Amine-Rich Coatings to Potentially Promote Cell Adhesion, Proliferation and Differentiation, and Reduce Microbial Colonization: Strategies for Generation and Characterization
Abstract
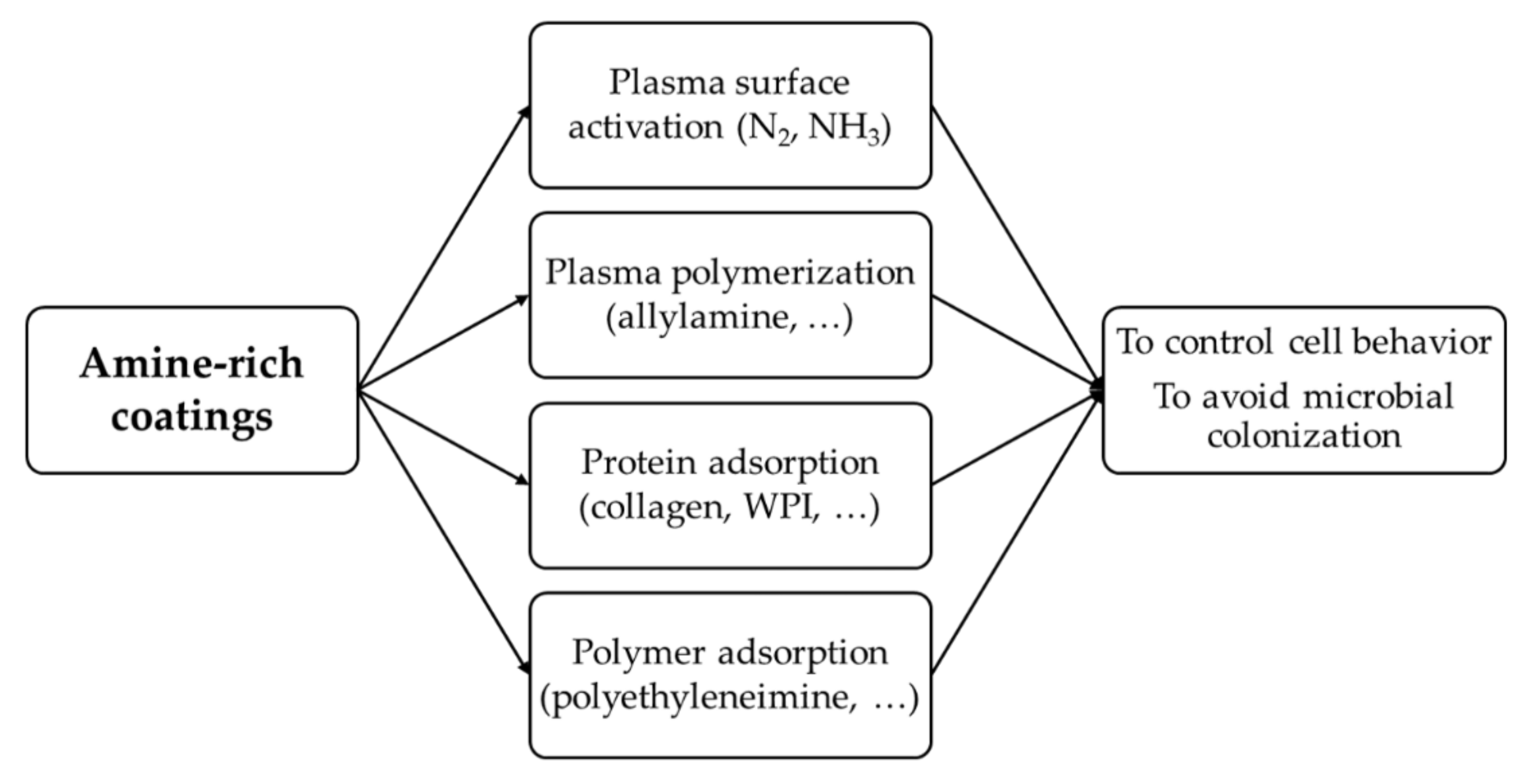
:1. Introduction
- -
- Application of plasma:
- Plasma surface activation with nitrogen (N2) or ammonia (NH3) gas;
- Plasma polymerization with a precursor containing –NH2 groups, such as allylamine.
- -
- Chemical modification of the surface by adsorption of molecules:
- Proteins such as collagen or whey protein isolate (WPI);
- Synthetic polymers that contain –NH2 groups, such as polyethyleneimine (PEI) or polydopamine (PDA).
- -
- Physicochemical characterization of the coatings: contact angle (CA) measurements, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), atomic force microscopy (AFM), Fourier-transform infrared spectroscopy (FTIR);
- -
- Amine groups quantification: dyes methods (Coomassie Brilliant Blue, Orange II) or chemical derivatization (with glutaraldehyde, or compounds in vapor phase).
2. Plasma Coatings
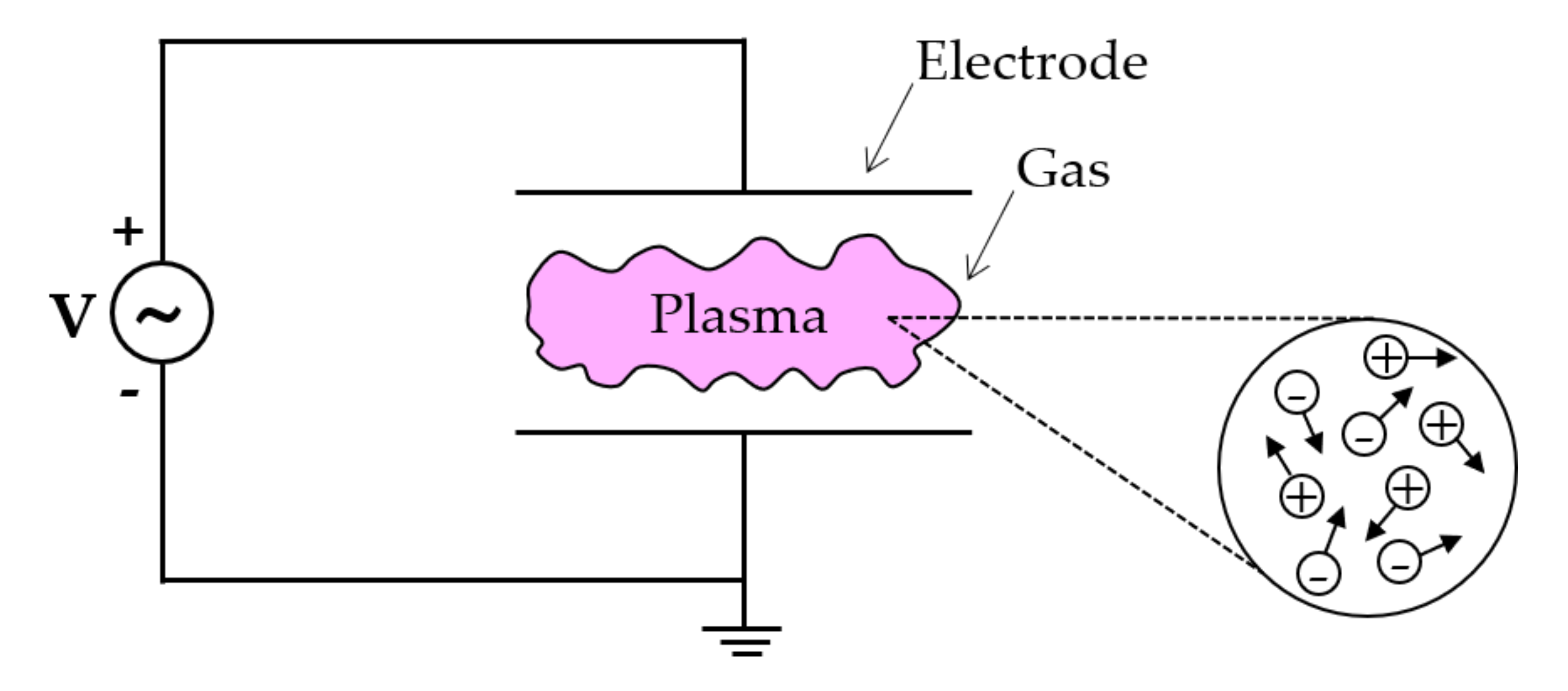
2.1. General Information on Plasma
2.2. Plasma Amine-Rich Coatings
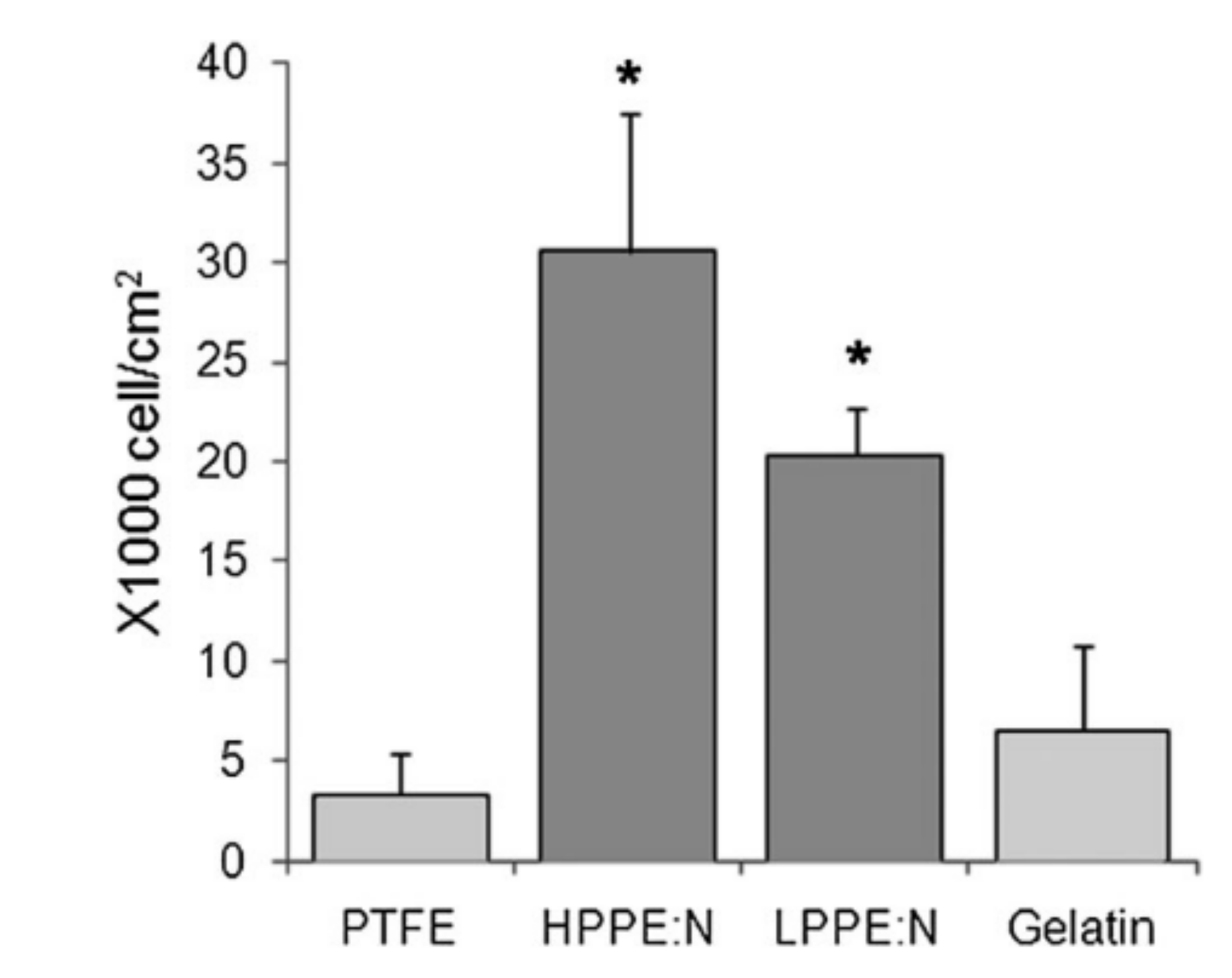
2.2.1. Effect on Cell Behavior
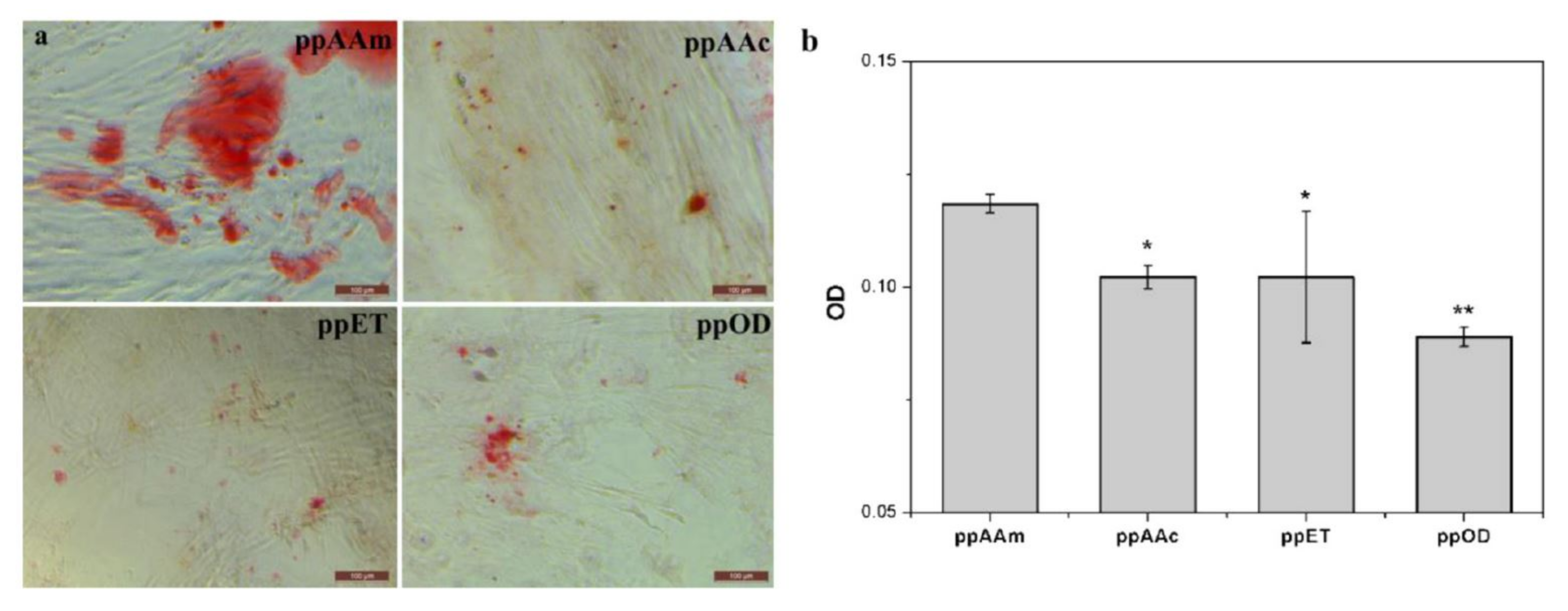
2.2.2. Molecules’ Immobilization
2.2.3. Antimicrobial Applications
3. Protein Coatings
3.1. Globular Protein Coatings
3.2. Fibrillar Coatings
3.2.1. Collagen Fibrillar Coatings
3.2.2. Amyloid Fibrillar Coatings
3.2.3. Other Fibrillar Coatings
4. Synthetic Polymer Coatings
5. Characterization of Amine-Rich Coatings
5.1. Physicochemical Characterization
5.2. Amine Groups Quantification
- -
- Dyes: A comparative study between Orange II and Coomassie Brilliant Blue dyes showed that Orange II dye seems to be the most appropriate in the case of primary amine grafted on polyethylene terephthalate (PET) [103]. In another study, the Orange II dye was used on PET membrane treated by allylamine plasma. A positive correlation was found between the results of the colorimetric staining and XPS and FTIR analyses. Coomassie Brilliant Blue is commonly used following the amino density estimation by colorimetric assay (ADECA) method based on the reversible formation of a complex of CBB with the N+ groups [104]. After staining and washing, the dye in solution is quantified, leading to an evaluation of the amine groups. The reversibility of the process provides an advantage to this method. However, this method seems to be less reliable than the Orange II quantification method due to steric hindrance that limits the interaction between the dye and amine groups [103].
- -
- Chemical derivatization: Chemical derivatization is widely used by grafting glutaraldehyde with an enzyme detectable by fluorescence spectroscopy or microscopy [30]. Regarding plasma deposition, in the vapor phase, amine groups may be identified via the grafting of compounds such as 4-trifluoromethyl-benzaldehyde (TFBA) as shown in Figure 8 [8] or pentafluorobenzaldehyde (PFBA) [97] in the vapor phase. For example, TFBA can be chemically grafted via imine bonds to –NH2 groups. Then, XPS analyses are performed to detect the presence of fluorine in the coating.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Health Care-Associated Infections—Fact Sheet. Available online: https://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf (accessed on 14 February 2020).
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: https://www.jpiamr.eu/wp-content/uploads/2014/12/AMR-Review-Paper-Tackling-a-crisis-for-the-health-and-wealth-of-nations_1-2.pdf (accessed on 14 February 2020).
- Monegro, A.F.; Regunath, H.; Hospital Acquired Infections. StatPearls. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441857/ (accessed on 14 February 2020).
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wertheimer, M.R.; St-Georges-Robillard, A.; Lerouge, S.; Mwale, F.; Elkin, B.; Oehr, C.; Wirges, W.; Gerhard, R. Fabrication and characterization of organic thin films for applications in tissue engineering: Emphasis on cell-surface interactions. MRS Online Proc. Libr. 2012, 1469, 43–48. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surface modification by allylamine plasma polymerization promotes osteogenic differentiation of human adipose-derived stem cells. ACS Appl. Mater. Interfaces 2014, 6, 9733–9741. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Meyer-Plath, A.A.; Schröder, K.; Finke, B.; Ohl, A. Current trends in biomaterial surface functionalization—Nitrogen-containing plasma assisted processes with enhanced selectivity. Vacuum 2003, 71, 391–406. [Google Scholar] [CrossRef]
- Bílek, F.; Křížová, T.; Lehocký, M. Preparation of active antibacterial LDPE surface through multistep physicochemical approach: I. Allylamine grafting, attachment of antibacterial agent and antibacterial activity assessment. Colloids Surf. B Biointerfaces 2011, 88, 440–447. [Google Scholar] [CrossRef]
- Reis, R.; Dumée, L.F.; He, L.; She, F.; Orbell, J.D.; Winther-Jensen, B.; Duke, M.C. Amine enrichment of thin-film composite membranes via low pressure plasma polymerization for antimicrobial adhesion. ACS Appl. Mater. Interfaces 2015, 7, 14644–14653. [Google Scholar] [CrossRef]
- Vasilev, K.; Sah, V.R.; Goreham, R.V.; Ndi, C.; Short, R.D.; Griesser, H.J. Antibacterial surfaces by adsorptive binding of polyvinyl-sulphonate- stabilized silver nanoparticles. Nanotechnology 2010, 21, 215102. [Google Scholar] [CrossRef]
- Aziz, G.; De Geyter, N.; Morent, R. Incorporation of primary amines via plasma technology on biomaterials. In Advances in Bioengineering; InTech: London, UK, 2015. [Google Scholar]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 9780471724254. [Google Scholar]
- Song, H.; Jung, S.C.; Kim, B.H. Focal adhesion of osteoblastic cells on titanium surface with amine functionalities formed by plasma polymerization. Jpn. J. Appl. Phys. 2012, 51, 08HE01. [Google Scholar] [CrossRef]
- Ren, T.B.; Weigel, T.; Groth, T.; Lendlein, A. Microwave plasma surface modification of silicone elastomer with allylamine for improvement of biocompatibility. J. Biomed. Mater. Res. Part A 2008, 86A, 209–219. [Google Scholar] [CrossRef]
- Aziz, G.; De Geyter, N.; Declercq, H.; Cornelissen, R.; Morent, R. Incorporation of amine moieties onto ultra-high molecular weight polyethylene (UHMWPE) surface via plasma and UV polymerization of allylamine. Surf. Coat. Technol. 2015, 271, 39–47. [Google Scholar] [CrossRef]
- Aziz, G.; Thukkaram, M.; De Geyter, N.; Morent, R. Plasma parameters effects on the properties, aging and stability behaviors of allylamine plasma coated ultra-high molecular weight polyethylene (UHMWPE) films. Appl. Surf. Sci. 2017, 409, 381–395. [Google Scholar] [CrossRef]
- Vasilev, K.; Michelmore, A.; Martinek, P.; Chan, J.; Sah, V.; Griesser, H.J.; Short, R.D. Early stages of growth of plasma polymer coatings deposited from nitrogen- and oxygen-containing monomers. Plasma Process. Polym. 2010, 7, 824–835. [Google Scholar] [CrossRef]
- Zhao, J.H.; Michalski, W.P.; Williams, C.; Li, L.; Xu, H.S.; Lamb, P.R.; Jones, S.; Zhou, Y.M.; Dai, X.J. Controlling cell growth on titanium by surface functionalization of heptylamine using a novel combined plasma polymerization mode. J. Biomed. Mater. Res. Part A 2011, 97A, 127–134. [Google Scholar] [CrossRef]
- Finke, B.; Hempel, F.; Testrich, H.; Artemenko, A.; Rebl, H.; Kylián, O.; Meichsner, J.; Biederman, H.; Nebe, B.; Weltmann, K.D.; et al. Plasma processes for cell-adhesive titanium surfaces based on nitrogen-containing coatings. Surf. Coat. Technol. 2011, 205. [Google Scholar] [CrossRef]
- Testrich, H.; Rebl, H.; Finke, B.; Hempel, F.; Nebe, B.; Meichsner, J. Aging effects of plasma polymerized ethylenediamine (PPEDA) thin films on cell-adhesive implant coatings. Mater. Sci. Eng. C 2013, 33, 3875–3880. [Google Scholar] [CrossRef]
- Gigout, A.; Ruiz, J.-C.; Wertheimer, M.R.; Jolicoeur, M.; Lerouge, S. Nitrogen-rich plasma-polymerized coatings on PET and PTFE surfaces improve endothelial cell attachment and resistance to shear flow. Macromol. Biosci. 2011, 11, 1110–1119. [Google Scholar] [CrossRef]
- Truica-Marasescu, F.; Wertheimer, M.R. Nitrogen-rich plasma-polymer films for biomedical applications. Plasma Process. Polym. 2008, 5, 44–57. [Google Scholar] [CrossRef]
- Ruiz, J.C.; St-Georges-Robillard, A.; Thérésy, C.; Lerouge, S.; Wertheimer, M.R. Fabrication and characterisation of amine-rich organic thin films: Focus on stability. Plasma Process. Polym. 2010, 7, 737–753. [Google Scholar] [CrossRef]
- Babaei, S.; Girard-Lauriault, P.L. Tuning the surface properties of oxygen-rich and nitrogen-rich plasma polymers: Functional groups and surface charge. Plasma Chem. Plasma Process. 2016, 36, 651–666. [Google Scholar] [CrossRef]
- Naderi, J.; Giles, C.; Saboohi, S.; Griesser, H.J.; Coad, B.R. Combatting fungal biofilm formation by diffusive release of fluconazole from heptylamine plasma polymer coating. Biointerphases 2020, 15, 061012. [Google Scholar] [CrossRef]
- Nemcakova, I.; Blahova, L.; Rysanek, P.; Blanquer, A.; Bacakova, L.; Zajíčková, L. Behaviour of vascular smooth muscle cells on amine plasma-coated materials with various chemical structures and morphologies. Int. J. Mol. Sci. 2020, 21, 9467. [Google Scholar] [CrossRef]
- Charbonneau, C.; Ruiz, J.-C.; Lequoy, P.; Hébert, M.-J.; De Crescenzo, G.; Wertheimer, M.R.; Lerouge, S. Chondroitin sulfate and epidermal growth factor immobilization after plasma polymerization: A versatile anti-apoptotic coating to promote healing around stent grafts. Macromol. Biosci. 2012, 12, 812–821. [Google Scholar] [CrossRef]
- Abbas, A.; Vercaigne-Marko, D.; Supiot, P.; Bocquet, B.; Vivien, C.; Guillochon, D. Covalent attachment of trypsin on plasma polymerized allylamine. Colloids Surf. B Biointerfaces 2009, 73, 315–324. [Google Scholar] [CrossRef]
- Gancarz, I.; Bryjak, J.; Poźniak, G.; Tylus, W. Plasma modified polymers as a support for enzyme immobilization II. Amines plasma. Eur. Polym. J. 2003, 39, 2217–2224. [Google Scholar] [CrossRef]
- Yang, J.; Bei, J.; Wang, S. Enhanced cell affinity of poly (D,L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 2002, 23, 2607–2614. [Google Scholar] [CrossRef]
- Simovic, S.; Losic, D.; Vasilev, K. Controlled drug release from porous materials by plasma polymer deposition. Chem. Commun. 2010, 46, 1317–1319. [Google Scholar] [CrossRef]
- Girard-Lauriault, P.-L.; Truica-Marasescu, F.; Petit, A.; Wang, H.T.; Desjardins, P.; Antoniou, J.; Mwale, F.; Wertheimer, M.R. Adhesion of human U937 monocytes to nitrogen-rich organic thin films: Novel insights into the mechanism of cellular adhesion. Macromol. Biosci. 2009, 9, 911–921. [Google Scholar] [CrossRef]
- Girard-Lauriault, P.-L.; Mwale, F.; Iordanova, M.; Demers, C.; Desjardins, P.; Wertheimer, M.R. Atmospheric pressure deposition of micropatterned nitrogen-rich plasma-polymer films for tissue engineering. Plasma Process. Polym. 2005, 2, 263–270. [Google Scholar] [CrossRef]
- Bílek, F.; Sulovská, K.; Lehocký, M.; Sáha, P.; Humpolíček, P.; Mozetič, M.; Junkar, I. Preparation of active antibacterial LDPE surface through multistep physicochemical approach II: Graft type effect on antibacterial properties. Colloids Surf. B Biointerfaces 2013, 102, 842–848. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Nourbakhsh, N.; Kenari, M.A.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021. [Google Scholar] [CrossRef]
- Hay, E. Cell Biology of Extracellular Matrix; Springer: New York, NY, USA, 1981; ISBN 978-1-4612-8226-6. [Google Scholar]
- Rabe, R.; Hempel, U.; Martocq, L.; Keppler, J.K.; Aveyard, J.; Douglas, T.E.L. Dairy-inspired coatings for bone implants from whey protein isolate-derived self-assembled fibrils. Int. J. Mol. Sci. 2020, 21, 5544. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Douglas, T.E.L.; Heinemann, S.; Scharnweber, D.; Dubruel, P.; Bacakova, L. Collagen-lactoferrin fibrillar coatings enhance osteoblast proliferation and differentiation. J. Biomed. Mater. Res. Part A 2015, 103, 525–533. [Google Scholar] [CrossRef]
- Douglas, T.; Heinemann, S.; Mietrach, C.; Hempel, U.; Bierbaum, S.; Scharnweber, D.; Worch, H. Interactions of collagen types I and II with chondroitin sulfates A-C and their effect on osteoblast adhesion. Biomacromolecules 2007, 8, 1085–1092. [Google Scholar] [CrossRef]
- Haynes, C.A.; Norde, W. Globular proteins at solid/liquid interfaces. Colloids Surf. B Biointerfaces 1994, 2, 517–566. [Google Scholar] [CrossRef]
- Norde, W. Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid Interface Sci. 1986, 25, 267–340. [Google Scholar] [CrossRef]
- Hempel, U.; Preissler, C.; Vogel, S.; Möller, S.; Hintze, V.; Becher, J.; Schnabelrauch, M.; Rauner, M.; Hofbauer, L.C.; Dieter, P. Artificial extracellular matrices with oversulfated glycosaminoglycan derivatives promote the differentiation of osteoblast-precursor cells and premature osteoblasts. Biomed. Res. Int. 2014, 2014, 938368. [Google Scholar] [CrossRef]
- Hempel, U.; Matthäus, C.; Preissler, C.; Möller, S.; Hintze, V.; Dieter, P. Artificial matrices with high-sulfated glycosaminoglycans and collagen are anti-inflammatory and pro-osteogenic for human mesenchymal stromal cells. J. Cell. Biochem. 2014, 115, 1561–1571. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Oppenheim, T.W.; Buell, A.K.; Chirgadze, D.Y.; Welland, M.E. Nanostructured films from hierarchical self-assembly of amyloidogenic proteins. Nat. Nanotechnol. 2010, 5, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, N.P.; Styan, K.E.; Easton, C.D.; Li, Y.; Waddington, L.; Lara, C.; Forsythe, J.S.; Mezzenga, R.; Hartley, P.G.; Muir, B.W. Nanotopographic surfaces with defined surface chemistries from amyloid fibril networks can control cell attachment. Biomacromolecules 2013, 14, 2305–2316. [Google Scholar] [CrossRef]
- Reynolds, N.P.; Charnley, M.; Mezzenga, R.; Hartley, P.G. Engineered lysozyme amyloid fibril networks support cellular growth and spreading. Biomacromolecules 2014, 15, 599–608. [Google Scholar] [CrossRef]
- Reynolds, N.P.; Charnley, M.; Bongiovanni, M.N.; Hartley, P.G.; Gras, S.L. Biomimetic topography and chemistry control cell attachment to amyloid fibrils. Biomacromolecules 2015, 16, 1556–1565. [Google Scholar] [CrossRef] [Green Version]
- Hindié, M.; Degat, M.C.; Gaudire, F.; Gallet, O.; Van Tassel, P.R.; Pauthe, E. Pre-osteoblasts on poly(l-lactic acid) and silicon oxide: Influence of fibronectin and albumin adsorption. Acta Biomater. 2011, 7, 387–394. [Google Scholar] [CrossRef]
- Sima, F.; Davidson, P.; Pauthe, E.; Sima, L.E.; Gallet, O.; Mihailescu, I.N.; Anselme, K. Fibronectin layers by matrix-assisted pulsed laser evaporation from saline buffer-based cryogenic targets. Acta Biomater. 2011, 7, 3780–3788. [Google Scholar] [CrossRef]
- Montaño-Machado, V.; Chevallier, P.; Mantovani, D.; Pauthe, E. On the potential for fibronectin/phosphorylcholine coatings on PTFE substrates to jointly modulate endothelial cell adhesion and hemocompatibility properties. Biomatter 2015, 5, e979679. [Google Scholar] [CrossRef] [Green Version]
- Geißler, U.; Hempel, U.; Wolf, C.; Scharnweber, D.; Worch, H.; Wenzel, K.W. Collagen type I-coating of Ti6A14V promotes adhesion of osteoblasts. J. Biomed. Mater. Res. 2000, 51, 752–760. [Google Scholar] [CrossRef]
- Roehlecke, C.; Witt, M.; Kasper, M.; Schulze, E.; Wolf, C.; Hofer, A.; Funk, R.H.W. Synergistic effect of titanium alloy and collagen type I on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs 2001, 168, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Mieszkowska, A.; Beaumont, H.; Martocq, L.; Koptyug, A.; Surmeneva, M.A.; Surmenev, R.A.; Naderi, J.; Douglas, T.E.L.; Gurzawska-Comis, K.A. Phenolic-enriched collagen fibrillar coatings on titanium alloy to promote osteogenic differentiation and reduce inflammation. Int. J. Mol. Sci. 2020, 21, 6406. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Cahalan, P.; Cahalan, L.; Fini, M.; Giardino, R. Surface engineering of titanium by collagen immobilization. Surface characterization and in vitro and in vivo studies. Biomaterials 2003, 24, 4639–4654. [Google Scholar] [CrossRef]
- Hsueh, Y.H.; Cheng, C.Y.; Chien, H.W.; Huang, X.H.; Huang, C.W.; Wu, C.H.; Chen, S.T.; Ou, S.F. Synergistic effects of collagen and silver on the deposition characteristics, antibacterial ability, and cytocompatibility of a collagen/silver coating on titanium. J. Alloys Compd. 2020, 830, 154490. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Vandrovcová, M.; Kročilová, N.; Keppler, J.K.; Zárubová, J.; Skirtach, A.G.; Bačáková, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamuła, E.; Reilly, G.C.; Douglas, T.E.L. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
- Gadang, V.P.; Hettiarachchy, N.S.; Johnson, M.G.; Owens, C. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, Malic Acid, and EDTA on a Turkey Frankfurter system. J. Food Sci. 2008, 73, M389–M394. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, Z.; Xiong, P.; Yan, J.; Li, Y.; Li, M.; Cheng, Y.; Zheng, Y. Bioinspired and biomimetic AgNPs/gentamicin-embedded silk fibroin coatings for robust antibacterial and osteogenetic applications. ACS Appl. Mater. Interfaces 2017, 9, 25830–25846. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Yang, Y.; Jiang, Z.; Deng, Y.; Song, S.; Yang, W.; Chen, Z.G. Bioinspired, biocompatible and peptide-decorated silk fibroin coatings for enhanced osteogenesis of bioinert implant. J. Biomater. Sci. Polym. Ed. 2018, 29, 1595–1611. [Google Scholar] [CrossRef]
- Wang, S.D.; Ma, Q.; Wang, K.; Chen, H.W. Improving antibacterial activity and biocompatibility of bioinspired electrospinning silk fibroin nanofibers modified by graphene oxide. ACS Omega 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Cestari, M.; Muller, V.; Rodrigues, J.H.D.S.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Preparing silk fibroin nanofibers through electrospinning: Further heparin immobilization toward hemocompatibility improvement. Biomacromolecules 2014, 15, 1762–1767. [Google Scholar] [CrossRef]
- Sottile, J.; Hocking, D.C. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell 2002, 13, 3546–3559. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.; Marcey, D. Fibronectin, an Extracellular Adhesion Molecule. Available online: http://biology.kenyon.edu/BMB/Chime/Fibronectin/frames/fibrotxt.htm (accessed on 27 April 2018).
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Hay, E. Cell Biology of Extracellular Matrix, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 1991; ISBN 9780306439513. [Google Scholar]
- Parisi, L.; Toffoli, A.; Ghezzi, B.; Mozzoni, B.; Lumetti, S.; Macaluso, G.M. A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn. Dent. Sci. Rev. 2020, 56, 50–55. [Google Scholar] [CrossRef]
- García, A.J.; Vega, M.D.; Boettiger, D. Modulation of cell proliferation and differentiation through substrate- dependent changes in fibronectin conformation. Mol. Biol. Cell 1999, 10, 785–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Brett, D. A review of collagen and collagen-based wound dressings. Wounds 2008, 20, 347–356. [Google Scholar]
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian Soc. Periodontol. 2013, 17, 539. [Google Scholar] [CrossRef]
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides are building blocks of heat-induced fibrillar protein aggregates of β-lactoglobulin formed at pH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef]
- Heyn, T.R.; Garamus, V.M.; Neumann, H.R.; Uttinger, M.J.; Guckeisen, T.; Heuer, M.; Selhuber-Unkel, C.; Peukert, W.; Keppler, J.K. Influence of the polydispersity of pH 2 and pH 3.5 beta-lactoglobulin amyloid fibril solutions on analytical methods. Eur. Polym. J. 2019, 120. [Google Scholar] [CrossRef]
- Keppler, J.K.; Heyn, T.R.; Meissner, P.M.; Schrader, K.; Schwarz, K. Protein oxidation during temperature-induced amyloid aggregation of beta-lactoglobulin. Food Chem. 2019, 289, 223–231. [Google Scholar] [CrossRef]
- Serfert, Y.; Lamprecht, C.; Tan, C.P.; Keppler, J.K.; Appel, E.; Rossier-Miranda, F.J.; Schroen, K.; Boom, R.M.; Gorb, S.; Selhuber-Unkel, C.; et al. Characterisation and use of β-lactoglobulin fibrils for microencapsulation of lipophilic ingredients and oxidative stability thereof. J. Food Eng. 2014, 143, 53–61. [Google Scholar] [CrossRef]
- Keppler, J.K.; Martin, D.; Garamus, V.M.; Berton-Carabin, C.; Nipoti, E.; Coenye, T.; Schwarz, K. Functionality of whey proteins covalently modified by allyl isothiocyanate: Part 1 physicochemical and antibacterial properties of native and modified whey proteins at pH 2 to 7. Food Hydrocoll. 2017, 65, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Loveday, S.M.; Wang, X.L.; Rao, M.A.; Anema, S.G.; Singh, H. β-Lactoglobulin nanofibrils: Effect of temperature on fibril formation kinetics, fibril morphology and the rheological properties of fibril dispersions. Food Hydrocoll. 2012, 27, 242–249. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Zhang, X.; Geng, H.; Xue, W.; Chen, Z. Assembly behavior, structural characterization and rheological properties of legume proteins based amyloid fibrils. Food Hydrocoll. 2021, 111, 106396. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.H.; Le, T.H.; Huynh, V.Q.N.; Vo, D.V.N.; Trinh, Q.T.; Kim, S.Y.; Van Le, Q. Silk fibroin-based biomaterials for biomedical applications: A review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Xia, T.; Zou, M.; Li, Z.; Peng, B.; Guo, D.; Deng, Z. Mussel-inspired polydopamine coating as a versatile platform for synthesizing polystyrene/Ag nanocomposite particles with enhanced antibacterial activities. J. Mater. Chem. B 2014, 2, 3450–3461. [Google Scholar] [CrossRef]
- Xu, H.; Shi, X.; Ma, H.; Lv, Y.; Zhang, L.; Mao, Z. The preparation and antibacterial effects of dopa-cotton/AgNPs. Appl. Surf. Sci. 2011, 257, 6799–6803. [Google Scholar] [CrossRef]
- Sileika, T.S.; Kim, H.-D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Su, L.; Yu, Y.; Zhao, Y.; Liang, F.; Zhang, X. Strong antibacterial polydopamine coatings prepared by a shaking-assisted method. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Dong, P.; Hao, W.; Xia, Y.; Da, G.; Wang, T. Comparison study of corrosion behavior and biocompatibility of polyethyleneimine (PEI)/heparin and chitosan/heparin coatings on NiTi alloy. J. Mater. Sci. Technol. 2010, 26, 1027–1031. [Google Scholar] [CrossRef]
- Dong, P.; Hao, W.; Wang, X.; Wang, T. Fabrication and biocompatibility of polyethyleneimine/heparin self-assembly coating on NiTi alloy. Thin Solid Films 2008, 516, 5168–5171. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Lucchesi, E.G.; Nascimento, V.F.; França, C.G.; Gonzalez, I.; Macedo, W.A.A.; Machado, D.; Lancellotti, M.; Moraes, A.M.; Beppu, M.M.; et al. Antibacterial and non-cytotoxic ultra-thin polyethylenimine film. Mater. Sci. Eng. C 2017, 71, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.L.; Koerber, J.T.; Pack, D.W. A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjug. Chem. 2003, 14, 934–940. [Google Scholar] [CrossRef]
- Brunot, C.; Ponsonnet, L.; Lagneau, C.; Farge, P.; Picart, C.; Grosgogeat, B. Cytotoxicity of polyethyleneimine (PEI), precursor base layer of polyelectrolyte multilayer films. Biomaterials 2007, 28, 632–640. [Google Scholar] [CrossRef]
- Liu, Z.M.; Lee, S.Y.; Sarun, S.; Peschel, D.; Groth, T. Immobilization of poly (ethylene imine) on poly (l-lactide) promotes MG63 cell proliferation and function. J. Mater. Sci. Mater. Med. 2009, 20, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Liudmila, M.M.; Dmitriy, A.R.; Maria, G.F.; Evgeniy, E.F. Ultrathin polyethyleneimine (PEI) films for culturing of the human mesenchymal stromal cells (hMSCs). J. Cardiovasc. Med. Cardiol. 2020, 255–261. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian cell behavior on hydrophobic substrates: Influence of surface properties. Colloids Interf. 2019, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Jung, D.; Park, Y.; Kim, Y.; Moon, D.W.; Lee, T.G. Quantitative analysis of surface amine groups on plasma-polymerized ethylenediamine films using UV-visible spectroscopy compared to chemical derivatization with FT-IR spectroscopy, XPS and TOF-SIMS. Appl. Surf. Sci. 2007, 253, 4112–4118. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E.S. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Norris, K.; Mishukova, O.I.; Zykwinska, A.; Colliec-Jouault, S.; Sinquin, C.; Koptioug, A.; Cuenot, S.; Kerns, J.G.; Surmeneva, M.A.; Surmenev, R.A.; et al. Marine polysaccharide-collagen coatings on Ti6Al4V alloy formed by self-assembly. Micromachines 2019, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A review (Review). Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef] [Green Version]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.; Vivien, C.; Bocquet, B.; Guillochon, D.; Supiot, P. Preparation and multi-characterization of plasma polymerized allylamine films. Plasma Process. Polym. 2009, 6, 593–604. [Google Scholar] [CrossRef]
- Noel, S.; Liberelle, B.; Robitaille, L.; De Crescenzo, G. Quantification of primary amine groups available for subsequent biofunctionalization of polymer surfaces. Bioconjug. Chem. 2011, 22, 1690–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussot, G.; Perrin, C.; Moreau, T.; Dobrijevic, M.; Le Postollec, A.; Vandenabeele-Trambouze, O. A rapid and reversible colorimetric assay for the characterization of aminated solid surfaces. Anal. Bioanal. Chem. 2011, 399, 1061–1069. [Google Scholar] [CrossRef]
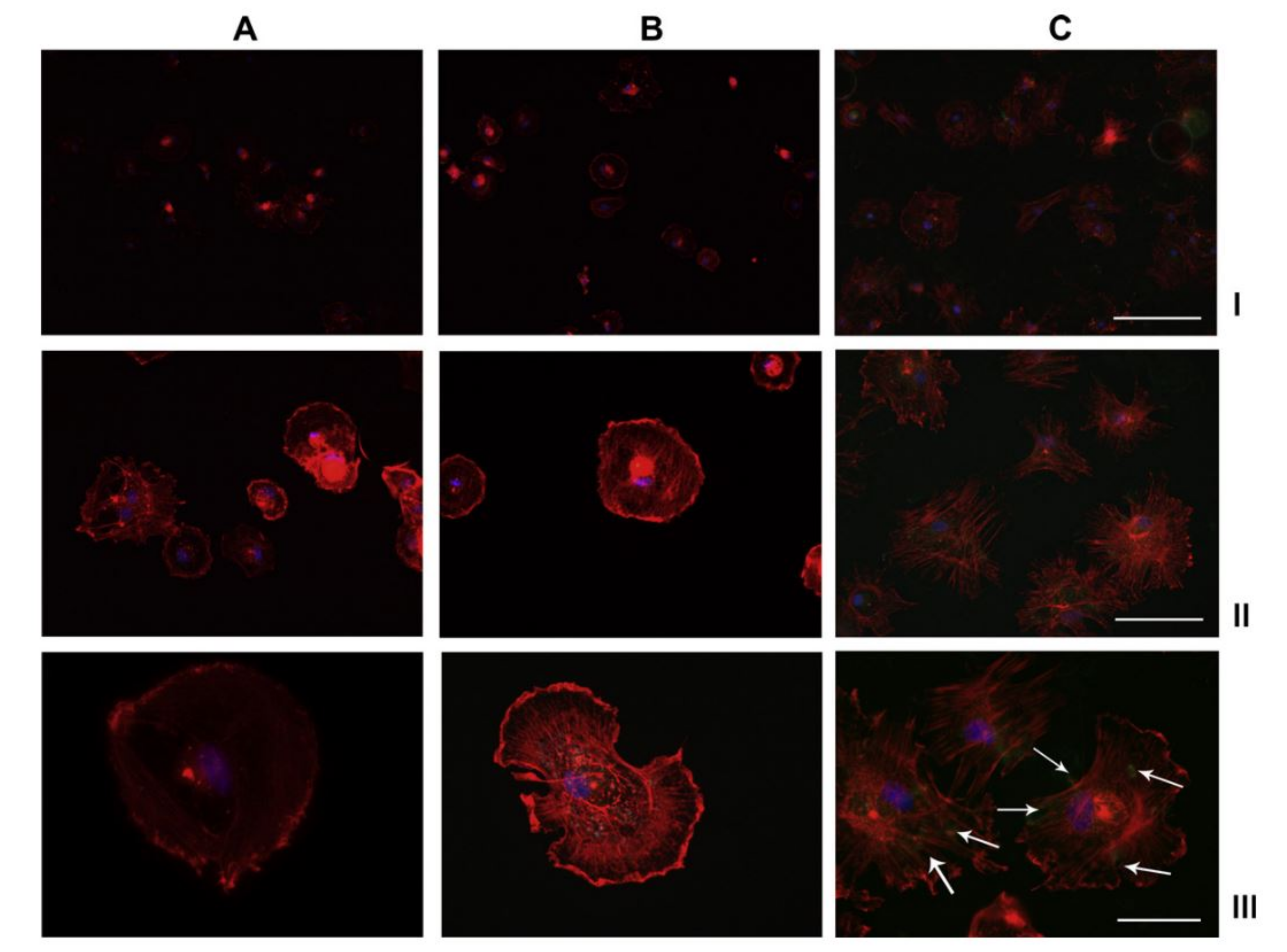

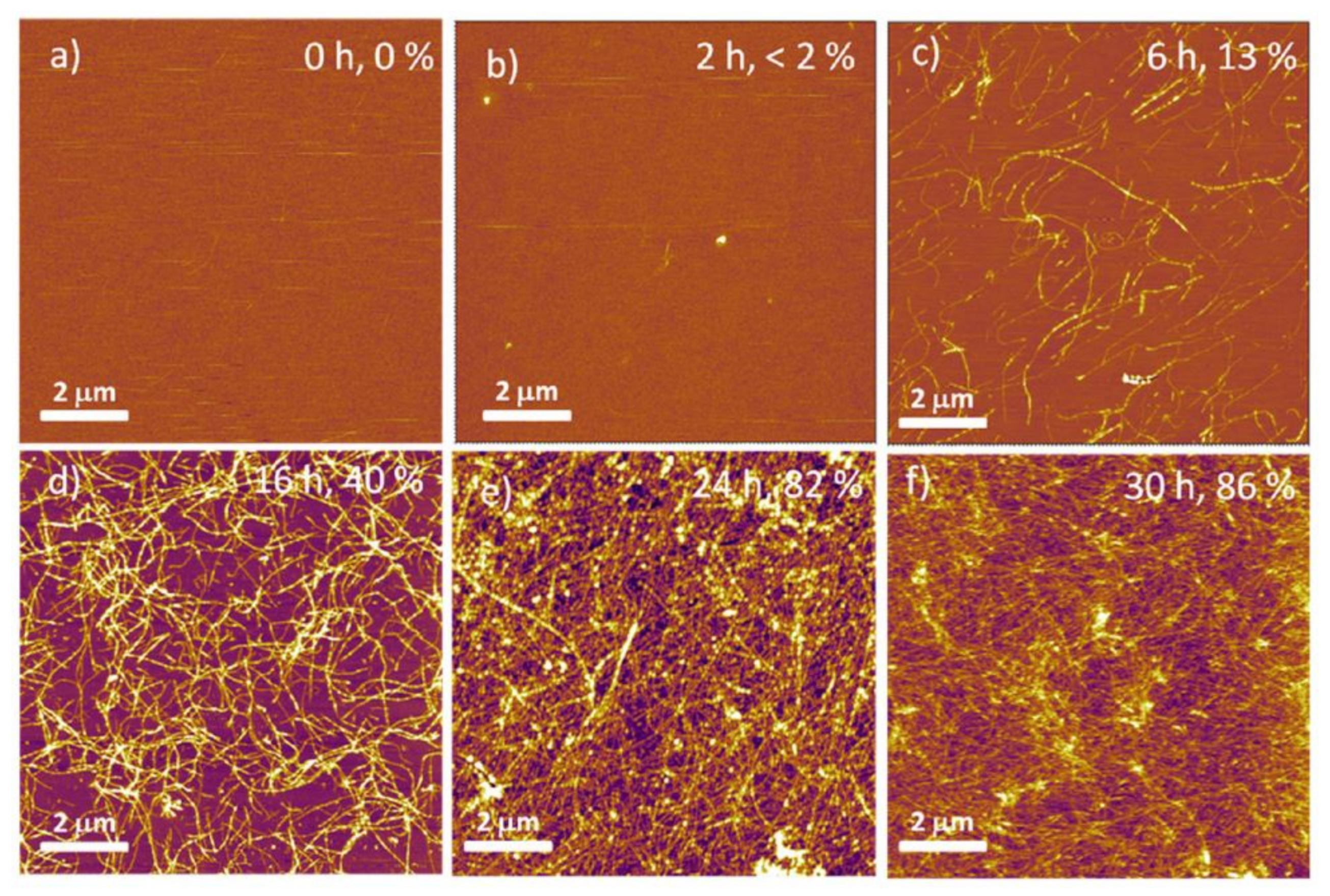
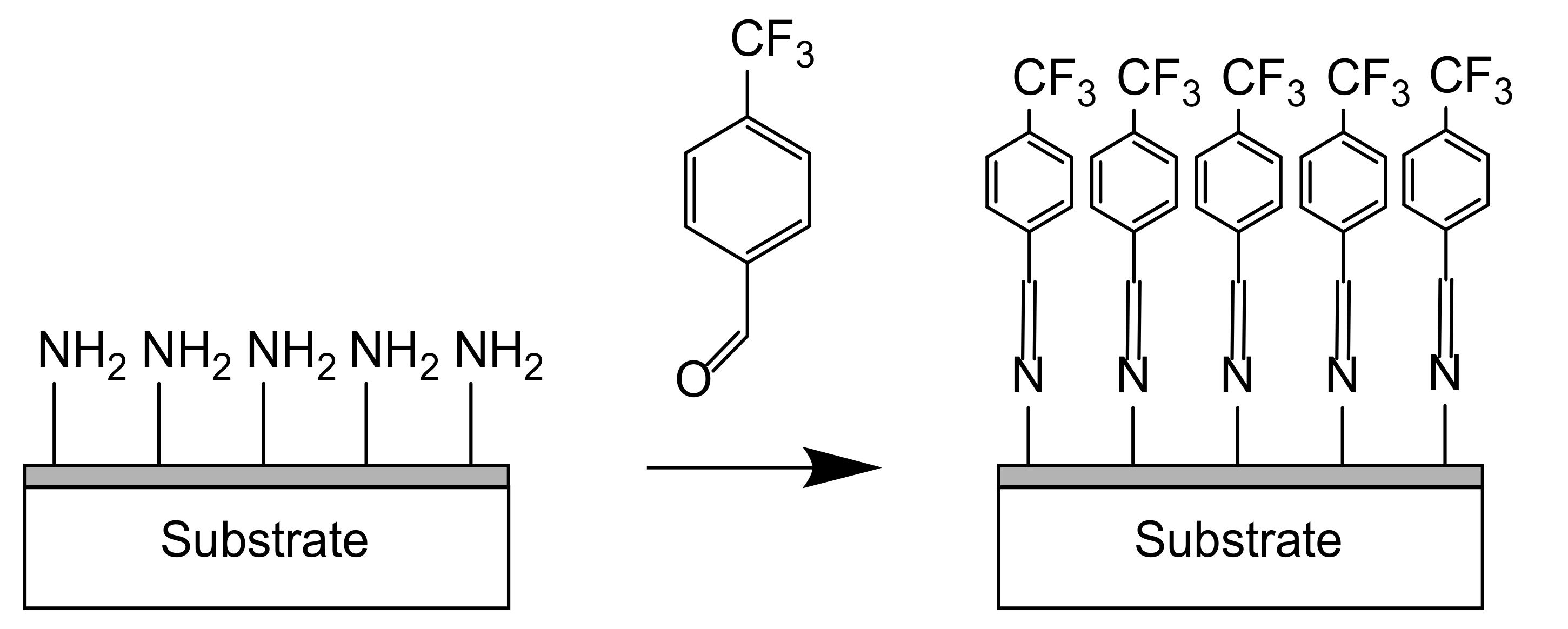
| Application | Precursor | Substrate | Findings | Ref. |
|---|---|---|---|---|
| Effect on cell behavior | Mixture of NH3/C2H4 | PTFE PET | Human umbilical vein endothelial cells’ adhesion and growth increased More resistant to shear flow | [23] |
| Mixture of N2/H2 | Ti6Al4V | Human osteoblast cells’ adhesion and spreading enhanced | [21] | |
| Heptylamine | Ti | Osteoblast-like cells’ attachment increased after 24 h Coating with high retention of –NH2 groups enhanced actin cytoskeleton formation | [20] | |
| Ethylenediamine | Ti6Al4V | Human osteoblast cells’ adhesion and spreading enhanced | [21,22] | |
| Allylamine | Ti6Al4V | Human osteoblast cells’ adhesion and spreading enhanced | [21] | |
| Glass coverslips | Human adipose-derived stem cells’ attachment, spreading, and proliferation enhanced Osteogenic differentiation improved | [6] | ||
| Silicone elastomer | Human skin fibroblasts’ adhesion and spreading increased | [16] | ||
| Cyclopropylamine | Tissue culture polystyrene dishes PCL nanofibers | Initial adhesion of vascular smooth muscle cells (VSMCs) improved for all plasma power settings tested Proliferation and metabolic activity of VSMCs after 7 days higher for coating made at 33 W No increase in inflammatory markers | [28] | |
| Molecules’ immobilization | Mixture of NH3/C2H4 | PET | Grafting of chondroitin sulfate and epidermal growth factor to –NH2Decrease in cell apoptosis VSMCs growth increased | [29] |
| Allylamine | Si | Grafting of trypsin | [30] | |
| NH3n-butyl amine Allylamine | Polysulfone films | Immobilization of glucose isomerase Activity highest for allylamine/Ar plasma coatings close to plasma edge | [31] | |
| NH3 | Poly (D,L-lactide) | Better anchoring of collagen on plasma-treated substrates and resistance to PBS rinsing Cell affinity of modified substrates improved | [32] | |
| Antimicrobial applications | Allylamine | Silver nanoparticles (AgNPs) coated with polyvinyl sulphonate (PVS) | PVS coated-AgNPs were bound to allylamine coatings Prevent attachment of S. epidermidis and biofilm formation | [11] |
| Anodic alumina oxide (AAO) | Substrate pores were loaded with vancomycin drug and were reduced by plasma polymer deposition to allow a controlled drug release | [33] | ||
| Low-density polyethylene | Bonding of antibacterial agents by immersion of the coatings in antibacterial solutions | [9] | ||
| 1-vinylimidazole | Thin-film composite membranes | Enhanced AgNPs binding onto the plasma-treated substrates which cause a decrease in E.coli growth | [10] | |
| Heptylamine | Si/Glass/Thermanox plastic/Cell culture plate | Release of antifungal drug | [27] |
| Protein | Substrate | Findings | Ref. |
|---|---|---|---|
| Fibronectin (FN) | Poly (lactic acid) (PLLA) Silicon oxide (SO) | Osteoblast-like cells’ adhesion and spreading enhanced after 3 days Cell proliferation and mitochondrial activity improved after 7 days | [50] |
| Si | Human osteoprogenitor cells’ attachment enhanced and formation of actin filaments Formation of dense stress fibers attached to FN coatings | [51] | |
| PTFE | FN combined with phosphorylcholine Endothelial cells’ adhesion and spreading enhanced Higher cell viability after 24 h | [52] | |
| Collagen | Ti6Al4V | Osteoblast cells’ initial adhesion enhanced | [53] |
| Osteoblast cells’ spreading, proliferation and differentiation improved | [54] | ||
| Collagen coupled with phloroglucinol (PG) Fibroblast- and osteoblast-like cells adhere and spread well Reduction in inflammatory response Osteogenic differentiation promoted with a high PG concentration Osteoclast activation reduced with a low PG concentration | [55] | ||
| Ti | Early osseointegration enhanced in vivo | [56] | |
| Porous Ti oxide | Collagen coating coupled with AgNPs Osteoblast cells’ adhesion improved Adhesion and proliferation of E.coli were reduced | [57] | |
| Amyloid fibrils from whey protein isolate (WPI) | Hydrogel (no substrate) | Osteoblast and fibroblast cells’ growth enhanced Calcium deposition of osteoblasts increased Osteogenic differentiation of human adipose-derived stem cells increased | [58] |
| WPI coupled with aragonite Osteoblast cells’ proliferation supported for 3 weeks | [59] | ||
| Turkey Frankfurter (food application) | Nisin, grape seed extract, malic aid, and ethylenediamine tetraacetic acid incorporated in WPI coatings Effective antimicrobial activity against different pathogens | [60] | |
| Films (no substrate) | Oregano, rosemary, and garlic essential oils incorporated in WPI films Film containing oregano and garlic essential oil most effective against S. aureus, S. enteritidis, L. monocytogenes, E. coli, and L. plantarum | [61] | |
| Glass | Resistance of WPI fibrillar coatings to autoclave sterilization Human bone marrow stromal cells’ spreading, and differentiation enhanced | [39] | |
| Amyloid fibrils from lysozyme | Mica | Fibroblast and epithelial cells’ spreading increased Increased of focal adhesion and associated stress fibers | [48] |
| Silk fibroin (SF) | Ti | Gentamycin and silver nanoparticles incorporated to SF coatings Antibacterial activity against S.aureus Osteoblast cells’ adhesion, growth, and osteogenic activities enhanced | [62] |
| PEEK | SF combined with bone-forming peptide Initial attachment and proliferation supported Osteoblast cell proliferation, spreading, and osteogenic differentiation enhanced for SF with bone-forming peptide coating | [63] | |
| Electrospun nanofibers | SF modified with graphene oxide which resulted in a Decrease in E. coli and S. aureus survival rates Osteoblast cells’ growth enhanced | [64] | |
| – | Electrospun nanofibers | SF combined with heparin Cell growth and proliferation improved | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martocq, L.; Douglas, T.E.L. Amine-Rich Coatings to Potentially Promote Cell Adhesion, Proliferation and Differentiation, and Reduce Microbial Colonization: Strategies for Generation and Characterization. Coatings 2021, 11, 983. https://doi.org/10.3390/coatings11080983
Martocq L, Douglas TEL. Amine-Rich Coatings to Potentially Promote Cell Adhesion, Proliferation and Differentiation, and Reduce Microbial Colonization: Strategies for Generation and Characterization. Coatings. 2021; 11(8):983. https://doi.org/10.3390/coatings11080983
Chicago/Turabian StyleMartocq, Laurine, and Timothy E. L. Douglas. 2021. "Amine-Rich Coatings to Potentially Promote Cell Adhesion, Proliferation and Differentiation, and Reduce Microbial Colonization: Strategies for Generation and Characterization" Coatings 11, no. 8: 983. https://doi.org/10.3390/coatings11080983
APA StyleMartocq, L., & Douglas, T. E. L. (2021). Amine-Rich Coatings to Potentially Promote Cell Adhesion, Proliferation and Differentiation, and Reduce Microbial Colonization: Strategies for Generation and Characterization. Coatings, 11(8), 983. https://doi.org/10.3390/coatings11080983







