Influence of Reaction pH towards the Physicochemical Characteristics of Phosphorylated Polyvinyl Alcohol-Aluminum Phosphate Nanocomposite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PPVA-AlPO4
2.3. Instrumental Analysis
3. Results and Discussion
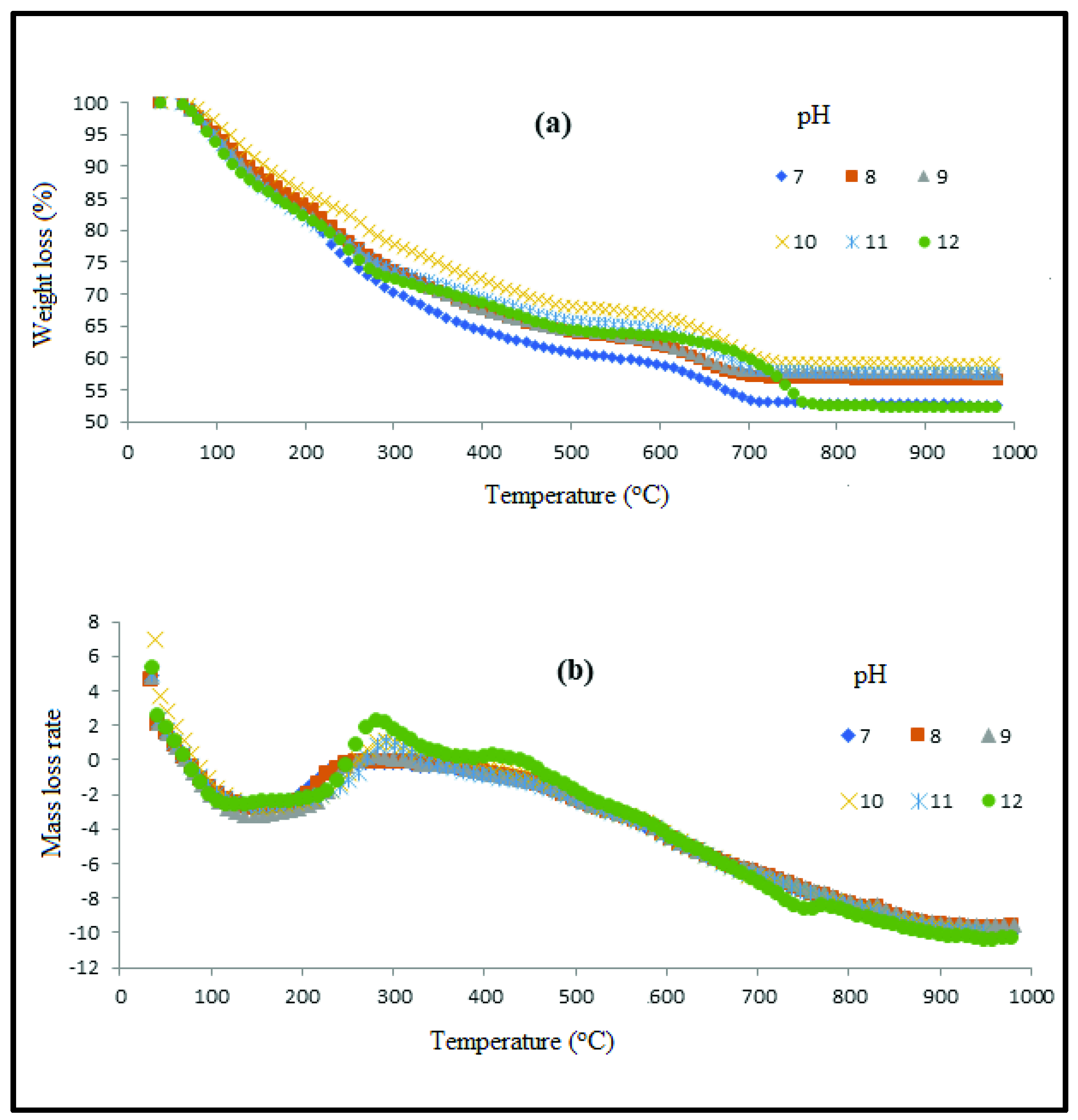
3.1. Thermal Analysis
3.2. Powdered XRD Analysis
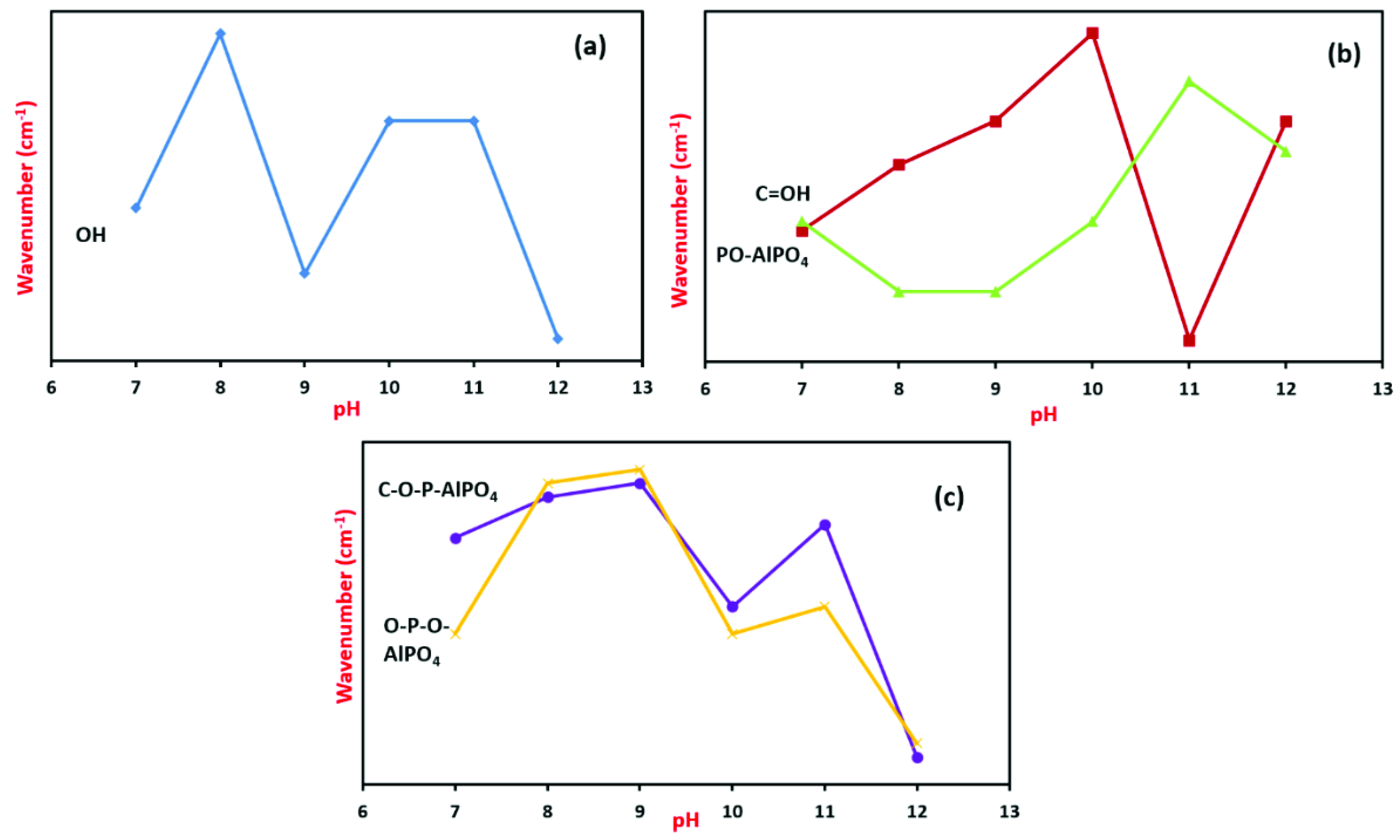
3.3. FTIR Analysis
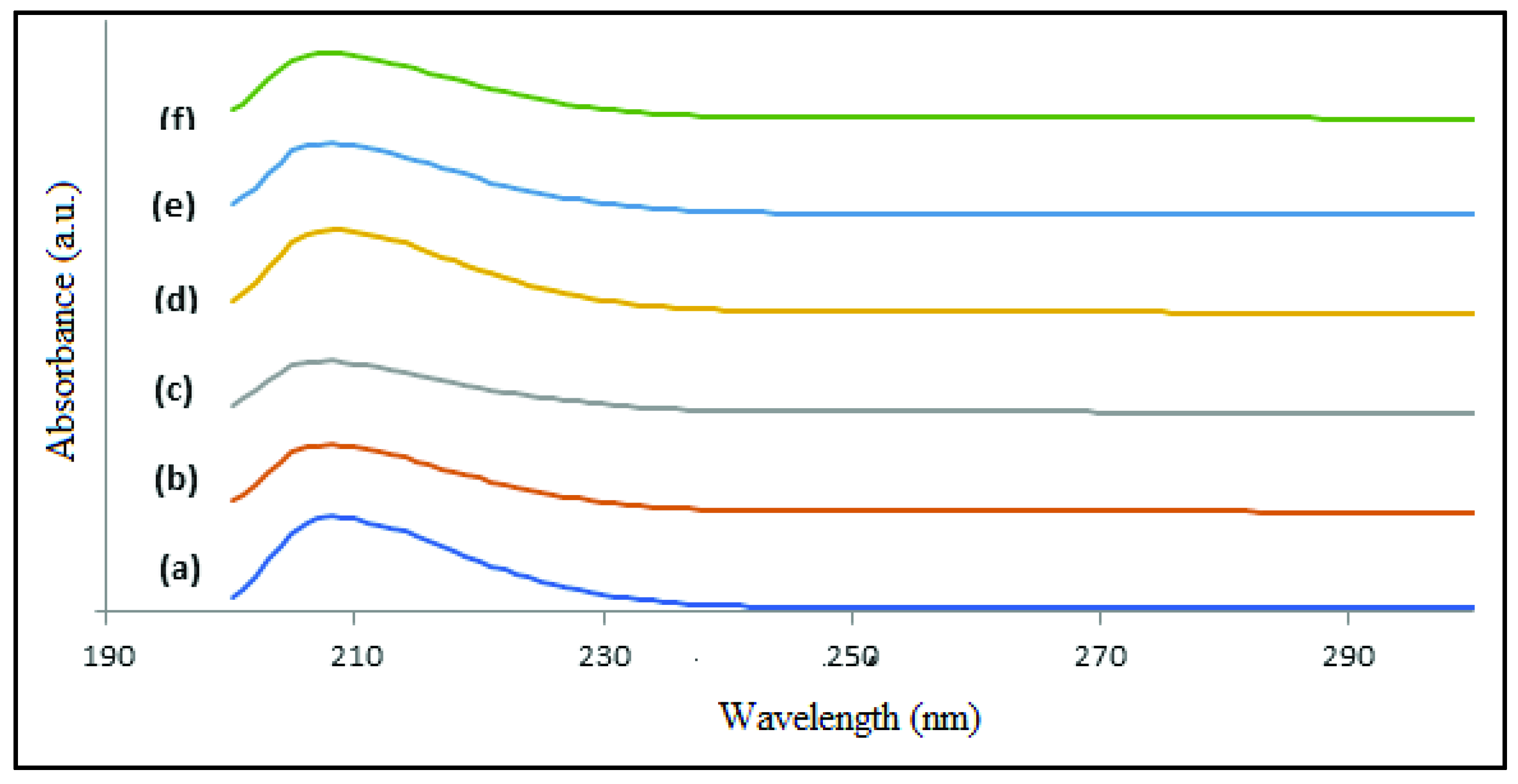
3.4. UV-Vis Analysis
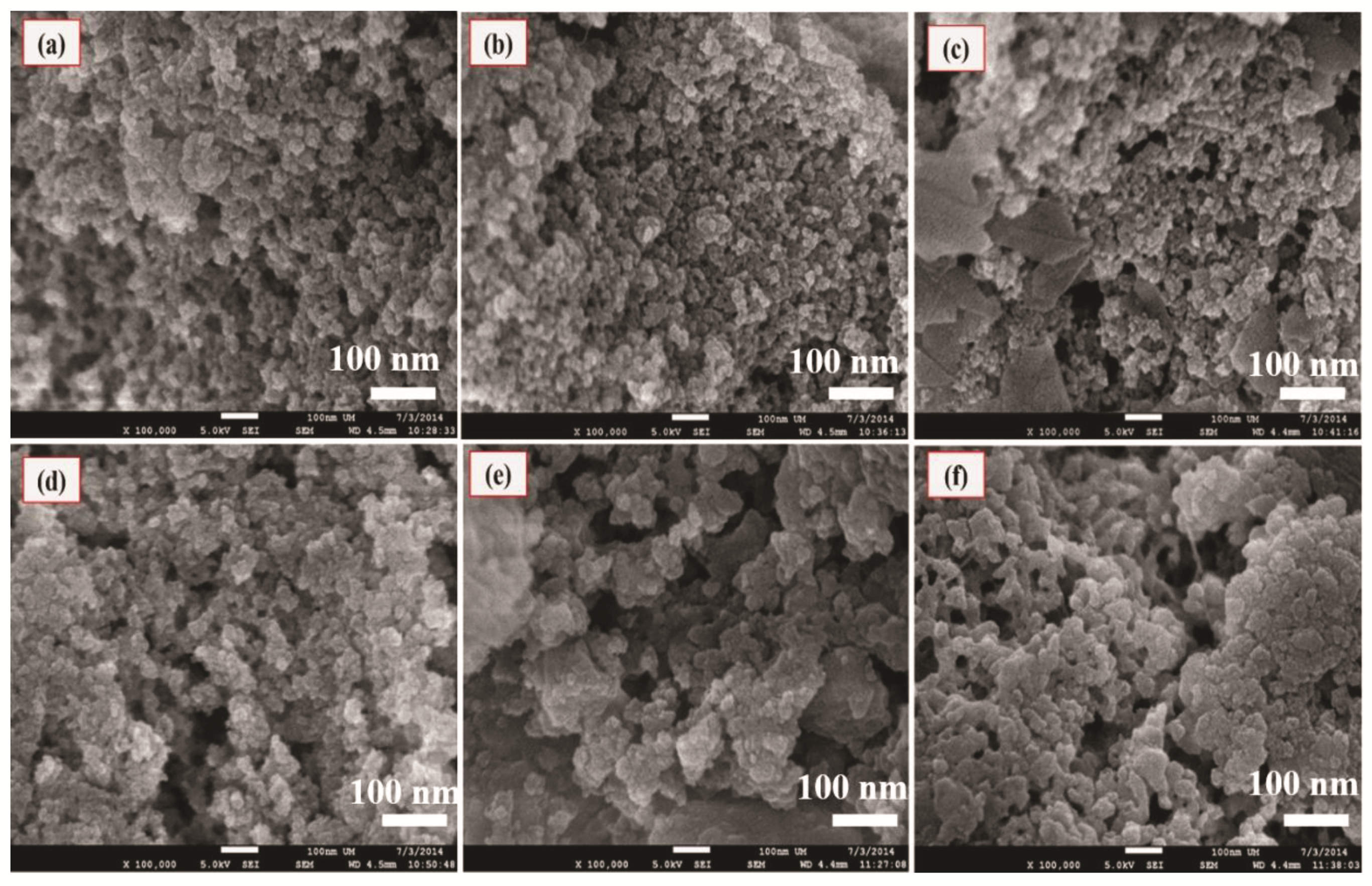
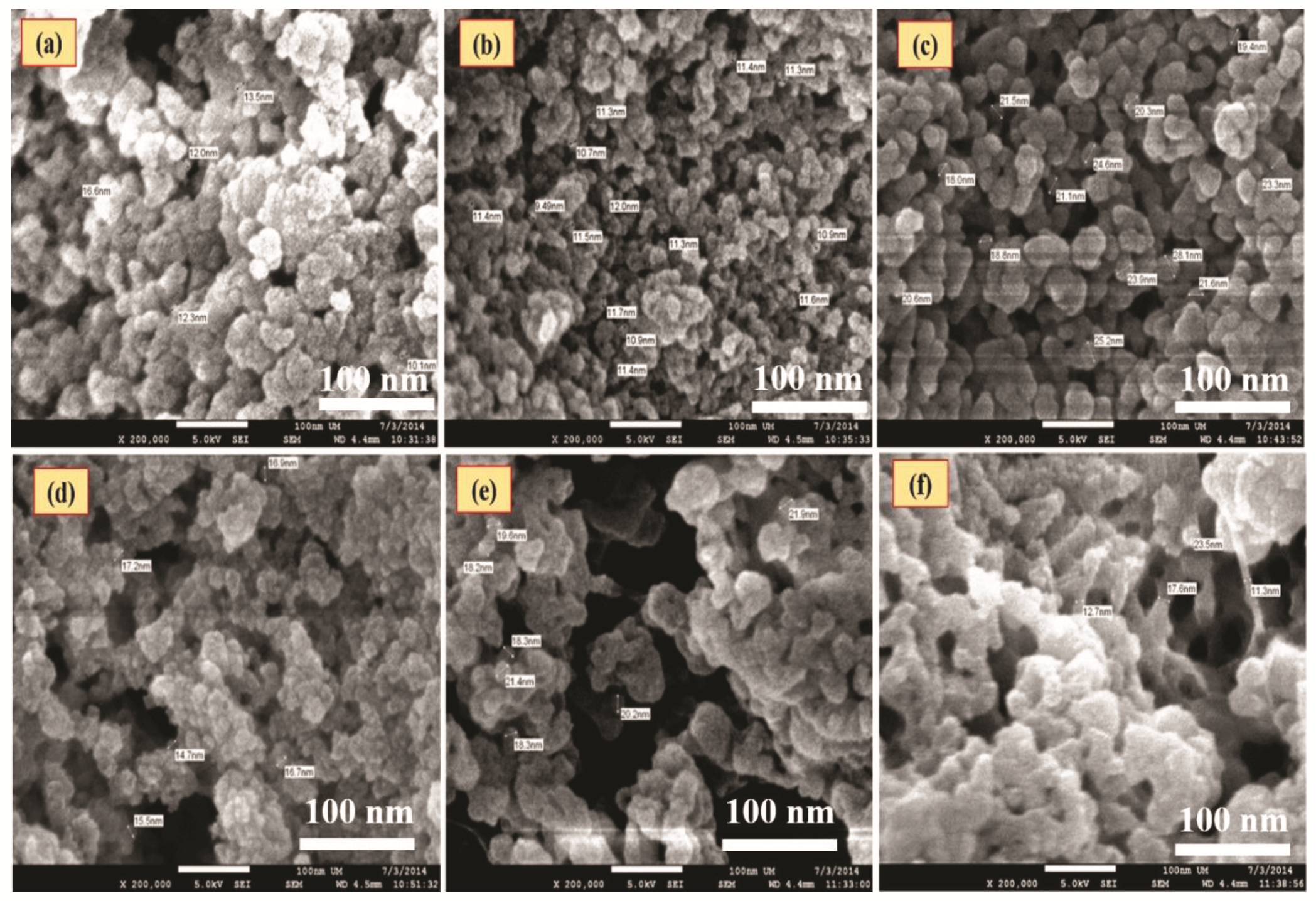
3.5. FESEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, J.; Zhang, P.; Song, L.; Li, J.; Ji, B.; Li, J.; Chen, L. Polyethylene glycol supported by phosphorylated polyvinyl alcohol/graphene aerogel as a high thermal stability phase change material. Compos. Part B Eng. 2019, 179. [Google Scholar] [CrossRef]
- Sayyedan, F.S.; Enayati, M.H. On structure and oxidation behaviour of non-stoichiometric amorphous aluminium phosphate coating. Surf. Eng. 2019, 35, 670–676. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Zhu, S.; Li, H.; Ma, Z.; Liu, Y.; Liu, L. Preparation of an aluminium phosphate binder and its influence on the bonding strength of coating. Bull. Mater. Sci. 2019, 42, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Khadom, A.A.; Farhan, S.N. Corrosion inhibition of steel in phosphoric acid. Corros. Rev. 2018, 36, 267–280. [Google Scholar] [CrossRef]
- Stöckel, S.; Ebert, S.; Böttcher, M.; Seifert, A.; Wamser, T.; Krenkel, W.; Schulze, S.; Hietschold, M.; Gnaegi, H.; Goedel, W.A. Coating of alumina fibres with aluminium phosphate by a continuous chemical vapour deposition process. Chem. Vap. Depos. 2014, 20, 388–398. [Google Scholar] [CrossRef]
- Saat, A.M.; Johan, M.R. The surface structure and thermal properties of novel polymer composite films based on partially phosphorylated poly (vinyl alcohol) with aluminum phosphate. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaseem, M.; Hussain, T.; Baek, S.H.; Ko, Y.G. Formation of stable coral reef-like structures via self-assembly of functionalized polyvinyl alcohol for superior corrosion performance of AZ31 Mg alloy. Mater. Des. 2020, 193, 108823. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Sanchez, C.; Rozes, L.; Ribot, F.; Laberty-Robert, C.; Grosso, D.; Sassoye, C.; Boissiere, C.; Nicole, L. Chimie douce: A land of opportunities for the designed construction of functional inorganic and hybrid organic-inorganic nanomaterials. Comptes Rendus Chim. 2010, 13, 3–39. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Liu, H.; Zhang, X.; Wen, R.; He, C.; Yin, Z.; Liu, Y.-G.; Fang, M.; Wu, X.; Min, X. Growth mechanism and synchronous synthesis of 1D β-sialon nanostructures and β-sialon-Si3N4 composite powders by a process of reduction nitridation. Mater. Res. Express 2019, 6, 065054. [Google Scholar] [CrossRef]
- Son, H.-W.; Berthebaud, D.; Yubuta, K.; Yoshikawa, A.; Shishido, T.; Suzuta, K.; Mori, T. New synthesis route for complex borides; rapid synthesis of thermoelectric yttrium aluminoboride via liquid-phase assisted reactive spark plasma sintering. Sci. Rep. 2020, 10, 8914. [Google Scholar] [CrossRef] [PubMed]
- Saat, A.M.; Johan, M.D. Enhanced thermal and structural properties of partially phosphorylated polyvinyl alcohol—Aluminum phosphate (PPVA-AlPO4) nanocomposites with aluminium nitrate source. AIP Conf. Proc. 2017, 1901, 130011. [Google Scholar]
- Chen, D.; He, L.; Shang, S. Study on aluminum phosphate binder and related Al2O3-SiC ceramic coating. Mater. Sci. Eng. A 2003, 384, 29–35. [Google Scholar] [CrossRef]
- Mekky, W.; Nicholson, P.S. Nano-aluminum-phosphate via a polymerized organic–inorganic complex route. J. Mater. Process. Technol. 2007, 190, 393–396. [Google Scholar] [CrossRef]
- Burrell, L.; Johnston, C.; Schulze, D.; Klein, J.; White, J.L.; Hem, S.L. Aluminium phosphate adjuvants prepared by precipitation at constant pH. Part II: Physicochemical properties. Vaccine 2001, 19, 282–287. [Google Scholar] [CrossRef]
- Palacios, E.; Leret, P.; María, J.; Fernández, J.F.; Antonio, H.; Rodríguez, M.A. Influence of the pH and ageing time on the acid aluminum phosphate synthesized by precipitation. CrystEngComm 2013, 15, 3359–3365. [Google Scholar] [CrossRef]
- Kandori, K.; Ikeguchi, N.; Yasukawa, A.; Ishikawa, T. Control of size and adsorptive properties of spherical aluminum phosphate particles. J. Colloid Interface Sci. 1996, 430, 425–430. [Google Scholar] [CrossRef]
- Kawamura, K.; Shibuya, K.; Okuwaki, A. Morphology of aluminum phosphate by the Al-EDTA mediated particle formation in aqueous solutions at high temperatures. Mater. Res. Bull. 2007, 42, 256–264. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, K.; Shi, Y.; Hong, N.; Lo, S.; Hu, Y.; Gui, Z. 2D Lamellar Aluminophosphate Nanolayers for Enhancing Flame Retardancy and Mechanical Properties of Polymers. Ind. Eng. Chem. Res. 2013, 52, 16766–16773. [Google Scholar] [CrossRef]
- Ganschow, M.; Schulz-Ekloff, G.; Wark, M.; Wendschuh-Josties, M.; Wöhrle, D. Microwave-assisted preparation of uniform pure and dye-loaded AlPO4-5 crystals with different morphologies for use as microlaser systems. J. Mater. Chem. 2001, 11, 1823–1827. [Google Scholar] [CrossRef]





| Samples | pH < 1 (Initial) | KOH Volume (mL) | pH (Intermediate) | Appearance | |
|---|---|---|---|---|---|
| As prepared | pH 7 | Colourless solution | 126.50 | 7.13 | White solution |
| pH 8 | Colourless solution | 129 | 8.16 | White solution | |
| pH 9 | Colourless solution | 131.20 | 9.22 | White solution | |
| pH 10 | Colourless solution | 132.90 | 10.17 | White solution | |
| pH 11 | Colourless solution | 135 | 11.18 | White solution | |
| pH 12 | Colourless solution | 139.90 | 12 | White solution | |
| pH 13 | Colourless solution | >150 | 13 | Colourless solution | |
| Thermal Properties | pH 7 | pH 8 | pH 9 | pH 10 | pH 11 | pH 12 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDTA | T (°C) | T (°C) | T (°C) | T (°C) | T (°C) | T (°C) | ||||||
| 1 | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | ||||||
| 2 | 250 weak broad (endo) | 250 weak broad (endo) | 250 weak broad (endo) | 285 intense (endo) | 285 intense (endo) | 280 intense (endo) | ||||||
| 3 | Broad less intense exothermic peak at 400–460 | Broad less intense exothermic peak at 400–460 | Broad less intense exothermic peak at 400–460 | Broad less intense exothermic peak at 400–460 (455) | Broad less intense exothermic peak at 400–460 | 410 small exothermic peak | ||||||
| 4 | 680 | 670 | 670 | 680 | 680 | 770(exo) | ||||||
| TGA | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) |
| 1st stage | 0–260 | 28 | 0–260 | 37 | 0–260 | 38 | 0–280 | 21 | 0–260 | 25 | 0–260 | 27 |
| 2nd stage | 260–450 | 11 | 260–450 | 10 | 260–450 | 11 | 280–480 | 9 | 260–480 | 8 | 260–480 | 9 |
| 3rd stage | 450–700 | 8 | 450–700 | 9 | 450–700 | 8 | 480–720 | 11 | 480–720 | 9 | 480–760 | 12 |
| Final Weight residue | 700–1000 | 53 | 700–1000 | 56 | 700–1000 | 57 | 720–1000 | 59 | 720–1000 | 58 | 760–1000 | 52 |
| Thermal Properties | pH 7 | pH 8 | pH 9 | pH 10 | pH 11 | pH 12 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDTA | T (°C) | T (°C) | T (°C) | T (°C) | T (°C) | T (°C) | ||||||
| 1 | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | 30–250(broad endothermic peak) | ||||||
| 2 | 250 weak broad (endo) | 250 weak broad (endo) | 250 weak broad (endo) | 285 intense (endo) | 285 intense (endo) | 280 intense (endo) | ||||||
| 3 | Broad less intense exothermic peak at 400–460 | Broad less intense exothermic peak at 400–460 | Broad less intense exothermic peak at 400–460 | Broad less intense exothermic peak at 400–460 (455) | Broad less intense exothermic peak at 400–460 | 410 small exothermic peak | ||||||
| 4 | 680 | 670 | 670 | 680 | 680 | 770(exo) | ||||||
| TGA | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) | T (°C) | Weight Residue (%) |
| 1st stage | 0–260 | 28 | 0–260 | 37 | 0–260 | 38 | 0–280 | 21 | 0–260 | 25 | 0–260 | 27 |
| 2nd stage | 260–450 | 11 | 260–450 | 10 | 260–450 | 11 | 280–480 | 9 | 260–480 | 8 | 260–480 | 9 |
| 3rd stage | 450–700 | 8 | 450–700 | 9 | 450–700 | 8 | 480–720 | 11 | 480–720 | 9 | 480–760 | 12 |
| Final Weight residue | 700–1000 | 53 | 700–1000 | 56 | 700–1000 | 57 | 720–1000 | 59 | 720–1000 | 58 | 760–1000 | 52 |
| Band Assignment and Wavenumber (cm−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Al−OH | OH | Al−O–P | H−O−H | C−OH@ H−O−H | P−O−AlPO4 | C−O−P−AlPO4 | HP−O−AlPO4 | O−P−O−AlPO4 | O−P−O−AlPO4 | |
| As prepared | pH 7 | 3377 | 2202 | 1640 | 1372 | 1043 | 822 | 541 | |||
| pH 8 | 3385 | 1643 | 1371 | 1046 | 823 | 552 | 418 | ||||
| pH 9 | 3374 | 2253 | 1645 | 1371 | 1047 | 823 | 553 | 435 | |||
| pH 10 | 3786 | 3381 | 1649 | 1372 | 1038 | 823 | 541 | 419 | |||
| pH 11 | 3381 | 2206 | 1730 | 1635 | 1374 | 1044 | 543 | 420 | |||
| pH 12 | 3371 | 1740 | 1645 | 1373 | 1027 | 824 | 533 | 422 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Saat, A.; Kamil, M.S.; Hamizi, N.A.; Badruddin, I.A.; Ghazali, N.; Sagadevan, S.; Kamangar, S.; Khan, T.M.Y.; Johan, M.R. Influence of Reaction pH towards the Physicochemical Characteristics of Phosphorylated Polyvinyl Alcohol-Aluminum Phosphate Nanocomposite. Coatings 2021, 11, 1105. https://doi.org/10.3390/coatings11091105
Mohamed Saat A, Kamil MS, Hamizi NA, Badruddin IA, Ghazali N, Sagadevan S, Kamangar S, Khan TMY, Johan MR. Influence of Reaction pH towards the Physicochemical Characteristics of Phosphorylated Polyvinyl Alcohol-Aluminum Phosphate Nanocomposite. Coatings. 2021; 11(9):1105. https://doi.org/10.3390/coatings11091105
Chicago/Turabian StyleMohamed Saat, Asmalina, Md Salim Kamil, Nor Aliya Hamizi, Irfan Anjum Badruddin, Nadiah Ghazali, Suresh Sagadevan, Sarfaraz Kamangar, T. M. Yunus Khan, and Mohd Rafie Johan. 2021. "Influence of Reaction pH towards the Physicochemical Characteristics of Phosphorylated Polyvinyl Alcohol-Aluminum Phosphate Nanocomposite" Coatings 11, no. 9: 1105. https://doi.org/10.3390/coatings11091105
APA StyleMohamed Saat, A., Kamil, M. S., Hamizi, N. A., Badruddin, I. A., Ghazali, N., Sagadevan, S., Kamangar, S., Khan, T. M. Y., & Johan, M. R. (2021). Influence of Reaction pH towards the Physicochemical Characteristics of Phosphorylated Polyvinyl Alcohol-Aluminum Phosphate Nanocomposite. Coatings, 11(9), 1105. https://doi.org/10.3390/coatings11091105







