Outstanding Performance of a New Exfoliated Clay Impregnated with Rutile TiO2 Nanoparticles Composite for Dyes Adsorption: Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Experimental
2.1. Reactants and Clay
2.2. Preparation of Rt/BC Adsorbent
2.3. Adsorbent Characterization
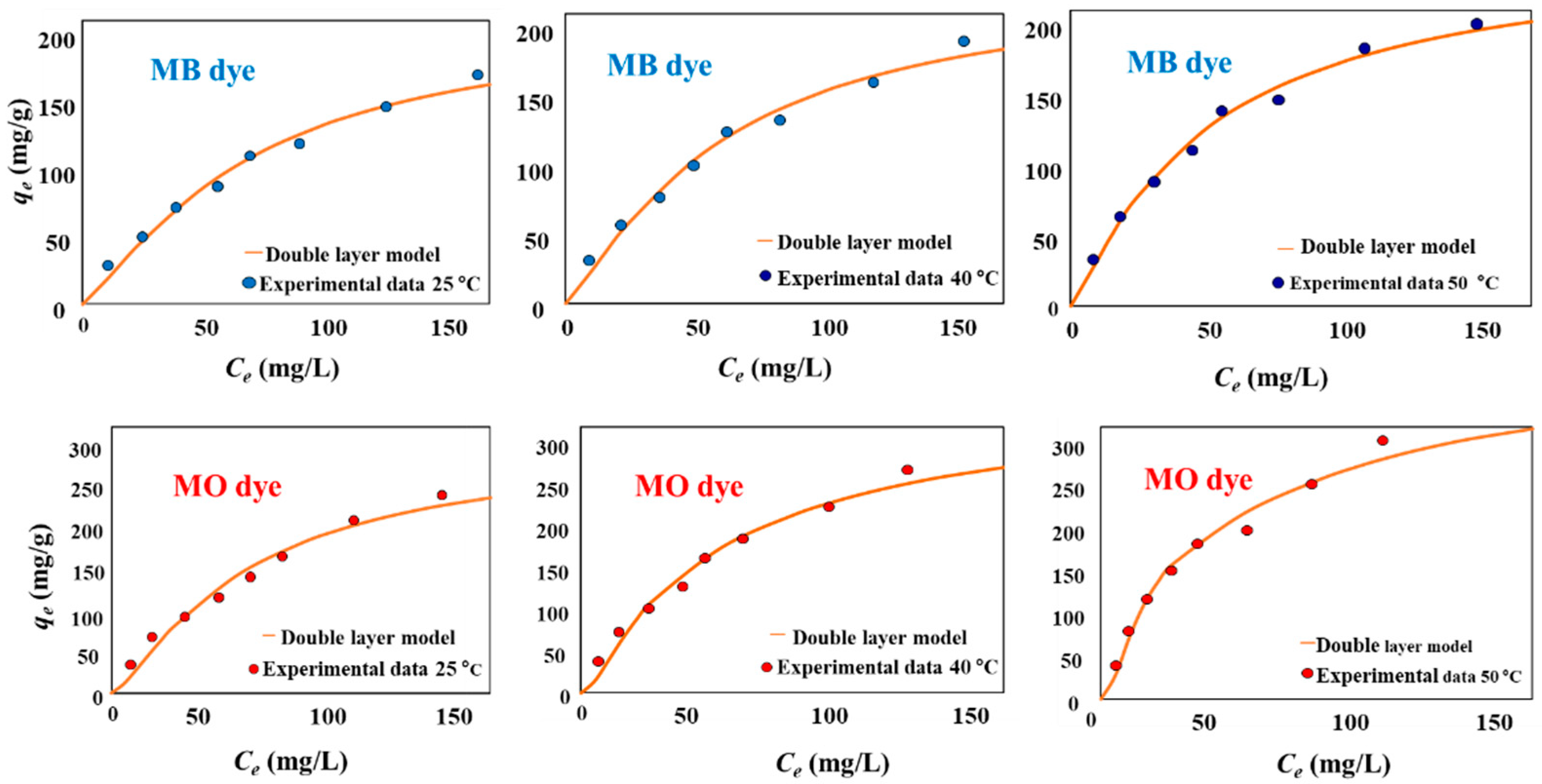
2.4. Isotherm Studies of MB and MO Adsorption
2.5. MB and MO Adsorption Modeling
- Advanced monolayer model (AMM)
- Advanced double layer model (ADM)
- A multilayer model, as the general form of ASPMs, was also implemented in this study to fit the MB and MO adsorption processes. The following equations (Equations (4)–(9) are used to determine the parameters of the multilayer model [9]:where the parameter N2 designates the number of removed dye layers with specified energy of adsorption. Numerous situations were anticipated depending on the multilayer adsorption model as given in Figure 1 [13,31,32]:
- Langmuir adsorption model: n parameter was fixed at 1, and N2 was fixed at 0;
- Monolayer adsorption model: n was the fitted parameter, and N2 was fixed at 0;
- Double-layer adsorption model: n was the fitted parameter, and N2 was fixed at 1;
- Triple-layer adsorption model: n was the fitted parameter, and N2 was fixed at 2;
- Multilayer adsorption model: n and N2 were the fitted parameters.
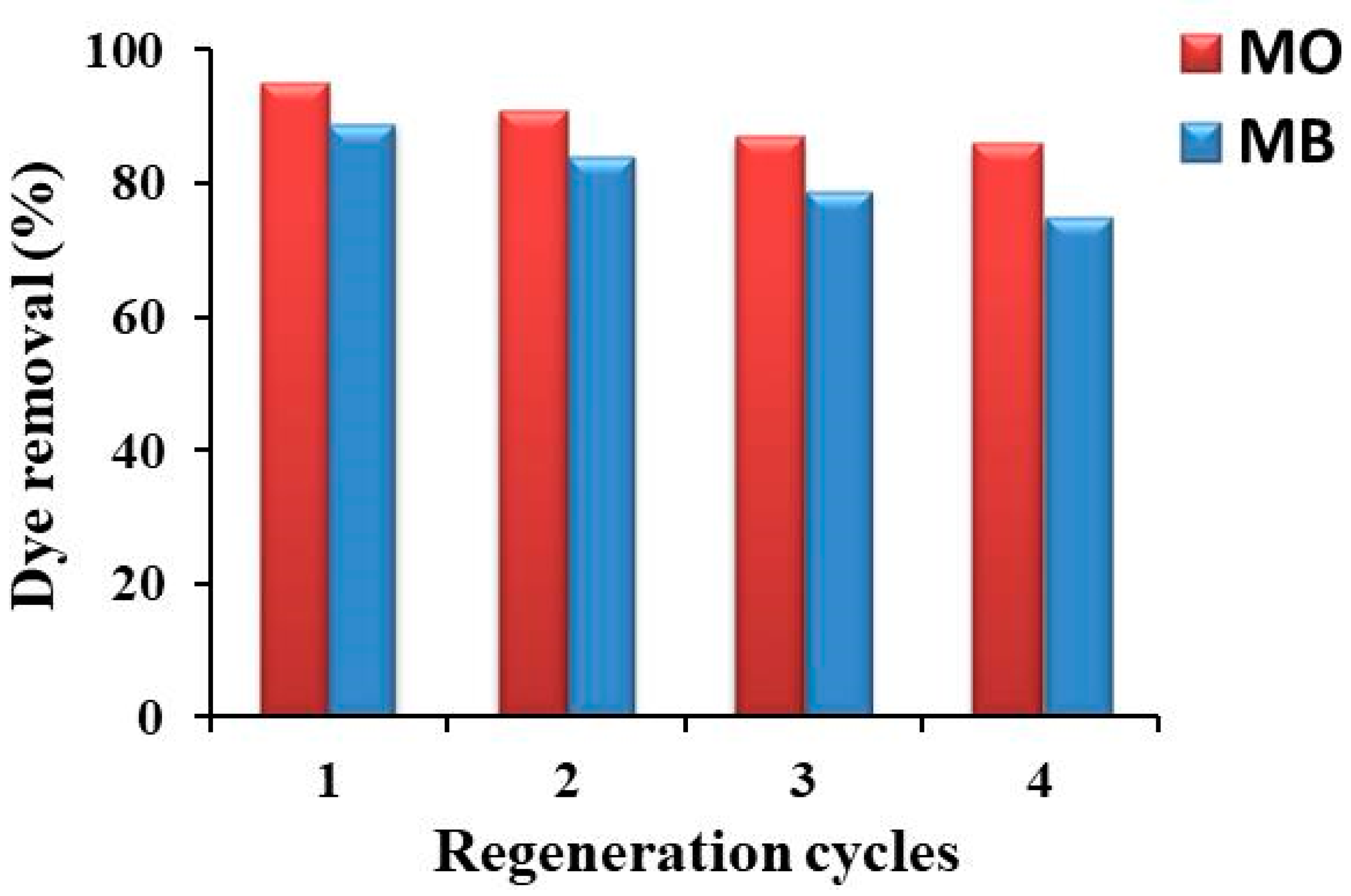
2.6. Regeneration of Rt/BC Adsorbent
3. Results and Discussion
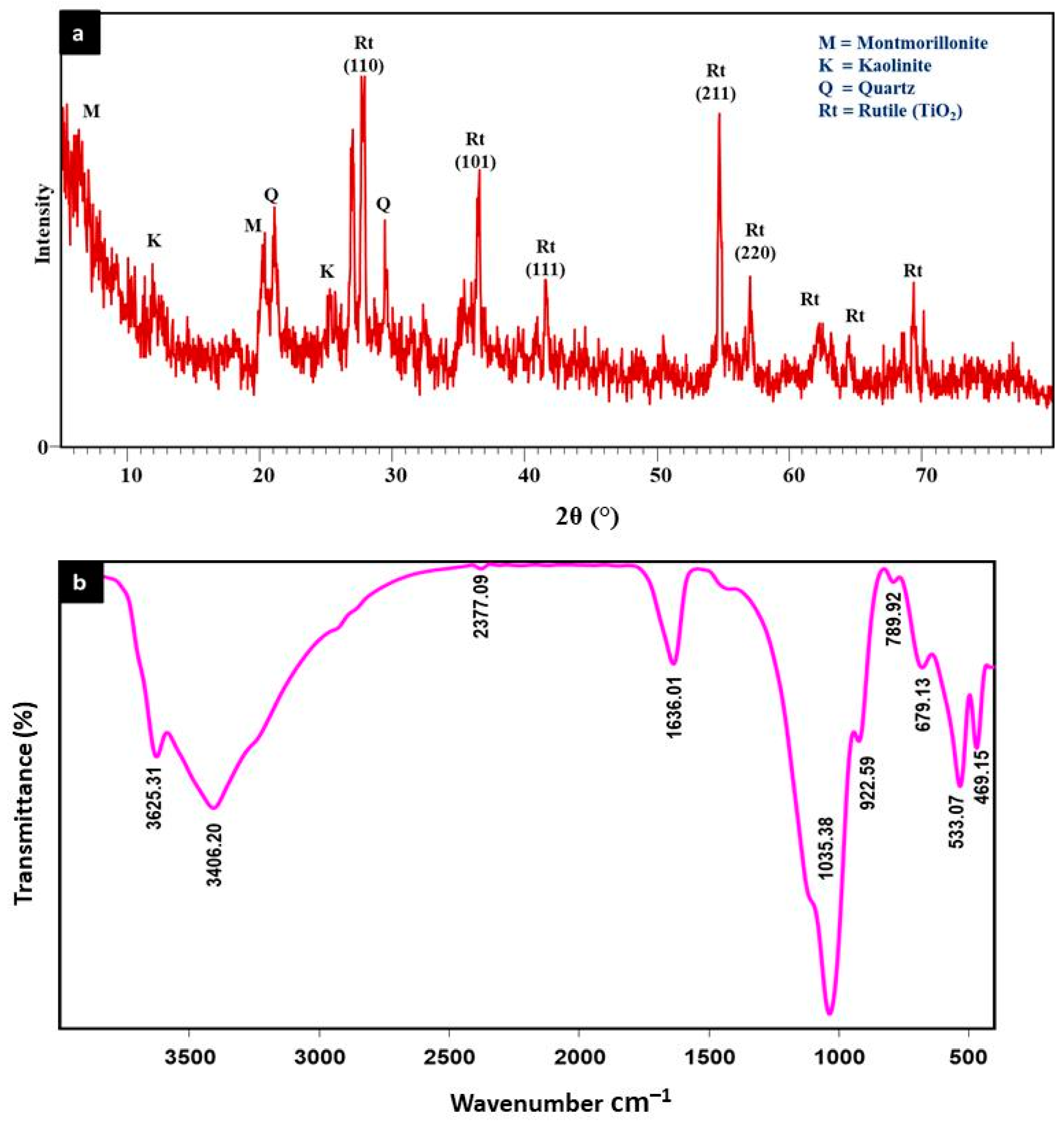
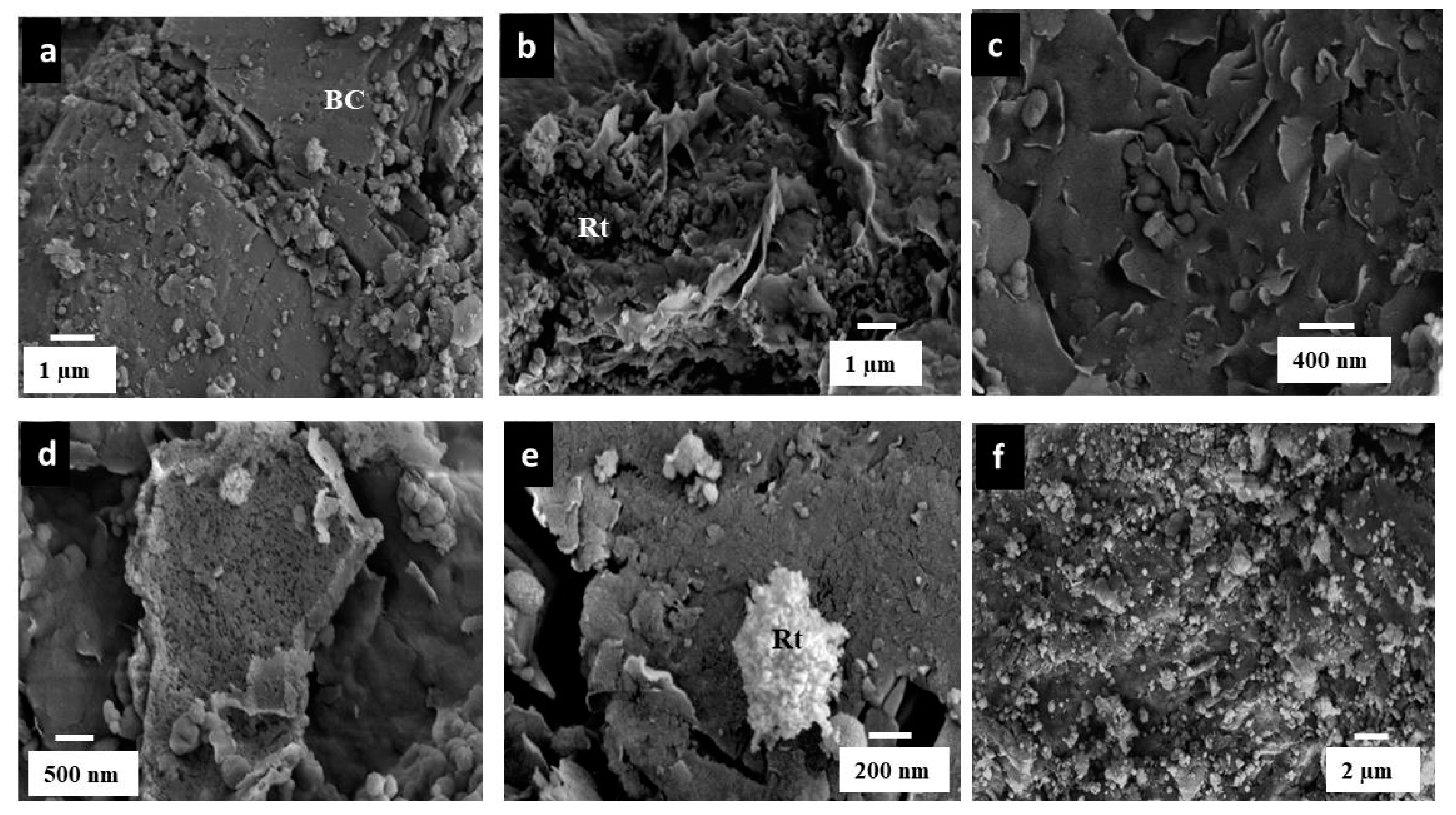
3.1. Characterization of the Rt/BC Material
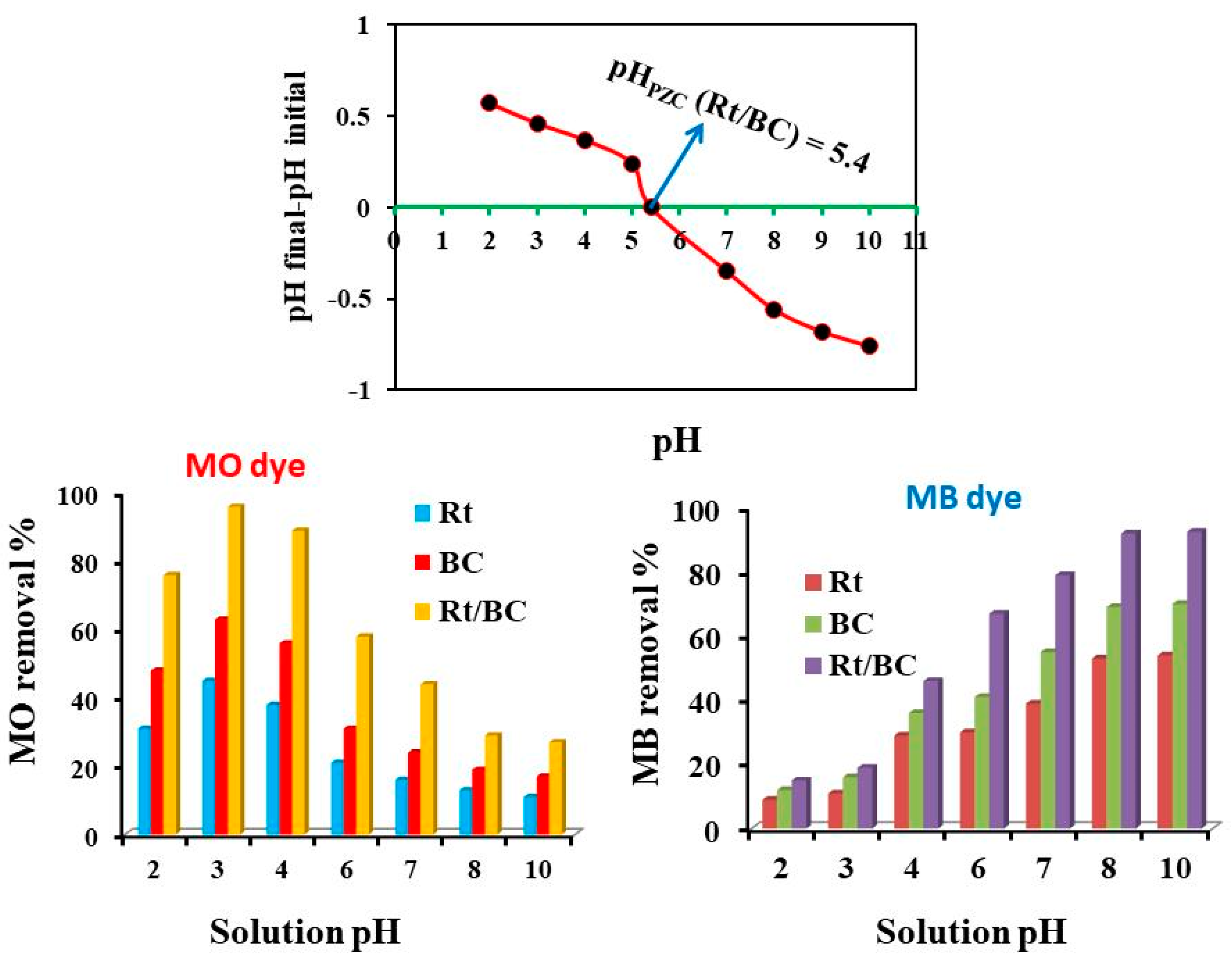
3.2. pH Effect on MB and MO Adsorption by Rt/BC
3.3. MB and MO Adsorption Modeling and Mechanism Interpretation
3.3.1. Steric Parameters
- In the first scenario: n ≤ 0.5, the molecules of each tested dye were linked to two or more active sites of the Rt/BC adsorbent (i.e., a horizontal adsorption geometry occurred).
- The second scenario: 0.5 < n < 1, this behavior indicated that Rt/BC could remove MB or MO molecules with both adsorption orientations (i.e., horizontal and vertical) but with various proportions.
- In the last scenario: n ≥ 1, this suggested that MB or MO dye can be adsorbed on Rt/BC through an entire vertical geometry.
| Adsorbent | Dye | Qmax (mg/g) | Reference |
|---|---|---|---|
| Graphene | MB | 153 | [38] |
| Kaolin | MB | 45 | [39] |
| Zeolite 4A | MB | 22 | [39] |
| Polydopamine microspheres | MB | 90.7 | [40] |
| Activated rice husk | MB | 65 | [41] |
| Ball clay | MB | 25 | [42] |
| Fe3O4/montmorillonite | MB | 69 | [43] |
| Rt/BC | MB | 214.52 | This study |
| Activated clay | MO | 16.78 | [44] |
| Treated coal powder | MO | 18.52 | [45] |
| Banana peel | MO | 21 | [46] |
| Nano-composite films | MO | 29.41 | [47] |
| Activated wheat straw | MO | 50.4 | [48] |
| CTAB/H2O2-clay | MO | 194.3 | [5] |
| Rt/BC composite | MO | 294.50 | The present study |
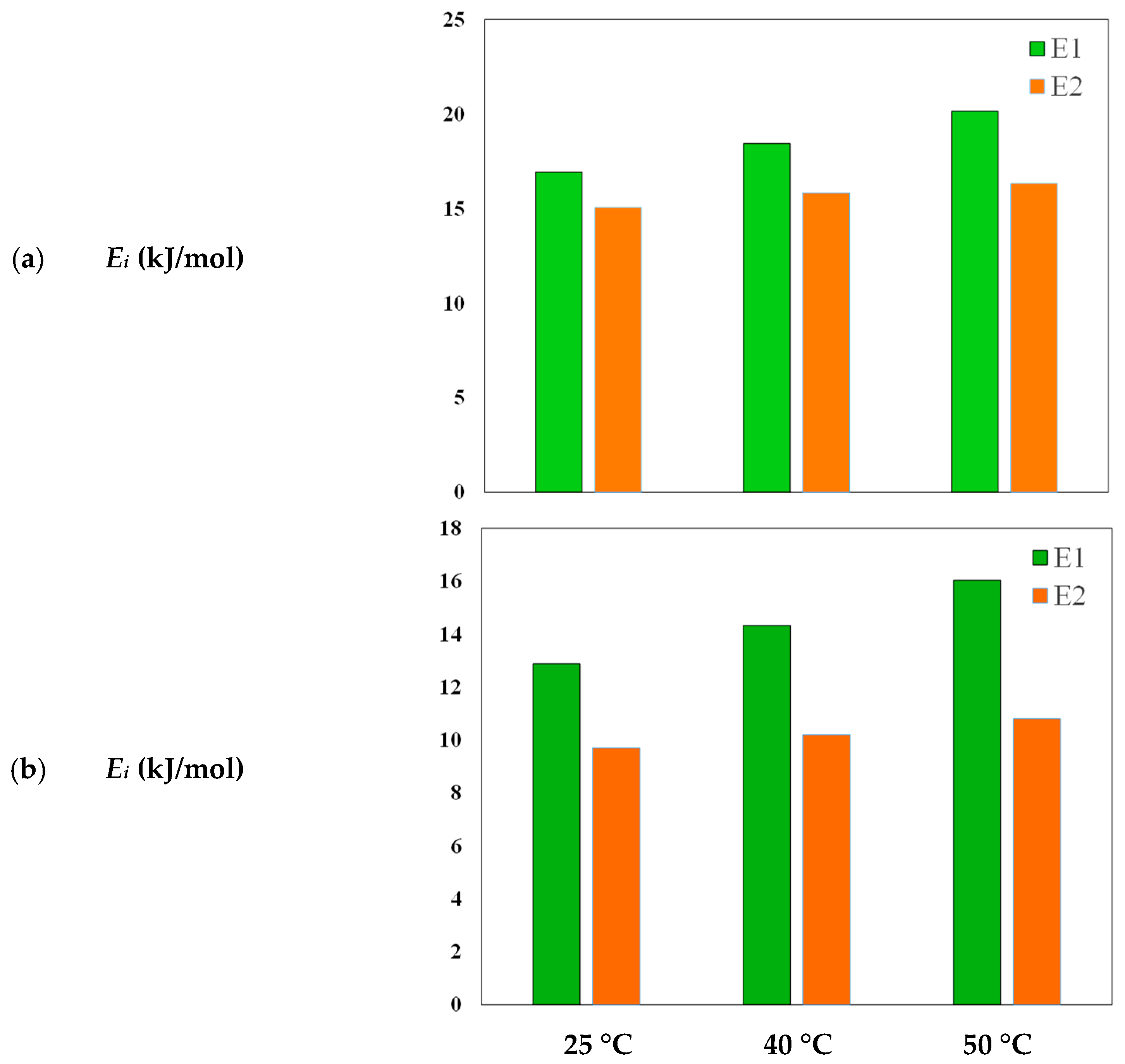
3.3.2. Energetic Parameters for MB and MO Adsorption
3.4. Regeneration of Rt/BC Adsorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Ben Lamine, A.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Kaledin, V.I.; Ilnitskaya, S.I.; Ovchinnikova, L.P.; Popova, N.A.; Bogdanova, L.A.; Morozkova, T.S. Mutagenic activation and carcinogenicity of aminoazo dyes ortho aminoazotoluene and 3′ methyl-4-dimethyl-amino-azobenzene in experiments on suckling mice. Biophysics 2014, 59, 431–435. [Google Scholar] [CrossRef]
- Alderete, B.L.; da Silva, J.; Godoi, R.; da Silva, F.R.; Taffarel, S.R.; da Silva, L.P.; Garcia, A.L.H.; Mitteregger, H., Jr.; de Amorim, H.L.N.; Picada, J.N. Evaluation of toxicity and mutagenicity of a synthetic effluent containing azo dye after advanced oxidation process treatment. Chemosphere 2021, 263, 128291. [Google Scholar] [CrossRef] [PubMed]
- Köktürk, M.; Altindağ, F.; Ozhan, G.; Çalimli, M.H.; Nas, M.S. Textile dyes Maxilon blue 5G and Reactive blue 203 induce acute toxicity and DNA damage during embryonic development of Danio rerio. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 242, 108947. [Google Scholar] [CrossRef]
- Mobarak, M.; Selim, A.Q.; Mohamed, E.A.; Seliem, M.K. A superior adsorbent of CTAB/H2O2 solution−modified organic carbon rich-clay for hexavalent chromium and methyl orange uptake from solutions. J. Mol. Liq. 2018, 259, 384–397. [Google Scholar] [CrossRef]
- Seliem, M.K.; Mobarak, M.; Selim, A.; Mohamed, E.; Halfaya, R.A.; Gomaa, H.K.; Anastopoulos, I.; Giannakoudakis, D.A.; Lima, E.C.; Bonilla-Petriciolet, A.; et al. A novel multifunctional adsorbent of pomegranate peel extract and activated anthracite for Mn(VII) and Cr(VI) uptake from solutions: Experiments and theoretical treatment. J. Mol. Liq. 2020, 311, 113169. [Google Scholar] [CrossRef]
- Jalil, A.A.; Triwahyono, S.; Adam, S.H.; Rahim, N.D.; Aziz, M.A.A.; Hairom, N.H.H.; Razali, N.A.M.; Abidin, M.A.; Mohamadiah, M.K.A. Adsorption of methyl orange from aqueous solution onto calcined Lapindo volcanic mud. J. Hazard. Mater. 2010, 181, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011, 362, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A.; Selim, A.Q.; Mobarak, M.; Kumar, R.; Anastopoulos, I.; Giannakoudakis, D.; Bonilla-Petriciolet, A.; Mohamed, E.A.; Seliem, M.K.; Komarneni, S. Experimental and Theoretical Studies of Methyl Orange Uptake by Mn–Rich Synthetic Mica: Insights into Manganese Role in Adsorption and Selectivity. Nanomaterials 2020, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Hui, C.; Khan, R.A.; Du, J.; Zhang, Q.; Zhao, Y.-H. Efficient removal of crystal violet using Fe3O4-coated biochar: The role of the Fe3O4 nanoparticles and modeling study their adsorption behavior. Sci. Rep. 2015, 5, 12638. [Google Scholar] [CrossRef] [Green Version]
- Seliem, M.K.; Komarneni, S.; Byrne, T.; Cannon, F.S.; Shahien, M.G.; Khalil, A.A.; AbdEl-Gaid, I.M. Removal of perchlorate by synthetic organosilicas and organoclay: Kineticsand isotherm studies. Appl. Clay Sci. 2013, 71, 21–26. [Google Scholar] [CrossRef]
- Li, Z.; Sellaoui, L.; Dotto, G.L.; Ben Lamine, A.; Bonilla-Petriciolet, A.; Hanafy, H.; Belmabrouk, H.; Netto, M.S.; Erto, A. Interpretation of the adsorption mechanism of Reactive Black 5 and Ponceau 4R dyes on chitosan/polyamide nanofibers via advanced statistical physics model. J. Mol. Liq. 2019, 285, 165–170. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Selim, A.Q.; Ahmed, S.A.; Sellaoui, L.; Bonilla-Petriciolet, A.; Erto, A.; Li, Z.; Li, Y.; Seliem, M.K. H2O2-activated anthracite impregnated with chitosan as a novel composite for Cr(VI) and methyl orange adsorption in single-compound and binary systems: Modeling and mechanism interpretation. Chem. Eng. J. 2020, 380, 122445. [Google Scholar] [CrossRef]
- Ramadan, H.; Mobarak, M.; Lima, E.C.; Bonilla-Petriciolet, A.; Li, Z.; Seliem, M.K. Cr(VI) adsorption onto a new composite prepared from Meidum black clay and pomegranate peel extract: Experiments and physicochemical interpretations. J. Environ. Chem. Eng. 2021, 9, 105352. [Google Scholar] [CrossRef]
- Barakat, M.A.; Kumar, R.; Lima, E.C.; Seliem, M.K. Facile synthesis of muscovite–supported Fe3O4 nanoparticles as an adsorbent and heterogeneous catalyst for effective removal of methyl orange: Characterisation, modelling, and mechanism. J. Taiwan Inst. Chem. Eng. 2021, 119, 146–157. [Google Scholar] [CrossRef]
- Hajjaji, M.; Alami, A. Influence of operating conditions on methylene blue uptake by a smectite rich clay fraction. Appl. Clay Sci. 2009, 44, 127–129. [Google Scholar] [CrossRef]
- Tehrani, A.; Nikkar, H.; Mahmoodi, N.M.; Markazi, M.; Menger, F. The sorption of cationic dyes onto kaolin: Kinetic, isotherm and thermodynamic studies. Desalination 2011, 266, 274–280. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hajjaji, M.; Alami, A.; El Bouadili, A. Removal of methylene blue from aqueous solution by fibrous clay minerals. J. Hazard. Mater. 2006, 135, 188–192. [Google Scholar] [CrossRef]
- Chen, Z.X.; Jin, X.Y.; Chen, Z.; Megharaj, M. Naidu, Removal of methyl orange from aqueous solution using benton-ite-supported nanoscale zero-valent iron. J. Colloid Interface Sci. 2011, 363, 601–607. [Google Scholar] [CrossRef]
- Ramadana, H.S.; Alia, R.A.M.; Mobarak, M.; Badawi, M.; Selim, A.Q.; Mohamed, E.A.; Bonilla-Petriciolet, A.; Seliem, M.K. One-step fabrication of a new outstanding rutile TiO2 nanoparticles/anthracite adsorbent: Modeling and physicochemical interpretations for malachite green removal. Chem. Eng. J. 2021, 426, 131890. [Google Scholar] [CrossRef]
- Wang, R.; Cai, X.; Shen, F. TiO2 hollow microspheres with mesoporous surface: Superior adsorption performance for dye removal. Appl. Surf. Sci. J. 2014, 305, 352–358. [Google Scholar] [CrossRef]
- Banerjee, S.; Benjwal, P.; Singh, M.; Kar, K.K. Graphene oxide (rGO)-metal oxide (TiO2/Fe3O4) based nanocomposites for the removal of methylene blue. Appl. Surf. Sci. 2018, 439, 560–568. [Google Scholar] [CrossRef]
- Quiñones, C.; Ayalaa, J.; Vallejo, W. Methylene blue photoelectrodegradation under UV irradiation on Au/Pd-modified TiO2 films. Appl. Surf. Sci. 2010, 257, 367–371. [Google Scholar] [CrossRef]
- Nawi, M.A.; Zain, S.M. Enhancing the Surface Properties of the Immobilized Degussa P-25 TiO2 for the Efficient Photocatalytic Removal of Methylene Blue from Aqueous Solution. Appl. Surf. Sci. 2012, 258, 6148–6157. [Google Scholar] [CrossRef]
- Devi, L.G.; Kumar, S.G. Exploring the critical dependence of adsorption of various dyes on the degradation rate using Ln3+-TiO2 surface under UV/solar light. Appl. Surf. Sci. 2012, 261, 137–146. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Yan, W. Excellent adsorption and desorption characteristics of polypyrrole/TiO2 composite for Methylene Blue. Appl. Surf. Sci. 2013, 279, 400–408. [Google Scholar] [CrossRef]
- Shahien, M.G.; Khedr, M.M.; Maurice, A.E.; Farghali, A.; Ali, R.A. Synthesis of high purity rutile nanoparticles from medium-grade Egyptian natural ilmenite. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Selim, A.Q.; Sellaoui, L.; Ahmed, S.A.; Mobarak, M.; Mohamed, E.A.; Ben Lamine, A.; Erto, A.; Bonilla-Petriciolet, A.; Seliem, M.K. Statistical physics-based analysis of the adsorption of Cu2+ and Zn2+ onto synthetic cancrinite in single-compound and binary systems. J. Environ. Chem. Eng. 2019, 7, 103217. [Google Scholar] [CrossRef]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Netto, M.S.; Oliveira, M.L.S.; Seliem, M.K.; Dotto, G.L.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Abu Sharib, A.A.A.; Bonilla-Petriciolet, A.; Selim, A.Q.; Mohamed, E.A.; Seliem, M.K. Utilizing modified weathered basalt as a novel approach in the preparation of Fe3O4 nanoparticles: Experimental and theoretical studies for crystal violet adsorp-tion. J. Environ. Chem. Eng. 2021, 9, 106220. [Google Scholar] [CrossRef]
- Amrhar, O.; El Gana, L.; Mobarak, M. Calculation of adsorption isotherms by statistical physics models: A review. Environ. Chem. Lett. 2021, 19, 4519–4547. [Google Scholar] [CrossRef]
- Tran, H.N.; Fen, Y.; You, W.S.J.; Chao, H.P. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of π–π interactions. Process Saf. Environ. Prot. 2017, 107, 168–180. [Google Scholar] [CrossRef]
- Shi, X.; Fu, H.; Li, Y.; Mao, J.; Zheng, S.; Zhu, D. Impact of coal structural heterogeneity on the nonideal sorption of organic contaminants. Environ. Toxicol. Chem. 2011, 30, 1310–1319. [Google Scholar] [CrossRef]
- Sellaoui, L.; Saha, B.; Wjihi, S.; Ben Lamine, A. Physicochemical parameters interpretation for adsorption equilibrium of ethanol on metal organic framework: Application of the multilayer model with saturation. J. Mol. Liq. 2017, 233, 537–542. [Google Scholar] [CrossRef]
- Hua, P.; Sellaoui, L.; Franco, D.; Netto, M.S.; Dotto, G.L.; Bajahzar, A.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of acid green and procion red on a magnetic geopolymer based adsorbent: Experiments, characterization, and theo-retical treatment. Chem. Eng. J. 2020, 383, 123113. [Google Scholar] [CrossRef]
- Gong, R.; Ye, J.; Dai, W.; Yan, X.; Hu, J.; Hu, X.; Li, S.; Huang, H. Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution with Finger-Citron-Residue-Based Activated Carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Rida, K.; Bouraoui, S.; Hadnine, S. Adsorption of methylene blue from aqueous solution by kaolin and zeolite. Appl. Clay Sci. 2013, 83–84, 99–105. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Franco, D.; Tanabe, E.H.; Bertuol, D.; Reis, G.; Lima, E.C.; Dotto, G.L. Alternative treatments to improve the potential of rice husk as adsorbent for methylene blue. Water Sci. Technol. 2016, 75, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Auta, M.; Hameed, B.H. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198–199, 219–227. [Google Scholar] [CrossRef]
- Cottet, L.; Almeida, C.A.P.; Naidek, N.; Viante, M.F.; Lopes, M.C.; Debacher, N.A. Adsorption characteristics of montmorillonite clay modified with iron oxide with respect to methylene blue in aqueous media. Appl. Clay Sci. 2014, 95, 25–31. [Google Scholar] [CrossRef]
- Ma, Q.; Shen, F.; Lu, X.; Bao, W.; Ma, H. Studies on the adsorption behavior of methyl orange from dye wastewater onto activated clay. Desalination Water Treat. 2013, 51, 3700–3709. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, A.; Wang, G.; Zhao, X. Adsorption Behavior of Methyl Orange onto Modified Ultrafine Coal Powder. Chin. J. Chem. Eng. 2009, 17, 942–948. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Jiang, R.; Fu, Y.Q.; Zhu, H.Y.; Yao, J.; Xiao, L. Removal of Methyl Orange from Aqueous Solutions by Magnetic Maghemite/Chitosan Nanocomposite Films: Adsorption Kinetics and Equilibrium. J. Appl. Polym. Sci. 2012, 125, 540–549. [Google Scholar] [CrossRef]
- Su, Y.; Jiao, Y.; Dou, C.; Han, R. Biosorption of methyl orange from aqueous solutions using cationic surfactant-modified wheat straw in batch mode. Desalination Water Treat. 2014, 52, 6145–6155. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Mobarak, M.; Kumar, R.; Barakat, M.; Bonilla-Petriciolet, A.; Seliem, M.K.; Selim, A.Q. Novel hybrid multifunctional composite of chitosan and altered basalt for barium adsorption: Experimental and theoretical studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124613. [Google Scholar] [CrossRef]
- Selim, A.Q.; Mohamed, E.A.; Seliem, M.K. Deep insights into the organic carbon role in selectivity and adsorption mechanism of phosphate and crystal violet onto low–cost black limestone: Modelling and physicochemical parameters interpretation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123755. [Google Scholar] [CrossRef]
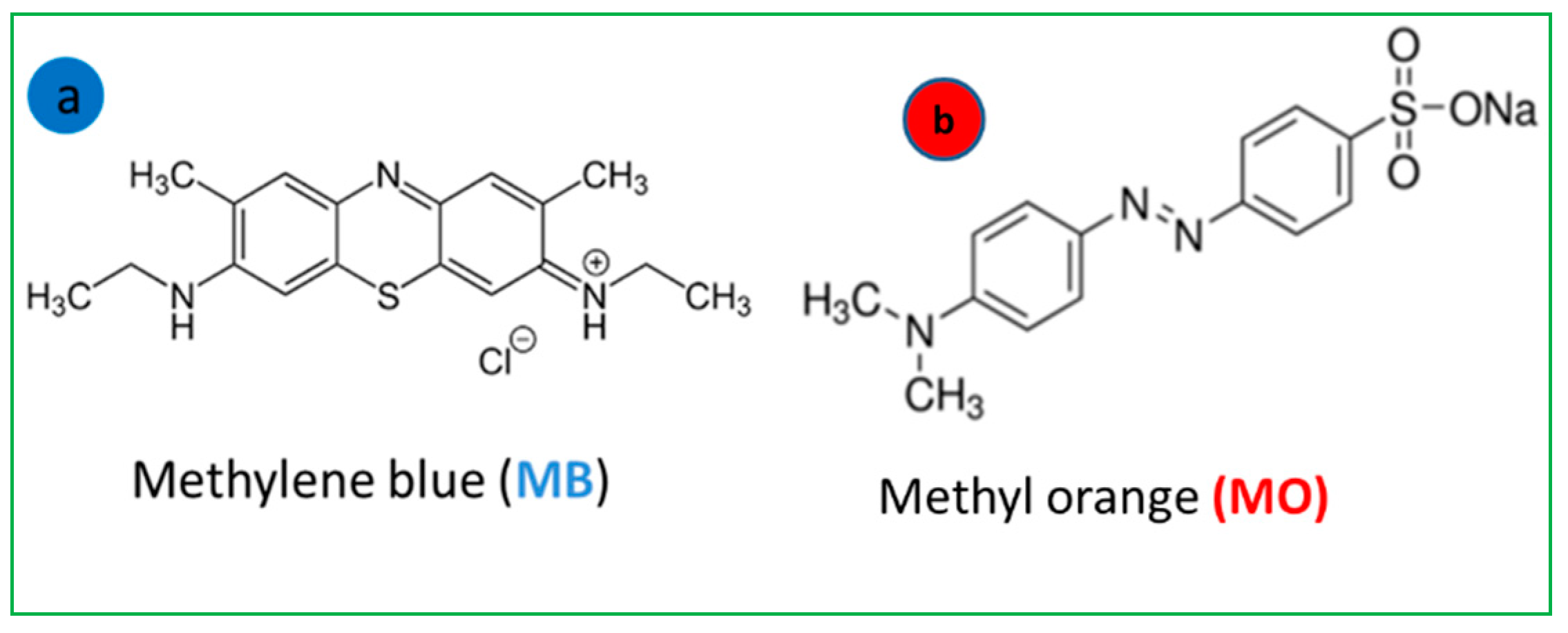

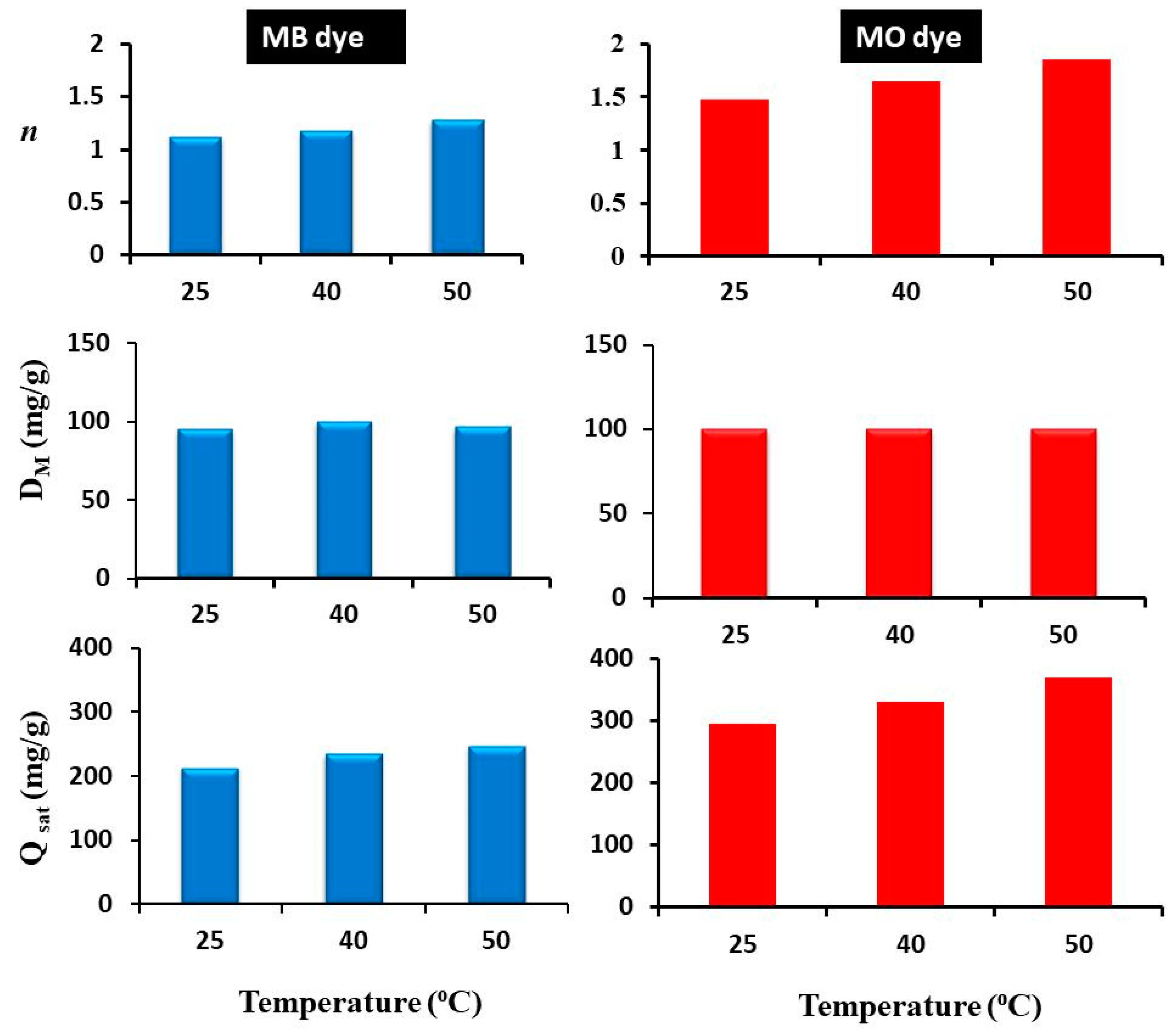
| T (°C) | Dye | n | DM (mg/g) | Qsat (mg/g) |
|---|---|---|---|---|
| 25 | MB | 1.12 | 95.76 | 214.52 |
| 40 | MB | 1.18 | 100 | 235.9 |
| 50 | MB | 1.29 | 96.66 | 249.03 |
| 25 | MO | 1.47 | 100 | 294.5 |
| 40 | MO | 1.65 | 100 | 330.1 |
| 50 | MO | 1.85 | 100 | 370.37 |
| T (°C) | Dye | C1 | C2 | ΔE1 (kJ/mol) | ΔE2 (kJ/mol) |
|---|---|---|---|---|---|
| 25 | MB | 47.13 | 99.99 | 16.93 | 15.07 |
| 40 | MB | 36.57 | 99.47 | 18.44 | 15.84 |
| 50 | MB | 24.07 | 99.53 | 20.15 | 16.342 |
| 25 | MO | 27.76 | 100 | 12.87 | 9.7 |
| 40 | MO | 20.4 | 99.39 | 14.32 | 10.2 |
| 50 | MO | 12.78 | 89.61 | 16.03 | 10.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, I.A.; Seliem, M.K.; Lima, E.C.; Badawi, M.; Li, Z.; Bonilla-Petriciolet, A.; Anastopoulos, I. Outstanding Performance of a New Exfoliated Clay Impregnated with Rutile TiO2 Nanoparticles Composite for Dyes Adsorption: Experimental and Theoretical Studies. Coatings 2022, 12, 22. https://doi.org/10.3390/coatings12010022
Ahmed IA, Seliem MK, Lima EC, Badawi M, Li Z, Bonilla-Petriciolet A, Anastopoulos I. Outstanding Performance of a New Exfoliated Clay Impregnated with Rutile TiO2 Nanoparticles Composite for Dyes Adsorption: Experimental and Theoretical Studies. Coatings. 2022; 12(1):22. https://doi.org/10.3390/coatings12010022
Chicago/Turabian StyleAhmed, Inas A., Moaaz K. Seliem, Eder C. Lima, Michael Badawi, Zichao Li, Adrián Bonilla-Petriciolet, and Ioannis Anastopoulos. 2022. "Outstanding Performance of a New Exfoliated Clay Impregnated with Rutile TiO2 Nanoparticles Composite for Dyes Adsorption: Experimental and Theoretical Studies" Coatings 12, no. 1: 22. https://doi.org/10.3390/coatings12010022
APA StyleAhmed, I. A., Seliem, M. K., Lima, E. C., Badawi, M., Li, Z., Bonilla-Petriciolet, A., & Anastopoulos, I. (2022). Outstanding Performance of a New Exfoliated Clay Impregnated with Rutile TiO2 Nanoparticles Composite for Dyes Adsorption: Experimental and Theoretical Studies. Coatings, 12(1), 22. https://doi.org/10.3390/coatings12010022











