Particle-Size-Dependent Anticorrosion Performance of the Si3N4-Nanoparticle-Incorporated Electroless Ni-P Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coatings on Low-Carbon Steel
- Ni-P → Nickel-Phosphorus coating.
- Ni-P-SN → Si3N4 submicron particles (200 nm) incorporated Ni-P-Si3N4 coating.
- Ni-P-nSN → Si3N4 nanoparticles (20 nm) incorporated Ni-P-Si3N4 coating.
2.2. Characterization
2.3. Anti-Corrosion Performance Evaluation
3. Results and Discussion
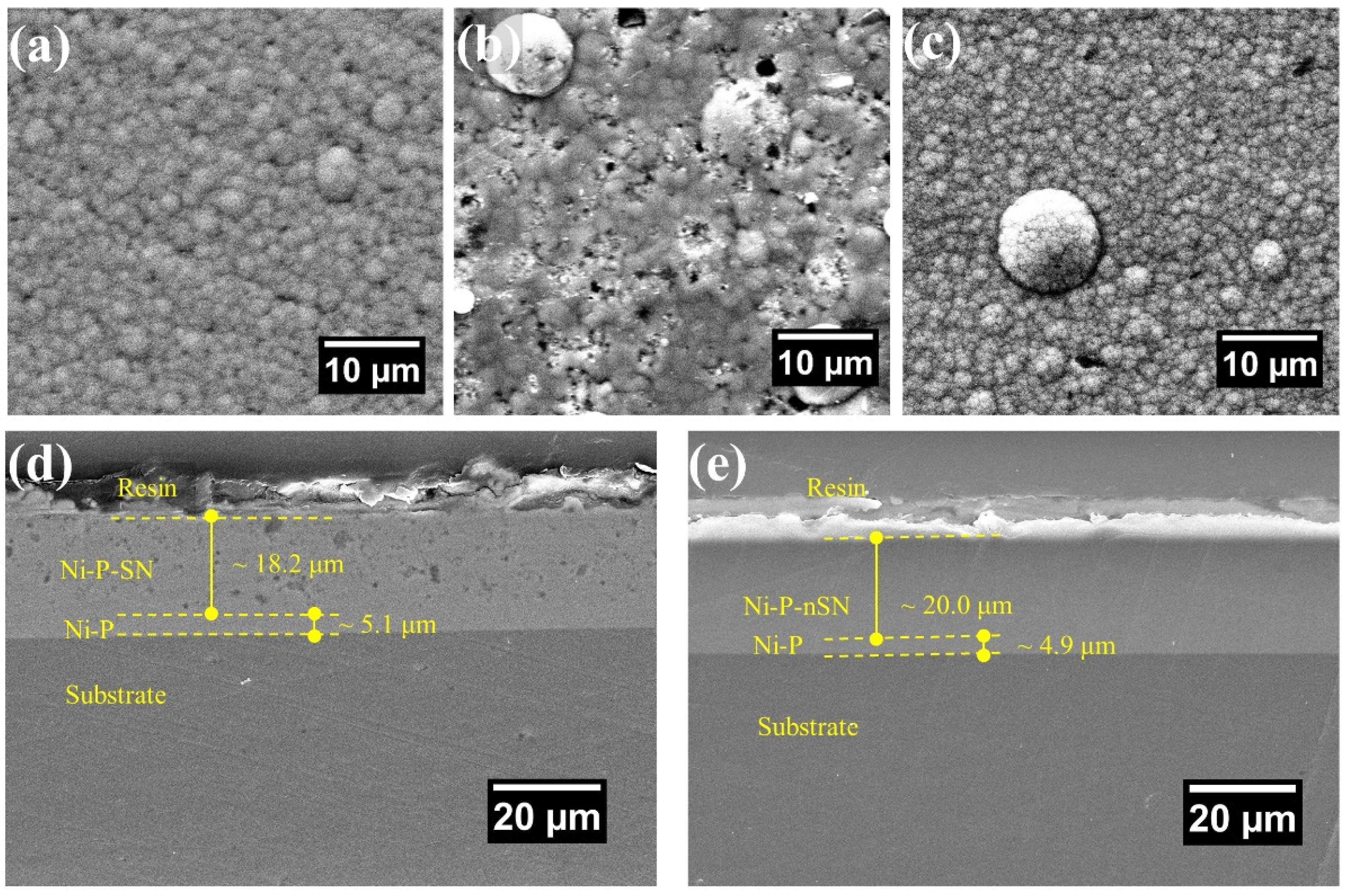
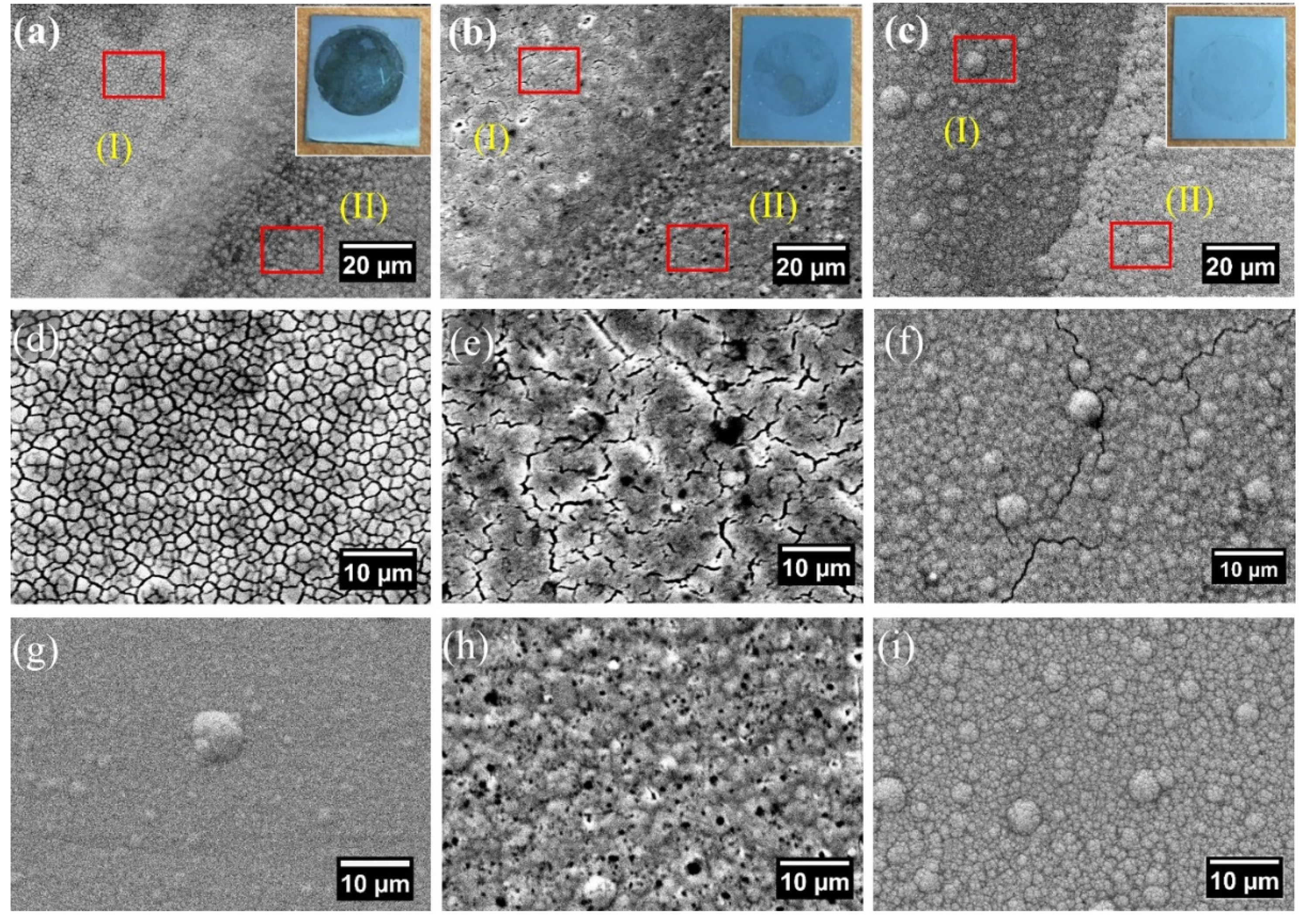
3.1. Coating Surface and Cross-Section
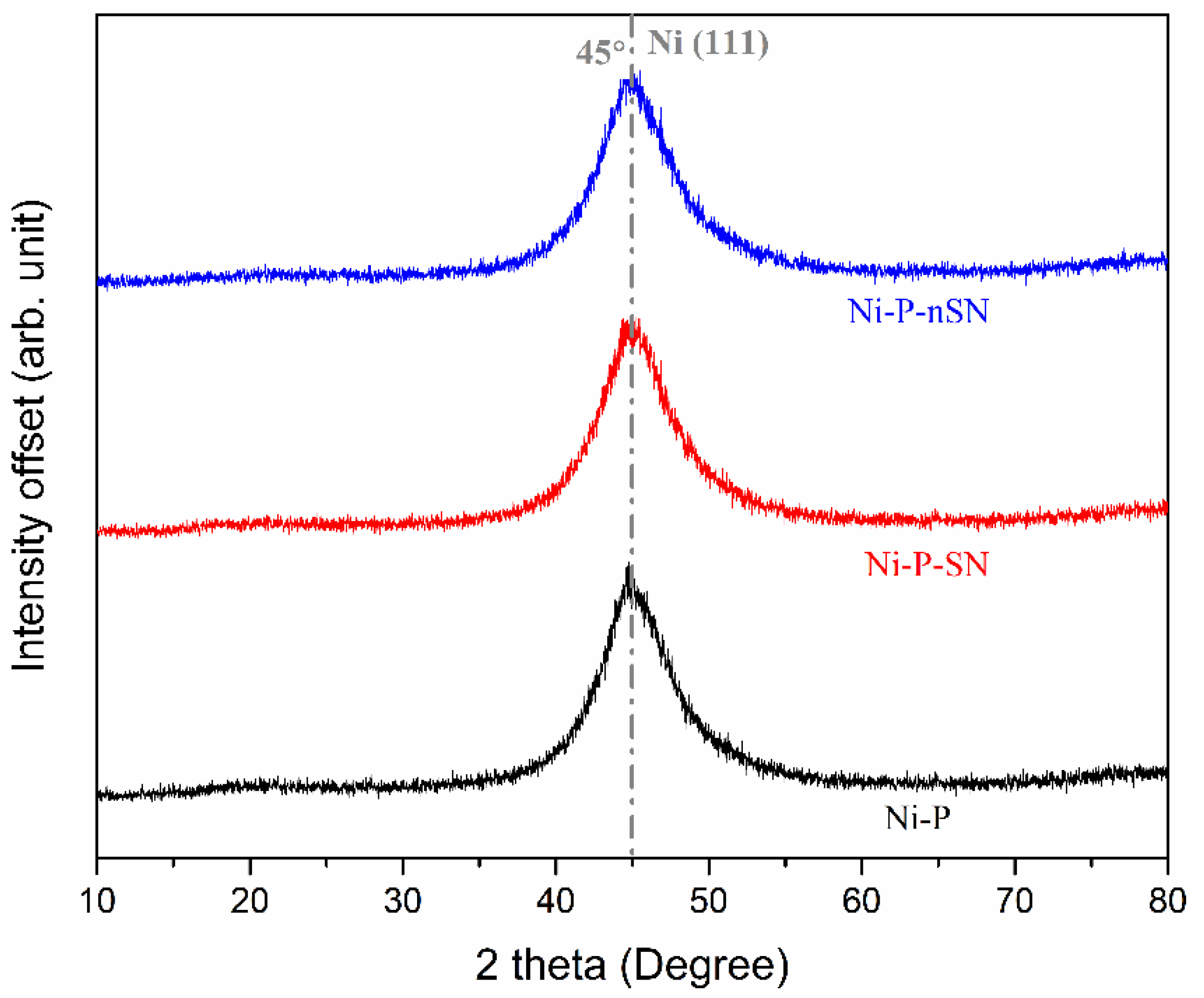
3.2. Phase and Microstructural Study
3.2.1. XRD Analysis
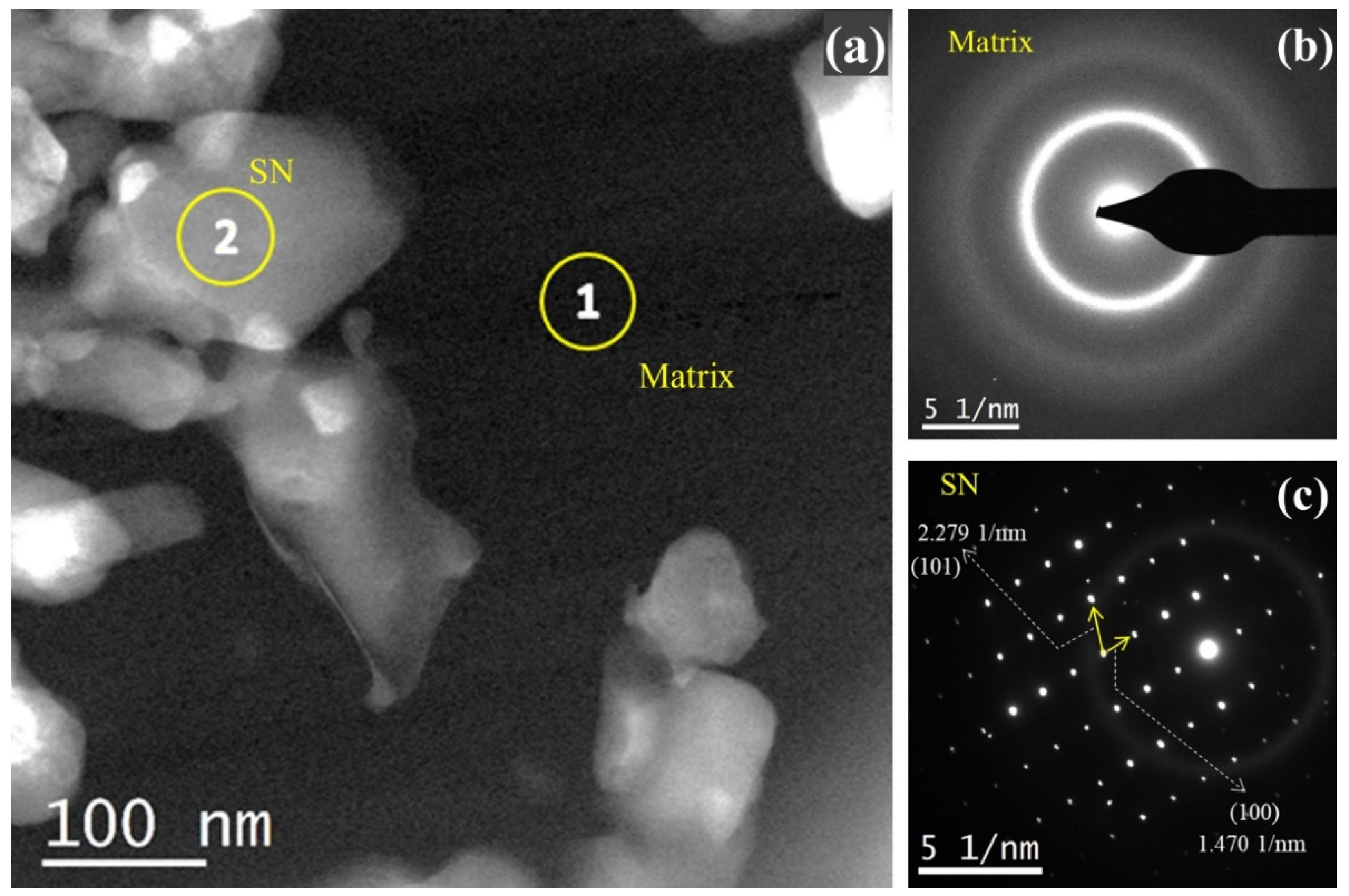
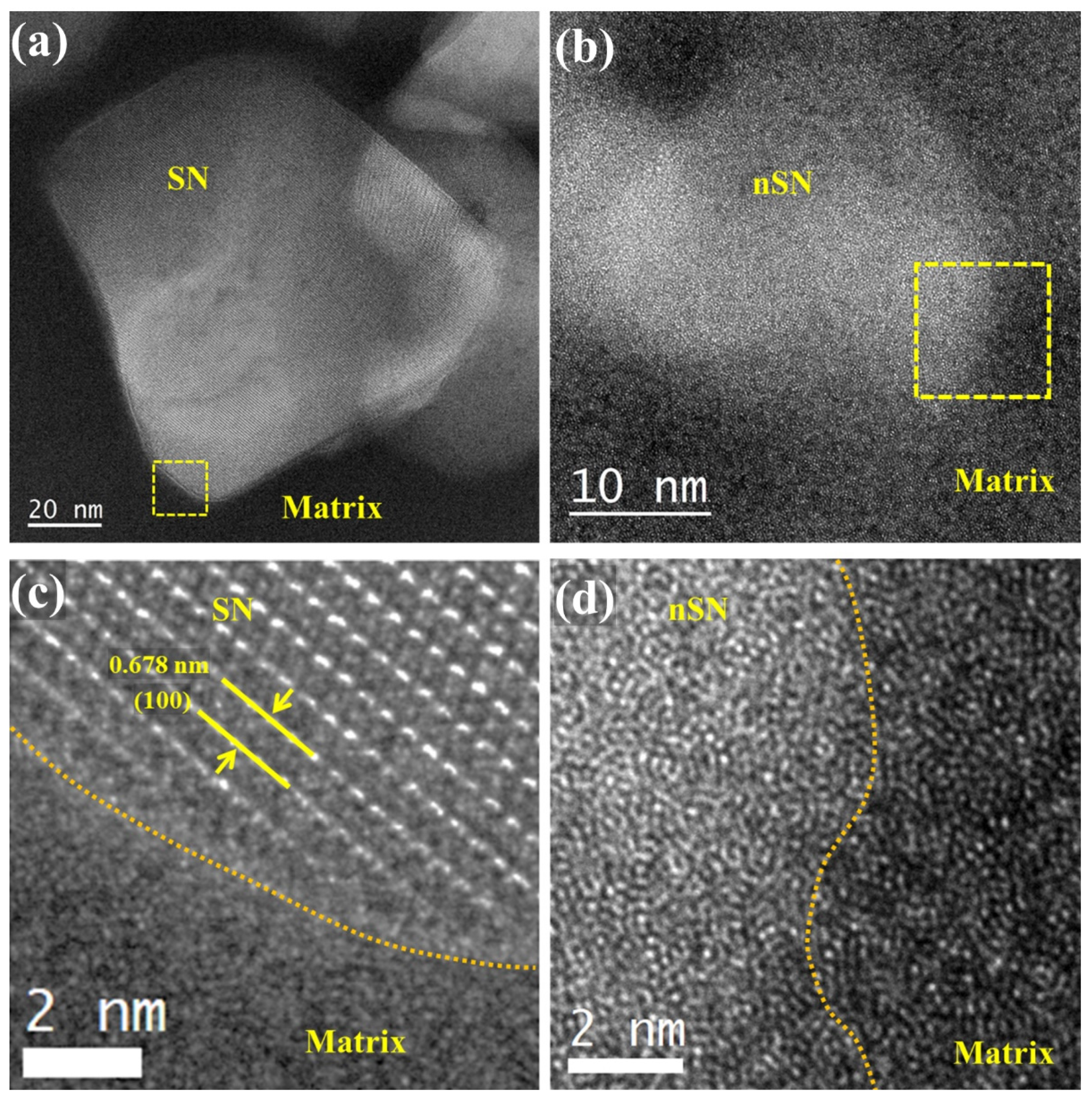
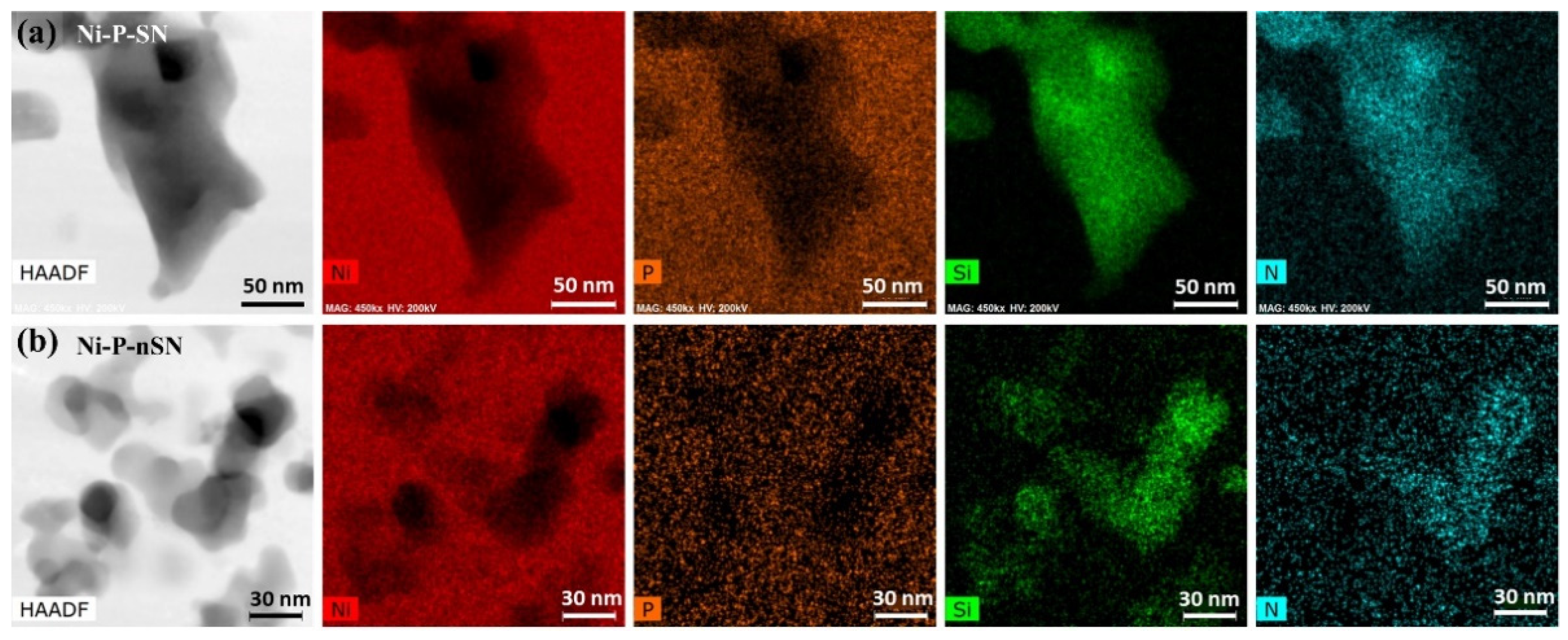
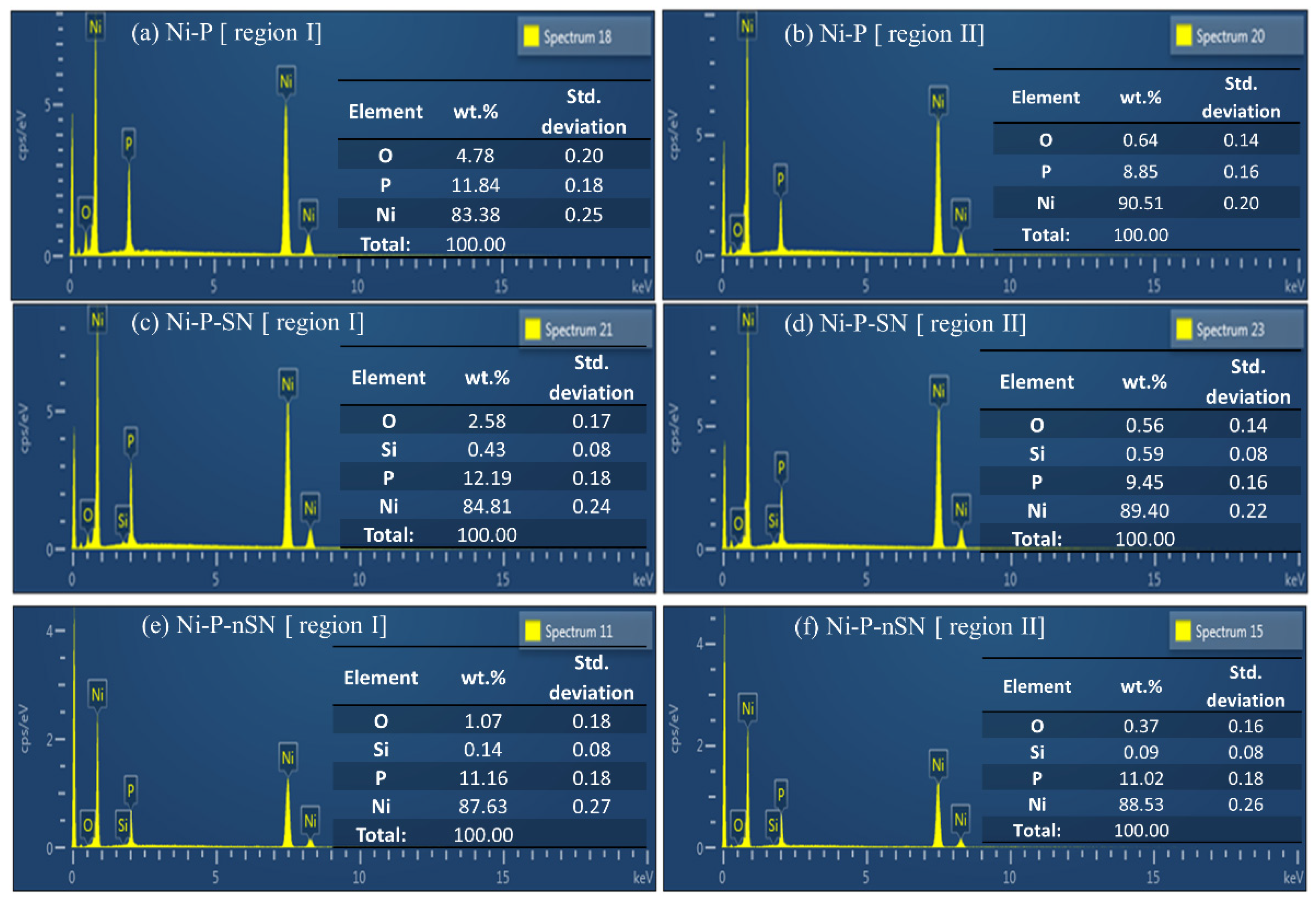
3.2.2. TEM, HRTEM, and EDS Analysis
3.3. Corrosion Behavior Evaluation
3.3.1. Tafel and Nyquist Curves
3.3.2. Corroded Surface Analysis
3.3.3. ICP-MS Measurement of the Corrosion Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spencer, L.F. Electroless Nickel Plating—A Review. Metal Finish. 1974, 72, 50–54. [Google Scholar]
- Loto, C.A. Electroless Nickel Plating—A Review. Silicon 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Reddy, V.V.N.; Ramamoorthy, B.; Nair, P.K. A Study on the Wear Resistance of Electroless Ni–P/Diamond Composite Coatings. Wear 2000, 239, 111–116. [Google Scholar] [CrossRef]
- Palaniappa, M.; Seshadri, S.K. Friction and Wear Behavior of Electroless Ni–P and Ni–W–P Alloy Coatings. Wear 2008, 265, 735–740. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Rafizadeh, S.H. Effect of Coating Time and Heat Treatment on Structures and Corrosion Characteristics of Electroless Ni-P Alloy Deposits. Surf. Coat. Technol. 2004, 176, 318–326. [Google Scholar] [CrossRef]
- Sun, C.; Li, J.; Shuang, S.; Zeng, H.; Luo, J.L. Effect of Defect on Corrosion Behavior of Electroless Ni-P Coating in CO2-Saturated NaCl Solution. Corros. Sci. 2018, 134, 23–37. [Google Scholar] [CrossRef]
- Cui, C.; Du, H.; Liu, H.; Xiong, T. Corrosion Behavior of the Electroless Ni-P Coating on the Pore Walls of the Lotus-Type Porous Copper. Corros. Sci. 2020, 162, 108202. [Google Scholar] [CrossRef]
- Das, C.M.; Limaye, P.K.; Grover, A.K.; Suri, A.K. Preparation and Characterization of Silicon Nitride Codeposited Electroless Nickel Composite Coatings. J. Alloy. Compd. 2007, 436, 328–334. [Google Scholar] [CrossRef]
- Singh, D.D.N.; Ghosh, R. Electroless Nickel-Phosphorus Coatings to Protect Steel Reinforcement Bars from Chloride Induced Corrosion. Surf. Coat. Technol. 2006, 201, 90–101. [Google Scholar] [CrossRef]
- Cheong, W.J.; Luan, B.L.; Shoesmith, D.W. The Effects of Stabilizers on the Bath Stability of Electroless Ni Deposition and the Deposit. Appl. Surf. Sci. 2004, 229, 282–300. [Google Scholar] [CrossRef]
- Gu, C.; Lian, J.; Li, G.; Niu, L.; Jiang, Z. High Corrosion-Resistant Ni-P/Ni/Ni-P Multilayer Coatings on Steel. Surf. Coat. Technol. 2005, 197, 61–67. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, R.; Liu, S.; Wu, D.; Zhang, Y.; Wang, T.; Zou, Y. Study on the Role of Element Mo in Improving Thermal Stability and Corrosion Resistance of Amorphous Ni-P Deposit. J. Non-Cryst. Solids 2020, 549, 120358. [Google Scholar] [CrossRef]
- Apachitei, I.; Duszczyk, J. Autocatalytic Nickel Coatings on Aluminium with Improved Abrasive Wear Resistance. Surf. Coat. Technol. 2000, 132, 89–98. [Google Scholar] [CrossRef]
- Yan, M.; Ying, H.G.; Ma, T.Y. Improved Microhardness and Wear Resistance of the As-Deposited Electroless Ni-P Coating. Surf. Coat. Technol. 2008, 202, 5909–5913. [Google Scholar] [CrossRef]
- Pezzato, L.; Lorenzetti, L.; Tonelli, L.; Bragaggia, G.; Dabalà, M.; Martini, C.; Brunelli, K. Effect of SiC and Borosilicate Glass Particles on the Corrosion and Tribological Behavior of AZ91D Magnesium Alloy after PEO Process. Surf. Coat. Technol. 2021, 428, 127901. [Google Scholar] [CrossRef]
- Agarwala, R.C.; Agarwala, V. Electroless Alloy/Composite Coatings: A Review. Sadhana 2003, 28, 475–493. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K. Development of Electroless Ni-Zn-P/Nano-TiO2 Composite Coatings and Their Properties. Appl. Surf. Sci. 2010, 256, 7377–7383. [Google Scholar] [CrossRef]
- Abdel Aal, A.; Hassan, H.B.; Abdel Rahim, M.A. Nanostructured Ni-P-TiO2 Composite Coatings for Electrocatalytic Oxidation of Small Organic Molecules. J. Electroanal. Chem. 2008, 619–620, 17–25. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Kshetri, Y.K.; Gyawali, G.; Kim, T.-H.; Choi, J.-H.; Lee, S.W. Understanding the Effect of Si3N4 Nanoparticles on Wear Resistance Behavior of Electroless Nickel-Phosphorus Coating through Structural Investigation. Appl. Surf. Sci. 2021, 541, 148403. [Google Scholar] [CrossRef]
- Ramesh, C.S.; Keshavamurthy, R.; Channabasappa, B.H.; Ahmed, A. Microstructure and Mechanical Properties of Ni-P Coated Si3N4 Reinforced Al6061 Composites. Mater. Sci. Eng. A 2009, 502, 99–106. [Google Scholar] [CrossRef]
- Ramesh, C.S.; Keshavamurthy, R.; Channabasappa, B.H.; Pramod, S. Friction and Wear Behavior of Ni-P Coated Si3N4 Reinforced Al6061 Composites. Tribol. Int. 2010, 43, 623–634. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Gong, M.; Huang, W. Microstructure and Mechanical Properties of Ni-P-Si3N4 Nanowire Electroless Composite Coatings. Appl. Surf. Sci. 2015, 357, 328–332. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Ezhil Selvi, V.; Rajam, K.S. Electrochemical Behavior of Low Phosphorus Electroless Ni-P-Si3N4 Composite Coatings. Mater. Chem. Phys. 2010, 120, 546–551. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Selvi, V.E.; Rajam, K.S. Electrochemical Behaviour of High Phosphorus Electroless Ni-P-Si3N4 Composite Coatings. Trans. Inst. Met. Finish. 2010, 88, 311–316. [Google Scholar] [CrossRef]
- Sarret, M.; Müller, C.; Amell, A. Electroless NiP Micro- and Nano-Composite Coatings. Surf. Coat. Technol. 2006, 201, 389–395. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Gyawali, G.; Kshetri, Y.K.; Choi, J.-H.; Lee, S.W. Microstructural and Electrochemical Corrosion Properties of Electroless Ni-P-TaC Composite Coating. Surf. Coat. Technol. 2020, 381, 125135. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Y.; Qin, W.; Li, Y. Microstructure and Corrosion Behavior of Electroless Ni-P on Sprayed Al-Ce Coating of 3003 Aluminum Alloy. Surf. Coat. Technol. 2015, 281, 176–183. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Crystallisation Kinetics and Phase Transformation Behaviour of Electroless Nickel–Phosphorus Deposits with High Phosphorus Content. J. Alloy. Compd. 2002, 334, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Apachitei, I.; Tichelaar, F.D.; Duszczyk, J.; Katgerman, L. Solid-State Reactions in Low-Phosphorus Autocatalytic NiP-SiC Coatings. Surf. Coat. Technol. 2001, 148, 284–295. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Roostaei, M.; Mahmoudi, M.; Fattahpour, V.; Zeng, H.; Luo, J.L. Role of Ca2+ in the CO2 Corrosion Behavior and Film Characteristics of N80 Steel and Electroless Ni–P Coating at High Temperature and High Pressure. Mater. Chem. Phys. 2021, 267, 124618. [Google Scholar] [CrossRef]
- Ogle, K. Atomic Emission Spectroelectrochemistry: Real-Time Rate Measurements of Dissolution, Corrosion, and Passivation. Corrosion 2019, 75, 1398–1419. [Google Scholar] [CrossRef] [Green Version]
- Pletcher, D.; Walsh, F.C. Industrial Electrochemistry; Springer: Berlin/Heidelberg, Germany, 1993; ISBN 978-0-7514-0148-6. [Google Scholar]
- Li, J.; Sun, C.; Roostaei, M.; Mahmoudi, M.; Fattahpour, V.; Zeng, H.; Luo, J.-L. Insights into the Electrochemical Corrosion Behavior and Mechanism of Electroless Ni-P Coating in the CO2/H2S/Cl− Environment. Corrosion 2020, 76, 578–590. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, H.J.; Hao, L.; Lin, A.; Gan, F.X. Electrochemical Corrosion Behavior of Electroless Ni-P Coating in NaCl and H2SO4 Solutions. Mater. Corros. 2011, 62, 1003–1007. [Google Scholar] [CrossRef]
- Soares, H.J.M.; Campos, O.S.; Dias, D.F.; Casciano, P.N.S.; de Lima-Neto, P.; Correia, A.N. Chemical, Morphological and Corrosion Characterisations of Electrodeposited Ni-Fe-P Coatings. Electrochim. Acta 2018, 284, 18–23. [Google Scholar] [CrossRef]
- Jie, X. Effect of CeO2 on Electrochemical Corrosion Resistance of Ni–P–CeO2 Composite Coatings Prepared by Submerged Jet Electrodeposition. Int. J. Electrochem. Sci. 2020, 15, 7559–7574. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A. Effects of Different Parameters on Initiation and Propagation of Stress Corrosion Cracks in Pipeline Steels: A Review. Metals 2019, 9, 590. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, W.; Wang, Y.; Mitsuzak, N.; Chen, Z. Ductile Electroless Ni-P Coating onto Flexible Printed Circuit Board. Appl. Surf. Sci. 2016, 367, 528–532. [Google Scholar] [CrossRef]
- Buchtík, M.; Kosár, P.; Wasserbauer, J.; Tkacz, J.; Doležal, P. Characterization of Electroless Ni–P Coating Prepared on a Wrought ZE10 Magnesium Alloy. Coatings 2018, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Chinchu, K.S.; Riyas, A.H.; Ameen Sha, M.; Geethanjali, C.V.; Saji, V.S.; Shibli, S.M.A. ZrO2–CeO2 Assimilated Electroless Ni–P Anti-Corrosion Coatings. Surf. Interfaces 2020, 21, 100704. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Sankara Narayanan, T.S.N.; Seshadri, S.K. Electroless Ni-P Composite Coatings. J. Appl. Electrochem. 2003, 33, 807–816. [Google Scholar] [CrossRef]
- Khazrayie, M.A.; Aghdam, A.R.S. Si3N4/Ni Nanocomposite Formed by Electroplating: Effect of Average Size of Nanoparticulates. Trans. Nonferrous Met. Soc. China 2010, 20, 1017–1023. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Ashrafi, A. Characterisations of Low Phosphorus Electroless Ni and Composite Electroless Ni-P-SiC Coatings on A356 Aluminium Alloy. Trans. IMF 2018, 96, 52–56. [Google Scholar] [CrossRef]
- Afroukhteh, S.; Dehghanian, C.; Emamy, M. Preparation of the Ni-P Composite Coating Co-Deposited by Nano TiC Particles and Evaluation of It’s Corrosion Property. Appl. Surf. Sci. 2012, 258, 2597–2601. [Google Scholar] [CrossRef]
- Kucernak, A.R.J.; Naranammalpuram Sundaram, V.N. Nickel Phosphide: The Effect of Phosphorus Content on Hydrogen Evolution Activity and Corrosion Resistance in Acidic Medium. J. Mater. Chem. A 2014, 2, 17435–17445. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zeng, H.; Luo, J.-L. Probing the Corrosion Resistance of a Smart Electroless Ni-P Composite Coating Embedded with PH-Responsive Corrosion Inhibitor-Loaded Nanocapsules. Chem. Eng. J. 2021, 421, 127752. [Google Scholar] [CrossRef]

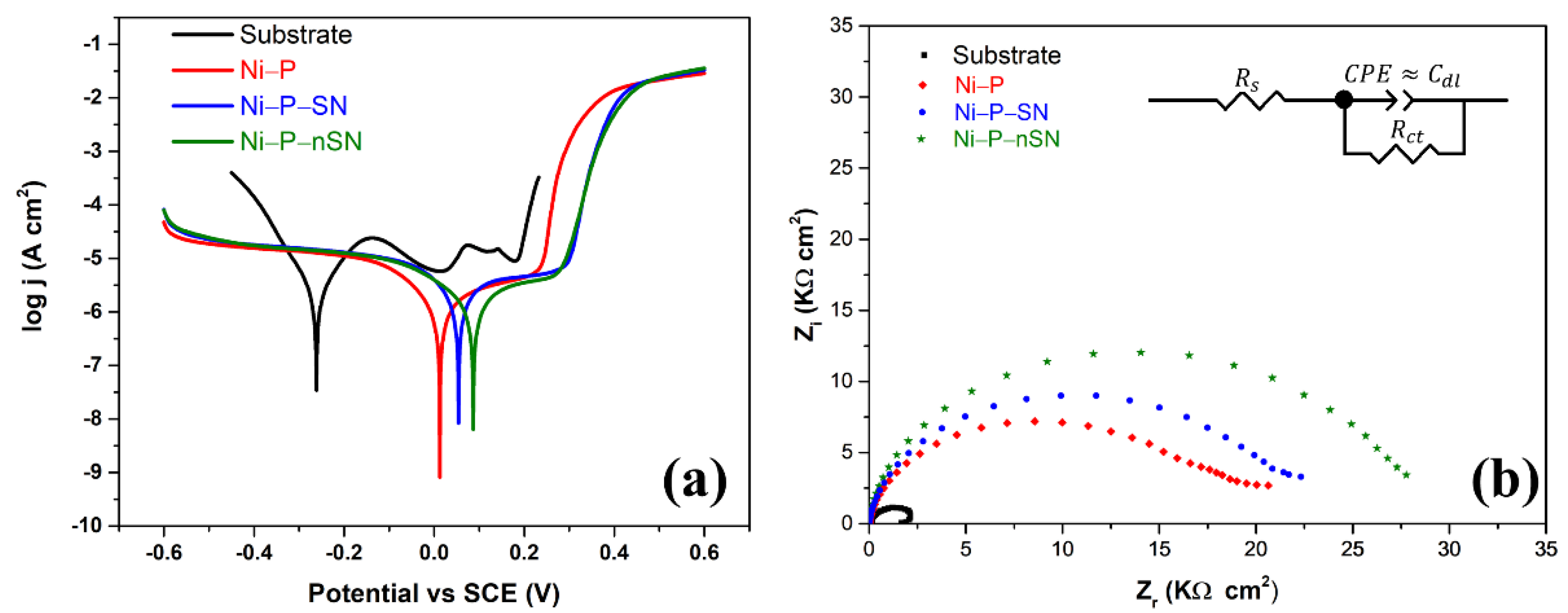
| Specimens | Ecorr (V) | jcorr (Acm−2) | Rp (kΩ) | Rs (Ωcm−2) | Rct (kΩcm−2) | Cdl (µFcm−2) | CR (mmy−1) |
|---|---|---|---|---|---|---|---|
| Steel Substrate | −0.260 | 2.61 × 10−6 | 7.3 | 15.02 | 2.5 | 52.05 | 0.053 |
| Ni-P | 0.024 | 1.19 × 10−6 | 29.4 | 15.51 | 18.26 | 39.31 | 0.014 |
| Ni-P-SN | 0.056 | 7.10 × 10−7 | 32.9 | 17.40 | 21.17 | 19.39 | 0.010 |
| Ni-P-nSN | 0.095 | 9.94 × 10−7 | 33.4 | 15.25 | 27.06 | 14.10 | 0.007 |
| Samples | Ni (ppm) | P (ppm) | Fe (ppm) | Si (ppm) |
|---|---|---|---|---|
| Ni-P | 385 | 2.6 | 0.2 | - |
| Ni-P-SN | 7.6 | 1.1 | <0.1 | 1.1 |
| Ni-P-nSN | 1.1 | <0.1 | <0.1 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, D.R.; Kshetri, Y.K.; Chaudhary, B.; Kim, T.-H.; Lee, S.W.; Kim, B.S.; Song, Y.; Kim, H.S.; Kim, H.H. Particle-Size-Dependent Anticorrosion Performance of the Si3N4-Nanoparticle-Incorporated Electroless Ni-P Coating. Coatings 2022, 12, 9. https://doi.org/10.3390/coatings12010009
Dhakal DR, Kshetri YK, Chaudhary B, Kim T-H, Lee SW, Kim BS, Song Y, Kim HS, Kim HH. Particle-Size-Dependent Anticorrosion Performance of the Si3N4-Nanoparticle-Incorporated Electroless Ni-P Coating. Coatings. 2022; 12(1):9. https://doi.org/10.3390/coatings12010009
Chicago/Turabian StyleDhakal, Dhani Ram, Yuwaraj K. Kshetri, Bina Chaudhary, Tae-Ho Kim, Soo Wohn Lee, Bum Sung Kim, Yoseb Song, Hak Soo Kim, and Hak Hee Kim. 2022. "Particle-Size-Dependent Anticorrosion Performance of the Si3N4-Nanoparticle-Incorporated Electroless Ni-P Coating" Coatings 12, no. 1: 9. https://doi.org/10.3390/coatings12010009
APA StyleDhakal, D. R., Kshetri, Y. K., Chaudhary, B., Kim, T.-H., Lee, S. W., Kim, B. S., Song, Y., Kim, H. S., & Kim, H. H. (2022). Particle-Size-Dependent Anticorrosion Performance of the Si3N4-Nanoparticle-Incorporated Electroless Ni-P Coating. Coatings, 12(1), 9. https://doi.org/10.3390/coatings12010009








