Insights on the Formation Mechanism of Ultra-Low Friction of Phenolic Resin Graphite at High Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Friction Tests
3. Results and Discussion
3.1. Characteristics of PRG
3.2. High Temperature Tribological Properties of PRG
3.3. Raman Spectroscopy
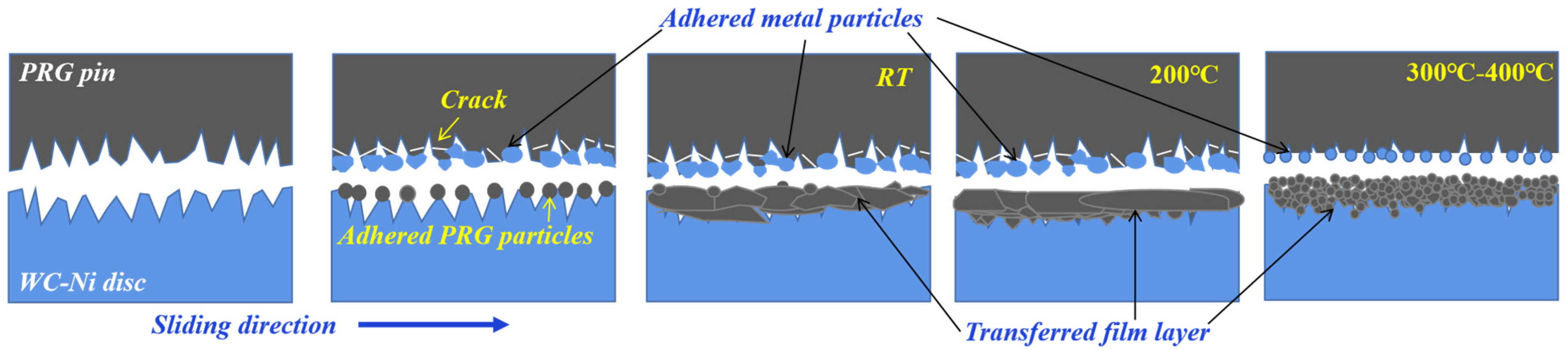
3.4. High Temperature Friction Mechanism of PRG
4. Conclusions
- (1)
- The PRG material can achieve ultra-low friction coefficient of 0.01–0.015 at 200, 300 and 400 °C. The differences are that with the increase of test temperature, the unstable running-in time to achieve the low friction coefficient decreases and the maintenance time of the low friction coefficient decreases.
- (2)
- At room temperature, the graphite material is mainly subjected to extrusion and shear force and crushed to form cracks, forming point, sheet and strip fragments with uneven size and shape. At 300 and 400 °C, the graphite tribofilms are formed and characterized by distributed granular graphite and layer by layer superposition. At 200 °C, the graphite material can form a compact and continuous transferred film and the friction coefficient is stable for a long time.
- (3)
- Under the combined action of thermal adhesion and shear force, graphite is formed at the transferred films on the surface of cemented carbide, and metal surface materials will also exist on the surface of graphite materials. The interaction between the graphite surface containing WC particles and the graphite tribofilms on the WC-Ni disk will form stable tribological behaviors. The oxide generated in the thermosetting process of polymers can fill the graphite crystal space and improve the strength and compactness of graphite tribofilms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, S.; Li, D.; Zhang, G.; Tieu, A.K.; Zhang, B. Comparison of the scuffing behaviour and wear resistance of candidate engineered coatings for automotive piston rings. Tribol. Int. 2017, 106, 10–22. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.; Su, H.; Lu, F.; Zhang, Y. Effect of temperature on the dynamic performance of C/C composite finger seal. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2016, 230, 2249–2264. [Google Scholar] [CrossRef]
- Liu, X.; Dong, H.; Lu, Z.; Zhang, J.; Liu, B. The influence mechanism of MoS2 and NiTi microparticles on the friction and wear properties of bearing steel. Tribol. Int. 2021, 160, 107033. [Google Scholar] [CrossRef]
- Qu, H.; Wen, Z.; Qu, J.; Song, B. Study on effects of ultrasonic vibration on sliding friction properties of PTFE composites/phosphor bronze under vacuum. Vacuum 2019, 164, 1–6. [Google Scholar] [CrossRef]
- Zhang, E.; Gao, F.; Fu, R.; Lu, Y.; Han, X.; Su, L. Tribological behavior of phenolic resin-based friction composites filled with graphite. Materials 2021, 14, 742. [Google Scholar] [CrossRef]
- Bonny, K.; De Baets, P.; Vleugels, J.; Huang, S.; Lauwers, B. Tribological characteristics of WC-Ni and WC-Co cemented carbide in dry reciprocating sliding contact. Tribol. Trans. 2009, 52, 481–491. [Google Scholar] [CrossRef]
- Cao, H.; Ma, W.; Chen, H.; Wang, Q.; Wang, Y. Core-rim microstructure and properties of WC/Ni composites. Int. J. Refract. Met. Hard Mater. 2019, 78, 170–177. [Google Scholar] [CrossRef]
- Sakka, M.M.; Antar, Z.; Elleuch, K.; Feller, J.F. Tribological response of an epoxy matrix filled with graphite and/or carbon nanotubes. Friction 2017, 5, 171–182. [Google Scholar] [CrossRef]
- Savchenko, D.; Serdan, A.; Morozov, V.; Van Tendeloo, G.; Ionov, S.G. Improvement of the oxidation stability and the mechanical properties of flexible graphite foil by boron oxide impregnation. New Carbon Mater. 2012, 27, 12–18. [Google Scholar] [CrossRef]
- Leonardi, M.; Alemani, M.; Straffelini, G.; Gialanella, S. A pin-on-disc study on the dry sliding behavior of a Cu-free friction material containing different types of natural graphite. Wear 2020, 442–443, 203157. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Lee, K.R. Fundamental understanding on low-friction mechanisms at amorphous carbon interface from reactive molecular dynamics simulation. Carbon 2020, 170, 621–629. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, S.; Wu, J.; Xu, L.; Li, T.; Ren, Y.; Liu, C. Friction and wear behavior of resin/graphite composite under dry sliding. J. Mater. Sci. Technol. 2015, 31, 325–330. [Google Scholar] [CrossRef]
- Jia, Q.; Yuan, X.; Zhang, G.; Dong, G.; Zhao, W. Dry friction and wear characteristics of impregnated graphite in a corrosive environment. Chin. J. Mech. Eng. 2014, 27, 965–971. [Google Scholar] [CrossRef]
- Hirani, H.; Goilkar, S.S. Formation of transfer layer and its effect on friction and wear of carbon-graphite face seal under dry, water and steam environments. Wear 2009, 266, 1141–1154. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Guo, F.; Liu, X.; Wang, Y. Friction characteristics of impregnated and non-impregnated graphite against cemented carbide under water lubrication. J. Mater. Sci. Technol. 2017, 33, 1203–1209. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Wang, Y.; Guo, F.; Liu, X.; Wang, Y. Wear behavior of WC-Ni sliding against graphite under water lubrication. J. Mater. Sci. Technol. 2017, 33, 1346–1352. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Li, S.; Li, S.; Luo, J. Influence of a carbon-based tribofilm induced by the friction temperature on the tribological properties of impregnated graphite sliding against a cemented carbide. Friction 2021, 9, 686–696. [Google Scholar] [CrossRef]
- Seleman, M.M.E.; Ahmed, M.M.Z.; AtayaLuo, S. Microstructure and mechanical properties of hot extruded 6016 aluminum alloy/graphite composites. J. Mater. Sci. Technol. 2018, 34, 1580–1591. [Google Scholar] [CrossRef]
- Ataya, S.; Alsaleh, N.; Seleman, M.M.E. Strength and wear behavior of Mg alloy AE42 reinforced with carbon short fibers. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F. Dry sliding wear mechanism of WC-13Ni hard alloy irradiated by high-intensity pulsed electron beam. Tribol. Lett. 2017, 65, 143. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Zeng, Q.; Eryilmaz, O.; Erdemir, A. Superlubricity of the DLC films-related friction system at elevated temperature. RSC Adv. 2015, 5, 93147–93154. [Google Scholar] [CrossRef]
- Zeng, Q.; Qin, L. High Temperature anti-friction behaviors of a-Si: H films and counterface material selection. Coatings 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Dong, Y.; Yin, H.; Li, Z.; Rui, Y.; Li, D.; Gu, Z.; Sun, X.; Lei, X.; Zhang, Z. Characterizing thermal-oxidation behaviors of nuclear graphite by combining O2 supply and micro surface area of graphite. Sci. Rep. 2018, 8, 13400. [Google Scholar] [CrossRef]
- Jiang, W.; Nadeau, G.; Zaghib, K.; Kinoshita, K. Thermal analysis of the oxidation of natural graphite-Effect of particle size. Acta 2000, 351, 85–93. [Google Scholar] [CrossRef]
- Guo, F.; Tian, Y.; Liu, Y.; Wang, Y. Ultralow friction between cemented carbide and graphite in water using three-step ring-on-ring friction test. Wear 2016, 352–353, 54–64. [Google Scholar] [CrossRef]
- Huai, W.; Zhang, C.; Wen, S. Graphite-based solid lubricant for high-temperature lubrication. Friction 2021, 9, 1660–1672. [Google Scholar] [CrossRef]
- Kumar, N.; Pandian, R.; Das, P.K.; Ravindran, T.R.; Dash, S.; Tyagi, A.K. High-temperature phase transformation and low friction behaviour in highly disordered turbostratic graphite. J. Phys. D Appl. Phys. 2013, 46, 395305. [Google Scholar] [CrossRef]
- Lafon-Placette, S.; Delbé, K.; Denape, J.; Ferrato, M. Tribological characterization of silicon carbide and carbon materials. J. Eur. Ceram. Soc. 2015, 35, 1147–1159. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Alsmeyer, D.C.; Mccreery, R.L. Raman-spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Cabrera-Sanfelix, P.; Arnau, A.; Darling, G.R.; Sanchez-Portal, D. On the structure of the first hydration layer on NaCl(100): Role of hydrogen bonding. J. Chem. Phys. 2007, 126, 11347. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Rossi di Montelera, L.; Camino, G.; Weil, E.D.; Pearce, E.M. Structure-charring relationship in phenol-formaldehyde type resins. Polym. Degrad. Stab. 1997, 56, 23–35. [Google Scholar] [CrossRef]
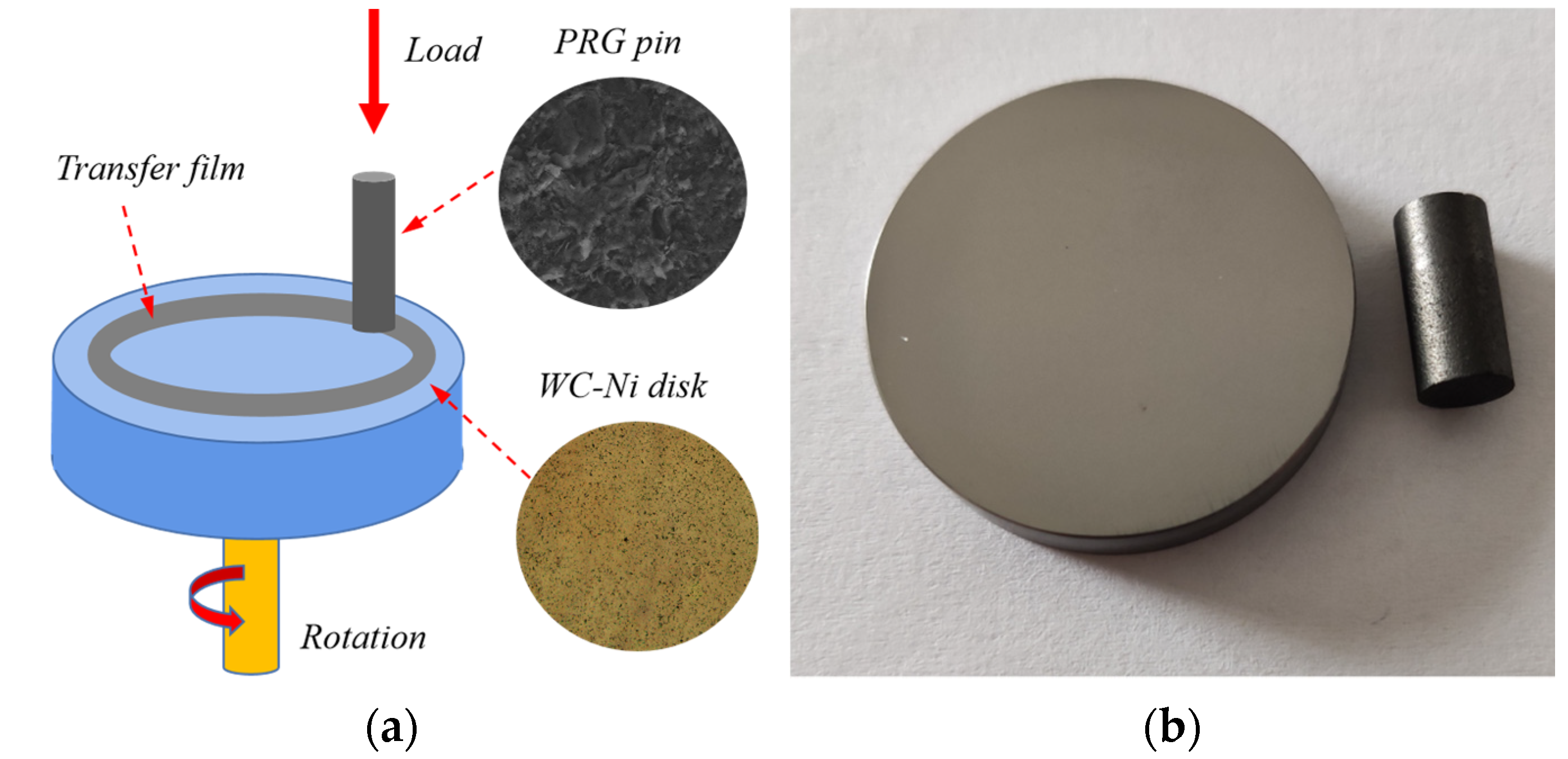

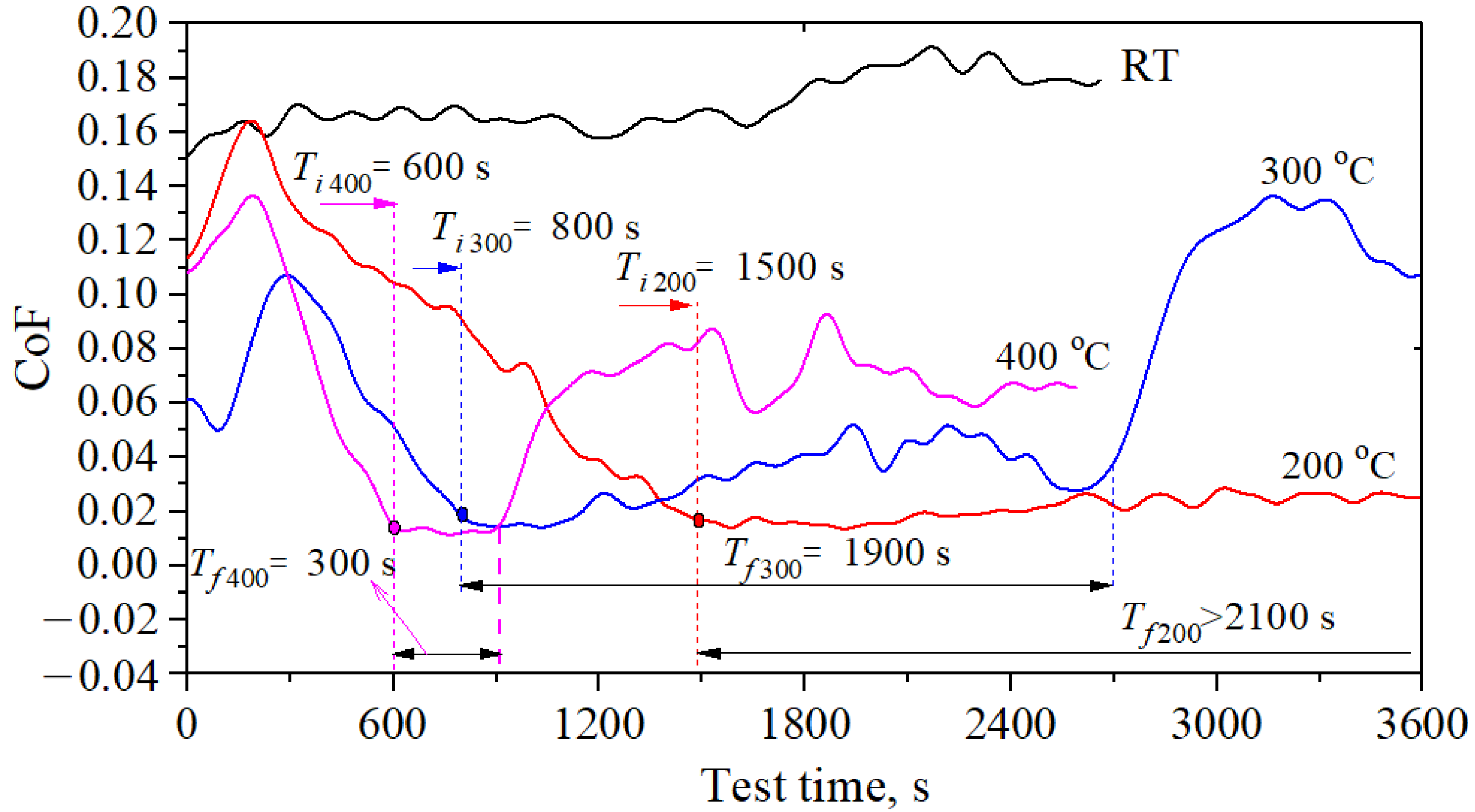


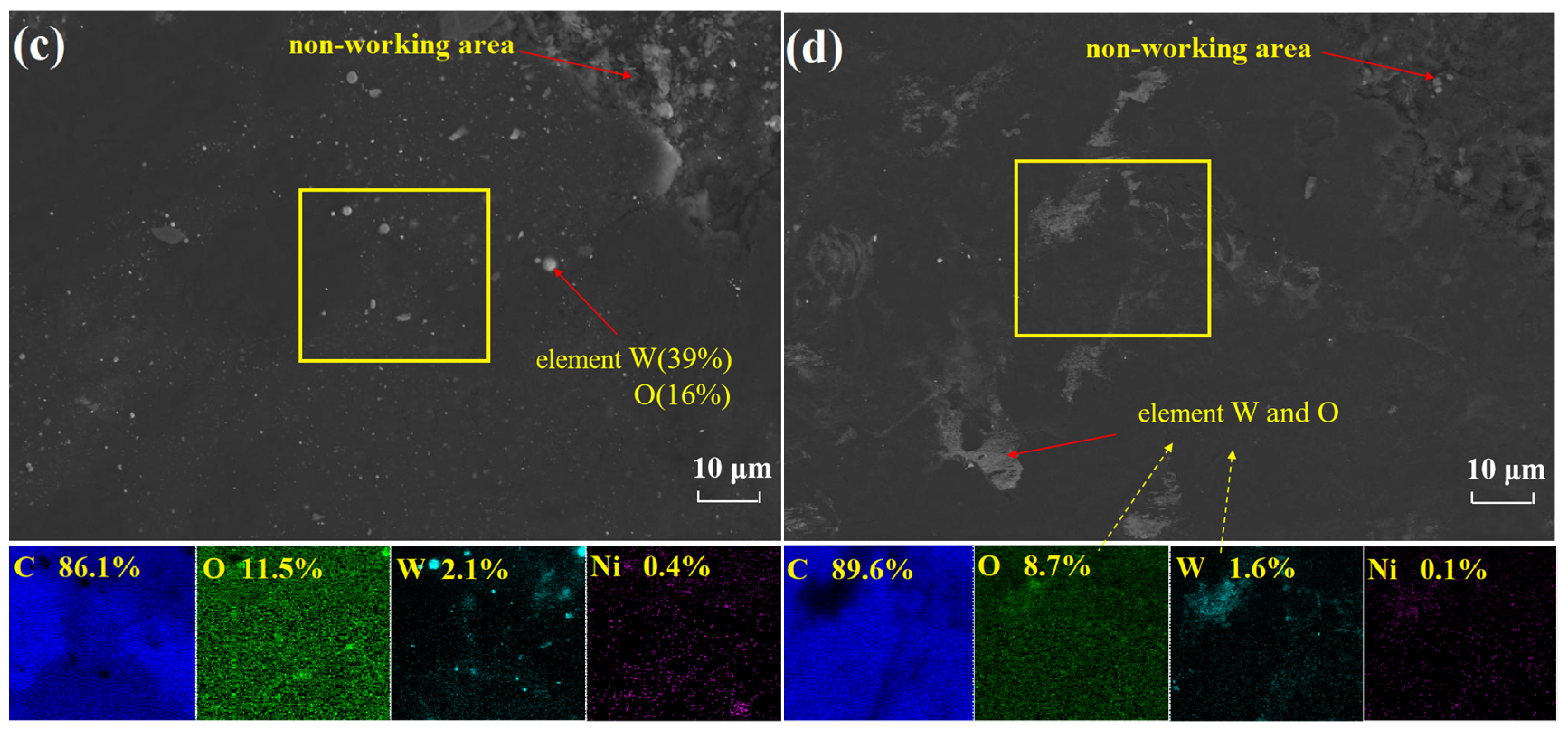

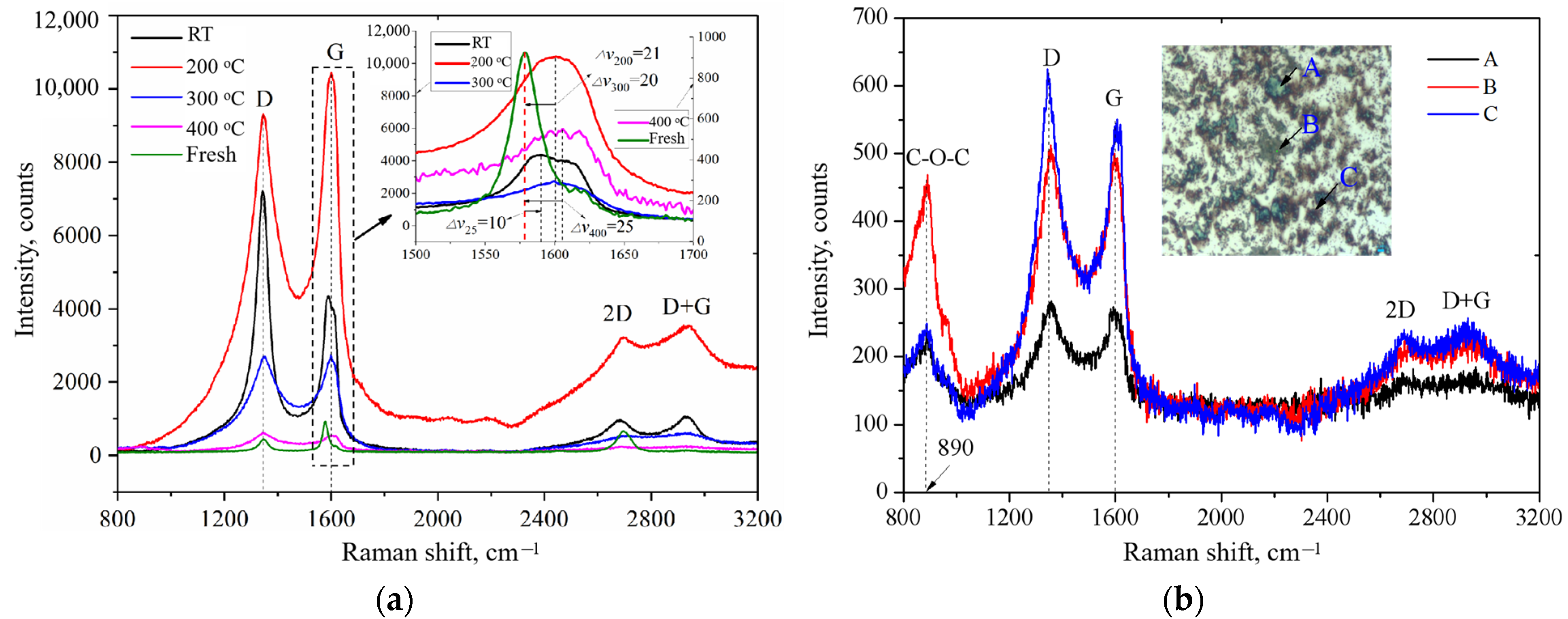
| Material | Roughness, Ra(nm) | Young’s Modulus (Gpa) | Hardness | Density (g/cm3) | Porosity (vol.%) | Graphitization (%) |
|---|---|---|---|---|---|---|
| WC-Ni | 13 | 550 | 1500 HV | 15 | \ | \ |
| PRG | 500 | 26 | 75 HS | 1.88 | 5.0 | 50–75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Yin, P.; Zeng, Q.; Wang, J. Insights on the Formation Mechanism of Ultra-Low Friction of Phenolic Resin Graphite at High Temperature. Coatings 2022, 12, 6. https://doi.org/10.3390/coatings12010006
Zhang F, Yin P, Zeng Q, Wang J. Insights on the Formation Mechanism of Ultra-Low Friction of Phenolic Resin Graphite at High Temperature. Coatings. 2022; 12(1):6. https://doi.org/10.3390/coatings12010006
Chicago/Turabian StyleZhang, Fan, Peng Yin, Qunfeng Zeng, and Jianmei Wang. 2022. "Insights on the Formation Mechanism of Ultra-Low Friction of Phenolic Resin Graphite at High Temperature" Coatings 12, no. 1: 6. https://doi.org/10.3390/coatings12010006
APA StyleZhang, F., Yin, P., Zeng, Q., & Wang, J. (2022). Insights on the Formation Mechanism of Ultra-Low Friction of Phenolic Resin Graphite at High Temperature. Coatings, 12(1), 6. https://doi.org/10.3390/coatings12010006







