The DLC Coating on 316L Stainless Steel Stochastic Voronoi Tessellation Structures Obtained by Binder Jetting Additive Manufacturing for Potential Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
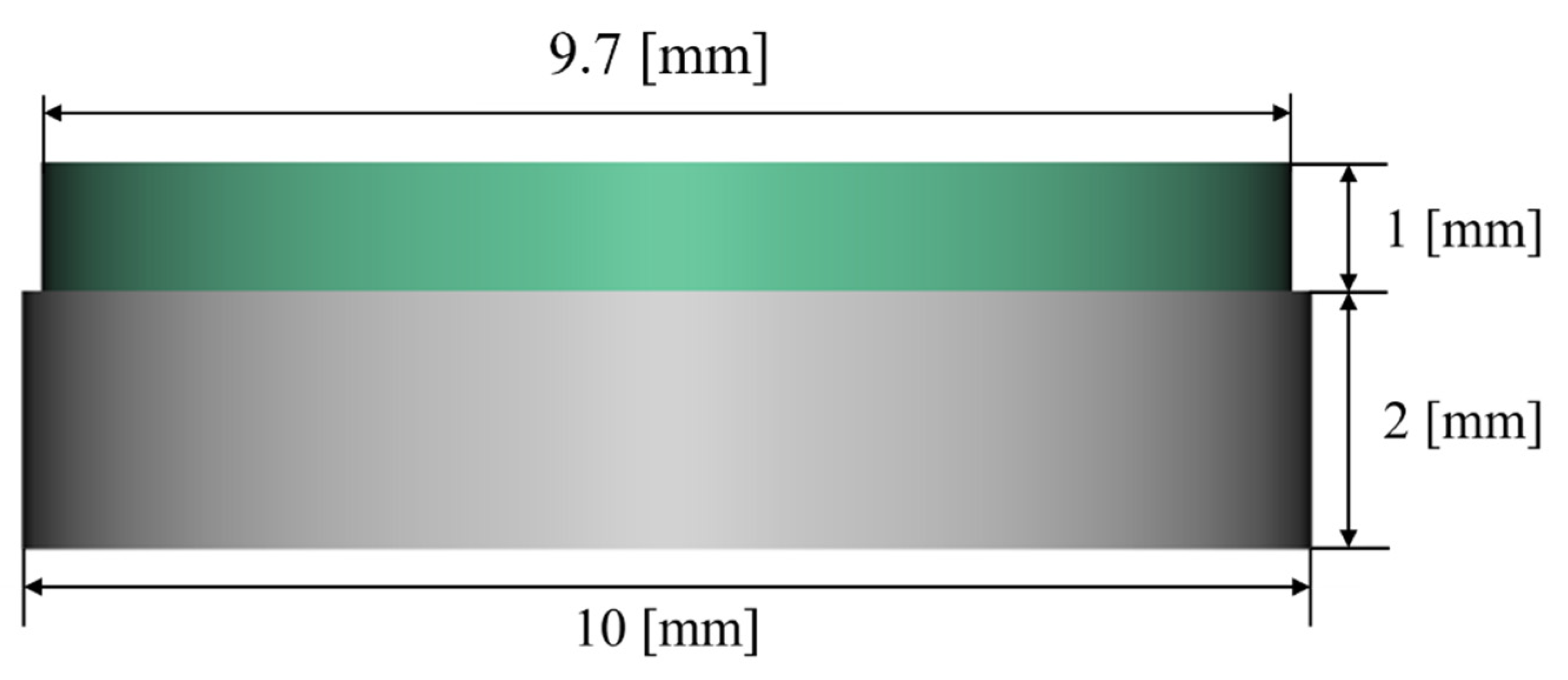
2.1. Samples Design
- p = {pi,…,p} is a set of distinct seed points located in the d-dimensional Euclidean space Rd,
- d(p,pi) represents the Euclidean distance between the location p and seed pi,
- V(pi) represents the ordinary Voronoi polygon associated with seed pi.
2.2. Powder Characterization
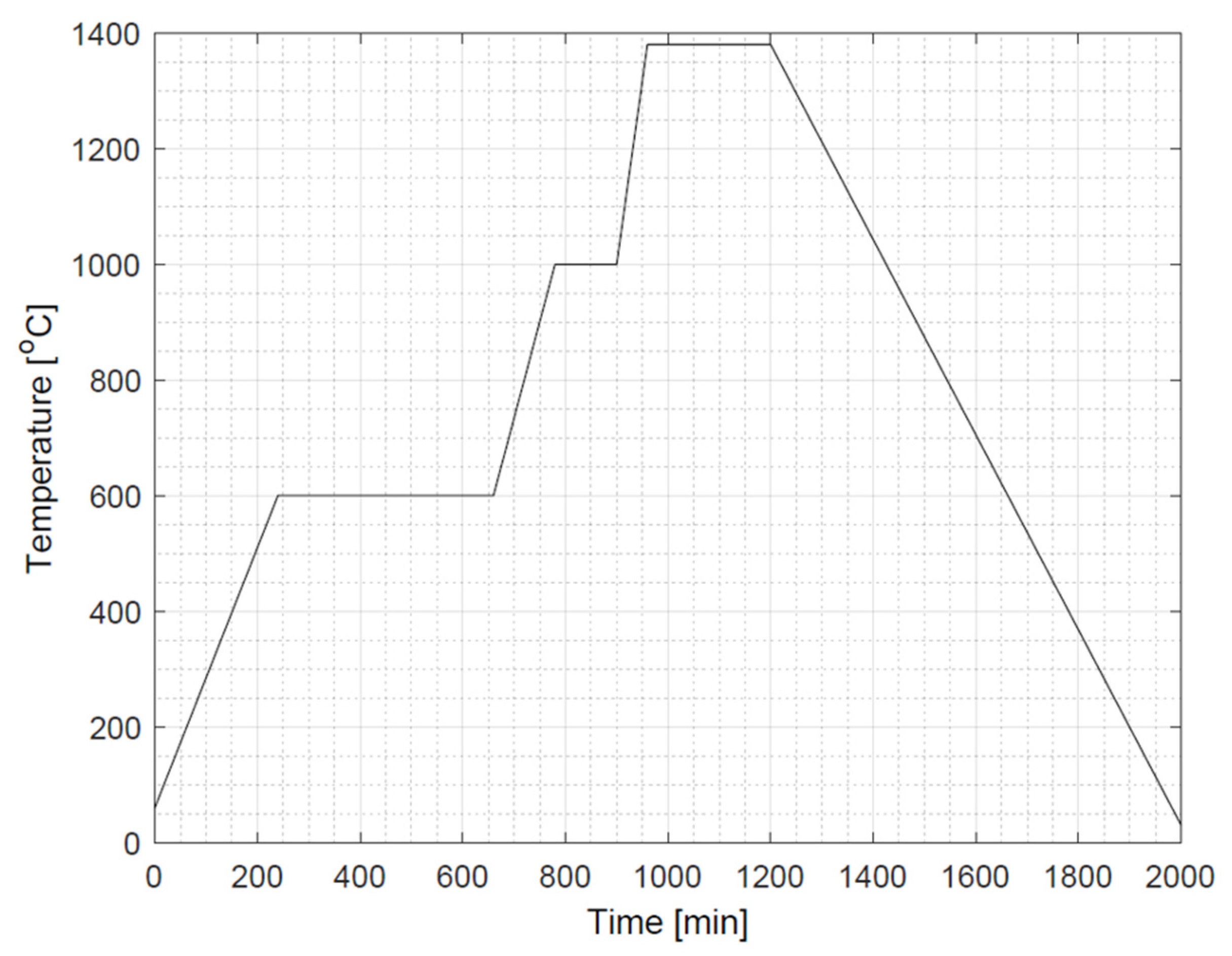
2.3. Fabrication Process
2.4. Diamond-Like Carbon Coating Deposition
2.5. Optical Microscopy
2.6. SEM Microscopy
2.7. Confocal Microscopy
2.8. Raman Spectroscopy
2.9. HRTEM Microscopy
3. Results and Discussion
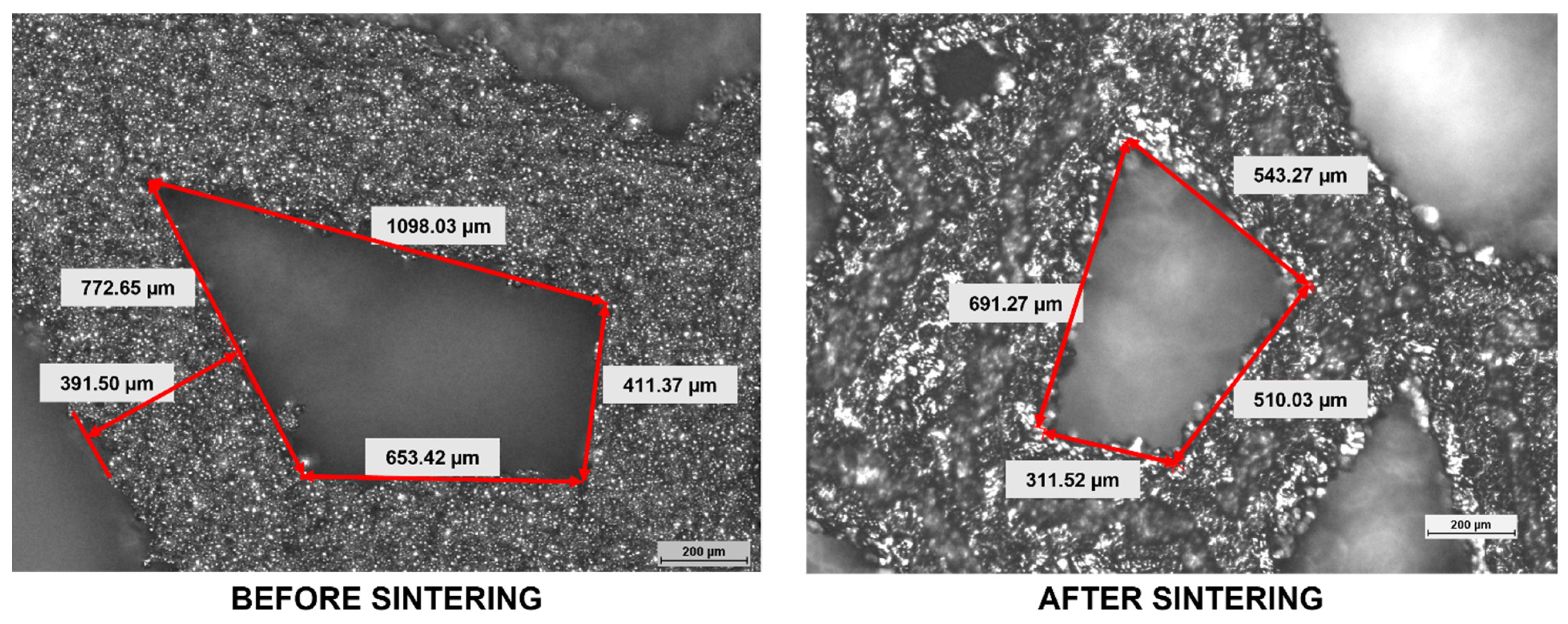
3.1. Dimensional Analysis
3.2. Surface Morphology
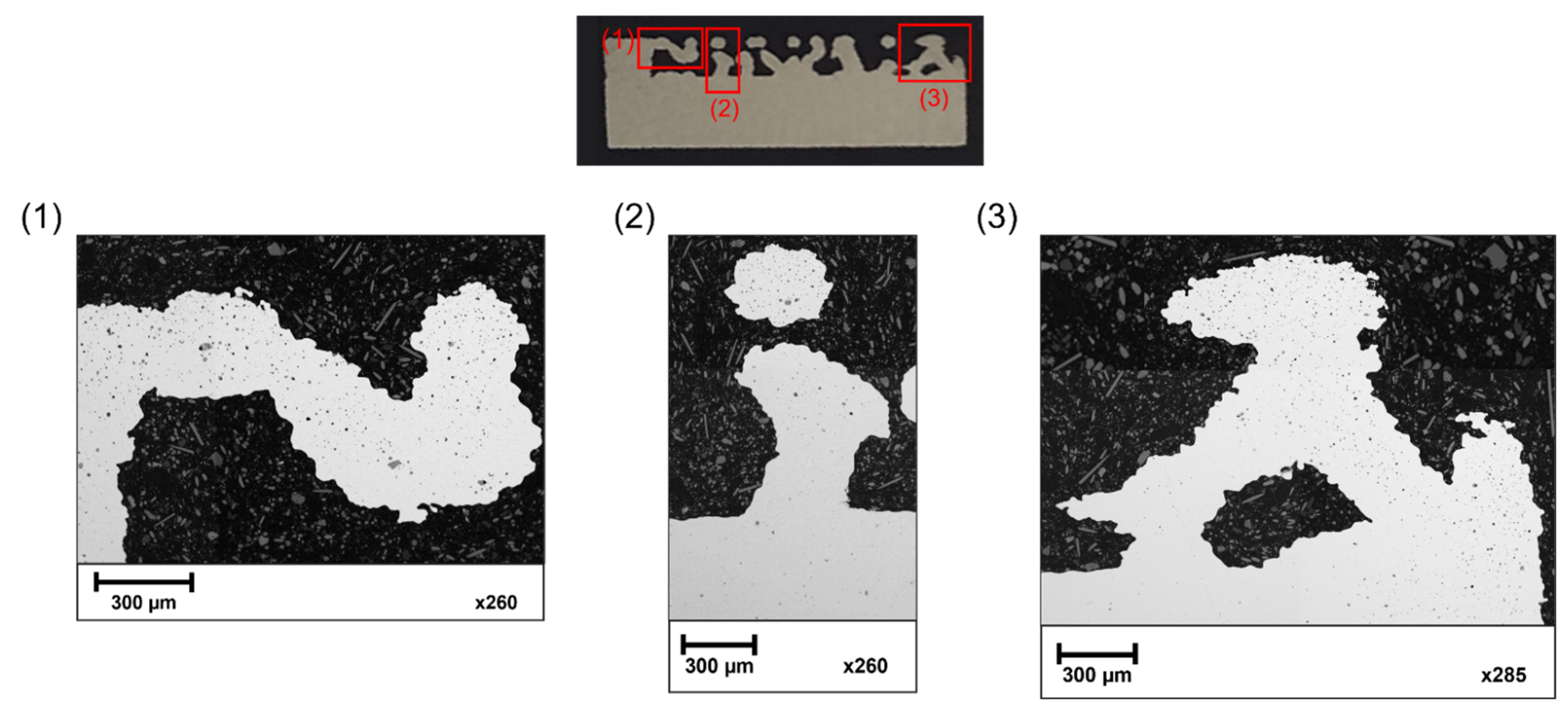
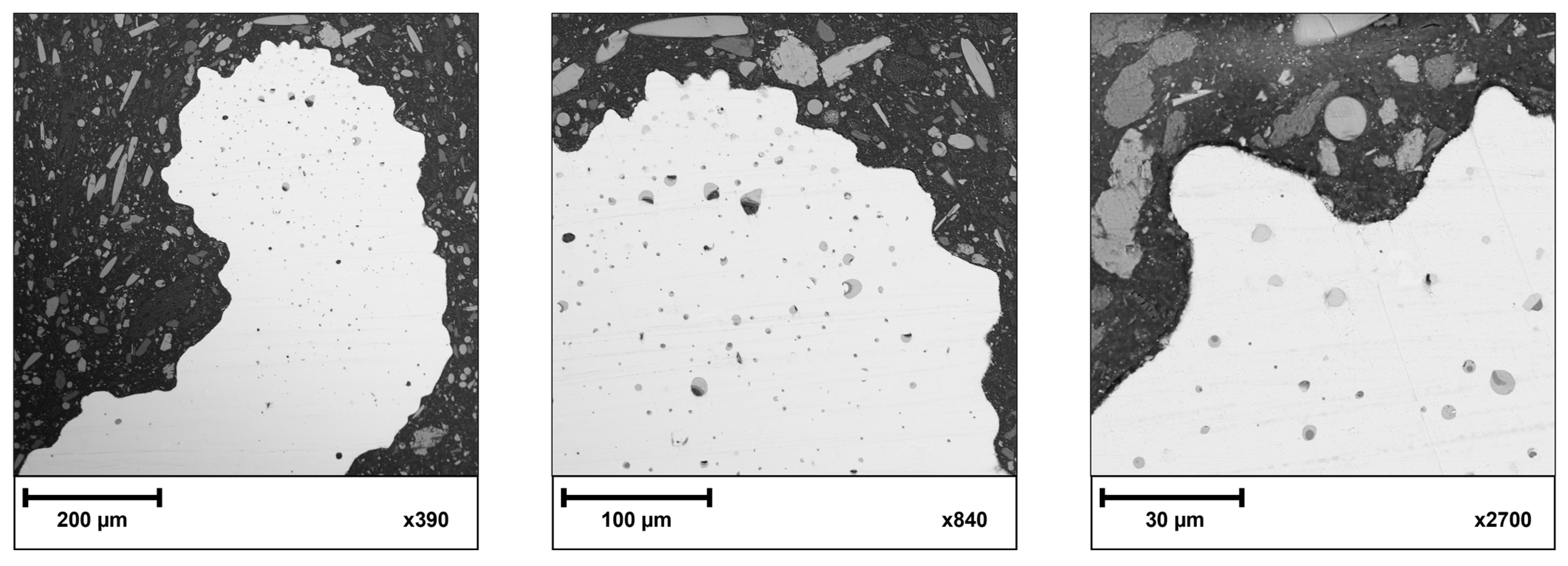
3.3. Internal Porosity
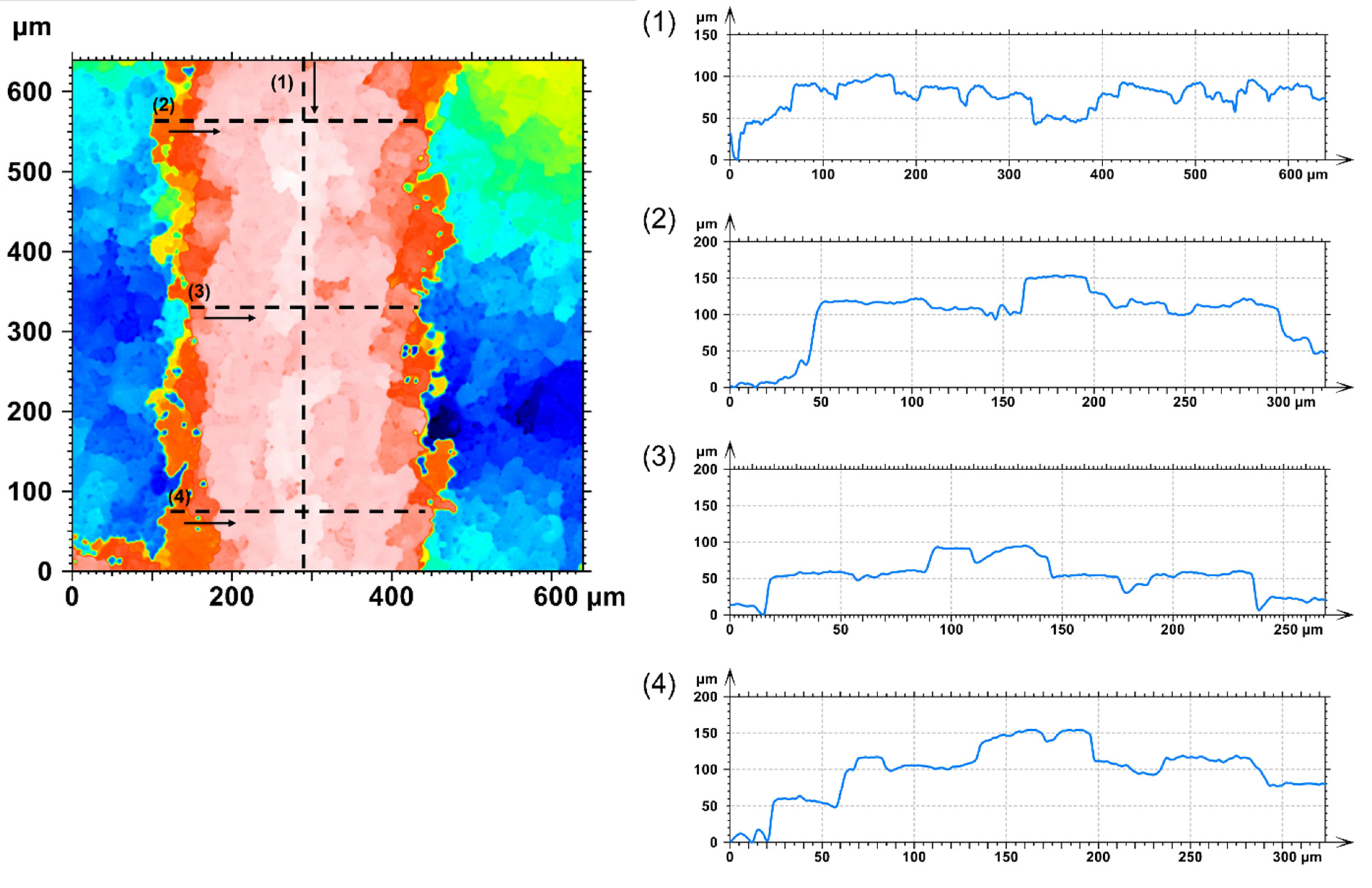
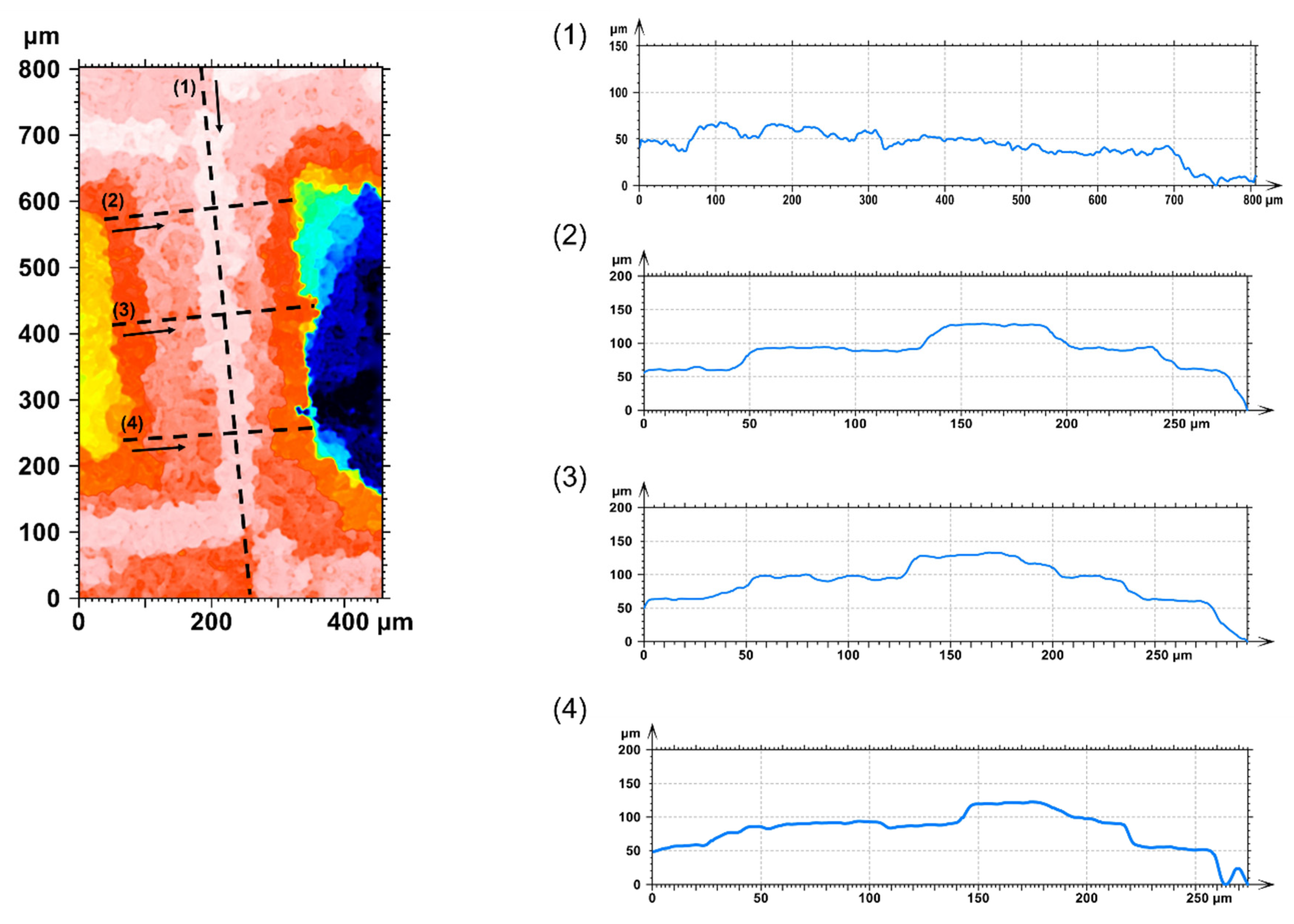
3.4. Surface Topography
3.5. Thickness and Continuity of the DLC Coating
3.6. The Chemical Structure of DLC Coating
3.7. Microstructure and Chemical Composition
4. Conclusions
- Compared to the design model, the interconnected complex spatial lattice of the Voronoi structure was accurately duplicated by the binder jetting technology;
- The dimensions of the struns and pores size of the Voronoi structure were larger than the design, which reduces its porosity, and affects the shape of the pores and connections between the struns;
- The specificity of the spatial structure design, the material used, and the manufacturing technology influenced the morphology and topography of the surface. In SEM analysis, it was observed that the rough surface morphology of the struns, was caused by powder grain agglomerates. The powder grains in the same agglomerate sintered to the final stages, but due to the large spacing, the diffusion and necking between adjacent agglomerates were limited;
- SEM images of DLC coatings obtained for different levels of the analyzed structure show that the DLC coating was successfully deposited on the surface of the Voronoi structure;
- The main challenge for additive manufacturing technologies is to produce elements with low initial porosity. The inner pores were located in the upper layers of the struns and at the periphery of the cylindrical base of the sample. In the near-surface area, no open pores were observed that could affect the coating deposition process. Most of the pores have a spherical morphology. The small number of internal pores in the cross-section may indicate a good packing density of the powder bed at the printing stage and the appropriate selection of the sinter parameters;
- Based on the data from the confocal microscopy analysis, it was observed that the mean roughness of the surface of the strun before modification with DLC coating was higher in comparison with the strun after modification. The DLC layer caused a partial smoothing of the structure by filling the space between adjacent agglomerates. This was highlighted in the cross-sectional analysis of SEM and HR TEM;
- SEM cross-section analysis and HR TEM analysis indicate a continuous DLC coating with thicknesses from 30 nm to 230 nm. It was observed that coating thickness depends on the radius of curvature of the surface;
- The result of Raman spectroscopy shows the presence of a typical DLC coating on the surface of the tested sample with ID/IG = 1.08;
- The HR TEM results indicate a continuous image of the DLC coating with content chromium oxide and silicon dioxide.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cullinane, D.M.; Einhorn, T.A. Biomechanics of Bone. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 17–32. [Google Scholar]
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Qu, H. Additive manufacturing for bone tissue engineering scaffolds. Mater. Today Commun. 2020, 24, 101024. [Google Scholar] [CrossRef]
- Merola, M.; Affatato, S. Materials for Hip Protheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Metals and Medicine. Mater. Trans. 2021, 62, 139–148. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Jamari, J. Tresca stress evaluation of Metal-on-UHMWPE total hip arthroplasty during peak loading from normal walking activity. Mater. Today Proc. 2022, 63, 143–146. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, W.; Li, Y. Fabrication and characterization of porous Ti6Al4V parts for biomedical applications using electron beam melting process. Matter. Lett. 2009, 63, 403–405. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef]
- Zaharin, H.A.; Rani, A.M.A.; Azam, F.I.; Gina, T.L.; Sallih, N.; Ahmad, A.; Yunus, N.A.; Zulkifli, T.Z.A. Effect of Unit Cell Type and Pore Size on Porosity and Mechanical Behaviour of Additively Manufactured Ti6Al4V Scaffold. Materials 2018, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Hao, L.; Hussein, A.; Young, P. Ti-6Al-4V triply periodic minimal surface structures for bone implants fabricated via selective laser melting. J. Mech. Behav. Biomed. Mater. 2015, 51, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Yanez, A.; Cuadrado, A.; Martel, O.; Alfonso, H.; Monopoli, D. Gyroid porous titanium structures: A versatile solution to be used as scaffolds in bone defect reconstruction. Mater. Des. 2018, 140, 21–29. [Google Scholar] [CrossRef]
- Murry, L.E. Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoconduction and vascularization: An overview. J. Mater. Sci. Technol. 2019, 35, 231–241. [Google Scholar] [CrossRef]
- Du, Y.; Liang, H.; Xie, D.; Mao, N.; Zhao, J.; Tian, Z.; Wnag, C.; Shen, L. Design and statistical analysis of irregular porous scaffolds for orthopedic reconstruction based on Voronoi tessellation and fabricated via selective laser melting (SLM). Mater. Chem. Phys. 2020, 239, 121968. [Google Scholar] [CrossRef]
- Kelly, A.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar]
- Heinl, P.; Muller, L.; Korner, C.; Singer, R.F.; Muller, F.A. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef]
- Victor, S.P.; Pillai, C.K.S.; Sharma, C.P. Biointegration: An introduction. In Biointegration of Medical Implant Materials, 2nd ed.; Sharma, C.P., Ed.; Elsevier, Woodhead Publishing: Duxford, UK, 2020; pp. 1–16. [Google Scholar]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef]
- Szatkiewicz, T.; Laskowska, D.; Bałasz, B.; Mitura, K. The Influence of the Structure Parameters on the Mechanical Properties of Cylindrically Mapped Gyroid TPMS Fabricated by Selective Laser Melting with 316L Stainless Steel Powder. Materials 2022, 15, 4352. [Google Scholar] [CrossRef]
- Bourell, D.; Kruth, J.P.; Leu, M.; Levy, G.; Rosen, D.; Beese, A.M.; Clare, A. Materials for additive manufacturing. CIRP Ann. 2017, 66, 659–681. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliot, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing-Process parameters, materials, properties, modeling, and challenges. Prog. Mater Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Brent, S. Binder Jetting. In Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Gibson, I., Rosen, D., Brent, S., Eds.; Springer: New York, NY, USA, 2015; pp. 205–208. [Google Scholar]
- Mirzababaei, S.; Pasebani, S. A Review on Binder Jet Additive Manufacturing of 316 Stainless Steel. J. Manuf. Mater. Process 2019, 3, 82. [Google Scholar]
- Mitura, K.; Niedzielski, P.; Bartosz, G.; Moll, J.; Walkowiak, B.; Pawłowska, Z.; Louda, P.; Kieć-Świerczyńska, M.; Stanisław, M. Interactions between carbon coatings and tissue. Surf. Coat. Technol. 2006, 201, 2117–2123. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.-R. Biomedical Applications of Diamond-Like Carbon Coatings: A Review. J. Biomed. Mater. Res. Part B 2007, 83, 72–84. [Google Scholar] [CrossRef]
- Niedzielski, P.; Mitura, E.; Mitura, S.; Dluzniewski, M.; Przymusiala, P.; Der Sahaguian, S.; Staryga, E.; Zak, J.; Sokolowska, A.; Szmidt, J.; et al. Comparison of the surface structure of carbon films deposited by different methods. Diam. Relat. Mater. 1997, 6, 721–724. [Google Scholar] [CrossRef]
- Kaczorowski, W.; Gajewski, K.; Szymanski, W.; Batory, D.; Wojciechowska, A.; Swiatek, L.; Gotszalk, T.; Niedzielski, P. Evaluation of mechanical properties of carbon coatings synthesised in radio frequency plasma on PDMS. Surf. Coat. Technol. 2018, 333, 220–228. [Google Scholar] [CrossRef]
- Kaczorowski, W.; Świątek, H.; Łuczak, K.; Głuszek, M.; Cłapa, M. Impact of plasma pre-treatment on the tribological properties of DLC coatings on PDMS substrates. Materials 2021, 14, 433. [Google Scholar] [CrossRef]
- Cui, F.Z.; Li, D.J. A review of investigations on biocompatibility of diamond-like carbon and carbon nitride films. Surf. Coat. Technol. 2000, 131, 481–487. [Google Scholar] [CrossRef]
- Bendavid, A.; Martin, P.J.; Comte, C.; Preston, E.W.; Haq, A.J.; Magdon Ismail, F.S.; Singh, R.K. The mechanical and biocompatibility properties of DLC-Si films prepared by pulsed DC plasma activated chemical vapor deposition. Diam. Relat. Mater. 2007, 16, 1616–1622. [Google Scholar] [CrossRef]
- Grill, A. Tribology of diamondlike carbon and related materials: An updated review. Surf. Coat. Technol. 1997, 94–95, 507–513. [Google Scholar] [CrossRef]
- Batory, D.; Blaszczyk, T.; Clapa, M.; Mitura, S. Investigation of anti-corrosion properties of Ti:C gradient layers manufactured in hybrid deposition system. J. Mater. Sci. 2008, 43, 3385–3391. [Google Scholar] [CrossRef]
- Bociąga, D.; Mitura, K. Biomedical effect of tissue contact with metallic material used for body piercing modified by DLC coatings. Diam. Relat. Mater. 2008, 17, 1410–1415. [Google Scholar] [CrossRef]
- Jakubowski, W.; Bartosz, G.; Niedzielski, P.; Szymanski, W.; Walkowiak, B. Nanocrystalline diamond surface is resistant to bacterial colonization. Diam. Relat. Mater. 2004, 13, 1761–1763. [Google Scholar] [CrossRef]
- Tillmann, W.; Dias, N.F.L.; Stangier, D.; Schaak, C.; Hoges, S. Coatability of diamond-like carbon on 316L stainless steel printed by binder jetting. Addit. Manuf. 2021, 44, 102064. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Hing, K.A.; Annaz, B.; Saeed, S.; Revell, P.A.; Buckland, T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J. Mater. Sci.-Mater. Med. 2005, 16, 467–475. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Wang, C.; Zhang, A.; Chen, B.; Han, Q.; Wang, J. Design and properties of biomimetic irregular scaffolds for bone tissue engineering. Comput. Biol. Med. 2021, 130, 104241. [Google Scholar] [CrossRef]
- Deering, J.; Dowling, K.I.; DiCecco, L.-A.; McLean, G.D.; Yu, B.; Grandfield, K. Selective Voronoi tessellation as a method to design anisotropic and biomimetic implants. J. Mech. Behav. Biomed. Mater. 2021, 116, 104361. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Xie, D.; Li, L.; Mao, N.; Wang, C.; Tain, Z.; Jiang, Q.; Shen, L. Trabecular-like Ti-6Al-4V scaffolds for orthopedic: Fabrication by selective laser melting and in vitro biocompatibility. J. Mater. Sci. Technol. 2019, 35, 1284–1297. [Google Scholar] [CrossRef]
- Jahani, B.; Wang, X. The Effects of Surface Roughness on the Functionality of Ti13Nb13Zr Orthopedic Implants. Biomed. J. Sci. Tech. Res. 2021, 38, 30058–30067. [Google Scholar] [CrossRef]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Alla, R.K.; Ginjupalli, K.; Upadhya, N.; Shammas, M.; Ravi, R.K.; Sekhar, R. Surface Roughness of Implants: A Review. Trends Biomater. Artif. Organs 2011, 25, 112–118. [Google Scholar]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surface. Biomaterials 2013, 34, 8018–8029. [Google Scholar] [CrossRef]
- Gallo, J.L.; Holinka, M.; Moucha, C.S. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef]
- Rajk, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Carvalho, I.; Rodrigues, L.; Lima, M.J.; Carvalho, S.; Cruz, S.M.A. Overview on the Antimicrobial Activity and Biocompatibility of Sputtered Carbon-Based Coatings. Processes 2021, 9, 1428. [Google Scholar] [CrossRef]
- Andrade-Del Olmo, J.; Ruiz-Rubio, L.; Perez-Alvarez, L.; Saez-Martinez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef]
- Kaneko, M.; Hiratsuka, M.; Alanazi, A.; Nakamori, H.; Namiki, K.; Hirakuri, K. Surface Reformation of Medical Devices with DLC Coating. Materials 2021, 14, 376. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Ashraf, M.N.; Timmermann, A.; Schmid, C.; Ensinger, W. The Influence of Preparation Conditions on the Structural Properties and Hardness of Diamond-Like Carbon Films, Prepared by Plasma Source Ion Implantation. Coatings 2020, 10, 360. [Google Scholar] [CrossRef]
- Bociaga, D.; Sobczyk-Guzenda, A.; Komorowski, P.; Balcerzak, J.; Jastrzebski, K.; Przybyszewska, K.; Kaczmarek, A. Surface Characteristics and Biological Evaluation of Si-DLC Coatings Fabricated Using Magnetron Sputtering Method on Ti6Al7Nb Substrate. Nanomaterials 2019, 9, 812. [Google Scholar] [CrossRef]
- Yonezawa, K.; Kawaguchi, M.; Kaneuji, A.; Ichiseki, T.; Iinuma, Y.L.; Kawamura, K.; Shintani, K.; Oda, S.; Taki, M.; Kawahara, N. Evaluation of Antibacterial and Cytotoxic Properties of a Fluorinated Diamond-Like Carbon Coating for the Development of Antibacterial Medical Implants. Antibiotics 2020, 9, 495. [Google Scholar] [CrossRef]
- nTopology. 2020. Available online: https://ntopology.com/ (accessed on 2 June 2022).
- Material Product Data Sheet, Osprey® 316L for Additive Manufacturing, Sandvik Osprey Ltd., UK. Available online: https://www.metalpowder.sandvik/siteassets/metal-powder/datasheets/osprey-316l-am-austenitic-stainless-steels.pdf (accessed on 29 March 2022).
- Do, T.; Kwon, P.; Shin, C.S. Process development toward full-density stainless steel part with binder jetting printing. Int. J. Mach. Tools Manuf. 2017, 121, 50–60. [Google Scholar] [CrossRef]
- ISO 4287. Geometrical Products Specifications (GPS)-Surface Texture: Profile Method-Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 1997.
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Casiraghi, C.; Piazza, F.; Ferrari, A.C.; Grambole, D.; Robertson, J. Bonding in hydrogenated diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2005, 14, 1098–1102. [Google Scholar] [CrossRef]
- Mitura, S.; Mitura, A.; Niedzielski, P.; Couvrat, P. Nanocrystalline Diamond Coatings. Chaos Solitons Fractals 1999, 10, 2165–2176. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Hameister, R.; Singh, G. Allergies in orthopaedic and trauma surgery. Orthop. Traumatol. Surg. Res. 2017, 103, S75–S81. [Google Scholar] [CrossRef]
- Matusewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles- A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Gutensohn, K.; Beythien, C.; Bau, J.; Fenner, T.; Grewe, P.; Koester, R.; Padmanaban, K.; Kuehnl, P. In Vitro Analyses of Diamond-like Carbon Coated Stents: Reduction of Metal Ion Release, Platelet Activation, and Thrombogenicity. Thromb. Res. 2000, 99, 577–585. [Google Scholar] [CrossRef]
- Mitura, K. HR TEM examinations of nanodiamond particles for biomedical application. J. Achiev. Mater. Manuf. Eng. 2009, 37, 317–322. [Google Scholar]
- Toboła, D.; Morgiel, J.; Maj, Ł. TEM analysis of surface layer of Ti-6Al-4V ELI alloy after slide burnishing and low-temperature gas nitriding. Appl. Surf. Sci. 2020, 515, 145942. [Google Scholar] [CrossRef]
- Morgiel, J.; Szymkiewicz, K.; Maj, Ł.; Tarnowski, M.; Wierzchoń, M. TEM studies of low temperaturę cathode-plasma nitrided Ti6Al7Nb alloy. Surf. Coat. Technol. 2019, 359, 183–189. [Google Scholar] [CrossRef]
- Szymkiewicz, K.; Morgiel, J.; Maj, Ł.; Pomorska, M.; Trnowski, M.; Wierzchoń, T. TEM investigations of active screen plasma nitride Ti6Al4V and Ti6Al7Nb alloys. Surf. Coat. Technol. 2020, 383, 125268. [Google Scholar] [CrossRef]









| Element | C | Si | Mn | P | S | Cr | Ni | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Weight percent [%] | ≤0.03 | ≤1 | ≤2 | ≤0.045 | ≤0.03 | 16–18 | 10–14 | 2–3 | Balance |
| Layer Thickness | Saturation | Recoat Speed | Roller Speed | Ultrasonic Intensity |
|---|---|---|---|---|
| 40 µm | 70% | 50 mm/s | 500 rpm | 100% |
| Pre-Treatment | Deposition of DLC Coating | |
|---|---|---|
| Pressure | 2 Pa | 40 Pa |
| RF generator | 500 W | 1000 W |
| Negative bias voltage | 1000 V | 700 V |
| Deposition time | 10 min | 5 min |
| Type of atmosphere | - | CH4 |
| S1 | S2 | S3 | AVRAGE [µm] | S1_DLC | S2_DLC | S3_DLC | AVRAGE [µm] | |
|---|---|---|---|---|---|---|---|---|
| ALONG ROUGHNESS PROFILE | ||||||||
| Ra [µm] | 7.18 | 8.61 | 9.07 | 8.29 | 3.10 | 5.40 | 1.58 | 3.36 |
| Rz [µm] | 44.10 | 37.80 | 69.80 | 50.57 | 15.00 | 25.00 | 16.90 | 18.97 |
| ACROSS ROUGHNESS PROFILE | ||||||||
| Ra [µm] | 3.54 | 7.65 | 6.28 | 6.43 | 4.45 | 2.96 | 4.39 | 4.04 |
| 6.17 | 6.84 | 6.69 | 4.08 | 3.68 | 5.53 | |||
| 8.18 | 8.50 | 3.99 | 3.59 | 2.97 | 4.68 | |||
| Rz [µm] | 18.70 | 41.70 | 34.20 | 32.76 | 20.90 | 14.90 | 16.20 | 18.89 |
| 28.50 | 32.10 | 27.40 | 20.90 | 18.30 | 24.90 | |||
| 53.60 | 36.60 | 22.00 | 16.20 | 13.10 | 24.60 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, D.; Bałasz, B.; Kaczorowski, W.; Grabarczyk, J.; Svobodova, L.; Szatkiewicz, T.; Mitura, K. The DLC Coating on 316L Stainless Steel Stochastic Voronoi Tessellation Structures Obtained by Binder Jetting Additive Manufacturing for Potential Biomedical Applications. Coatings 2022, 12, 1373. https://doi.org/10.3390/coatings12101373
Laskowska D, Bałasz B, Kaczorowski W, Grabarczyk J, Svobodova L, Szatkiewicz T, Mitura K. The DLC Coating on 316L Stainless Steel Stochastic Voronoi Tessellation Structures Obtained by Binder Jetting Additive Manufacturing for Potential Biomedical Applications. Coatings. 2022; 12(10):1373. https://doi.org/10.3390/coatings12101373
Chicago/Turabian StyleLaskowska, Dorota, Błażej Bałasz, Witold Kaczorowski, Jacek Grabarczyk, Lucie Svobodova, Tomasz Szatkiewicz, and Katarzyna Mitura. 2022. "The DLC Coating on 316L Stainless Steel Stochastic Voronoi Tessellation Structures Obtained by Binder Jetting Additive Manufacturing for Potential Biomedical Applications" Coatings 12, no. 10: 1373. https://doi.org/10.3390/coatings12101373
APA StyleLaskowska, D., Bałasz, B., Kaczorowski, W., Grabarczyk, J., Svobodova, L., Szatkiewicz, T., & Mitura, K. (2022). The DLC Coating on 316L Stainless Steel Stochastic Voronoi Tessellation Structures Obtained by Binder Jetting Additive Manufacturing for Potential Biomedical Applications. Coatings, 12(10), 1373. https://doi.org/10.3390/coatings12101373








