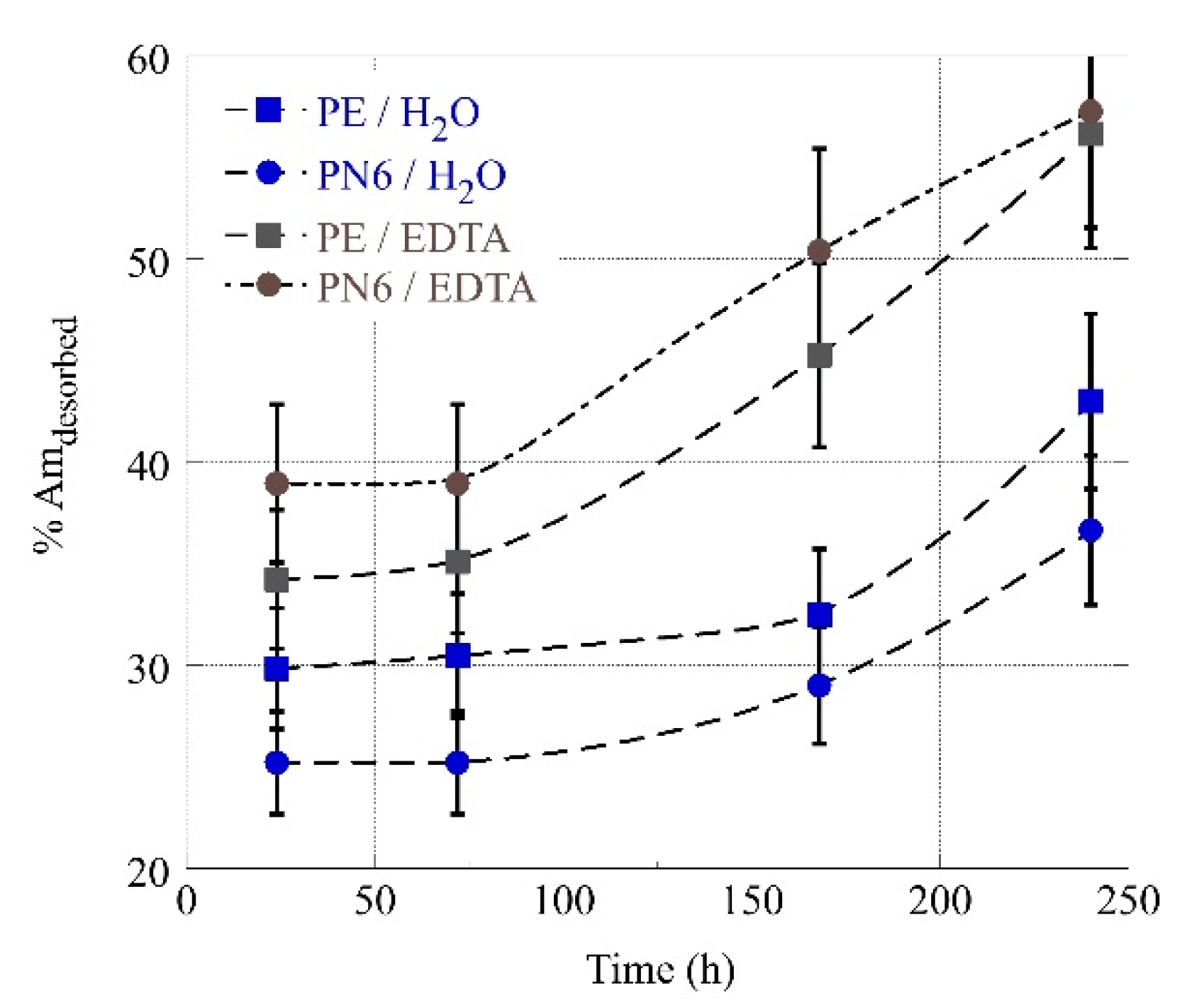
3.4. Desorption of Am-241 from MP’s in Deionized and EDTA Water Solutions
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Aryal, N.; Wood, J.; Rijal, I.; Deng, D.; Jha, M.K.; Ofori-Boadu, A. Fate of environmental pollutants: A review. Water Environ. Res. 2020, 92, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Horton, A.; Rose, N.L.; Hal, C. A temporal sediment record of microplastics in an urban lake, London, UK. J. Paleolimnol. 2019, 61, 449–462. [Google Scholar] [CrossRef]
- Prunier, J.; Maurice, L.; Perez, E.; Gigault, J.; Wickmann, A.C.P.; Davranche, M.; Halle, A. Trace metals in polyethylene debris from the North Atlantic subtropical gyre. Environ. Pollut. 2019, 245, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Lo, H.S.; Wong, H.M.; Zhou, M.; Wong, C.Y.; Tam, N.F.Y.; Cheung, S.G. Heavy metals contamination of sedimentary microplastics in Hong Kong. Mar. Pollut. Bull. 2020, 153, 110977. [Google Scholar] [CrossRef]
- Xie, Q.; Li, H.X.; Lin, L.; Li, Z.L.; Huang, J.S.; Xu, X.R. Characteristics of expanded polystyrene microplastics on island beaches in the Pearl River Estuary: Abundance, size, surface texture and their metals-carrying capacity. Ecotoxicology 2021, 30, 1632–1643. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Single-use surgical face masks as radionuclide (U-232 and Ra-226) carriers. J. Mol. Liq. 2021, 342, 117578. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Neptunium interaction with microplastics in aqueous solutions. J. Mol. Liq. 2022, 356, 119056. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Microplastics as radionuclide (U-232) carriers. J. Mol. Liq. 2022, 351, 118641. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Kayan, B.; Kalderis, D. Microplastics as carriers of hydrophilic pollutants in an aqueous environment. J. Mol. Liq. 2021, 350, 118182. [Google Scholar] [CrossRef]
- Spyridakis, I.; Tzanakakis, V.A.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Polyamide nylon 6 as a potential carrier of nitrate anions in aqueous environments. J. Mol. Liq. 2022, 352, 118706. [Google Scholar] [CrossRef]
- Öz, N.; Kadizade, G.; Yurtsever, M. Investigation of heavy metal adsorption on microplastics. Appl. Ecol. Environ. Res. 2019, 17, 7301–7310. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.L.; Hoel, D.; Glines, W.M. Radiobiology of Select Radionuclides in Hanford Site Tank Waste. Health Phys. 2022, 123, 99–192. [Google Scholar] [CrossRef]
- Johansen, M.P.; Prentice, E.; Cresswell, T.; Howell, N. Initial data on adsorption of Cs and Sr to the surfaces of microplastics with biofilm. J. Environ. Radioact. 2018, 190–191, 130–133. [Google Scholar] [CrossRef]
- Rout, S.; Yadav, S.; Joshi, V.; Karpe, R.; Pulhani, V.; Kumar, A.V. Microplastics as vectors of radioiodine in the marine environment: A study on sorption and interaction mechanism. Environ. Pollut. 2022, 307, 119432. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.; Sun, C.; Zhang, L.; Jiang, F.; Cao, W.; Zheng, L. Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar. Pollut. Bull. 2019, 144, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Adyel, T.M.; Miao, L.; You, G.; Liu, S.; Hou, J. Biofilm influenced metal accumulation onto plastic debris in different freshwaters. Environ. Pollut. 2021, 285, 117646. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 24792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hou, X.; Qiao, J.; Lin, J. Determination of 241Am in Environmental Samples. A Review. Molecules 2022, 27, 4536. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.O.; Silva, F. Management of radioactive disused lightning rods. In Proceedings of the 2013 International Nuclear Atlantic Conference—INAC 2013, Recife, PE, Brazil, 24–29 November 2013; pp. 24–29, ISBN 978-85-99141-05-2. [Google Scholar]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef]
- Naqash, N.; Prakash, S.; Kapoor, D.; Singh, R. Interaction of freshwater microplastics with biota and heavy metals: A review. Environ. Chem. Lett. 2020, 18, 1813–1824. [Google Scholar] [CrossRef]
- Nash, K.L. A Review of the basic chemistry and recent developments in trivalent f-elements separations. Solvent Extr. Ion Exch. 1993, 11, 729–768. [Google Scholar] [CrossRef]
- Choppin, G.R. Environmental behavior of actinides. Czech J. Phys. 2006, 56, 13–21. [Google Scholar] [CrossRef]
- Stadler, S.; Kim, J.I. Hydrolysis reactions of Am(III) and Am(V). Radiochim. Acta 1988, 44/45, 39–44. [Google Scholar] [CrossRef]
- Meinrath, G.; Kim, J.I. The carbonate complexation of the Am(III) ion. Radiochim. Acta 1991, 52/53, 29–34. [Google Scholar] [CrossRef]
- Runde, W.; Meinrath, G.; Kim, J.I. A study of solid-liquid phase equilibria of trivalent lanthanide and actinide ions in carbonate systems. Radiochim. Acta 1992, 58/59, 93–100. [Google Scholar] [CrossRef]
- Choppin, G.R. Actinide speciation in the environment. J. Radioanal. Nucl. Chem. 2007, 273, 695–703. [Google Scholar] [CrossRef]
- Kim, J.I. The Chemical Behavior of Transuranium Elements and Barrier Functions in Natural Aquifer Systems. MRS Online Proc. Libr. 1992, 294, 3–21. [Google Scholar] [CrossRef]
- Piringer, G.O.; Baner, A.L. Plastic Packaging: Interactions with Food and Pharmaceuticals, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-31455-3. [Google Scholar]
- Bochicchio, D.; Panizon, E.; Monticelli, L.; Rossi, G. Interaction of hydrophobic polymers with model lipid bilayers. Sci. Rep. 2017, 7, 6357. [Google Scholar] [CrossRef] [PubMed]
- Cernochova, K.; Mathur, J.; Choppin, G. Chemical speciation of Am, Cm and Eu with EDTA at high ionic strength: Thermodynamics and laser fluorescence spectroscopy studies. Radiochim. Acta 2005, 93, 733–739. [Google Scholar] [CrossRef]
- Kiliari, T.; Pashalidis, I. Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat. Meas. 2010, 45, 966–968. [Google Scholar] [CrossRef]
- Prodromou, M.; Pashalidis, I. Radium removal from aqueous solutions by adsorption on non-treated and chemically modified biomass by-product. J. Radioanal. Nucl. Chem. 2013, 295, 2095–2102. [Google Scholar] [CrossRef]
- Stasi, C.; Georgiou, E.; Ioannidis, I.; Pashalidis, I. Uranium removal from laboratory and environmental waters by oxidised biochar prepared from palm tree fibres. J. Radioanal. Nucl. Chem. 2022, 331, 375–381. [Google Scholar] [CrossRef]
- Beneš, P.; Paulenová, M. Surface charge and adsorption properties of polyethylene in aqueous solutions of inorganic electrolytes. Kolloid-Zeitschrift und Zeitschrift für Polymere 1973, 251, 766–771. [Google Scholar] [CrossRef]
- Hurwitz, G.; Guillen, G.R.; Hoek, E.M.V. Probing polyamide membrane surface charge, zeta potential, wettability, and hydrophilicity with contact angle measurements. J. Membr. Sci. 2010, 349, 349–357. [Google Scholar] [CrossRef]
- Ramírez-Guinart, O.; Kaplan, D.; Rigol, A.; Vidal, M. Deriving probabilistic soil distribution coefficients (Kd). Part 3: Reducing variability of americium Kd best estimates using soil properties and chemical and geological material analogues. J. Environ. Radioact. 2020, 223–224, 106378. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.N.; Choppin, G.R. Sorption of Am3+ cations on suspended silicate: Effects of pH, ionic strength, complexing anions, humic acid and metal ions. J. Radioanal. Nucl. Chem. 2007, 274, 517–523. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidis, I.; Xenofontos, A.; Anastopoulos, I.; Pashalidis, I. Americium Sorption by Microplastics in Aqueous Solutions. Coatings 2022, 12, 1452. https://doi.org/10.3390/coatings12101452
Ioannidis I, Xenofontos A, Anastopoulos I, Pashalidis I. Americium Sorption by Microplastics in Aqueous Solutions. Coatings. 2022; 12(10):1452. https://doi.org/10.3390/coatings12101452
Chicago/Turabian StyleIoannidis, Ioannis, Andrea Xenofontos, Ioannis Anastopoulos, and Ioannis Pashalidis. 2022. "Americium Sorption by Microplastics in Aqueous Solutions" Coatings 12, no. 10: 1452. https://doi.org/10.3390/coatings12101452
APA StyleIoannidis, I., Xenofontos, A., Anastopoulos, I., & Pashalidis, I. (2022). Americium Sorption by Microplastics in Aqueous Solutions. Coatings, 12(10), 1452. https://doi.org/10.3390/coatings12101452







