Abstract
Nanostructured noble metal-semiconductor materials have been attracting increasing attention because of their broad application in the field of environmental remediation, sensing and photocatalysis. In this study, a facile approach for fabricating silver@mesoporousanataseTiO2 (Ag@mTiO2) core-shell nanoparticles employing sol-gel and hydrothermal reaction is demonstrated. The Ag@mTiO2nanoparticles display excellent surface-enhanced Raman scattering (SERS) sensitivity and they can detect the methylene blue (MB) molecules with the concentration of as low as 10−8 M. They also exhibit outstanding photocatalytic activity compared with mTiO2, due to the efficient separation and recombination restrain of electron–hole pairs under ultraviolet light. The Ag@mTiO2nanoparticles also present good stability and they can achieve recyclable photocatalytic degradation experiments for five times without loss of activity. Subsequently, the nanoparticles with dual functions were successfully used to in situ monitor the photodegradation process of MB aqueous solution. These results, demonstrating the multifunctional Ag@mTiO2 nanoparticles, hold promising applications for simultaneous SERS analysis and the removal of dye pollutants in environmental field.
1. Introduction
With the expansion of the manufacturing industry, there is an increase in the total amount of organic pollutants. In particular, organic dye pollutants are highly soluble in water exhausted by printing sewage, paper mills and leather manufacture [1,2]. In addition, various pathogens, such as bacteria, virus, and microbes in dye wastewater may react with the organics, which will lead to more harmful contaminations and even produce toxicity. Their accumulation in the environment has caused substantial damage to the aquatic ecosystem and poses a threat to human safety, as they may lead to chronic or immediate illness [3]. For the purpose of treating dye contaminations in wastewater, their accurate detection [4,5,6] and degradation are one of the expressed problems to be solved. Different kinds of physical, chemical and biochemical measures have been devoted to deal with these problems, including membrane separation [7], advanced oxidation [8], photocatalytic degradation [9,10] and anaerobic treatment [11]. Among them, photocatalysis technique employed in wastewater treatment has great promise, because it can create strong oxidation agents to break down organic matters into carbon dioxide and water. In addition, this simple process has no need for sophisticated equipment or expensive cost. It is observed that TiO2, as a semiconductor, has received a great deal of attention, owing to its excellent photocatalytic activity, good chemical inertia, thermal stability and non-toxicity, as well as biocompatibility and low cost [12,13]. Nevertheless, as a single component, TiO2 has a low capacity to exploit solar energy due to its intrinsically wide bandgap, which is 3.2 eV [14,15]. In order to advance photocatalyst applications and meet more functional requirements, modifications of TiO2 forming a heterostructure are being investigated. Many research groups demonstrated the beneficial effects of the integration of noble metals in TiO2-based photocatalysts, such as Au, Ag, Pt and Pd [16,17,18,19,20,21]. Cheng et al. [22] fabricated 3D Ag/ZnO bifunctional composite, acting as a SERS-active substrate and photocatalyst, to detect and degrade organic dye pollutant. Wang et al. [23] prepared Ag/GO/TiO2nanorod arrays that displayed excellent photocatalytic properties and SERS sensitivities. Their SERS enhancement factor was determined to be as high as 5.86 × 105 and was employed to degrade rhodamine 6G under solar light illumination.
In addition, the morphology of nanomaterials is also an important factor to promote photocatalytic property [24,25,26]. Yolk-shell structured Fe3O4@TiO2 as a Photo-Fenton-like catalyst, which contains the Fe3O4 core inside a void space surrounded by a TiO2 shell, has been developed for the highly efficient elimination of tetracycline, because of its high and specific surface area and abundant active reaction sites [27]. Du et al. [28] prepared Au@TiO2 hollow submicrospheres with controllable size and shell thickness; their photocatalytic activity increased by 30% compared with that of P25. Zhao et al. [29] synthesized sea urchin-like Fe3O4@TiO2@Ag nanocomposites with tunable cavity sizes. The hybrid via localized surface plasmon resonance (LSPR) absorption of noble metals enhanced the bandgap absorption of TiO2; the special structure also provided many active sites for redox reactions during the photocatalysis. More importantly, mesoporous TiO2 has larger specific surface area and relatively large pore volume compared with the amorphous structure, which can ensure large enrichment capability and high mass transport efficiency [30]. However, there are few reports on the photocatalytic performance of the mesoporousTiO2 shell-coated Ag(Ag@mTiO2), which may also provide a great quantity of active sites as well as “hot spots” for different usages.
In this report, the Ag@mTiO2core-shell nanostructures are fabricated; they are composed of an Ag core and a mesoporous TiO2 shell. The TiO2 shell is capable of protecting Ag from corrosion and the mesoporous morphology may adsorb many target molecules, while also being beneficial for the Ag core to exhibit its local surface plasmon resonance effect. The nanocomposites served not only as SERS substrates, but also as reusable photocatalysts, and were successfully applied to the in situ SERS monitor photocatalytic degradation process of organic dyes.
2. Experiment
2.1. Chemicals
Acetonitrile (C2H6N, AR), trisodium citrate (Na3C6H5O7·2H2O, AR), silvernitrate (AgNO3, 99.0%), methyl orange (C14H14N3NaO3S), ethanol (EtOH, 99.7%) and ammonia (NH3·H2O, 28%)were obtained from Sinopharm Chemical Reagent Co. Ltd. Shanghai, China. Titaniumbutoxide (TBOT, 98%), methylene blue (C16H18ClN3S·3H2O) and rhodamine 6G (C28H31N2O3Cl, 95%) were bought from Aladdin Reagent Co. Ltd. Shanghai, China. All chemicals were applied without any purification. Deionized water purified by a Millipore-Q System (18.2 MΩ/cm) was used in all experiments.
2.2. Preparation of Ag Nanoparticles
Ag nanoparticles were prepared using a similar approach to the previous report [21]. A total of 90 mg of solid AgNO3were charged into 250 mL of deionized water and heated to a boil. Afterwards, 10 mL of trisodium citrate (1 wt%) aqueous solution were introduced into the above solution and boiled at 100 °C for an hour. Then, the reaction solution was cooled to room temperature by an ice bath. The resultant Ag nanoparticles were obtained by centrifuging at 8000 rpm for 10 min. Finally, the prepared Ag nanoparticles were dispersed in 10 mL of deionized water to obtain the concentration of 0.5 mg/mL for the following experiments.
2.3. Synthesis of Ag@TiO2 Core-Shell Nanoparticles
The Ag@TiO2 nanoparticles were synthesized according to the following method. In a typical experiment, 2 mL of the prepared Ag dispersion solution, 35 mL of acetonitrile, 105 mL of ethanol and 0.5 mL of ammonia were introduced into a 250 mL glass beaker. The mixture was magnetically stirred for 20 min. After that, 0.5 mL of TBOT was dissolved in 15 mL of ethanol and 5 mL of acetonitrile, and then the mixed solution was slowly added into the above reaction solution. The reaction was allowed to proceed for 1.5 h at room temperature under continuous magnetic stirring. The resultant products were collected and washed 3 times using ethanol by centrifuging at 6000 rpm, and finally dispersed in 5 mL of ethanol for further use.
2.4. Preparation of Ag@mTiO2 Nanoparticles
Ag@TiO2 nanoparticles were subjected to a simple hydrothermal process to obtain Ag@mTiO2 nanoparticles. In a typical procedure, 5 mL of the prepared Ag@TiO2nanoparticleswere mixed with 10 mL of deionized water and 20 mL of ethanol. After ultrasonication for 5 min, the mixture was sealed into a Teflon-lined stainless-steel autoclave (100 mL capacity). The autoclave was heated at 160 °C for 8 h. Then, it was allowed to naturally cool to room temperature. The resulting products were collected and washed using ethanol by centrifuging for several times. Finally, the prepared Ag@mTiO2nanoparticles were dispersed in 5 mL of deionized water for further examinations.
2.5. Photocatalytic Property of Ag@mTiO2 Nanoparticles
The photocatalytic activity of the nanoparticles was investigated by the degradation of methylene blue aqueous solution at ambient conditions under ultraviolet lamp light. A commercial Hg lamp (350 W, CEAULIGHT, Beijing, China) with λ ≈ 254 nm (light intensity of 12 mW/cm2) was used to supply this light. Specifically, Ag@mTiO2 nanoparticles and 40 mL of 10−5 M MB aqueous solution were added into a 50 mL quartz vessel, and then magnetically stirred in a dark condition for 60 min to reach the adsorption and desorption equilibrium of the MB with the nanoparticles. Afterwards, the solution was exposed to UV radiation with a Hg lamp located 10 cm away from the solution surface to initiate the degradation reaction. During the photodecomposition experiment, 3 mL of the reaction solution for different time intervals was taken out from the reaction suspension and centrifuged to separate the catalyst. The supernatant was analyzed with a UV-vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) to monitor the residual concentration of MB. Degradation ratio of MB was calculated through the intensities of absorbance at 664 nm according to the following equation:
where C0 represents the initial concentration of MB, Ct is the concentration of MB at time t. Control experiments of different catalysts and various concentrations of MB were carried out using the same steps and conditions in order to eliminate the influence of different parameters on the photocatalytic experiments.
Degradation ratio (%) = [(C0 − Ct)/C0] × 100%
2.6. SERS Property of Ag@mTiO2 Nanoparticles
MB is used as a model Raman molecule to assess the SERS activity of Ag@mTiO2 nanoparticles. Specifically, 0.5 mL of Ag@mTiO2 dispersion was mixed with 3 mL of MB aqueous solution at different concentrations and then the mixed solution was kept in the dark for 24 h to achieve adsorption equilibrium. The MB functionalized nanoparticles were collected by centrifuging, and then they were washed twice with deionized water. Afterwards, the MB functionalized composites were suspended in 0.5 mL of deionized water and put into a quartz cuvette with a 1 cm path length for SERS detection.
2.7. In Situ SERS Monitoring of Photocatalytic Reactions by Ag@mTiO2 Nanoparticles
Photocatalytic experiments monitored by in situ SERS were conducted in a liquid system at room temperature and the detailed procedure was described as follows. Firstly, Ag@mTiO2nanoparticlesand 20 mL of 10−5 M MB aqueous solution were added into a 50 mL centrifuge tube, and kept in a dark condition for 24 h to attain the adsorption equilibrium of the MB with the nanoparticles. Next, the MB functionalized nanoparticles were collected by centrifuging and washed twice with deionized water. Afterwards, the MB functionalized nanoparticles were suspended in 10 mL of deionized water; then, the solution was transferred to a quartz vessel of 20 mL and exposed to the ultraviolet irradiation. A total of 2 mL of reaction solution was taken out every 3 min and placed in a quartz cuvette with a 1 cm path length for SERS analysis to monitor the progress of the reaction.
2.8. Characterizations
The ultraviolet-visible (UV-vis) spectra were collected using a Cary 60 spectrometer (Agilent Technologies, Santa Clara, CA, USA). X-ray diffraction (XRD) patterns were carried out on a Bruker AXS D8 Advance diffractometer at a scanning rate of 0.02 (Cu-Kα radiation, λ = 1.54056 Å, Bruker, Nasdaq, MA, USA). The 2θ angles are in a range of 20° to 80°. Surface morphology and size analysis of samples were obtained with a transmission electron microscopy (TEM, JEM-2100, JEOL, Tokyo, Japan) at an accelerating voltage of 200 kV and field-emission scanning electron microscopy (FESEM, ZEISS, Oberkochen, German). The specific surface area was determined using N2 adsorption-desorption isotherms at 77 K on the basis of Brunauer–Emmett–Teller (BET, ASAP 2020, Quantachrome, FL, USA). Energy dispersive X-ray (EDX, ZEISS, Oberkochen, German) analysis was recorded on a Gemini-SEM 300 microscope. Photoluminescence spectroscopy (PL, Edinburgh Instruments, Edinburgh, England) was employed to explore the charge–carrier generation, transfer and separation efficiency of Ag@mTiO2 samples by using Edinburgh FLS1000. Raman spectra were recorded through a portable Raman spectrometer (Ocean Optics, London, England) with a red laser (785 nm) and the laser power was 392 mW. The acquisition time was 30 s for one time of scanning. The photocatalytic experiments were carried out in the CEL-LB70 photochemical reaction box (CEAULIGHT, Beijing, China).
3. Results and Discussion
3.1. Synthesis of Ag@mTiO2 Nanoparticles
The Ag@mTiO2 nanoparticle consists of an Ag nanoparticle as a core and a mesoporous TiO2 shell, and the fabrication process is depicted in Scheme 1. The Ag nanoparticles were prepared by a wet chemistry approach and their TEM image in Figure 1a demonstrates that they are homogeneous spheres with an average size of 70 nm. Afterwards, the Ag@TiO2 core-shell nanoparticles were synthesized via the sol-gel method using TBOT as the Ti source to form TiO2 shells encapsulating around Ag nanoparticles [31]. As can be observed in the TEM image (Figure 1b), the differentiation of the TiO2 shell from the Ag nanoparticle in a Ag@TiO2 core-shell nanoparticle is obvious, owing to the higher electron density of Ag relative to TiO2 and allowing for fewer electrons to penetrate. The TEM image of Ag@TiO2 presents a distinct contrast between the lighter outer shell and the dark inner core, confirming that the TiO2 was successfully encapsulated over the Ag nanoparticles; the shell thickness is about 20 nm. Figure 1c shows the representative TEM image of Ag@mTiO2 obtained by the hydrothermal treatment of Ag@TiO2 nanoparticles. It can be observed that the compact TiO2 shell changed into porous morphology, suggesting that the Ag@mTiO2 nanoparticles were obtained, which offers a large surface area beneficial for photocatalysis. We used UV-vis spectroscopy to characterize the optical property of Ag, Ag@TiO2 and Ag@mTiO2 nanoparticles. As shown in Figure 1d, the Ag nanoparticles display a narrow LSPR peak positioned at 408 nm. The LSPR peak of the Ag@TiO2 nanoparticles is shifted to a higher wavelength (452 nm), together with a broadening in peak width, resulting from the increase of the refractive index around the Ag nanoparticles [32]. Upon hydrothermal reaction, the LSPR peak of the Ag@mTiO2 nanoparticles is further shifted from 452 to 504 nm, indicating the formation of mesoporous TiO2 shell. It is worth mentioning that the strong LSPR intensities of the Ag@TiO2 and Ag@mTiO2 nanoparticles greatly verifies that the Ag nanoparticles are kept in good condition during the coating of TiO2 layer and hydrothermal reaction. The high-resolution TEM (HRTEM) image in the Figure 1eexhibits clear lattice fringes with spacings of 0.204 and 0.235 nm corresponding to the (200) and (111) planes of Ag, as well as the spacing of 0.352 nm corresponding to the (101) plane of anatase TiO2.
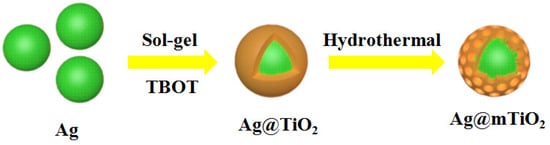
Scheme 1.
The formation of Ag@mTiO2 core-shell nanoparticles.
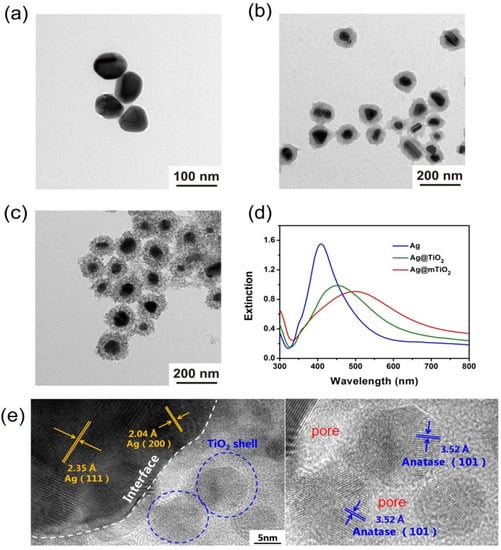
Figure 1.
Typical TEM images of (a) Ag, (b) Ag@TiO2 and (c) Ag@mTiO2 nanoparticles. (d) UV-vis spectra of Ag (blue line), Ag@TiO2 (green line) and Ag@mTiO2 (red line). (e) The HRTEM image of an Ag@mTiO2 nanoparticle.
Energy dispersive X-ray (EDX) was employed to identify the composition of the Ag@mTiO2 nanoparticles. As shown in Figure 2a, the spectrum clearly displays strong peaks of the elements Ag, Ti and O, confirming the successful coating of the TiO2layer onto the Ag nanoparticles. Of note, the Si element detected in the sample originates from silicon wafer.
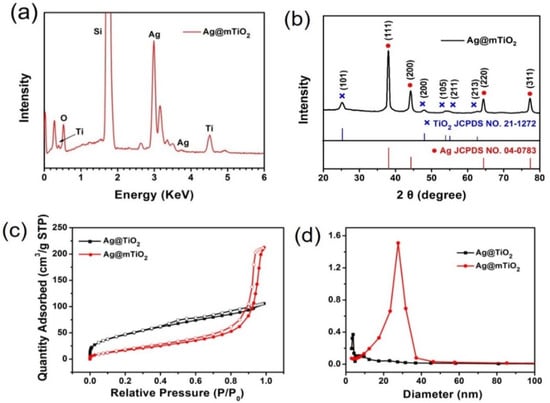
Figure 2.
(a) EDX spectrum and (b) XRD pattern of Ag@mTiO2 nanoparticles. (c) Nitrogen adsorption–desorption isotherms and (d) the Barrett–Joyner–Halenda (BJH) pore diameter distribution curves of Ag@TiO2 and Ag@mTiO2.
The crystalline nature of Ag@mTiO2 nanoparticles was further characterized by XRD. The XRD profile in Figure 2b illustrates strong diffraction peaks with 2θ values of 38.1°, 44.3°,64.4°, and, 77.3°, which can be indexed to the (111), (200), (220), and, (311) crystalline planes of faced and centered cubic Ag (JCPDS card No. 04-0783), respectively, verifying the presence of Ag nanoparticles. We also observe the peaks located at 25.3°,48.09°, and, 53.9°, which correspond to the (101), (200), and, (105) planes of anatase TiO2 (JCPDS card No. 21-1272), indicating the formation of anatase TiO2 [33]. Figure 2c,d exhibits the N2 adsorption–desorption isotherms and pore diameter distribution curves of Ag@TiO2 and Ag@mTiO2. It is clear that the surface area of the Ag@TiO2 nanoparticles is 80.7 m2/g and the average diameter is 3.8 nm, while after the hydrothermal reaction, the obtained Ag@mTiO2 nanoparticles present a large surface area of 84 m2/g; the average pore diameter is 27.7 nm, suggesting a mesoporous structure. Importantly, the large surface area and characteristic pore properties of Ag@mTiO2nanoparticles make them advantageous for promoting adsorption performance.
3.2. Photocatalytic Property of Ag@mTiO2 Nanoparticles
3.2.1. Comparison of Photocatalytic Property of Various Nanoparticles
The photocatalytic degradation of MB under UV light was investigated using Ag, mTiO2 (the preparation method is described in Supporting Information, and FESEM images are shown in Figures S1 and S2) and Ag@mTiO2 nanoparticles. The results in Figure 3a display the Ag@mTiO2nanoparticles that exhibit the highest degradation ratio compared with pure mTiO2 and Ag nanoparticles, which are 91%, 88% and 60% in 21 min, respectively. This result demonstrating the Ag nanoparticles is significantly helpful for enhancing the photo catalytic activity of mTiO2. In order to acquire further insight about the enhancement mechanism of Ag nanoparticles, the photoluminescence (PL) spectra of mTiO2 and Ag@mTiO2 with an excitation wavelength of 225 nm were obtained (Figure 3b). PL spectroscopy can clearly demonstrate the persistence of the charge recombination process and migration behavior of the electrons. As observed in Figure 3b, for Ag@mTiO2 nanoparticles, the PL emission intensity located at 397 nm is reduced, which demonstrates that the electron–hole recombination process is delayed [34,35]. Hence, the PL spectra indicate that the prepared Ag@mTiO2nanoparticles experience a delay of the electron–hole recombination process due to the presence of Ag nanoparticles. This is in good agreement with previous reports [16,23]. The Fermi level of Ag is lower than that of anatase TiO2; therefore, a Schottky barrier can form between the Ag and TiO2 interface, which could act as an efficient electron trap, thus preventing a photo-excited electron–hole recombination.
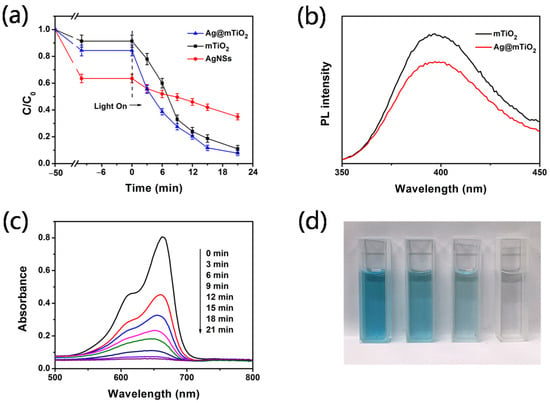
Figure 3.
(a) Photocatalytic degradation of MB by different catalysts. (b) PL spectra of mTiO2 and Ag@mTiO2nanoparticles (excitation wavelength is 225 nm). (c) UV-vis absorption spectra of MB solution for different times. (d) Photograph of MB solution at different times catalyzed by Ag@mTiO2 nanoparticles.
It is well known that the photocatalytic activity is primarily influenced by the effective separation and transportation of photogenerated electron–hole pairs. For Ag@mTiO2 nanoparticles, with the decrease of the recombination of electron–hole pairs, more electrons and holes will be involved in the photocatalytic reaction of MB. We proposed the photocatalytic mechanism of Ag@mTiO2 nanoparticles, as shown in Scheme 2. Figure 3c displays UV-vis spectra of MB with Ag@mTiO2 nanoparticles as photocatalysts; Figure 3d shows photographs of the MB aqueous during the photocatalytic reaction. It is obvious that the absorption intensities of MB at 664 nm gradually decline, and finally disappear in 21 min. This is in agreement with the photographs shown in Figure 3d, with the color of MB fading away during the reaction, reaching colorless after a 21 min illumination.
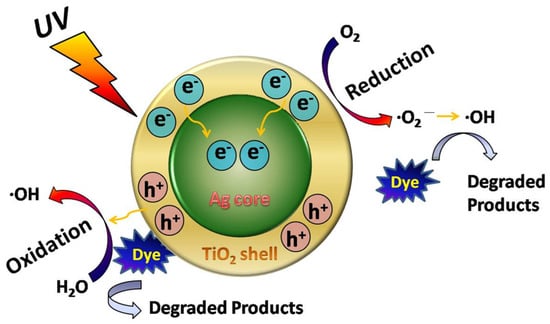
Scheme 2.
Proposed photocatalytic mechanism of Ag@mTiO2 nanoparticles.
3.2.2. Effect of Ag@mTiO2 Nanoparticle Dosage
The effect of the Ag@mTiO2 dosage on MB degradation is depicted in Figure 4. The experiments were carried out using a different mass of Ag@mTiO2 when fixing the initial volume and concentration of MB and the reaction time. It can be observed that with increasing the nanoparticle mass from 2 to 4 mg, the degradation ratio (calculated by Equation (1)) increases from 67.3% to 91% (Figure 4a). This is because more Ag@mTiO2 nanoparticles provide more active sites, resulting in higher photocatalytic activity. However, when the nanoparticle mass increases to 6 mg, the degradation ratio decreases to 84.2%. This result indicates more nanoparticles may block light scattering and transmittance, and thus decrease the photocatalytic behavior. In addition, the aggregation of the nanoparticles occurs at a high dosage, leading to a decrease in the number of active sites [33].
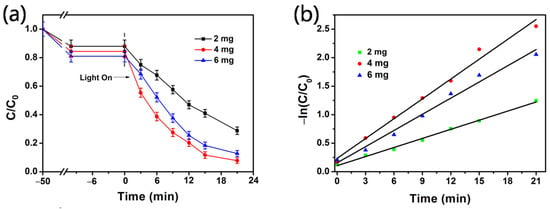
Figure 4.
(a) Effect of Ag@mTiO2dosage on photocatalytic degradation of MB. (b) Kinetic plots of −ln(C/C0) versus time.
To better elucidate the photocatalytic degradation of MB, the reaction kinetics are described in Figure 4b in following the kinetics equation:
where C0 refers to the initial concentration of MB, C represents the concentration of MB at reaction time t, and k is the degradation kinetic rate constant obtained from the slope of the linear plot. As observed in Figure 4b, the catalytic dose of 4 mg obviously exhibits the largest k value, which is about 2.18 folds and 1.23 folds higher than the values at 2 and 6 mg. All of the above results suggest that the catalytic dose of 4 mg presents the best photocatalytic activity.
−ln(C/C0) = kt
3.2.3. Effect of Initial Concentration
The effect of the initial MB concentration is investigated in a range of 2 × 10−5 to 5 × 10−6 M with 4 mg of Ag@mTiO2 nanoparticles. Figure 5a depicts the photodegradation of MB in different concentrations. It is clear that the degradation ratio(calculated by Equation (1)) of MB increases from 59.3% to 91%, when the MB concentrations drop from 2 × 10−5 to 1 × 10−5 M, because photocatalyst active sites are occupied by excess MB molecules, which lead to the deactivation of the active sites and reduces the generation of active species [36,37]. Additionally, the obstruction of light reaching the nanoparticles may occur resulting from the increased density of MB molecules [22]. When the MB concentrations further decreases to 8 × 10−6 and 5 × 10−6 M, the degradation ratios of MB, as expected, reduce to 83.8% and 85.5%, due to the lower MB concentration, resulting in a lower probability of reaction with the rapid generation of active species [38]. The degradation rates are exhibited straight in Figure 5b and the k values are 0.0425, 0.11593, 0.08772, and 0.09358 min−1, respectively, for 2 × 10−5 to 5 × 10−6 M.
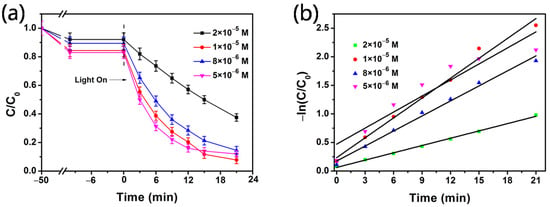
Figure 5.
(a) Effect of MB initial concentrations on photocatalytic degradation. (b) Kinetic plots of −ln(C/C0) versus time.
3.2.4. Recyclability of Ag@mTiO2 Nanoparticles
The photocatalytic stability is an important parameter of property evaluation for its practical application or commercial use; therefore, a recycle experiment was performed to evaluate the recyclable stability of Ag@mTiO2 nanoparticles under the same conditions for decomposing MB in 25 min. The UV-vis spectra of the XRD profiles of the Ag@mTiO2 nanoparticles after five cyclic photodegradation experiments were collected in Figure 6a,b, demonstrating no obvious change in the LSPR peak intensity and diffraction peaks of the Ag@mTiO2 nanoparticles after five cycles, compared with the original samples. The results suggest that Ag@mTiO2 nanoparticles have wonderful structural stability during the ultraviolet light illumination. Figure 6c displays the degradation efficiency of MB with five cycles. We can observe that the degradation efficiency of MB is still high, up to 94%, after five runs. Consequently, the prepared Ag@mTiO2 nanoparticles could act as reversible photocatalysts with high robustness.
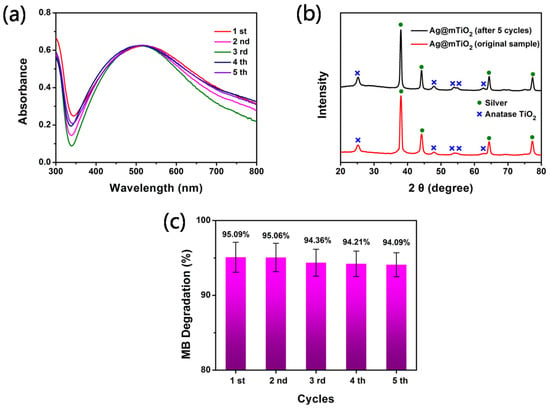
Figure 6.
(a) UV-vis spectra of Ag@mTiO2 dispersion in water after MB degradation reaction for 5 consecutive cycles. (b) XRD patterns of Ag@mTiO2 nanoparticles before and after 5 consecutive cycles. (c) The degradation ratio of MB with 5 consecutive cycles.
3.2.5. Photodegradation of Various Organic Dyes
To confirm the universal applicability of Ag@mTiO2 as photocatalysts, they were employed to photocatalytically decompose various organic dyes, for example, the methyl orange (MO) and rhodamine 6G (R6G) aqueous solutions (both of the initial concentrations of the organic dyes were 1 × 10−5 M) under UV irradiation. Figure 7a,b displays the UV-vis absorption spectra of dye solution after UV irradiation for different times and the relationships of the concentration of dye versus reaction time, using Ag@mTiO2 as a photocatalyst. The results show that MO and R6G are completely degraded in 15 and 36 min, respectively. Figure 7c,d depicts the linear relationship of −ln(C/C0) versus reaction time, indicating that all the photocatalytic reactions obey pseudo-first-order kinetics. The apparent first-order rate constants are 0.179 and 0.056 min−1, respectively, for R6G and MO.
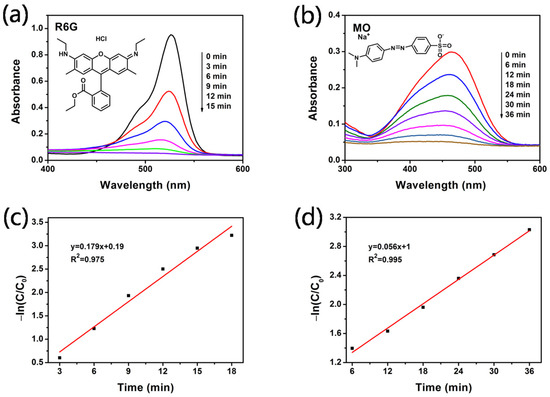
Figure 7.
UV-vis absorption spectra of (a) R6G and (b) MO solution under UV irradiation for different times. Kinetic plots of −ln(C/C0) versus time of (c) R6G and (d) MO.
3.3. SERS Property of Ag@mTiO2 Nanoparticles
To demonstrate the SERS sensitivity of Ag@mTiO2 nanoparticles, MB is chosen as a probe molecule in our experiment. Figure 8 reveals the SERS signals of MB, with different concentrations ranging from 10−5 to 10−8 M. All of them clearly exhibit two dominant Raman peaks of MB, positioned at 1396 and 1621 cm−1, which are assigned for C–N symmetrical stretching and C–C ring stretching [39], respectively. The detection limit of MB is as low as 10−8 M. The intensive SERS enhancement can be ascribed to the strong surface plasmon resonance effect resulting from Ag nanoparticles [40,41,42]. Moreover, the mesoporous TiO2 shell may also adsorb more MB molecules [30].
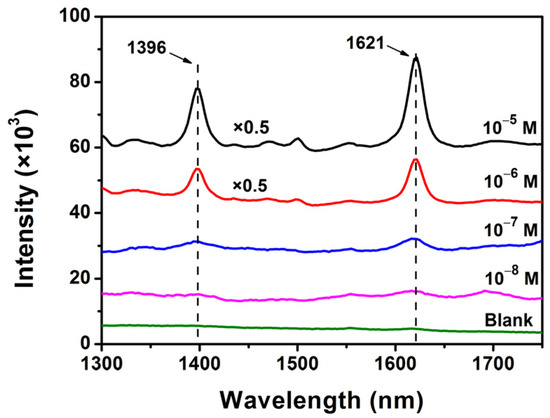
Figure 8.
SERS spectra of MB with different concentrations enhanced by Ag@mTiO2nanoparticles.
3.4. In situ SERS Monitoring Photodegradation of MB by Ag@mTiO2 Nanoparticles
In light of the excellent SERS and photocatalytic activity of Ag@mTiO2nanoparticles, they can serve as SERS active substrates to monitor the photodegradation process of MB under ultraviolet light illumination in real time [43,44,45,46]. Figure 9a shows the SERS signals of MB in the period of the degradation reaction. It is clear that the SERS characteristic peaks of MB at 1396 and 1621 cm−1 gradually reduce with the increasing irradiation time. After 18 min, the primary bands of MB almost disappeared without the appearance of new peaks because the degradation products are Raman inactive. To determine the kinetic parameters accurately, the peaks at 1396 and 1621 cm−1 were selected to fit the linear relationship of −ln(It/I0) versus the reaction time, as displayed in Figure 9b. The action rate constants are determined from the slope to be 0.127 and 0.12 min−1 with an R2 as high as 0.968 and 0.95, respectively.
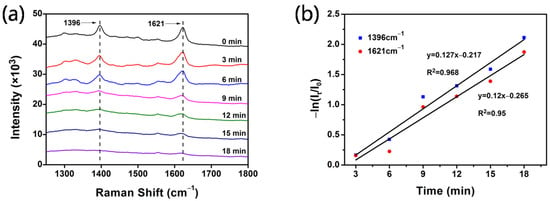
Figure 9.
(a) SERS signals of MB molecules during the photodegradation reaction by Ag@mTiO2nanoparticles. (b) The −ln (It/I0) against reaction time for SERS bands of MB at 1396 and 1621 cm−1.
4. Conclusions
In conclusion, we have successfully synthesized bifunctional Ag@mTiO2 core-shell nanoparticles combined with SERS and photocatalytic activity. They presented excellent LSPR properties resulting from the Ag cores and a large surface area due to the mesoporous TiO2shells. They were used as SERS substrates to detect MB molecules with a limit of detection of 10−8 M. In addition, the optical properties of Ag@mTiO2 nanoparticles indicated that electron–hole recombination can be effectively inhibited due to the presence of Ag. Their photocatalytic activity was demonstrated and the rate of the photodegradation of MB was superior to that of pure TiO2 nanoparticles. Furthermore, due to their excellent catalytic and SERS properties, Ag@mTiO2 nanoparticles, in situ, effectively monitored the SERS photodegradation process of MB. Therefore, the Ag@mTiO2 nanoparticles can be used as convenient SERS and photocatalytic materials in the detection and degradation of organic dye contaminants.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/coatings12010064/s1, Figure S1. FESEM image of TiO2 nanospheres (the size is about 90 nm), Figure S2. FESEM image of mTiO2 nanospheres (the size is about 90 nm).
Author Contributions
Funding acquisition and writing—original draft, Y.M.; funding acquisition, software, and writing—original draft, G.S.; software and writing—review and editing, L.Z.; formal analysis and writing—review and editing, X.W.; resources, P.L.; formal analysis, funding acquisition, and writing—original draft, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Natural Science Foundation of China (Grant Nos. 21976142, 51601080, 21903039) and the Innovative Team Program of Natural Science Foundation of Hubei Province (2021CFA032).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
There are no conflict to declare.
References
- Lin, X.; Rong, F.; Fu, D.; Yuan, C. Enhanced photocatalytic activity of fluorine doped TiO2 by loaded with Ag for degradation of organic pollutants. Powder Technol. 2012, 219, 173–178. [Google Scholar] [CrossRef]
- Chen, J.J.; Su, H.L.; You, X.L.; Gao, J.; Lau, W.M.; Zhang, D. 3D TiO2 submicrostructures decorated by silver nanoparticles as SERS substrate for organic pollutants detection and degradation. Mater. Res. Bull. 2014, 49, 560–565. [Google Scholar] [CrossRef]
- He, J.; Song, G.; Wang, X.Y.; Zhou, L.; Li, J.M. Multifunctional magnetic Fe3O4/GO/Ag composite microspheres for SERS detection and catalytic degradation of methylene blue and ciprofloxacin. J. Alloys Compd. 2022, 893, 162226. [Google Scholar] [CrossRef]
- Wu, Y.R.; Sun, X.J.; Yang, Y.; Li, J.M.; Zhang, Y.; Qin, D. Enriching silver nanocrystals with a second noble metal. Acc. Chem. Res. 2017, 50, 1774–1784. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, J.; Zhou, L.; Song, G.; Lu, F.; You, L.J.; Li, J.M. Rapid and ultrasensitive surface enhanced Raman scattering detection of hexavalent chromium using magnetic Fe3O4/ZrO2/Ag composite microsphere substrates. Colloids Surf. A 2021, 610, 125414. [Google Scholar] [CrossRef]
- Yang, J.; Song, G.; Zhou, L.; Wang, X.Y.; You, L.J.; Li, J.M. Highly sensitively detecting tetramethylthiuram disulfide based on synergistic contribution of metal and semiconductor in stable Ag/TiO2 core-shell SERS substrates. Appl. Surf. Sci. 2021, 539, 147744. [Google Scholar] [CrossRef]
- Juang, Y.J.; Nurhayati, E.; Huang, C.P.; Pan, J.R.; Huang, S.M. A hybrid electrochemical advanced oxidation/microfiltration system using BDD/Ti anode for acid yellow 36 dye wastewater treatment. Sep. Purif. Technol. 2013, 120, 289–295. [Google Scholar] [CrossRef]
- Thiam, A.; Zhou, M.; Brillas, E.; Sires, I. A first pre-pilot system for the combined treatment of dye pollutants by electrocoagulation/EAOPs. J. Chem. Technol. Biotechnol. 2014, 89, 1136–1144. [Google Scholar] [CrossRef]
- Segundo, I.R.; Freitas, E.; Landi, S.; Costa, M.E.M.; Carneiro, J.O. Smart, photocatalytic and self-cleaning asphalt mixtures: A literature review. Coatings 2019, 9, 696. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Y.; You, J.; Li, Q.W.; Dong, Z.H.; Zhong, Y.J.; Han, Y.L.; You, Y.H. Preparation and photocatalytic property of ag modified titanium dioxide exposed high energy crystal plane (001). Coatings 2020, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, S.; Venkatesh, K.; Quaff, A.R. Dye decomposition by combined ozonation and anaerobic treatment: Cost effective technology. J. Appl. Res. Technol. 2017, 15, 340–345. [Google Scholar] [CrossRef]
- McManamon, C.; Holmes, J.D.; Morris, M.A. Improved photocatalytic degradation rates of phenol achieved using novel porous ZrO2-doped TiO2 nanoparticulate powders. J. Hazard. Mater. 2011, 193, 120–127. [Google Scholar] [CrossRef]
- Ding, Q.Q.; Zhang, L.; Yang, L.B. A simple approach for the synthesis of Ag-coated Ni@TiO2 nanocomposites as recyclable photocatalysts and SERS substrate to monitor catalytic degradation of dye molecules. Mater. Res. Bull. 2014, 53, 205–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Li, Y.; Zhang, D.; Chen, Y. One-Step synthesis of Ag@TiO2 nanoparticles for enhanced photocatalytic performance. Nanomaterials 2018, 8, 1032. [Google Scholar] [CrossRef] [Green Version]
- Khalid, N.R.; Mazia, U.; Tahir, M.B.; Niaz, N.A.; Javid, M.A. Photocatalytic degradation of RhB from an aqueous solution using Ag3PO4/N-TiO2 heterostructure. J. Mol. Liq. 2020, 313, 113522. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhu, Y.H.; Yang, X.T.; Zhou, Y.; Yao, Y.F.; Li, C.Z. Multifunctional Fe3O4@TiO2@Au magnetic microspheres as recyclable substrates for surface-enhanced Raman scattering. Nanoscale 2014, 6, 5971–5979. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, Y.; Chen, F.; Xu, P.; Li, M. Facile synthesis of mesoporous titanium dioxide doped by Ag-coated graphene with enhanced visible-light photocatalytic performance for methylene blue degradation. Rsc Advances 2017, 7, 25314–25324. [Google Scholar]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef]
- Liu, E.; Fan, J.; Hu, X.; Hu, Y.; Li, H.; Tang, C.; Sun, L.; Wan, J. A facile strategy to fabricate plasmonic Au/TiO2 nano-grass films with overlapping visible light-harvesting structures for H2 production from water. J. Mater. Sci. 2015, 50, 2298–2305. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Barba-Nieto, I.; Gomez-Cerezo, M.N.; Martinez-Arias, A.; Fernandez-Garcia, M.; Kubacka, A. Toward the green production of H2: Binary Pt-Ru promoted Nb-TiO2 based photocatalysts. ACS Sustainable Chem. Eng. 2019, 7, 15671–15683. [Google Scholar] [CrossRef]
- Hu, Z.W.; Xie, M.S.; Yang, D.T.; Chen, D.; Jian, J.Y.; Li, H.B.; Yuan, K.S.; Jiang, Z.J.; Zhou, H.B. A simple, fast, and sensitive colorimetric assay for visual detection of berberine in human plasma by NaHSO4-optimized gold nanoparticles. Rsc Advances 2017, 7, 34746–34754. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, W.; Yao, L.; Wang, J.; Han, H.; Zhu, T.; Liang, Y.; Fu, J.; Wang, Y. 3D Ag/ZnO microsphere SERS substrate with ultra-sensitive, recyclable and self-cleaning performances: Application for rapid in site monitoring catalytic dye degradation and insight into the mechanism. Colloids Surf. A 2020, 607, 125507. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, M.; Yu, H.; Zuo, Y.; Gao, J.; He, G.; Sun, Z.Q. Facile fabrication of Ag/graphene oxide/TiO2 nanorod array as a powerful substrate for photocatalytic degradation and surface-enhanced Raman scattering detection. Appl. Catal. B 2019, 252, 174–186. [Google Scholar] [CrossRef]
- Amoli-Diva, M.; Anvari, A.; Sadighi-Bonabi, R. Synthesis of magneto-plasmonic Au-Ag NPs-decorated TiO2-modified Fe3O4 nanocomposite with enhanced laser/solar-driven photocatalytic activity for degradation of dye pollutant in textile wastewater. Ceram. Int. 2019, 45, 17837–17846. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent advances in the synthesis and application of photocatalytic metal-metal oxide core-shell nanoparticles for environmental remediation and their recycling process. Rsc Advances 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Lu, F.F.; Dong, A.Q.; Ding, G.J.; Xu, K.; Li, J.M.; You, L.J. Magnetic porous polymer composite for high performance adsorption of acid red 18 based on melamine resin and chitosan. J. Mol. Liq. 2019, 294, 111515. [Google Scholar] [CrossRef]
- Du, D.; Shi, W.; Wang, L.Z.; Zhang, J.L. Yolk-shell structured Fe3O4@void@TiO2 as a photo-Fenton-like catalyst for the extremely efficient elimination of tetracycline. Appl. Catal. B 2017, 200, 484–492. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, C.Z.; Chen, A.Y.; Pu, W.H.; Gong, J.Y. Influence of yolk-shell Au@TiO2 structure induced photocatalytic activity towards gaseous pollutant degradation under visible light. Appl. Catal. B 2019, 251, 57–65. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Tao, C.R.; Xiao, G.; Wei, G.P.; Li, L.H.; Liu, C.X.; Su, H.J. Controlled synthesis and photocatalysis of sea urchin-like Fe3O4@TiO2@Ag nanocomposites. Nanoscale 2016, 8, 5313–5326. [Google Scholar] [CrossRef]
- Ma, W.F.; Zhang, Y.; Li, L.L.; You, L.J.; Zhang, P.; Zhang, Y.T.; Li, J.M.; Yu, M.; Guo, J.; Lu, H.J.; et al. Tailor-made magnetic Fe3O4@mTiO2 microspheres with a tunable mesoporous anatase shell for highly selective and effective enrichment of phosphopeptides. Acs Nano 2012, 6, 3179–3188. [Google Scholar] [CrossRef]
- Wang, P.; Chen, D.; Tang, F.Q. Preparation of titania-coated polystyrene particles in mixed solvents by ammonia catalysis. Langmuir 2006, 22, 4832–4835. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, X.L.; Zhou, J.; Chen, D.; Zhao, Z.Q. Hydrothermal synthesis of Ag@MSiO2@Ag three core-shell nanoparticles and their sensitive and stable SERS properties. Nanoscale 2016, 8, 4908–4914. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, F.; Khezri, S.M.; Kalantary, R.R.; Kakavandi, B. Enhanced photocatalytic degradation of metronidazole by TiO2 decorated on magnetic reduced graphene oxide: Characterization, optimization and reaction mechanism studies. J. Mol. Liq. 2020, 314, 113608. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Reddy, M.V.R.; Sayanna, R. Photoluminescence and photocatalytic activity of spin coated Ag+ doped anatase TiO2 thin films. Opt. Mater. 2020, 108, 110401. [Google Scholar] [CrossRef]
- Fang, Z.; Li, Q.; Su, L.; Chen, J.; Chou, K.C.; Hou, X. Efficient synergy of photocatalysis and adsorption of hexavalent chromium and rhodamine B over Al4SiC4/rGO hybrid photocatalyst under visible-light irradiation. Appl. Catal. B 2019, 241, 548–560. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Margiotta, V.; Lasorella, G.; Giotta, L.; Giannini, C.; Sibillano, T.; Murgolo, S.; Mascolo, G.; et al. Gram-scale synthesis of UV-vis light active plasmonic photocatalytic nanocomposite based on TiO2/Au nanorods for degradation of pollutants in water. Appl. Catal. B 2019, 243, 604–613. [Google Scholar] [CrossRef]
- Yang, X.H.; Fu, H.T.; Wong, K.; Jiang, X.C.; Yu, A.B. Hybrid Ag@TiO2 core-shell nanostructures with highly enhanced photocatalytic performance. Nanotechnology 2013, 24, 415601. [Google Scholar] [CrossRef]
- Lin, X.Q.; Kong, W.M.; Lin, X. Degradation of high-concentration p-nitrophenol by Fenton oxidation. Water Sci. Technol. 2020, 81, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Thi Thu Ha, P.; Xuan Hoa, V.; Nguyen Dac, D.; Tran Thu, T.; Nguyen Van, T.; Tran Dang, T.; Pham Minh, T.; Nguyen Xuan, C. The structural transition of bimetallic Ag-Au from core/shell to alloy and SERS application. Rsc Advances 2020, 10, 24577–24594. [Google Scholar]
- Yang, J.; Wang, X.Y.; Zhou, L.; Lu, F.; Cai, N.; Li, J.M. Highly sensitive SERS monitoring of catalytic reaction by bifunctional Ag-Pd triangular nanoplates. J. Saudi Chem. Soc. 2019, 23, 887–895. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.; Wang, X.Y.; Song, G.; You, L.J.; Li, J.M. Core-satellite Ag/TiO2/Ag composite nanospheres for multiple SERS applications in solution by a portable Raman spectrometer. Colloids Surf. A 2020, 584, 124013. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, J.; Wang, X.Y.; Song, G.; Lu, F.; You, L.J.; Li, J.M. Ag nanoparticles decorated Ag@ZrO2 composite nanospheres as highly active SERS substrates for quantitative detection of hexavalent chromium in waste water. J. Mol. Liq. 2020, 319, 114158. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, A.; Wang, J.; He, Q.; Sun, H.; Wang, D.; Sun, M.; Guo, H. In-situ monitoring reversible redox reaction and circulating detection of nitrite via an ultrasensitive magnetic Au@Ag SERS substrate. Sens. Actuators B 2018, 256, 107–116. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Liu, Y.; Zhang, J.; Sung, H.; Yang, J.; Zhu, J.; Liu, J.; Wu, Y. Plasmonic 3d semiconductor-metal nanopore arrays for reliable surface-enhanced raman scattering detection and in-site catalytic reaction monitoring. ACS Sens. 2018, 3, 2446–2454. [Google Scholar] [CrossRef]
- Cao, Q.; Yuan, K.; Liu, Q.; Liang, C.; Wang, X.; Cheng, Y.-F.; Li, Q.; Wang, M.; Che, R. Porous Au-Ag alloy particles inlaid AgCl membranes as versatile plasmonic catalytic interfaces with simultaneous, in Situ SERS monitoring. ACS Appl. Mater. Interfaces 2015, 7, 18491–18500. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Niu, W.; Li, C.; Tan, C.; Yin, P.; Cheng, H.; Hu, Z.; Yang, N.; He, Q.; Nam, G.H. In-situ probing of crystal-phase-dependent photocatalytic activities of Au nanostructures by Surface-Enhanced Raman spectroscopy. ACS Mater. Lett. 2020, 2, 409–414. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).